Abstract
Treatment of Epstein–Barr virus (EBV)-related lymphomas with lytic-inducing agents is an attractive targeted approach for eliminating virus-infected tumor cells. Zidovudine (AZT) is an excellent substrate for EBV-thymidine kinase: it can induce EBV lytic gene expression and apoptosis in primary EBV+ lymphoma cell lines. We hypothesized that the combination of AZT with lytic-inducing chemotherapy agents would be effective in treating EBV+ lymphomas. We report a retrospective analysis of 19 patients with aggressive EBV+ non-Hodgkin lymphoma, including nine cases of acquired immune deficiency syndrome-associated primary central nervous system lymphoma (AIDSPCNSL) treated with AZT-based chemotherapy. Our results demonstrate that high-dose AZT–methotrexate is efficacious in treating highly aggressive systemic EBV+ lymphomas in the upfront setting. In primary EBV+ lymphoma cell lines, the combination of AZT with hydroxyurea resulted in synergistic EBV lytic induction and cell death. Further, AZT–hydroxyurea treatment resulted in dramatic responses in patients with AIDSPCNSL. The combination of AZT with chemotherapy, especially lytic-inducing agents, should be explored further in clinical trials for the treatment of EBV-related lymphomas.
Introduction
Epstein–Barr virus (EBV) is associated with a wide range of lymphoproliferative disorders including endemic Burkitt lymphoma (BL), classical Hodgkin lymphoma (HL), natural killer (NK)–T-cell lymphoma and lymphomas arising in immunocompromised individuals. The EBV genome is present in up to 66% of acquired immune deficiency syndrome (AIDS)-related lymphomas (ARLs), including nearly all cases of primary central nervous system lymphoma (PCNSL), 80% of plasmablastic lymphoma (PBL), 80% of HL, 20 – 34% of BL and 30% of diff use large B-cell lymphoma (DLBCL) [1 – 3]. Primary effusion lymphoma (PEL) is co-infected with EBV and oncogenic Kaposi sarcoma-associated herpesvirus (KSHV) in approximately 80% of cases [4]. EBV tends to be latent in infected tumor cells. Depending on EBV promoter usage, it expresses only a restricted number of genes, which help maintain its own latency and evade the immune response against infected host cells. Depending on the particular, tumor-specific, latency program, the EBV proteins expressed include the EBV-encoded nuclear antigens (EBNAs) and latent membrane proteins (LMPs), which are transforming proteins that help drive tumor cell growth and survival [5,6].
Rationale for the use of zidovudine in EBV+ lymphomas
The presence of EBV in tumor cells can be exploited therapeutically. Pharmacologic induction of EBV from latency can render virus-infected lymphomas susceptible to antiviral nucleoside analogs, such as ganciclovir (GCV), cidofovir and zidovudine (AZT), which can be readily phosphorylated by the viral-encoded kinases and serve as highly specific therapeutic agents [7 – 10]. As compared to other herpesvirus polymerase inhibitors, which are purine analogs, AZT is a thymidine analog and appears to be a superior substrate for EBV and KSHV thymidine kinases [11]. Furthermore, we have previously demonstrated that AZT, but not GCV, inhibited nuclear factor κB (NF- κB) activity, thus inducing EBV lytic gene expression and apoptosis in primary type I latency EBV+ BL cell lines [12,13]. The drug methotrexate (MTX) inhibits thymidylate synthase, thus blocking de novo synthesis of dTMP and increasing the likelihood of AZT incorporation into DNA [14]. MTX and other chemotherapy drugs that form the backbone of standard lymphoma treatment regimens, such as doxorubicin, cyclophosphamide and etoposide, also disrupt EBV latency [10,15]. Therefore, the combination of AZT with MTX and other lytic-inducing drugs can be advantageous for targeting EBV+ lymphomas. Moreover, both AZT and MTX penetrate well into the central nervous system (CNS) [16], which is particularly advantageous in ARLs with a higher incidence of CNS spread [17]. In fact, AZT in combination with MTX has been previously used with success in patients with relapsed/refractory aggressive ARLs [18]. The combination of AZT with other antiviral drugs has also shown clinical activity in patients with γ-herpesvirus-infected ARLs, including PCNSL, PEL, BL and multicentric Castleman disease (MCD) [19 – 23].
Here, we report the long-term outcome of 19 patients (18 human immunodeficiency virus positive [HIV+]) with various types of aggressive EBV-related lymphomas, including nine patients with AIDS-PCNSL, treated with high-dose AZT-containing regimens as first-line therapy. Patients with systemic non-Hodgkin lymphoma (NHL) (PBL = 4, BL = 3, DLBCL = 2 and solid PEL = 1) were treated with high-dose AZT–MTX combination alone, or alternated with conventional doxorubicin-based regimens (etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin [EPOCH] or hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone [hCVAD]), while patients with PCNSL were treated with high-dose AZT in combination with the ribonucleotide reductase hydroxyurea (HU). We found that AZT-based treatment induced impressive clinical responses in multiple EBV-related lymphoma subtypes. Further, the combination of AZT – HU was clinically active in AIDS-related PCNSL. The in vivo effect of AZT – HU could be recapitulated in primary EBV+ lymphoma cell lines established from HIV+ patients, in which the drug HU potentiated the lytic and apoptotic-inducing capability of AZT. Our results provide clinical proof that AZT in combination with drugs that potentiate its lytic-inducing effects can be an efficacious treatment strategy for EBV-related lymphomas, particularly in the HIV setting.
Methods
Patients
After approval by our institutional review board (20090442, 20090888), we identified retrospectively patients with EBV+ lymphomas who received AZT-based chemotherapy as first-line treatment between 2004 and 2009 at Jackson Memorial Hospital and Sylvester Comprehensive Cancer Center through our tumor registry. Ten patients with EBV+ NHL were treated with first-line MTX (3.0 – 4.5 g/m 2 IV on day 1) and AZT 1.5 g IV q12 h (days 2 – 5) every 3 weeks or upon recovery of blood counts at the discretion of the physician. This regimen was used alone, or given as odd cycles (i.e. 1, 3, 5) when alternated with dose adjusted EPOCH (DA-EPOCH) [24] or hCVAD [25] chemotherapy every other cycle. EBER (EBV encoded small RNA) was detected in all cases by routine in situ hybridization performed during lymphoma tissue characterization. Nine additional cases of AIDS-associated PCNSL who were treated with first-line high-dose AZT and HU were also identified and described. The diagnosis of PCNSL was based on a positive single photon emission computed tomography (SPECT) thallium-201 study and exclusion of toxoplasmosis (n = 3) or the presence of EBVDNA in cerebrospinal fluid by polymerase chain reaction (PCR) and brain lesion(s) on radiologic imaging (n = 6). Additionally, diagnosis was confirmed with brain biopsy in two patients. Patients with AIDS-PCNSL were treated at the discretion of the physician with initial high doses of AZT (1.2 – 1.5 g intravenously every 12 h) and oral HU 500 mg every 12 h for at least 2 weeks as inpatients. Those who were found to respond to treatment on repeat magnetic resonance imaging (MRI) and/or SPECT imaging continued to receive inpatient treatment during their hospitalization until a maximal response was achieved, up to 4 weeks total. Imaging studies were performed every 2 weeks on average. Those patients who had an adequate or complete response were continued on oral AZT (a maximum of 1.2 g daily in divided doses) and HU (500 – 1000 mg) as outpatients for up to several months. The AZT doses were titrated down in patients who developed cytopenias. Using a standardized data collection form, the following data were gathered from the patient medical records: age, sex, HIV status, CD4 cell count at the time of lymphoma diagnosis, treatment with highly active antiretroviral therapy (HAART), Eastern Cooperative Group (ECOG) performance status (PS), serum lactate dehydrogenase (LDH) level, staging information including imaging studies, bone marrow biopsy and cerebrospinal fluid (CSF) analysis, response to treatment and follow-up data. Complete remission (CR) was defined as complete disappearance of all lesions by computed tomography (CT) and normal bone marrow (BM) biopsy if initially involved. Partial remission (PR) was defined as ≥ 50% decrease in the greatest dimension of the lesions by CT. Progressive disease (PD) was defined as the appearance of new lesions or an increase in the size of existing lymph nodes. Toxicity data were gathered from physician notes according to Common Terminology Criteria for Adverse Events (CTCAE v4.0).
Overall survival (OS) and progression-free survival (PFS) were calculated from the time of chemotherapy initiation until death or disease progression. Kaplan–Meier survival curves were constructed for survival analyses. PASW 17.0 software (SPSS Inc., Chicago, IL) was used for all statistical analyses.
Cell lines
Primary EBV+ cell lines BL-8 and IBL-4 were previously described [21,26]. BL-8 was isolated from a patient with EBV+ BL, and carries the typical t(8:14) c-myc chromosomal translocation [26]. IBL-4 was derived from the pleural fluid of a patient with AIDS and EBV+ immunoblastic lymphoma unresponsive to AZT and interferon-α treatment [21]. IBL-4 cells are CD19+ and carry a balanced 2:8 (c-myc) translocation. The cell lines have been submitted to the AIDS and cancer specimen registry (ACSR). Cells were maintained in suspension culture in Iscove modified Dulbecco medium (IMDM; GIBCO-BRL, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin and streptomycin, 1% glutamine and 1 mM Na-pyruvate at 37°C in a 5% CO2 humidified atmosphere.
RNA isolation and qRT-PCR
Total RNA was isolated from cells using an RNeasy kit (Qiagen, Valencia, CA) as recommended by the manufacturer and reverse transcribed using Superscript-II reverse transcriptase (Life Technologies, Rockville, MA) according to the manufacturer ’ s recommendations. Quantitative real-time PCR (qRT-PCR) was performed using cDNA as a template and TaqMan Master mix or SYBR Green (Roche Diagnostics, Indianapolis, IN), in a LightCycler 2.0 instrument (Roche Diagnostics) under standard conditions. The Taqman probe for BZLF1 was described by Ryan et al. [27]; BXLF1 primers used were as follows: forward, 5’-gtgggatccatggctggatt-3’; and reverse, 5’-gctacccggagagtttccagt-3’, and were synthesized by Invitrogen (Grand Island, NY). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control for relative gene expression quantification and for each gene amplification; Cp values (reading obtained at 530 nm) were normalized to the Cp of GAPDH (reading obtained at 560 nm) using calibrator normalized relative quantification in LightCycler analysis software. The BZLF1 primer sets were synthesized according to Tang et al. [28]; BXLF1 and GAPDH primer sets were purchased from Applied Biosystems.
Annexin V/propidium iodide
Altogether 5 × 105 cells were treated or not with AZT (2.5 µg/mL), HU (2 µM) and their combination for 48 h. Cells were then harvested and washed twice with cold phosphatebuffered saline (PBS), and incubated with annexin V–fluorescein isothiocyanate (FITC; BD Pharmingen, San Diego, CA) and propidium iodide (PI) for 15 min at room temperature. Cells were acquired with the use of an LSR II flow cytometer (BD Biosciences). A total of 10 000 events were analyzed for each sample. Live cells were gated for analysis based on forward angle light scatter (FSC) and side angle light scatter (SSC), and then analyzed using a FACSDiva Version 6.1.3.
Immunofluorescence assays
BL-8 and IBL-4 cells were plated 48 h after the indicated treatments onto poly-L-lysine-coated coverslips (BD Biosciences) and fixed with 4% paraformaldehyde for 1 h at room temperature. Cells were permeabilized with 0.2% Triton X-100 in PBS for 20 min at 4°C and blocked with 10% normal donkey serum (Jackson Immunoresearch, West Grove, PA) in PBS with 0.1% Triton X-100 for 1 h at room temperature. Fixed cells were immunostained with anti-BZLF-1 antibody (Antibodies-online Inc.), diluted in PBS with 0.1% Triton X-100, and primary antibody was detected with donkey anti-mouse immunoglobulin G (IgG) conjugated to AlexaFluor 555 (Molecular Probes, Invitrogen). DNA fragmentation, a characteristic of apoptotic cells, was detected using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, which was performed as per the manufacturer’ s instructions (In Situ Cell Death Detection Kit, Fluorescein; Roche). Nuclei were stained with DAPI (4’,6-diamidino-2-phenylindole; EMD Biosciences, San Diego, CA). Coverslips were mounted onto slides using Aqua Polymount (Polysciences, Warrington, PA) and analyzed using a Zeiss Axiovision 4.8.2 with a Hamamatsu ORCA-R2 CCD camera and Zeiss Axiovert 200M inverted fluorescence microscope, saved in Zeiss ZVI fi le format, and batch exported to single channel monochrome 16-bit TIFF format using the Axiovision File - Export - Batch command.
Results
Efficacy of AZT–MTX-based chemotherapy in systemic EBV+ lymphomas
Patients
Ten patients with various aggressive EBV+ NHL subtypes were treated with first-line AZT – MTX alone (n = 3), or AZT–MTX alternated with standard doxorubicin-based regimens DA-EPOCH or hCVAD (n = 7) at the discretion of the treating physician. Four patients had PBL, three had BL, two had DLBCL and one had a solid PEL variant. The demographics, clinical characteristics, response and survival data of the patients are summarized in Table I. Of nine patients who were HIV+, seven had a CD4 cell count of less than 200/µL at the time of lymphoma diagnosis. Seven patients had stage IV disease. The solid PEL was positive for both KSHV latency-associated nuclear antigen (LANA) and EBV EBER. All HIV+ patients received HAART during treatment. Two patients received radiotherapy in addition to chemotherapy: one for consolidation after the end of chemotherapy due to bulky disease and the other for presumed local disease recurrence in the maxillary sinus after four cycles of chemotherapy. The latter patient in retrospect was deemed to have sinusitis, since radiological and clinical findings were stable over the 6 weeks without any therapy, and he received an additional four cycles of chemotherapy after the end of radiotherapy. All patients, except the one with solid PEL, received CNS prophylaxis with intrathecal chemotherapy at the discretion of the treating physician.
Table I.
Demographic and clinical characteristics of patients who were treated with high-dose AZT–MTX alone or in combination with dose dense regimens.
| Age | Sex | HIV | HIV- lymphoma* |
Lymphoma type |
PS | CD4 | LDH | Stage | Ki-67 (%) |
Extranodal sites |
Number of AZT– MTX cycles |
Alternating chemotherapy with AZT–MTX and no. of cycles |
RT | Adverse events (grade ≥ 3) |
Response† | Survival† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 34 | M | + | 1 | LCL | 3 | 4 | 1359 | IVB | 50 | Lung, CNS | 2 | --- | − | NF, T | Not evaluable |
Hospice |
| 51 | F | + | 14 | LCL | 2 | 47 | 11 506 | IVB | --- | Stomach | 3 | EPOCH x3 | − | --- | CR (95) | Alive (95) |
| 49 | M | + | 0 | BL | 2 | 91 | 4601 | IVB | --- | BM | 3 | EPOCH x1 | − | --- | CR (77) | Alive (77) |
| 40 | F | − | NA | BL | 2 | --- | 3155 | IIA | 100 | --- | 2 | hCVADx3 | + | --- | CR (87) | Alive (87) |
| 40 | M | + | 0 | BL | 2 | 214 | 3424 | IVB | 95 | OSM | 2 | hCVADx1 | − | --- | PD | Dead (9) |
| 34 | M | + | 9 | PBL | 2 | 16 | 689 | IVB | 96 | OSM | 1 | --- | − | Diarrhea, mucositis |
PD | Dead (4) |
| 33 | F | + | 13 | PBL | 1 | 166 | 418 | IIA | --- | OSM | 3 | EPOCH x3 | − | --- | CR (42) | Alive (42) |
| 44 | M | + | 0 | PBL | 1 | 458 | 470 | IVA | 100 | Bone, OSM | 3 | EPOCH x3 | − | --- | CR (46) | Alive (46) |
| 52 | M | + | 0 | PBL | 1 | 57 | 1005 | IIB | 95 | OSM | 4 | EPOCH x4 | + | --- | CR (49) | Alive (49) |
| 51 | M | + | 20 | Solid PBL | 2 | 113 | 710 | IVB | --- | Pericardium, OSM |
4 | --- | − | --- | CR (31) | Alive (68) |
PS, Eastern Cooperative Oncology Group performance score; LDH, serum lactate dehydrogenase level at diagnosis (normal range 313 – 618 U/L); MTX, methotrexate; AZT, zidovudine; RT, radiotherapy; NA, not available; LCL, large cell lymphoma; PEL, primary effusion lymphoma; PBL, plasmablastic lymphoma; CNS, central nervous system; BM, bone marrow; OSM, orosinusoidal mucosa; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; hCVAD, hyperfractionated cyclophosphamide with vincristine, doxorubicin and dexamethasone; NF, neutropenic fever; T, thrombocytopenia; CR, complete remission; PD, progressive disease.
Interval between human immunodeficiency virus (HIV) and lymphoma diagnosis in years.
Duration in months in parentheses.
One patient with DLBCL presented with lethargy, poor performance status (ECOG 3) and secondary CNS involvement. He was receiving antibiotics for bacterial pneumonia at the time of chemotherapy initiation. Although he had almost complete resolution of his brain lesions by thallium-SPECT after two cycles, chemotherapy was discontinued because his overall condition deteriorated. He was transferred to hospice care as per proxy wishes before staging studies were completed, to evaluate his response. Therefore, the patient was excluded from response analysis and censored in survival analyses.
Treatment response and survival
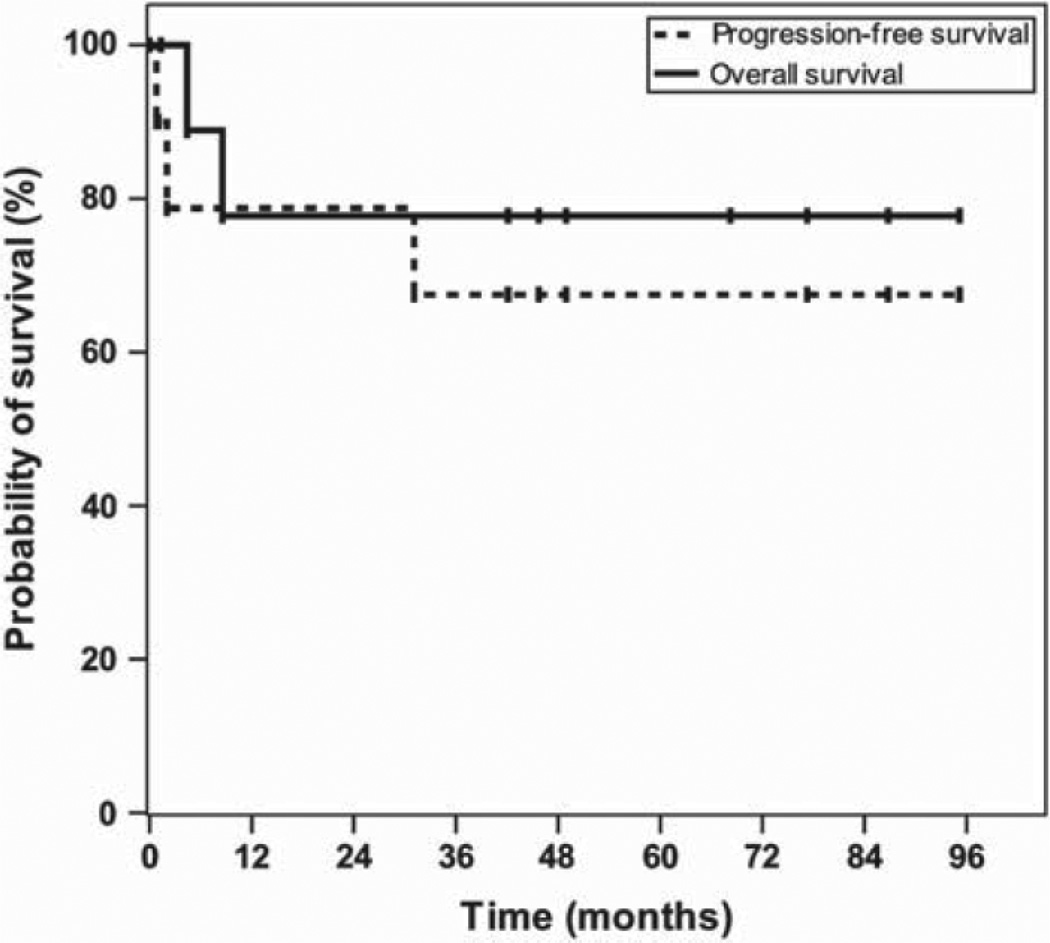
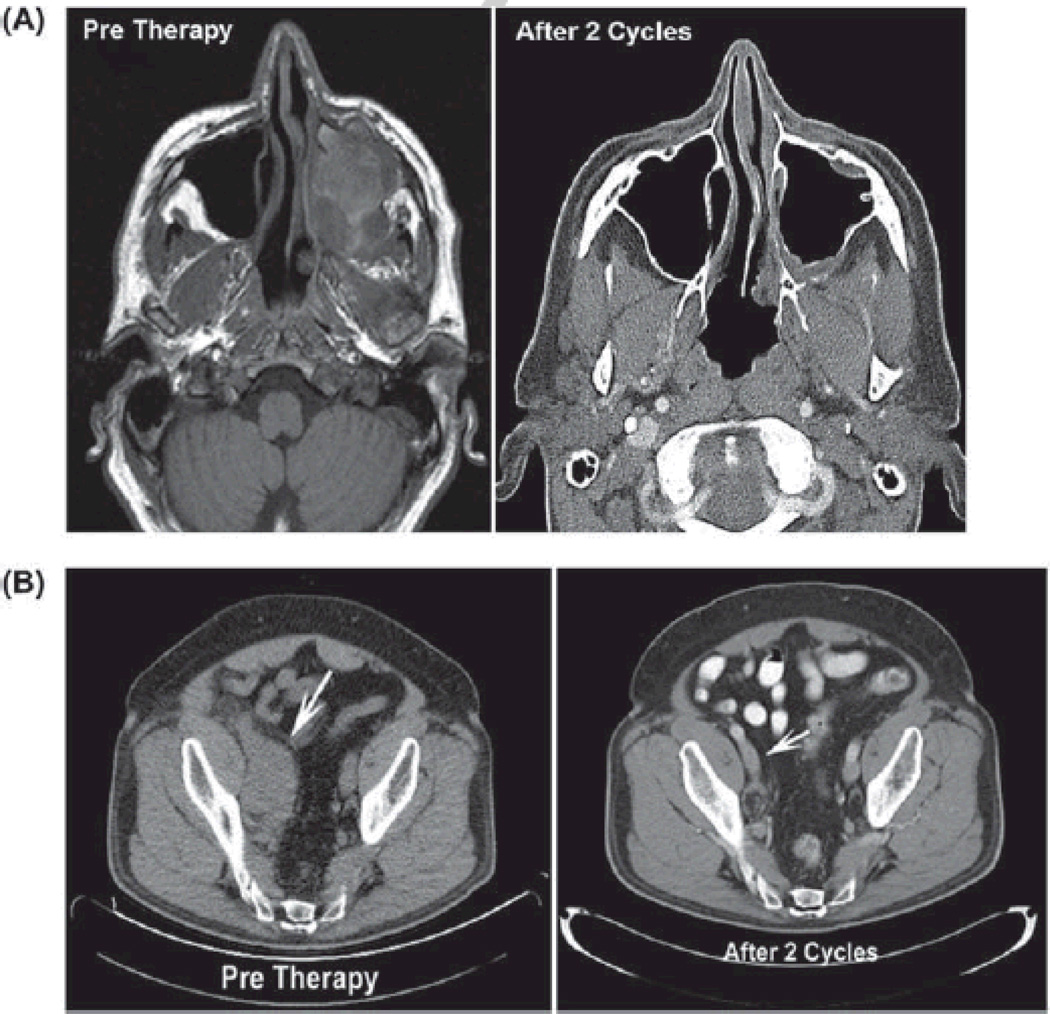
Of nine patients with systemic EBV+ lymphoma who were evaluable for response, seven (78%) achieved a CR and two (22%) had refractory disease. During a median follow-up period of 47.3 months (range 1.3–95.2), 3-year OS and PFS were 77.8% (95% confidence interval [CI]: 54.8–100.0%) and 67.5% (95% CI, 43.0–100.0%, Figure 1). Figure 2 demonstrates complete resolution of an invasive PBL oral mass involving the left maxillary sinus in one subject with AIDS by CT imaging after two cycles of AZ –MTX alone. Another patient with solid PEL variant treated with four cycles of AZT–MTX alone achieved a CR, and refused further chemotherapy thereafter. This patient relapsed 31 months later, was treated a second time with the combination of high-dose AZT–MTX, HU and low-dose doxorubicin under a new study protocol currently ongoing at our institution, and achieved a CR once again after two cycles [Figure 2(B)]. However, after receiving four cycles, he refused further chemotherapy and autologous stem cell transplant, and had another relapse 16 months later. He then received two additional cycles of high-dose AZT–MTX achieving a partial response, but eventually progressed. Most recently, he received five cycles of EPOCH chemotherapy followed by a CR, and continues to be progression-free at the time of this report. Three patients received solely high-dose AZT–MTX without alternating hCVAD or DA-EPOCH. One patient was not evaluable for response/survival (outlined above), and one achieved CR that lasted for 31 months (outlined above). The last patient had progressive disease after the first cycle of chemotherapy and did not respond to an anthracycline-based regimen later.
Figure 1.
Survival of patients with EBV+ systemic NHL treated with high-dose AZT – MTX-based chemotherapy. Kaplan– Meier plots showing overall and progression-free survival in 10 patients with EBV+ systemic non-Hodgkin lymphoma (four plasmablastic lymphoma, three Burkitt lymphoma, two diff use large B-cell lymphoma, one solid primary effusion lymphoma) treated with high-dose AZT – MTX alone (n = 2) or alternating with dose-intense chemotherapy (n =7).
Figure 2.
High-dose AZT – MTX alone, or in combination with hydroxyurea and doxorubicin, is highly efficacious against aggressive AIDS-related EBV+ lymphomas. (A) Computed tomography (CT) images show large invasive PBL mass in maxillary sinus at baseline, followed by complete resolution after two courses of high-dose AZT – MTX. (B) CT images of a 52-year-old HIV+ male with relapsed EBV+ KSHV+ solid primary effusion (PEL) variant in the abdomen (indicated by arrows) treated with high-dose AZT – MTX with doxorubicin and hydroxyurea before and after two treatment cycles.
Safety of high-dose AZT-MTX
In total, 27 cycles of AZT – MTX were administered. The regimen was highly tolerable, with only two patients (20%) experiencing grade 3 – 4 adverse events (Table I). The patient with systemic DLBCL, CNS involvement, pneumonia and poor performance status at the time of chemotherapy initiation developed grade 3 thrombocytopenia and neutropenic fever, while another patient with PBL developed grade 3 mucositis and diarrhea. There were no toxic deaths.
Efficacy using combination of AZT with hydroxyurea (AZT – HU) in EBV+ lymphomas
Preclinical studies using AZT in EBV+ lymphoma cell lines
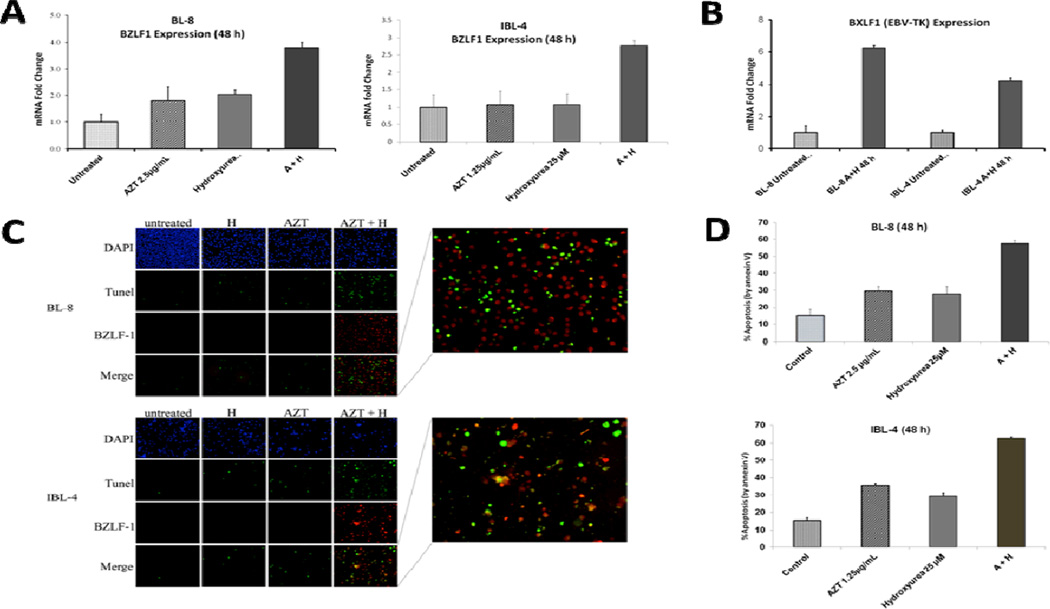
We showed that sublethal doses of AZT (1.25 – 2.5 µg/mL) and HU (25 µM) synergized to induce the EBV-lytic cycle (BZLF1 and BXLF1/EBV-thymidine kinase [TK] mRNA expression shown) with concomitant commitment to apoptosis as compared to either drug alone after 48 h in primary EBV+ BL (BL-8) and immunoblastic lymphoma (IBL-4) cell lines established from HIV+ patients, as demonstrated by qRTPCR and immunofluorescence assays [Figures 3(A) – 3(D)]. Notably, AZT and HU simultaneously induced BZLF1 gene expression and DNA fragmentation, a marker for apoptosis, in the same cells [immunofluorescence (IF) and TUNEL images merged, Figure 3(C)]. Similar laboratory observations made in the past using these and other cell lines available at our laboratory formed the clinical basis for using AZT – HU in patients with EBV+ lymphomas.
Figure 3.
AZT-induced EBV-lytic reactivation and apoptosis in lymphoma cell lines is potentiated by hydroxyurea (HU). Shown is the synergistic effect of AZT and HU in inducing the EBV-lytic cycle and apoptosis in Burkitt (BL-8) and immunoblastic (IBL-4) lymphoma cell lines at specified times and treatments. (A) Graphs depict relative BZLF1 gene expression by Taqman PCR. Error bars are representative of standard deviation of three separate experiments. (B) Graph depicts relative BXLF1 gene expression by PCR assay in untreated and AZT – HU-treated cell lines. Error bars are representative of standard deviation of three separate experiments. (C) Nuclei stained with DAPI (blue signal), TUNEL (green signal, indicative of fragmented DNA, a marker of apoptosis) and EBV early lytic protein BZLF-1 (red signal) are shown in BL-8 and IBL-4 cells (A, AZT; H, hydroxyurea). Cytospins used for these experiments were prepared from cells treated in (A). Right amplified panels show numerous co-stained TUNEL and BZLF-1 positive cells (red-green or orange color) after treatment with AZT plus hydroxyurea. (D) Graphs depict percentage of apoptosis by Annexin V/PI assays done after 48 h of incubation with control, AZT, HU or their combination. Error bars are representative of standard deviation of three separate experiments.
Efficacy of AZT–HU in AIDS-PCNSL
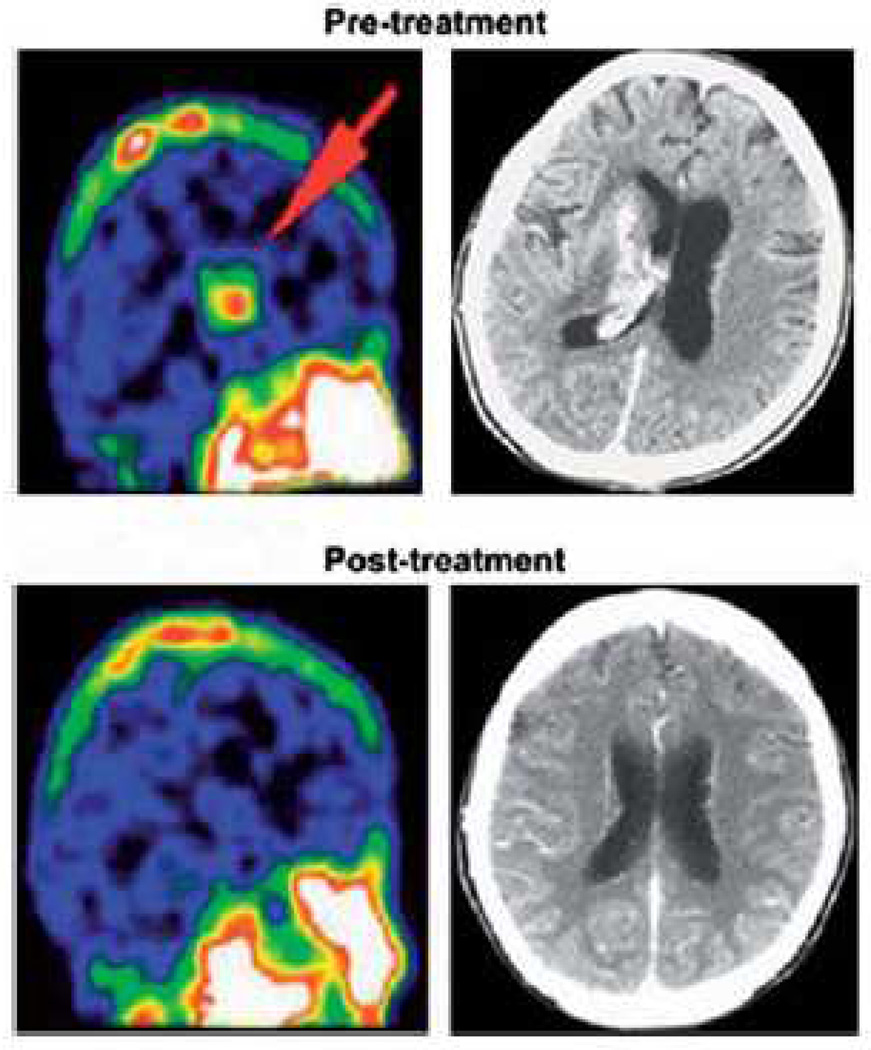
Nine patients diagnosed with AIDS-PCNSL (five males, median age: 48, range: 41 – 56 years) were treated with high-dose AZT – HU as first-line therapy (Table II). Only one patient had a CD4 count > 200/µL and three patients had a CD4 count < 50/µL at the time of PCNSL diagnosis. All but one patient received HAART during treatment. Two patients had very poor performance status (Karnofsky [KPS]: 40 and 20) at the time of diagnosis. Four patients had more than one brain lesion. Five patients achieved a response (three CR, two PR) after AZT – HU alone, while two had progressive disease. Five patients who either progressed or had an inadequate response after AZT – HU therapy received whole brain radiotherapy (WBRT). One of these patients with PR later achieved a CR after WBRT, and continues to be disease-free. Two patients were lost to follow-up before the end of their treatment and any response evaluation. Figure 4 illustrates PCNSL response by nuclear imaging, with complete resolution of the brain mass within 3 weeks of AZT – HU initiation. During this period, the patient did not have a significant change in absolute CD4 count, but eventually relapsed and died of disease. At the time of this analysis, three patients remained alive, 1, 4 and 8 years after chemotherapy commencement.
Table II.
Demographic and clinical characteristics of HIV+ patients with primary central nervous system lymphoma (PCNSL) who were treated with high-dose AZT and hydroxyurea (HU).
| Age | Sex | HIV- lymphoma* |
PS | CD4 | Lesion location |
Radiological response to AZT–HU† |
WBRT dose |
Radiological response after WBRT† |
Survival† |
|---|---|---|---|---|---|---|---|---|---|
| 42 | F | 6 | 90 | 37 | PV | CR (2) | Dead (8) | ||
| 53 | M | 8 | 90 | 48 | PV | CR (92) | Alive (92) | ||
| 44 | M | 9 | 90 | 374 | MB | CR (48) | Alive (48) | ||
| 56 | M | 1 | 90 | 64 | FR | PR (3) | Dead (4) | ||
| 48 | F | 0 | 90 | 55 | FR | PR (12) | 30 | CR (12) | Alive (12) |
| 41 | M | 10 | 90 | 5 | PV | NE | 40 | NE | Dead (2) |
| 51 | M | 0 | 40 | 151 | FR, PV | PD | 30 | PD | Dead (6) |
| 46 | F | 11 | 70 | 3 | FR | NE | 30 | NE | Dead (2) |
| 53 | F | 15 | 20 | 4 | FR | PD | 12 | PD | Dead (1) |
PS, Eastern Cooperative Oncology Group performance score; WBRT, whole brain radiotherapy; PV, periventricular; MB, midbrain; FR, frontal lobe; CR, complete remission; PR, partial response; PD, progressive disease; NE, not evaluable.
Interval between human immunodeficiency virus (HIV) and lymphoma diagnosis in years.
Duration in months in parentheses.
Figure 4.
Clinical efficacy of high-dose AZT–HU in HIV-associated primary CNS lymphoma (HIV-PCNSL). SPECT images before and after 4 weeks of AZT–HU treatment in an HIV+ patient with PCNSL are shown.
Discussion
In this study, we present our experience using high-dose AZT-based chemotherapy regimens as first-line treatment for EBV+ NHL. With a CR rate of 78% and 3-year OS of 77.8% among 10 patients (nine HIV+) with systemic aggressive EBV+ lymphoma, our results suggest that AZT in combination with MTX is an effective chemotherapy option, particularly when alternated with hCVAD or EPOCH and in the AIDS setting. The long-term results from nine HIV+ patients with PCNSL treated with AZT in combination with HU are promising, and support our preclinical studies demonstrating the cooperative effect between these two drugs in inducing the EBV-lytic cycle and apoptosis in EBV+ ARLs.
Treatment of systemic EBV+ lymphomas with AZT–MTX-based chemotherapy
The treatment of highly aggressive EBV+ lymphomas can be challenging, especially in severely immunocompromised patients. Although survival of patients with some ARLs, particularly DLBCL, has improved in the HAART era [29], the prognosis of ARL subtypes commonly associated with EBV, such as PBL, PCNSL and PEL, remains poor [30,31]. Patients with HIV+ BL have also experienced poor outcomes with conventional chemotherapy until just recently. Our results using high-dose AZT–MTX-based chemotherapy for EBV+ NHLs are comparable to those reported in prospective chemotherapy trials for ARLs. A CR can be achieved in up to 70 – 80% of patients with ARL treated with CHOP or DAEPOCH plus HAART [24,29,32]. By comparison with these studies, our patient cohort included a higher proportion of more aggressive lymphomas (four PBL, three BL, one PEL and two advanced stage DLBCL). A lower CR rate (46%) and a worse DFS (~20%) were reported with a similar AZT–MTX regimen in a series of 29 relapsed ARLs of unknown EBV status [18], suggesting that the combination is more effective in the upfront setting and when used specifically in EBV+ tumors. However, because we alternated AZT–MTX with conventional regimens in seven patients, a head-to-head comparison of AZT–MTX and the standard lymphoma regimens was not possible. Additionally, whether the AZT–MTX combination is efficacious in other EBV-related lymphoid malignancies, such as post-transplant lymphoproliferative disorder, as well as in HIV-negative patients, remains to be determined.
The efficacy of alternating AZT–MTX with DA-EPOCH in patients with EBV+ PBL is noteworthy. Notoriously, PBL carries a poor prognosis in HIV+ patients [2]. PBL is an aggressive and rare type of B-cell malignancy that is usually associated with HIV infection. Most PBL cases reported in the literature have been patients treated with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), with approximately two-thirds of them achieving a CR [31]. Despite a reasonable initial response to conventional chemotherapy, many patients with PBL relapse early in the disease. In a recent review, the median OS for PBL was reported to be 15 months [31]. The survival is likely to be grimmer considering that this analysis included only published PBL cases who received treatment. In our present study, three of four patients with PBL achieved a CR, and all remain disease-free 3 years after treatment initiation.
Regarding safety, the high-dose AZT–MTX combination was tolerated well in our patients, except one who had presented with a very poor PS and serious infectious comorbidity. Grade 3 – 4 neutropenia occurred in two patients (20%), in contrast to 52% of patients previously reported by Tosi et al. [18], and we did not observe any grade 3 – 4 anemia. The lesser hematologic toxicity observed in our study compared to that observed by Tosi et al. could be due to lower AZT doses per cycle (12 vs. 36 g/m 2) and concurrent use of HAART in our study.
One limitation of our study is that the AZT–MTX regimen was combined with other cytotoxic regimens in some patients, which did not allow individual assessment of regimen efficacies. This study is also limited by its retrospective nature and relatively small number of patients. However, considering the relative paucity of data on EBV _ lymphomas in the AIDS setting, we believe our results remain valuable to the scientific community.
Treatment of AIDS-PCNSL with AZT–HU combination
AIDS-PCNSL is strongly associated with EBV [33], and has a poorer prognosis than PCNSL occurring in HIV-negative patients [30]. Median survival in patients with AIDS-PCNSL is less than 6 months, even with WBRT [34,35]. Patients with AIDS-PCNSL tend to present with poor performance status, CD4 counts below 100/µL and high HIV viral load, rendering clinical studies and aggressive chemotherapy, such as conventional high-dose MTX [30], difficult in this population [36]. As a result, prospective studies conducted in the past were terminated early due to lack of accrual [19,37], and there is no agreed upon standard approach to these patients.
The efficacy of AZT in combination with GCV and recombinant human interleukin-2 (r-IL2) had been previously demonstrated in HIV-PCNSL [19,37]. However, in those studies it was unclear as to which drug (AZT, GCV or r-IL-2) was the active agent. We observed a clinical and radiologic response in five of nine patients with HIVPCNSL treated with AZT–HU, including three who had a CR. Two patients who received AZT-HU and no radiation remain disease-free 4 and 8 years later. Therefore, high-dose AZT alone or in combination with HU can be an alternative regimen when more aggressive chemotherapy is contraindicated in patients with advanced AIDS due to anticipated intolerance. Given our clinical observations, AZT-HU should be explored for the treatment of HIV-PCNSL either in combination with chemotherapy or standard brain irradiation, as HU is a well-known radio-sensitizer [38]. Alternatively, in patients with good performance status who can tolerate chemotherapy, high-dose AZT–MTX may be considered.
One limitation of this study is that data on EBV DNA in cerebrospinal fluid was not available in three patients with HIV-PCNSL. However, those were diagnosed by positive imaging studies and after ruling out toxoplasmosis. Given the rarity of AIDS-PCNSL, we believe that the results from this retrospective analysis are highly valuable.
Biological role of AZT in treatment of EBV+ lymphomas
The use of antiviral drugs such as AZT and GCV is appealing for treating γ-herpesvirus-associated lymphomas. These agents are preferentially phosphorylated by EBV-encoded kinases, thus potentiating their own cytostatic or anti-tumor effects [9,11,39,40]. Their activity depends on induction of the viral lytic genes TK and/or orf36/BGLF4/PK [41]. AZT has been shown to be preferentially phosphorylatyed by EBV-TK, while GCV is dependent on EBV-protein kinase (PK) for its anti-viral function [11,42]. Other investigators have demonstrated that histone deacetylase inhibitors can sensitize EBV+ lymphoma cells to GCV [7,43,44]. In a phase II trial, the combination of GCV with arginine butyrate was found to be effective in patients with refractory EBV+ lymphoid diseases [45]. AZT and GCV in combination have shown efficacy in EBV+ HIV-related PCNSL and KSHV associated MCD [19,22,37]. We have previously shown that AZT but not GCV inhibited NF-κB activity, resulting in induction of the full EBV lytic cycle and apoptosis in primary EBV+ BL cells [12]. The AZT apoptotic effect in BL cells was caspasemediated and independent of Fas [21]. Further, AZT and HU synergized to induce apoptosis in primary EBV+ lymphoma cell lines [12]. Here, we confirmed that the EBV lytic-inducing effect of AZT is potentiated by HU in primary EBV+ BL and IBL lines (Figure 3). The mechanism by which AZT and HU induce the EBV lytic cycle in EBV+ lymphomas remains to be elucidated, and is beyond the scope of this study.
Summary
In conclusion, high-dose AZT-based chemotherapy appears to be an efficacious approach for the treatment of EBV-related lymphomas, particularly in the HIV/AIDS setting. This is supported by our preclinical studies demonstrating the biological role of AZT in inducing the viral lytic cycle and apoptosis in aggressive EBV+ lymphoma cells. Based on our clinical experience, we have designed a phase II clinical trial using the lytic-inducing combination of high-dose AZT–MTX, HU and low-dose doxorubicin in relapsed/refractory EBV related lymphomas, which is currently recruiting patients. These AZT-based combination approaches could add efficacy to standard lymphoma chemotherapy and improve the treatment of γ-herpesvirus-related lymphomas.
Acknowledgments
This work was supported by a translational award from the Leukemia and Lymphoma Society to J.C.R. (initially to the late William Harrington) and D.P.D., NIH-NCI 5R01CA112217 to J.C.R. (initially to the late William Harrington), NIH-NCI 2U01CA121947 (AIDS Malignancy Consortium) to J.C.R., PHS grant CA019014 to D.P.D., NIH-Center for AIDS Research grant 5P30AI073961-05 to J.C.R., NIH-NCI PO1-CA-128115- 01A2 grant to J.C.R. and by the University of Miami Sylvester Comprehensive Cancer Center. We would also like to especially recognize the late William J. Harrington Jr. for his inspiring translational work with AZT as an effective lytic-inducing agent with anti-neoplastic activity in EBV+ lymphomas.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Shibata D, Weiss LM, Hernandez AM, et al. Epstein-Barr virus-associated non-Hodgkin’s lymphoma in patients infected with the human immunodeficiency virus. Blood. 1993;81:2102–2109. [PubMed] [Google Scholar]
- 2.Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413–1420. [PubMed] [Google Scholar]
- 3.Carbone A, Cesarman E, Spina M, et al. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009;113:1213–1224. doi: 10.1182/blood-2008-09-180315. [DOI] [PubMed] [Google Scholar]
- 4.Cesarman E, Mesri EA. Pathogenesis of viral lymphomas. Cancer Treat Res. 2006;131:49–88. doi: 10.1007/978-0-387-29346-2_2. [DOI] [PubMed] [Google Scholar]
- 5.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 6.Kulwichit W, Edwards RH, Davenport EM, et al. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci USA. 1998;95:11963–11968. doi: 10.1073/pnas.95.20.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mentzer SJ, Fingeroth J, Reilly JJ, et al. Arginine butyrate-induced susceptibility to ganciclovir in an Epstein-Barr-virus-associated lymphoma. Blood Cells Mol Dis. 1998;24:114–123. doi: 10.1006/bcmd.1998.0178. [DOI] [PubMed] [Google Scholar]
- 8.Di Renzo L, Avila-Carino J, Klein E. Induction of the lytic viral cycle in Epstein Barr virus carrying Burkitt lymphoma lines is accompanied by increased expression of major histocompatibility complex molecules. Immunol Lett. 1993;38:207–214. doi: 10.1016/0165-2478(93)90008-p. [DOI] [PubMed] [Google Scholar]
- 9.Moore SM, Cannon JS, Tanhehco YC, et al. Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues. Antimicrob Agents Chemother. 2001;45:2082–2091. doi: 10.1128/AAC.45.7.2082-2091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng WH, Hong G, Delecluse HJ, et al. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J Virol. 2004;78:1893–1902. doi: 10.1128/JVI.78.4.1893-1902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustafson EA, Chillemi AC, Sage DR, et al. The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase. Antimicrob Agents Chemother. 1998;42:2923–2931. doi: 10.1128/aac.42.11.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurokawa M, Ghosh SK, Ramos JC, et al. Azidothymidine inhibits NF-kappaB and induces Epstein-Barr virus gene expression in Burkitt lymphoma. Blood. 2005;106:235–240. doi: 10.1182/blood-2004-09-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos JC, Sin SH, Staudt MR, et al. Nuclear factor kappa B pathway associated biomarkers in AIDS defining malignancies. Int J Cancer. 2012;130:2728–2733. doi: 10.1002/ijc.26302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tosi P, Calabresi P, Goulette FA, et al. Azidothymidine-induced cytotoxicity and incorporation into DNA in the human colon tumor cell line HCT-8 is enhanced by methotrexate in vitro and in vivo. Cancer Res. 1992;52:4069–4073. [PubMed] [Google Scholar]
- 15.Lima RT, Seca H, Bras S, et al. Treatment of Akata EBV-positive cells with doxorubicin causes more EBV reactivation than treatment with etoposide. Chemotherapy. 2011;57:195–203. doi: 10.1159/000323627. [DOI] [PubMed] [Google Scholar]
- 16.Wynn HE, Brundage RC, Fletcher CV. Clinical implications of CNS penetration of antiretroviral drugs. CNS Drugs. 2002;16:595–609. doi: 10.2165/00023210-200216090-00002. [DOI] [PubMed] [Google Scholar]
- 17.Sarker D, Thirlwell C, Nelson M, et al. Leptomeningeal disease in AIDS-related non-Hodgkin’s lymphoma. AIDS. 2003;17:861–865. doi: 10.1097/00002030-200304110-00011. [DOI] [PubMed] [Google Scholar]
- 18.Tosi P, Gherlinzoni F, Mazza P, et al. 3’-Azido 3’-deoxythymidine+ methotrexate as a novel antineoplastic combination in the treatment of human immunodeficiency virus-related non-Hodgkin’s lymphomas. Blood. 1997;89:419–425. [PubMed] [Google Scholar]
- 19.Raez L, Cabral L, Cai JP, et al. Treatment of AIDS-related primary central nervous system lymphoma with zidovudine, ganciclovir, and interleukin 2. AIDS Res Hum Retroviruses. 1999;15:713–719. doi: 10.1089/088922299310809. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh SK, Wood C, Boise LH, et al. Potentiation of TRAIL-induced apoptosis in primary effusion lymphoma through azidothymidine-mediated inhibition of NF-kappa B. Blood. 2003;101:2321–2327. doi: 10.1182/blood-2002-08-2525. [DOI] [PubMed] [Google Scholar]
- 21.Lee RK, Cai JP, Deyev VV, et al. A zidothymidine and interferonalpha induce apoptosis in herpesvirus-associated lymphomas. Cancer Res. 1999;59:5514–5520. [PubMed] [Google Scholar]
- 22.Uldrick TS, Polizzotto MN, Aleman K, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood. 2011;117:6977–6986. doi: 10.1182/blood-2010-11-317610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roychowdhury S, Peng R, Baiocchi RA, et al. Experimental treatment of Epstein-Barr virus-associated primary central nervous system lymphoma. Cancer Res. 2003;63:965–971. [PubMed] [Google Scholar]
- 24.Little RF. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101:4653–4659. doi: 10.1182/blood-2002-11-3589. [DOI] [PubMed] [Google Scholar]
- 25.Cortes J, Thomas D, Rios A, et al. Hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone and highly active antiretroviral therapy for patients with acquired immunodeficiency syndrome-related Burkitt lymphoma/leukemia. Cancer. 2002;94:1492–1499. doi: 10.1002/cncr.10365. [DOI] [PubMed] [Google Scholar]
- 26.Toomey NL, Deyev VV, Wood C, et al. Induction of a TRAIL-mediated suicide program by interferon alpha in primary effusion lymphoma. Oncogene. 2001;20:7029–7040. doi: 10.1038/sj.onc.1204895. [DOI] [PubMed] [Google Scholar]
- 27.Ryan JL, Fan H, Glaser SL, et al. Epstein-Barr virus quantitation by real-time PCR targeting multiple gene segments: a novel approach to screen for the virus in paraffin-embedded tissue and plasma. J Mol Diagn. 2004;6:378–385. doi: 10.1016/S1525-1578(10)60535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang W, Harmon P, Gulley ML, et al. Viral response to chemotherapy in endemic burkitt lymphoma. Clin Cancer Res. 2010;16:2055–2064. doi: 10.1158/1078-0432.CCR-09-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navarro JT, Lloveras N, Ribera JM, et al. The prognosis of HIV-infected patients with diffuse large B-cell lymphoma treated with chemotherapy and highly active antiretroviral therapy is similar to that of HIV-negative patients receiving chemotherapy. Haematologica. 2005;90:704–706. [PubMed] [Google Scholar]
- 30.Bayraktar S, Bayraktar UD, Ramos JC, et al. Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J Neurooncol. 2011;101:257–265. doi: 10.1007/s11060-010-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: Lessons learned from 112 published cases. Am J Hematol. 2008;83:804–809. doi: 10.1002/ajh.21250. [DOI] [PubMed] [Google Scholar]
- 32.Weiss R, Mitrou P, Arasteh K, et al. Acquired immunodeficiency syndrome-related lymphoma: Simultaneous treatment with combined cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy and highly active antiretroviral therapy is safe and improves survival. Cancer. 2006;106:1560–1568. doi: 10.1002/cncr.21759. [DOI] [PubMed] [Google Scholar]
- 33.MacMahon EM, Glass JD, Hayward SD, et al. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet. 1991;338:969–973. doi: 10.1016/0140-6736(91)91837-k. [DOI] [PubMed] [Google Scholar]
- 34.Baumgartner JE, Rachlin JR, Beckstead JH, et al. Primary central nervous system lymphomas: natural history and response to radiation therapy in 55 patients with acquired immunodeficiency syndrome. J Neurosurg. 1990;73:206–211. doi: 10.3171/jns.1990.73.2.0206. [DOI] [PubMed] [Google Scholar]
- 35.Skiest DJ, Crosby C. Survival is prolonged by highly active antiretroviral therapy in AIDS patients with primary central nervous system lymphoma. AIDS. 2003;17:1787–1793. doi: 10.1097/00002030-200308150-00007. [DOI] [PubMed] [Google Scholar]
- 36.Kreisl T, Panageas KS, Elkin EB, et al. Treatment patterns and prognosis in patients with human immunodeficiency virus and primary central nervous system lymphoma. Leuk Lymphoma. 2008;49:1710–1716. doi: 10.1080/10428190802238560. [DOI] [PubMed] [Google Scholar]
- 37.Aboulafia DM, Ratner L, Miles SA, et al. Antiviral and immunomodulatory treatment for AIDS-related primary central nervous system lymphoma: AIDS Malignancies Consortium pilot study 019. Clin Lymphoma Myeloma. 2006;6:399–402. doi: 10.3816/clm.2006.n.017. [DOI] [PubMed] [Google Scholar]
- 38.Shewach DS, Lawrence TS. Antimetabolite radiosensitizers. J Clin Oncol. 2007;25:4043–4050. doi: 10.1200/JCO.2007.11.5287. [DOI] [PubMed] [Google Scholar]
- 39.Cannon JS, Hamzeh F, Moore S, et al. Human herpesvirus 8-encoded thymidine kinase and phosphotransferase homologues confer sensitivity to ganciclovir. J Virol. 1999;73:4786–4793. doi: 10.1128/jvi.73.6.4786-4793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin JC, Dvorak CA, Smee DF, et al. 9-[(1,3-Dihydroxy-2-propoxy)methyl]guanine: a new potent and selective antiherpes agent. J Med Chem. 1983;26:759–761. doi: 10.1021/jm00359a023. [DOI] [PubMed] [Google Scholar]
- 41.Staudt MR, Kanan Y, Jeong JH, et al. The tumor microenvironment controls primary effusion lymphoma growth in vivo. Cancer Res. 2004;64:4790–4799. doi: 10.1158/0008-5472.CAN-03-3835. [DOI] [PubMed] [Google Scholar]
- 42.Meng Q, Hagemeier SR, Fingeroth JD, et al. The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production. J Virol. 2010;84:4534–4542. doi: 10.1128/JVI.02487-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westphal EM, Blackstock W, Feng W, et al. Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV-positive malignancies. Cancer Res. 2000;60:5781–5788. [PubMed] [Google Scholar]
- 44.Ghosh SK, Perrine SP, Williams RM, et al. Histone deacetylase inhibitors are potent inducers of gene expression in latent EBV and sensitize lymphoma cells to nucleoside antiviral agents. Blood. 2012;119:1008–1017. doi: 10.1182/blood-2011-06-362434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perrine SP, Hermine O, Small T, et al. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood. 2007;109:2571–2578. doi: 10.1182/blood-2006-01-024703. [DOI] [PMC free article] [PubMed] [Google Scholar]