Abstract
Background
Human-induced pluripotent stem cells (iPSCs) are a potentially unlimited source for generation of cardiomyocytes (iPSC-CMs). However, current protocols for iPSC-CM derivation face a number of challenges, including variability in somatic cell sources and inconsistencies in cardiac differentiation efficiency.
Objectives
We aimed to assess the effect of epigenetic memory on differentiation and function of iPSC-CMs generated from somatic cell sources of cardiac versus non-cardiac origins.
Methods
Cardiac progenitor cells (CPCs) and skin fibroblasts from the same donors were reprogrammed into iPSCs and differentiated into iPSC-CMs via embryoid body and monolayer-based differentiation protocols.
Results
Differentiation efficiency was found to be higher in CPC-derived iPSC-CMs (CPC-iPSC-CMs) than in fibroblast-derived iPSC-CMs (Fib-iPSC-CMs). Gene expression analysis during cardiac differentiation demonstrated upregulation of cardiac transcription factors in CPC-iPSC-CMs, including NKX2-5, MESP1, ISL1, HAND2, MYOCD, MEF2C, and GATA4. Epigenetic assessment revealed higher methylation in the promoter region of NKX2-5 in Fib-iPSC-CMs compared to CPC-iPSC-CMs. Epigenetic differences were found to dissipate with increased cell passaging, and a battery of in vitro assays revealed no significant differences in their morphological and electrophysiological properties at early passage. Finally, cell delivery into small animal myocardial infarction (MI) model indicated that CPC-iPSC-CMs and Fib-iPSC-CMs possess comparable therapeutic capabilities in improving functional recovery in vivo.
Conclusions
This is the first study to compare differentiation of iPSC-CMs from human CPCs versus human fibroblasts from the same donors. We demonstrate that while epigenetic memory improves differentiation efficiency of cardiac versus non-cardiac somatic cell source in vitro, it does not contribute to improved functional outcome in vivo.
Keywords: induced pluripotent stem cells, epigenetic memory, DNA methylation, cardiac differentiation
INTRODUCTION
Coronary heart disease is the leading cause of death in developed countries, causing approximately 550,000 deaths annually in the United States alone (1). As the only option for end-stage treatment, heart transplantation excludes many patients who are not suitable candidates due to preexisting comorbidities and is furthermore limited by the low number of available organ donors. In recent years, cell-based therapies have emerged as a promising alternative (2). Human-induced pluripotent stem cells (iPSCs), in particular, are an attractive donor cell source due to their capacity for unlimited self-renewal and pluripotency. In addition, because iPSCs are derived from somatic tissue, their use largely circumvents ethical and immunological concerns associated with embryonic stem cell (ESC)-based therapies.
However, several recent studies have demonstrated that iPSCs are not as similar to ESCs as initially believed (5). Newer literature suggests that epigenetic memory has the potential to influence the differentiation potential and functional maturity of iPSC-derived cell types (6).
In this study, we aimed to assess the contributions of epigenetic memory to the differentiation potential, function, and maturity of human iPSCs derived from cardiac and non-cardiac sources. To do so, we generated human iPSCs from cardiac progenitor cells (CPC-iPSCs) and dermal fibroblasts (Fib-iPSCs) from the same donors, and differentiated these cells to iPSC-CMs for in vitro and in vivo characterization. As human CPCs have been shown to give rise to multiple lineages of cells found in the heart (7-9), we also compared the ability of CPC-iPSCs and Fib-iPSCs to differentiate into other cell types found in the heart.
METHODS
A detailed Methods section is available in the Online Data Supplement. Stanford University Human Subjects Research Institutional Review Board approved all the protocols in this study. iPSCs were generated using lentiviral transduction, as previously described (4). A modified protocol from Yang et al. was followed to differentiate iPSC-CMs by three-dimensional (3D) embryoid body (EB) formation (10). As an alternate method of cardiac differentiation, a two-dimensional (2D) monolayer protocol from Lian et al. was also employed (11). Following differentiation, iPSC-CMs were cultured in vitro using SCT Cardiac Maintenance Media (Menlo Park, CA). A detailed description is included in the Supplemental Methods.
Statistical analysis
Normality distribution was studied with the Kolmogorov-Smirnov test (p<0.05). Statistically significant differences were determined using the Mann-Whitney test or Wilcoxon signed rank test, with α set to 0.05 for genes not displaying a normal distribution, and with Student's t-test or paired Student's t-test, with α set to 0.05 for genes with a normal distribution. Unless specified, data are expressed as average ± SEM. All statistical analysis was carried out using Prism5 (GraphPad Software, La Jolla, CA).
RESULTS
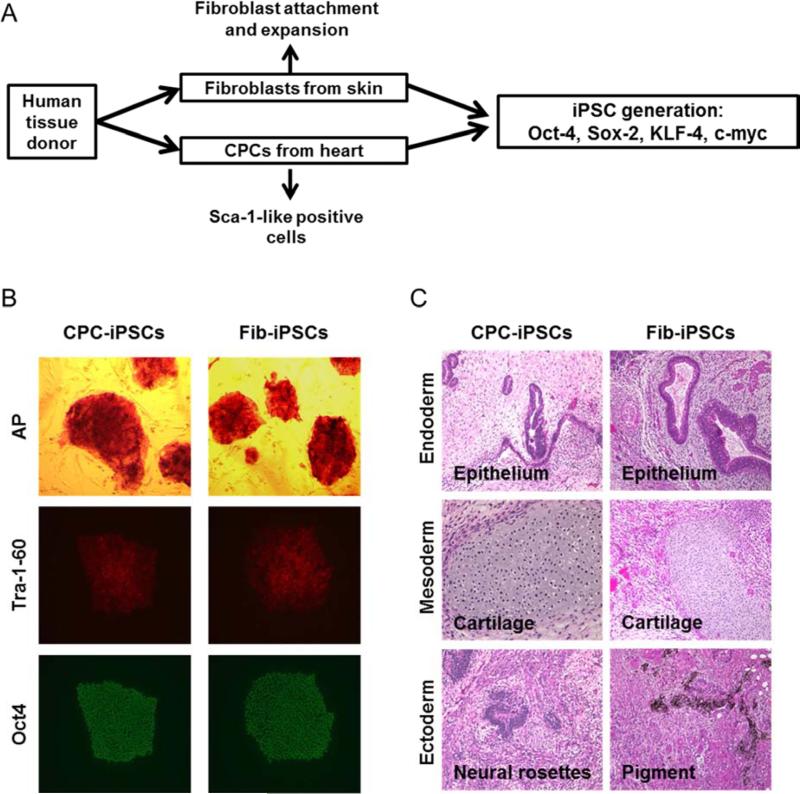
Skin fibroblasts and CPCs were isolated from two matched human fetal donors (Figure 1A). To perform CPC isolation, heart tissue was digested to a single cell suspension and labeled with Sca-1 antibody for magnetic cell sorting (12). Following a brief period of cell expansion, fetal CPCs and fibroblasts were characterized for gene expression. Fetal CPCs were found to express genes associated with cardiac lineage, such as KDR, NKX2-5, TBX18, WT1 MEF2C, and GATA4, whereas fibroblasts only expressed TBX18 (Supplemental Figure 1A). Neither the CPCs nor fibroblasts were found to express genes associated with pluripotency such as NANOG and OCT4, both of which were highly expressed in iPSCs (Supplemental Figure 1B). CPCs and fibroblasts were subsequently reprogrammed through lentiviral infection using OCT4, SOX2, KLF4, and MYC (3). After approximately 3 weeks, colonies positive for alkaline phosphatase (Figure 1B) with ESC-like morphology were mechanically isolated and expanded on Matrigel-coated dishes. No differences in reprogramming efficiency were observed between the two cell types. Both CPC-iPSCs and Fib-iPSCs exhibited identical morphologies and presence of pluripotency markers such as Tra-1-60, and Oct4 (Figure 1B). Teratoma formation assays using CPC-iPSCs and Fib-iPSCs produced derivatives from all 3 germ layers (Figure 1C). Paired CPC-iPSCs and Fib-iPSCs also were generated from an adult 65-year old donor as an additional control. Reprogramming was conducted in an identical manner to fetal donor sources. Adult CPC-iPSCs and Fib-iPSCs similarly exhibited ESC-like morphologies and markers of pluripotency (Supplemental Figure 2).
Figure 1. iPSC generation and characterization.
(A) Skin fibroblast and CPC primary cultures were established from the same donors and reprogrammed with the pluripotency transcription factors Oct4, Sox2, Klf4, and c-Myc. (B) Successfully reprogrammed iPSCs express standard markers of pluripotency such as alkaline phosphatase (AP), Tra-1-60 (red), and Oct4 (green). (C) Following transplantation into immunodeficient mice, CPC-iPSCs and Fib-iPSCs give rise to three-germ layer teratomas containing endoderm (epithelium), mesoderm (cartilage), and ectoderm (neural rosettes and pigments).
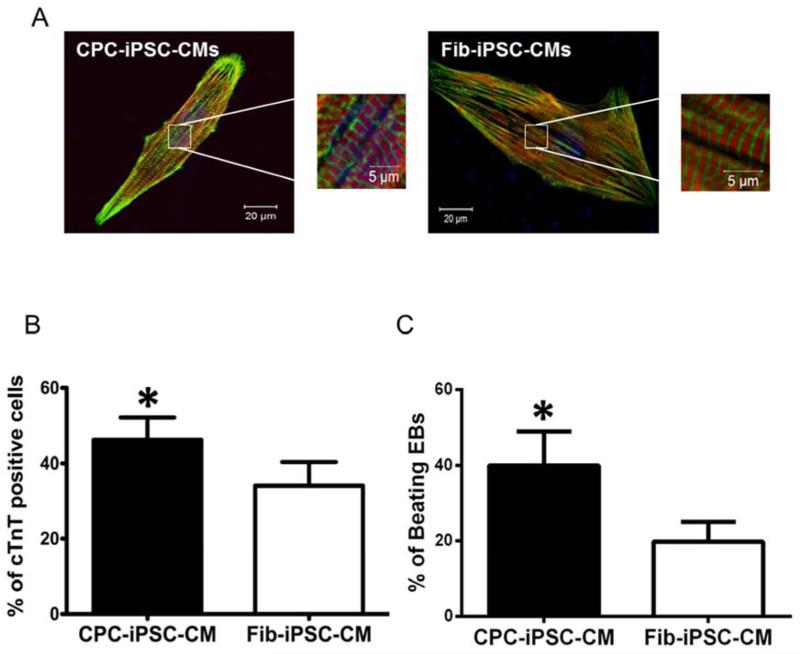
Following iPSC characterization and a brief period of cell expansion, CPC-iPSCs and Fib-iPSCs were differentiated into iPSC-CMs via 3D EB formation (Supplemental Figure 3A) (15). At day 15 following induction of cardiac differentiation, spontaneously beating EBs were observed under brightfield microscopy. Beating EBs were dissociated into single cells and characterized by confocal microscopy for immunostaining against cardiac-specific markers, such as cardiac troponin T (cTnT) and sarcomeric α-actinin (Figure 2A; Supplemental Figure 3B). A fluorescence-activated cell sorting (FACS) analysis of dissociated EBs confirmed a significantly higher percentage of cTnT-positive cells in CPC-iPSC-CMs than in Fib-iPSC-CMs from the same donor (46.2±5.9% vs 34.0±6.4%, n=12; p<0.05; Figure 2B). Quantification of beating EBs between passages 15-30 also revealed a higher number of beating EBs for CPC-iPSC-CMs as compared to Fib-iPSC-CMs (40.0±8.9% vs 19.8±5.2%, n=10; p<0.05; Figure 2C), indicating higher cardiac differentiation efficiencies for CPC-iPSC-CMs.
Figure 2. Characterization of induced pluripotent stem cell-derived cardiomyocytes.
(A) Immunostaining of CPC-iPSC-CMs and Fib-iPSC-CMs for cardiac specific markers. Pictures show cardiac troponin T (red), sarcomeric a-actinin (green), and DAPI (blue). (B) Quantification of the percentage of cells positive for cardiac troponin T (cTnT) as determined by FACS (n=12) at day 15 after cardiac differentiation. The percentage of cTnT positive cells is significantly (*p<0.05) higher in CPC-iPSC-CMs compared to Fib-iPSC-CMs. (C) Quantification of the percentage of beating EBs at day 15 post cardiac differentiation of CPC-iPSCs and Fib-iPSCs (n=10). The percentage of CPC-iPSC beating EBs is significantly higher (*p<0.05) than Fib-iPSC beating EBs.
To confirm findings that elevated cardiac differentiation efficiency in CPC-iPSC-CMs was not specific to EB-based methods of cardiac differentiation, we also employed a 2D monolayer differentiation protocol based on Lian et al. (Supplemental Figure 4A) (11). Fetal CPC-iPSC-CMs and Fib-iPSC-CMs generated through monolayer differentiation exhibited the same cardiac markers as iPSC-CMs generated through 3D EB differentiation (Supplemental Figure 4B). FACS analysis of dissociated monolayers demonstrated a significantly higher percentage of cTnT-positive cells in fetal CPC-iPSC-CMs compared to Fib-iPSC-CMs from the same donor (57.2±0.9% vs 51.7±0.9%, n=14; p<0.05; Supplemental Figure 5A-B). Immunostaining quantification for cTnT-positive cells using 2-D monolayer protocol also revealed significantly higher cardiac differentiation efficiencies for CPC-iPSC-CMs compared to Fib-iPSC-CMs (64.7±1.3% vs 54.5±1.4%, n=20; p<0.05; Supplemental Figure 6A-B). Analysis of paired CPC-iPSC-CMs and Fib-iPSC-CMs derived from a 65-year-old adult donor further confirmed results observed in fetal lines. FACS of adult lines demonstrated increased cardiac differentiation for CPC-iPSC-CMs versus Fib-iPSC-CMs (43.1±3.7% vs 35.2±3.5%, n=7; p<0.05; Supplemental Figure 7A-B). Immunomicroscopy quantification for cTnT-positive cells also yielded similar results in CPC-iPSC-CMs versus Fib-iPSC-CMs (64.2±2.7% vs 45.0±1.5%, n=10; p<0.05; Supplemental Figure 8A-B). Interestingly, prolonged culture of iPSCs for greater than 40 passages negated any differences between CPC-iPSC and Fib-iPSC lines (Supplemental Figure 9A-B). Taken together, these results suggest that at low passages (up to passage 30), derivation of iPSCs from cardiac sources such as CPCs helps enhance the ability of iPSCs to differentiate into cardiac lineages, but this effect is lost with time passage.
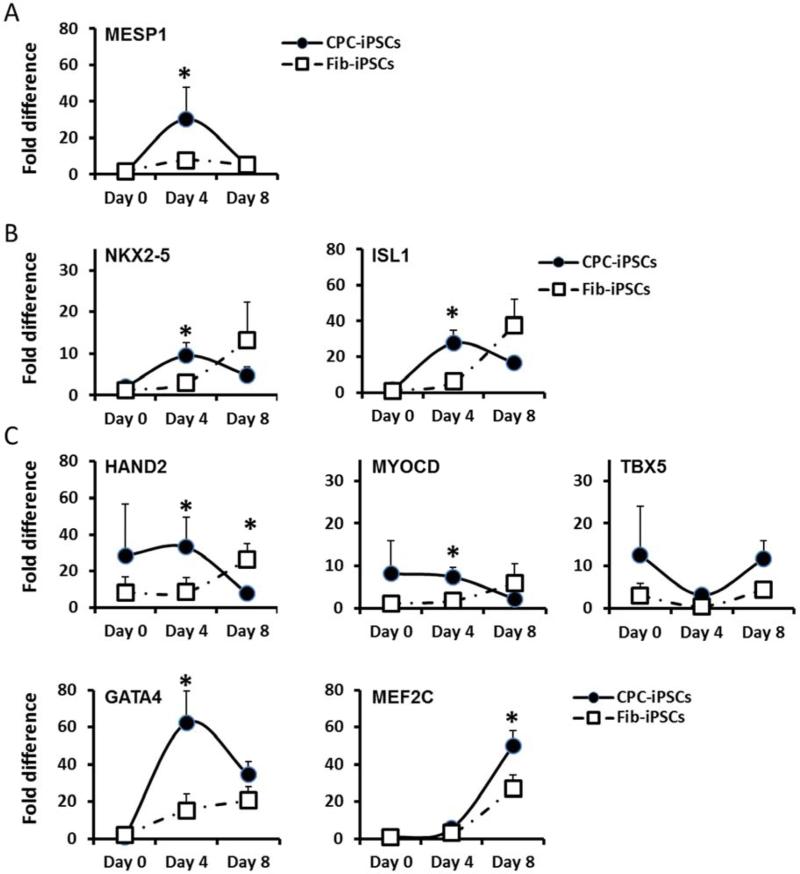
During generation of cardiomyocytes from iPSCs, pluripotent cells go through several distinct stages of differentiation, including the creation of an early mesoderm, followed by a cardiac mesoderm specific to different established cardiovascular lineages (10). To improve our understanding of cardiac differentiation in CPC-iPSC-CMs and Fib-iPSC-CMs, we grouped specific markers into 1) early cardiac mesodermal transcription factors, 2) cardiovascular progenitor transcription factors, and 3) late-stage cardiogenic transcription factors (Supplemental Figure 10A). We then charted their expression over the cardiac differentiation process. Specifically, we chose MESP1 as a marker for early cardiac mesoderm, NKX2-5 and ISL1 as markers for cardiovascular progenitors, and HAND2, MYOCD, TBX5, GATA4, and MEF2C as markers for more established cardiac lineages. MESP1 is a transcription factor necessary for the specification of multipotent cardiovascular progenitors and the expression of cardiovascular transcription factors (13). HAND2 is a transcription factor involved in heart development (14), whereas MYOCD is a transcriptional co-activator of Serum Response Factor (SRF), which interacts with TBX5 to promote cardiac gene expression (15). GATA4 is a co-activator of NKX2-5 (16), a transcription factor involved in heart formation and development (17). ISL1 is a transcription factor expressed with NKX2-5 in cardiac progenitor cells (18) and is necessary for the formation of the right ventricle (19). Cardiac gene expression was assessed during the differentiation process at day 0 pre-induction, day 4, and day 8 after initiation of differentiation (10). Quantitative polymerase chain reaction demonstrated significantly increased expression of the early cardiac mesodermal marker MESP1 and of cardiovascular progenitor transcription factors NKX2-5 and ISL1 at day 4 of induction in CPC-iPSC-CMs, but not in Fib-iPSC-CMs (Figure 3A-B and Supplemental Figure 10B-C). Cardiogenic transcription factors HAND2, MYOCD, and GATA4 were also found to be expressed at significantly higher levels in CPC-iPSCs than in Fib-iPSCs at day 4 of cardiac differentiation (Figure 3C and Supplemental Figure 10D). By day 8 of induction, only HAND2 and MEF2C presented significant differences. At day 8, HAND2 was higher in Fib-iPSCs, whereas MEF2C was higher in CPC-iPSCs. These findings suggest that the higher efficiency of cardiac differentiation observed for CPC-iPSC-CMs is likely due to heightened gene expression of cardiac mesodermal and cardiac transcription factors early in the differentiation process.
Figure 3. Gene expression levels during cardiac differentiation.
Graphs represent expression levels of (A) early mesodermal transcription factor, (B) early cardiovascular transcription factors, and (C) late-stage cardiogenic transcription factors during the process of cardiac differentiation. Gene expression levels are normalized to expression levels in Fib-iPSCs (day 0) to demonstrate expression change over time (n=2 for day 0 and n=5 for day 4 and day 8). NKX2-5 and ISL1 levels at day 4 of the cardiac differentiation were significantly higher (p<0.05) in CPC-iPSCs than Fib-iPSCs.
DNA methylation of transcription factor promoter region
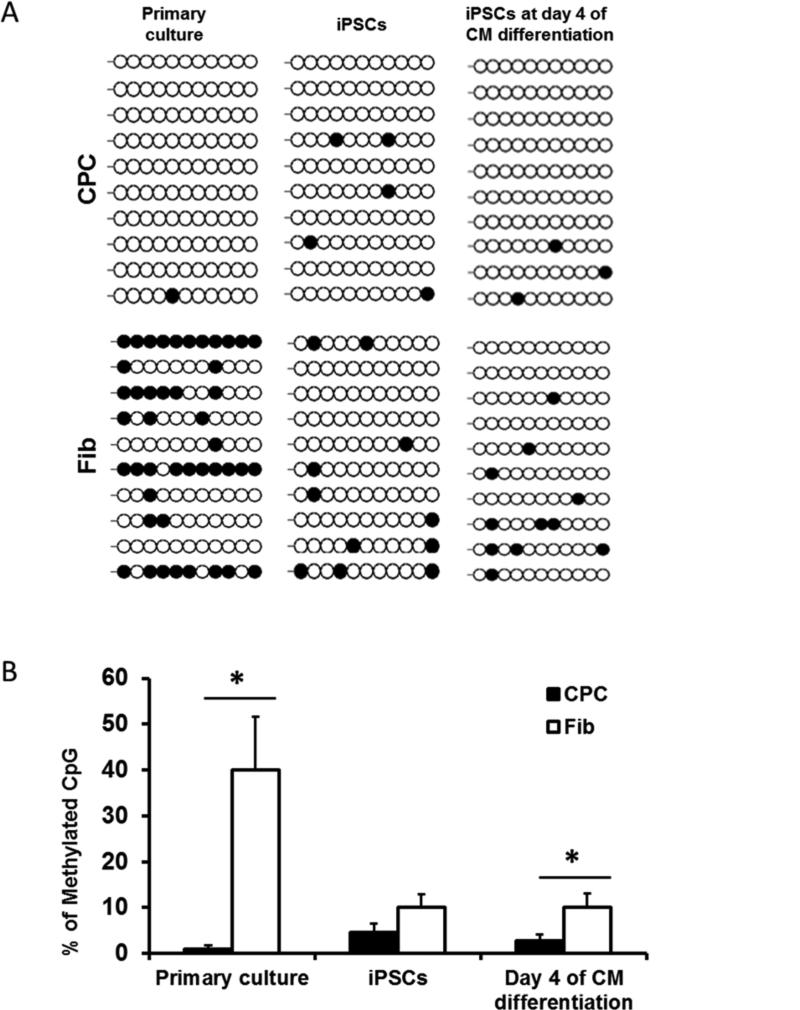
Several recent studies have demonstrated that the epigenetic memory of somatic donor cells can result in persistent gene expression, promoting iPSC differentiation toward somatic cell lineages of origin (20-22). To assess the contribution of epigenetic memory toward differences in gene expression observed during the cardiac differentiation process of CPC-iPSCs and Fib-iPSCs, we investigated DNA methylation patterns for the promoter regions of cardiac transcription factors in both cell types. DNA methylation is one of several epigenetic mechanisms by which gene regulation is regulated. Methylation of CpGs in the promoter region of a gene typically downregulates its expression, whereas efficient promoter demethylation of pluripotency genes enhances expression and is necessary for gene reactivation during reprogramming (20,23). Bisulfite sequencing demonstrated higher methylation of a region within 2,000 base pairs immediately upstream of the first coding exon of NKX2-5 in primary fibroblast cultures and Fib-iPSCs, compared with primary CPC cultures and CPC-iPSCs (Figure 4A). Differences in methylation persisted into day 4 of cardiac differentiation (Figure 4B). The decreased expression of NKX2.5 found in conjunction with heightened methylation of the NKX2.5 promoter in Fib-iPSCs suggests that incomplete resetting of the pre-existing epigenetic state is a strong contributor to the enriched cardiac gene expression patterns and differentiation efficiencies observed in CPC-iPSCs.
Figure 4. Analysis of DNA methylation pattern.
(A) DNA methylation pattern at the proximal promoter of NKX2-5 after bisulphite pyrosequencing. Black circles indicate methylated CpGs, whereas white circles indicate unmethylated CpGs. (B) Graph represents the percentage of methylated CpGs in primary cultures, undifferentiated iPSCs, and at day 4 of CM differentiation from both CPCs and fibroblasts. Percentage of methylated CpGs is significantly higher in primary fibroblast cultures and in Fib-iPSCs at day 4 of cardiac differentiation.
In vitro characterization
Cells generated from iPSCs such as cardiomyocytes are typically developmentally immature in comparison to their adult counterparts (24). Recent reports have shown that epigenetic memory can improve maturity and function of hematopoietic cells generated from blood cell-derived iPSCs, as compared with iPSCs derived from other cell types (22). We therefore assessed whether epigenetic memory from CPC-iPSC generation would result in functional or developmental differences for Fib-iPSC-CMs derived from the same donor. Following completion of cardiac differentiation, electrophysiological properties of CPC-iPSC-CMs and Fib-iPSC-CMs were characterized by Ca2+ imaging, whole-cell patch clamp, and multi-electrode array (MEA).
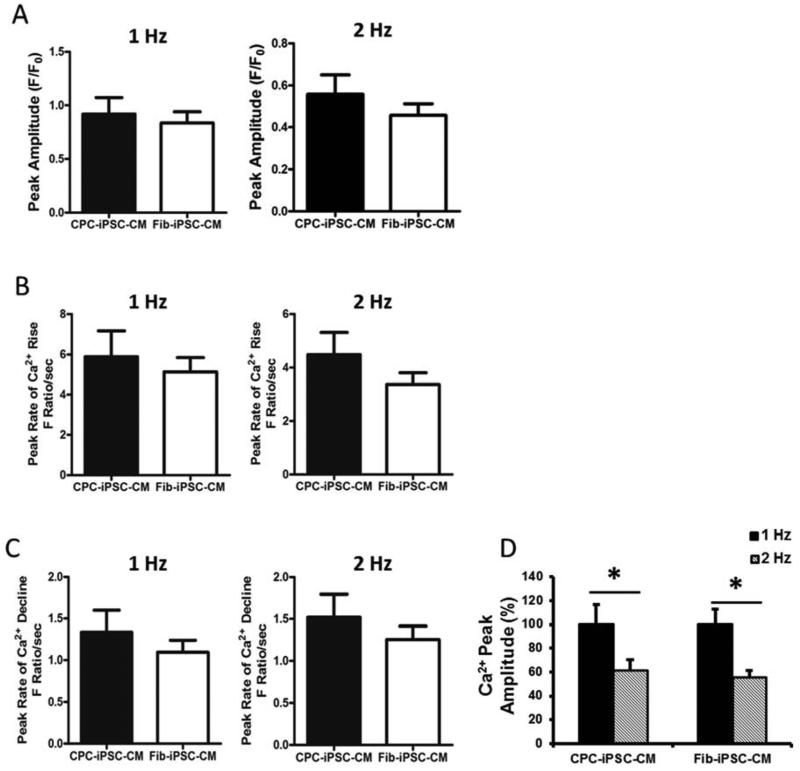
Quantification of Ca2+ is particularly important as Ca2+ is one of the major players in excitation-contraction coupling of the heart (25). Furthermore, iPSC-CMs are characterized by immature Ca2+ handling properties and incompletely developed sarcoplasmic reticulum (26). To assess whether epigenetic memory contributed toward maturity of CPC-iPSC-CMs, beating EBs were dissociated into single cells or small clumps of CMs for analysis by the Ca2+ binding dye Fluo-4 AM. Fluorescence amplitude resulting from Fluo-4 binding to intracellular Ca2+ was recorded in both 1 Hz (Supplemental Figure 11A-B) and 2 Hz stimulated beating CMs (Supplemental Figure 11C-D and Videos 1S-2S). Peak fluorescence amplitude was roughly the same for both somatic cell sources at 1 Hz and 2 Hz (Figure 5A), indicating that intracellular Ca2+ levels were not significantly different between the two groups. Other measures of Ca2+ handling such as peak amplitude, rate of Ca2+ rise (Figure 5B-C), and time needed to reach 50% and 90% Ca2+ decline also did not exhibit any significant differences between CPC-iPSC-CMs and Fib-iPSC-CMs (Supplemental Figure 11E-F). Both CPC-iPSC-CMs and Fib-iPSC-CMs exhibited immature calcium handling properties as increases in beating rate were found to result in significant decreases in cytosolic calcium transient amplitudes, which is a hallmark of developmentally immature cardiac cells (CPC-iPSC: 100±16.5% vs 60.6±9.9%, n=20; Fib-iPSC: 100±12.6% vs 54.7±6.5%, n=24, p<0.05, Figure 5D) (27).
Figure 5. Characterization of intracellular calcium handling.
Calcium handling properties of single cardiomyocytes after Fluo-4 AM imaging at day 28±2 days post-differentiation. Cytoplasmic calcium levels are directly proportional to fluorescence intensity. (A) Quantification of peak amplitude of Fluo-4 AM dye calcium transients in CPC-iPSC-CMs and Fib-iPSC-CMs when paced at 1 Hz and 2 Hz. (B) Quantification of the peak rate of Ca2+ increase in CPC-iPSC-CMs and Fib-iPSC-CMs at 1 Hz and 2 Hz pacing. (C) Quantification of peak rate of Ca2+ decline presented as fluorescence ratio per second when iPSC-CMs are paced at 1 Hz and 2 Hz. No statistically significant differences were observed between both cell types in any of the studied parameters (CPC-iPSC-CM: n=20; Fib-iPSC-CM n=24). (D) Graph shows the significant differences between 1 Hz and 2 Hz of the Ca2+ peak amplitude in both cell types (CPC-iPSC: 100±16.5% vs. 60.6±9.9%, n=20; Fib-iPSC: 100±12.6% vs. 54.7±6.5%, n=24, p<0.05)
Electrophysiological properties
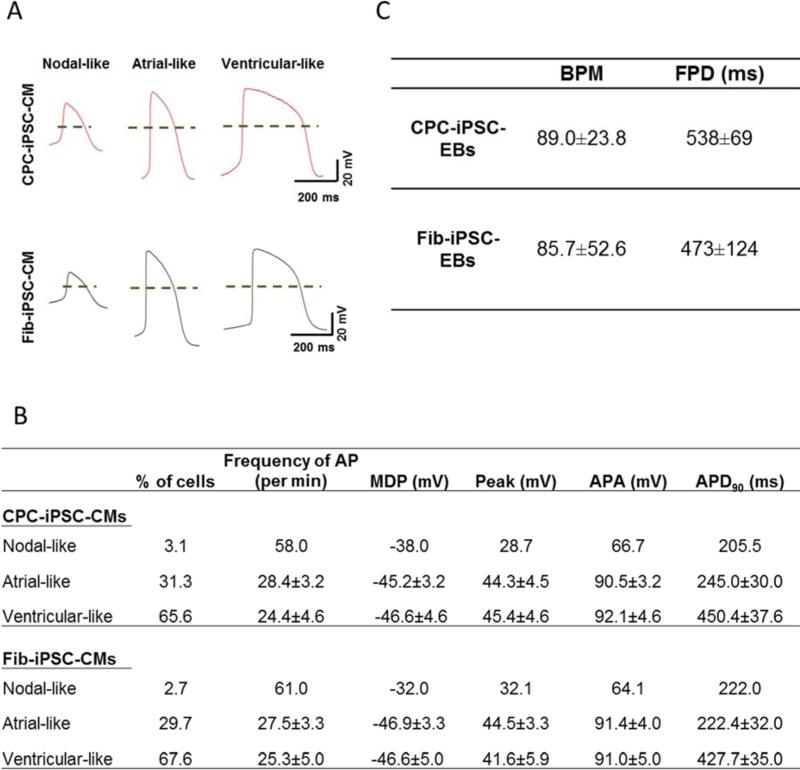
Lastly, electrophysiological properties of CPC-iPSC-CMs and Fib-iPSC-CMs were studied using patch clamp and MEA. Beating EBs were dissociated into single cells and seeded on Matrigel-coated coverslips for patch clamp. Action potential (AP) recordings at day 30±2 identified nodal-, atrial-, and ventricular-like CMs among the dissociated cells (Figure 6A). Measurement of key AP parameters, such as the frequency of AP, peak of AP, maximum diastolic potential, AP amplitude, and AP duration, did not uncover statistically significant differences between CPC-iPSC-CM and Fib-iPSC-CM subtypes (Figure 6B). MEA recordings show that spontaneous beating and electrical activity were present in all plated EBs regardless of the somatic cell origin (Supplemental Figure 12A-B and Videos 3S-4S). Both CPC-iPSC-CM and Fib-iPSC-CM beating EBs responded to 1 μM of isoproterenol with increased beating frequency (Supplemental Figure 12C-D). Electrophysiological parameters recorded at day 21±2 demonstrated similar average beating frequencies as indicated by the number of beats per minute (Figure 6C). Baseline field potential duration was slightly higher without reaching statistical significance in CPC-iPSC-EBs compared with Fib-iPSC-EBs (Figure 6C). Beating duration suggested that ventricular-like CMs were the predominant cell type in both types of EBs, consistent with single-cell patch clamp data. Taken together, our in vitro characterization results show no significant functional differences between CPC-iPSC-CMs and Fib-iPSC-CMs.
Figure 6. Electrophysiological characterization of CPC-iPSC-CMs and Fib-iPSC-CMs.
(A) Representative action potential (AP) recordings of nodal-, atrial-, and ventricular-like CPC-iPSC-CMs and Fib-iPSC-CMs. Dashes indicate 0 mV. (B) Comparison of key AP parameters between CPC-iPSC-CMs and Fib-iPSC-CMs. No significant differences in the analyzed parameters were observed between both groups. Data are presented as average ± SEM (CPC-iPSC-CMs, n=32; Fib-iPSC-CMs, n=37). (C) Baseline MEA electrophysiological parameters for beating embryoid bodies (EBs). Table depicts the comparison between CPC- and Fib-iPSC-EBs of beats per minute and field potential duration. Parameters are shown as average ± SD (n=10).
Endothelial and vascular smooth muscle differentiation efficiency
Cardiac progenitor cells give rise not only to CMs, but also to other cell types found normally in the heart (7-9). To investigate whether differences in epigenetic memory could contribute toward differentiation into cell fates other than CMs, we next differentiated both early passage CPC-iPSCs and Fib-iPSCs into endothelial cell (EC) and vascular smooth muscle cell (SMC) lineages using defined protocols (28,29). For endothelial cell differentiation, CD31 was used as a selection marker (18). FACS analysis revealed that the percentage of CD31+ cells resulting from differentiation was significantly higher in CPC-iPSCs when compared with Fib-iPSCs from the same donor (17.2±4.2% vs 9.7±1.9%, n=6; p<0.05; Supplemental Figure 13A). Immunostaining showed characteristic patterns of CD31 and von Willebrand factor in both CPC-iPSC-ECs and Fib-iPSC-ECs (Supplemental Figure 13B). For smooth muscle differentiation, α-smooth muscle actin (SMA) was used to identify SMCs. FACS analysis demonstrated that CPC-iPSCs produced higher numbers of SMCs than Fib-iPSCs (41.4±8.3% vs. 37.3±8.5%, n=6; p<0.05; Supplemental Figure 14A) when both lines were differentiated in parallel. Immunostaining showed characteristic patterns of α-SMA in both CPC-iPSC-SMCs and Fib-iPSC-SMCs (Supplemental Figure 14B).
Assessment of functional recovery after injection
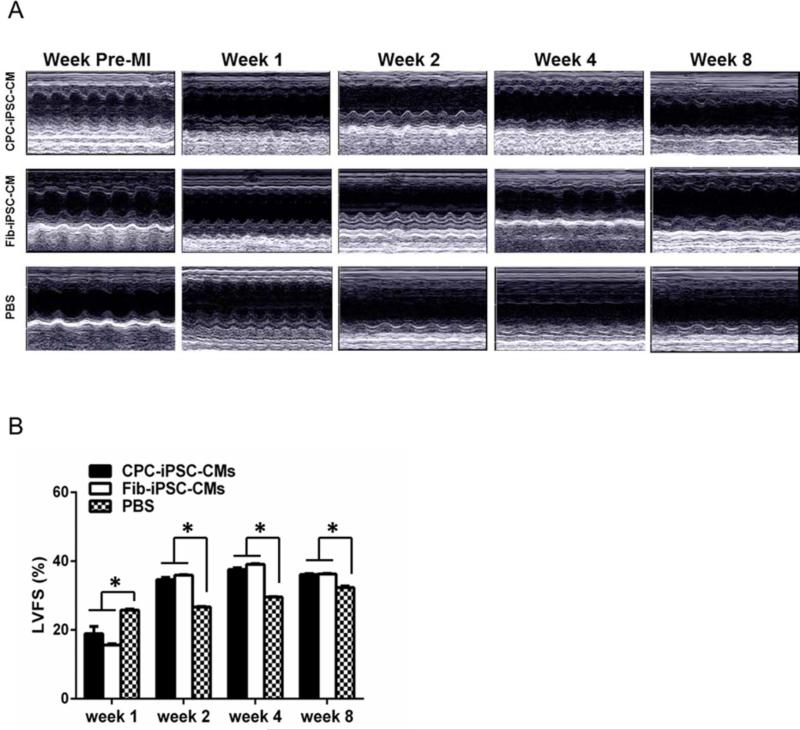
As in vitro characterization revealed no significant disparities in CPC-iPSC-CM and Fib-iPSC-CM function, we next compared in vivo therapeutic capacity of both cell types in a murine model of myocardial infarction (30). To track survival and engraftment of CPC-iPSC-CMs and Fib-iPSC-CMs in vivo, we first transduced CPC-iPSCs and Fib-iPSCs with a lentiviral construct driving constitutive expression of firefly luciferase and green fluorescent protein under the control of the ubiquitin promoter. CPC-iPSCs and Fib-iPSCs stably expressing the lentiviral construct were selected by FACS for isolation and expansion. Firefly luciferase expression in sorted CPC-iPSCs and Fib-iPSCs demonstrated no significant differences (Supplemental Figure 15A-B). For delivery into small animal models, approximately 1 million differentiated iPSC-CMs were further enriched by TMRM cell sorting and injected into the myocardium of SCID beige mice following ligation of the LAD artery, as previously described (30). Cell engraftment was measured noninvasively by bioluminescence imaging up to 42 days post-surgery (Supplemental Figure 16). Interestingly, CPC-iPSC-CMs demonstrated mildly higher levels of engraftment as compared to Fib-iPSC-CMs although both groups exhibited high levels of donor cell death. Assessment of cardiac functional recovery by echocardiography, however, revealed no significant differences between the CPC-iPSC-CM and Fib-iPSC-CM groups at weeks 1, 2, 4, and 8 after MI (Figure 7A). Measurements of the left ventricular wall thickness during systole and diastole did reveal that percentages of left ventricular fractional shortening (LVFS) were not significantly different between CPC-iPSC-CMs and Fib-iPSC-CMs over the 8 weeks, but were significantly higher in both compared to PBS control group (n=8; p<0.05; Figure 7B). The infarction area itself also was assessed with postmortem triphenyltetrazolium chloride staining (Supplemental Figure 17A-C). Hearts of animals receiving CPC-iPSC-CMs and Fib-iPSC-CMs exhibited smaller infarction sizes than PBS controls. However, no statistically significant differences in infarct size were observed between animals receiving CPC-iPSC-CMs versus those receiving Fib-iPSC-CMs (infarct size for CPC-iPSC-CMs: 21.9±2.4%, n=5; Fib-iPSC-CMs: 27.7±3.3%, n=5; PBS: 42.1±4.2%, n=3; p<0.05; Supplemental Figure 17D).
Figure 7. Echocardiographic evaluation of cardiac contractility.
(A) Representative images of infarcted hearts pre-MI and at weeks 1, 2, 4, and 8 post-infarction. (B) Graph shows average percentage of left ventricular fractional shortening (LVFS) respectively (n= 8) for the three groups at weeks 1, 2, 4, and 8 post-MI.
Taken together, these multiple levels of functional analysis indicate that animals receiving CPC-iPSC-CMs and Fib-iPSC-CMs exhibited greater recovery of cardiac function compared to the PBS controls; however, no significant differences were observed between the CPC-iPSC-CM and Fib-iPSC-CM cohorts.
Discussion
As cell fate decisions are largely controlled by epigenetic processes, understanding the epigenetic mechanisms underlying stem cell differentiation is crucial for clinical translation of reprogrammed cells. Previous reports have demonstrated that perturbation of epigenetic pathways can alter differentiation potential, skewing differentiation toward particular cell lineages and restricting alternative cell fates (20,31,32). Thus, characterization of the epigenetic processes involved in differentiation of pluripotent stem cells to cardiac fate would provide valuable insight into more efficient applications of iPSCs to treat heart disease. In this study, we demonstrate for the first time that human iPSCs derived from cardiac sources exhibit low levels of methylation on the early mesodermal cardiac promoter NKX2-5, and that these CPC-iPSCs are characterized by earlier and higher expression of genes involved in cardiomyocyte generation during differentiation, which leads to preferential differentiation of cell fate to cardiac lineages. Furthermore, we demonstrate that epigenetic memory of cardiac cell source derivation is eliminated over time (after 40 passages) and does not result in functional differences in vitro or in vivo. Our results suggest that differences in CpG methylation in the NKX2-5 promoter may constitute a crucial mechanism that contributes to different efficiencies observed during cardiac differentiation. Taken together, these findings form a broader understanding of epigenetic regulation of cardiac cell fate and offer a mechanism to improve cardiac differentiation of pluripotent stem cells.
To explain the higher cardiac differentiation efficiency observed in CPC-iPSCs, we examined gene expression levels of representative markers from each stage of cardiac differentiation. The markers studied included MESP1, HAND2, MYOCD, TBX5, GATA4, MEF2C, NKX2-5, and ISL1. Expression levels of cardiovascular progenitor cell markers NKX2-5 and ISL1 (17) in particular were found to be significantly higher in CPC-iPSCs at day 4, but not significantly different from Fib-iPSC expression by day 8 of differentiation. We believe that the differences in expression of NKX2-5 and ISL1 contribute to the disparity in efficiency of cardiac, endothelial, and smooth muscle differentiation in CPC-iPSCs and Fib-iPSCs, particularly in light of the critical role that both genes play in the specification and development of cardiomyocytes.
To elucidate the mechanism underlying the differential expression of transcription factors and cardiac markers, we compared methylation patterns of NKX2-5 in CPC-iPSC-CMs and Fib-iPSC-CMs. Our analysis focused on the methylation patterns of 11 CpGs within the CpG island located 1,150 bp upstream of the first exon of NKX2-5. Consistent with the gene expression levels presented for primary cultures, we observed significantly higher CpG methylation in the original fibroblasts compared to the CPCs. While tissue-specific methylation signatures diminished after reprogramming, silencing remained higher in Fib-iPSCs than in CPC-iPSCs. These findings suggest that certain methylation patterns in iPSCs remain close to the somatic cell types from which they were derived, providing evidence of somatic memory after reprogramming (20-22). Furthermore, these results demonstrate that somatic memory in iPSCs is retained during differentiation (21), and therefore can be observed during cardiac differentiation. Despite differences in methylation and cardiac differentiation efficiency, CPC-iPSC-CMs and Fib-iPSC-CMs were found to exhibit similar sarcomeric structures and in vitro characteristics when assessed by patch clamp, calcium handling, and MEA recording. Calcium handling recordings of both CPC-iPSC-CMs and Fib-iPSC-CMs produced comparable results in all the analyzed parameters. CPC-iPSC-CMs and Fib-iPSC-CMs demonstrated equally immature calcium-handling patterns as higher beating frequencies were not accompanied by increases in calcium transient amplitude (27). These results point to an immature state of iPSC-CMs, consistent with previous reports (33,34), and suggest that no residual somatic memory from CPCs contributes to CM maturation, which is a current limitation of iPSC-CMs.
To date, no functional comparison between iPSC-CMs derived from different somatic cell sources from the same human donor has been performed. Using small animal echocardiography, we found that the injection of iPSC-CMs improved functional recovery post MI as compared to animals receiving saline alone. However, significant differences were not observed between mice receiving CPC-iPSC-CMs versus Fib-iPSC-CMs. Taken together, these results prompt us to conclude that although injection of iPSC-CMs improves cardiac function after MI, the somatic origin from which iPSC-CMs are derived may not be critical.
Limitations
First, as reported in other manuscripts, the therapeutic cells used in our animal models of MI were observed to undergo high levels of acute donor cell death following in vivo delivery (35). This suggests that long-term functional benefits observed in animals receiving CPC-iPSC-CM and Fib-iPSC-CMs may not be so much the result of persistent donor iPSC-CM integration as due to the release of paracrine factors in the area of acute ischemic injury (36). Second, it is important to recognize that the methylation anaylsis in this study only has a correlative association with the differentiation efficiencies that we observed. Methylation assessment demostrated incomplete silencing of NKX2-5 in CPC-iPSCs as compared to Fib-iPSCs, which in turn was associated with higher levels of cardiac gene expression in CPC-iPSCs during the cardiac differentiation process, as well as higher yields of iPSC-CMs. These findings strongly suggest that epigenetic memory contributes to differentiation potential in generation of iPSC-CMs from somatic cell sources, but does not rule out other regulatory mechanisms of gene expression.
Conclusions
We report for the first time successful generation of iPSCs from CPCs and skin fibroblasts of the same human donors. Through close observation of iPSC development under directed cardiac differentiation protocols, we show that CPC-iPSCs demonstrate a greater efficiency of differentiation into CMs, ECs, and SMCs as compared to Fib-iPSCs. This is most likely due to incomplete epigenetic silencing of early cardiac mesodermal transcription factors such as NKX2-5. Interestingly, we did not observe downstream epigenetic effects upon the structure or function of CPC-iPSC-CMs and Fib-iPSC-CMs in vitro and in vivo. These findings identify an epigenetic regulatory mechanism that is important for differentiation of iPSCs and that may potentially be harnessed to accelerate clinical translation of iPSCs for treatment of cardiovascular diseases.
Supplementary Material
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE 1: The somatic source of induced human pluripotent stem cells is an important factor influencing their potential to differentiate into specific cell types.
COMPETENCY IN MEDICAL KNOWLEDGE 2: Epigenetic memory in human induced human pluripotent stem cells influences the efficiency of cardiac differentiation through regulation of gene expression without appreciably affecting the function of generated cardiomyocytes.
TRANSLATIONAL OUTLOOK: Better understanding of the effects of somatic cell source selection upon cardiac differentiation could accelerate clinical application of induced human pluripotent stem cells.
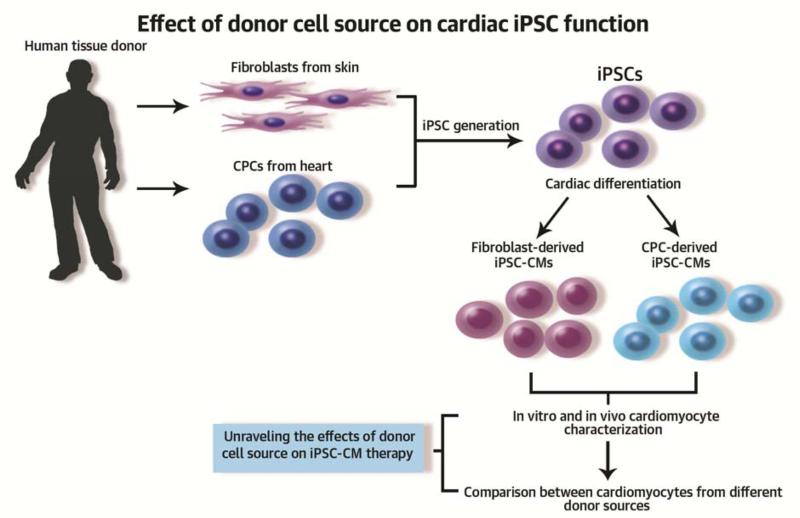
Central Illustration: Skin fibroblast and cardiac progenitor cell (CPC) primary cultures were established from the same human donors.
Somatic cells were reprogrammed into induced pluripotent stem cells (iPSCs) by overexpression of the pluripotency transcription factors Oct4, Sox2, Klf4, and c-Myc. Established iPSCs derived from fibroblasts (Fib-iPSC) and CPCs (CPC-iPSC) were differentiated into cardiomyocytes. Differentiation efficiency was found to be higher in cardiomyocytes derived from CPC-iPSC (CPC-iPSC-CM) compared to Fib-iPSC (Fib-iPSC-CM). In vitro experiments demonstrated that CPC-iPSCs express higher levels of cardiac transcription factors during cardiac differentiation compared to Fib-iPSCs. Epigenetic assessment showed higher methylation in the promoter region of the cardiac transcription factor NKX2-5 in Fib-iPSC-CMs compared to CPC-iPSC-CMs. However, no significant differences in their morphological and electrophysiological properties were observed. Interestingly, in vivostudies using mouse models of myocardial infarction showed comparable therapeutic effects between CPC-iPSC-CM and Fib-iPSC-CM. In summary, while epigenetic memory improves cardiac differentiation efficiency in vitro, the in vivo functional recovery following cell transplantation is equivalent
Acknowledgements
We would like to thank Dr. Enrique G. Navarrete for his assistance with the MEA system. We are grateful to Stanford Neuroscience Microscopy Service, for assistance with the confocal microscope. We thank Dr. Joseph Gold for critical reading of the manuscript.
Funding Sources: We thank the Swiss National Science Foundation SNF PBBEP3_129803 (V.S.F.), German Research Foundation DFG-Hu-1857/1-1 (B.C.H.), National Institutes of Health grants R01HL113006, U01HL099776, P01GM099130, and R24HL117756, American Heart Association Established Investigator Award 14420025, and California Institute of Regenerative Medicine grants DR2-05394 and TR3-05556 (J.C.W.) for funding for this study.
Abbreviations List
- CM
Cardiomyocyte
- CPC
Cardiac progenitor cell
- cTnT
Cardiac troponin T
- EB
Embryoid body
- EC
Endothelial cell
- Fib
Fibroblast
- iPSC
Induced pluripotent stem cell
- MI
Myocardial infarction
- Sca
Stem cell antigen
- SMC
Smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: Dr. Lee and Dr. Wu hold shares in Stem Cell Theranostics.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohsin S, Siddiqi S, Collins B, et al. Empowering adult stem cells for myocardial regeneration. Circ Res. 2011;109:1415–28. doi: 10.1161/CIRCRESAHA.111.243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Sun N, Panetta NJ, Gupta DM, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci. 2009;106:15720–5. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narsinh KH, Sun N, Sanchez-Freire V, et al. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121:1217–21. doi: 10.1172/JCI44635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang CP, Bruneau BG. Epigenetics and cardiovascular development. Annual review of physiology. 2012;74:41–68. doi: 10.1146/annurev-physiol-020911-153242. [DOI] [PubMed] [Google Scholar]
- 7.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh PC, Segers VF, Davis ME, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–4. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuura K, Nagai T, Nishigaki N, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–91. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 11.Lian X, Hsiao C, Wilson G, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci. 2012;109:E1848–57. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urbanek K, Torella D, Sheikh F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci. 2005;102:8692–7. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bondue A, Tannler S, Chiapparo G, et al. Defining the earliest step of cardiovascular progenitor specification during embryonic stem cell differentiation. J Cell Biol. 2011;192:751–65. doi: 10.1083/jcb.201007063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincentz JW, Barnes RM, Firulli AB. Hand factors as regulators of cardiac morphogenesis and implications for congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2011;91:485–94. doi: 10.1002/bdra.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Cao D, Wang Q, Wang DZ. Synergistic activation of cardiac genes by myocardin and Tbx5. PLoS One. 2011;6:e24242. doi: 10.1371/journal.pone.0024242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Li T, Liu Y, et al. WNT signaling promotes Nkx2.5 expression and early cardiomyogenesis via downregulation of Hdac1. Biochim Biophys Acta. 2009;1793:300–11. doi: 10.1016/j.bbamcr.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Scott IC. Life before Nkx2.5: cardiovascular progenitor cells: embryonic origins and development. Curr Top Dev Biol. 2012;100:1–31. doi: 10.1016/B978-0-12-387786-4.00001-4. [DOI] [PubMed] [Google Scholar]
- 18.Moretti A, Caron L, Nakano A, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Cai CL, Liang X, Shi Y, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–90. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lister R, Pelizzola M, Kida YS, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim K, Zhao R, Doi A, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:1117–9. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 24.Davis RP, Casini S, van den Berg CW, et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation. 2012;125:3079–91. doi: 10.1161/CIRCULATIONAHA.111.066092. [DOI] [PubMed] [Google Scholar]
- 25.Greenstein JL, Winslow RL. Integrative systems models of cardiac excitation-contraction coupling. Circ Res. 2011;108:70–84. doi: 10.1161/CIRCRESAHA.110.223578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu JD, Li J, Tweedie D, et al. Crucial role of the sarcoplasmic reticulum in the developmental regulation of Ca2+ transients and contraction in cardiomyocytes derived from embryonic stem cells. FASEB. 2006;20:181–3. doi: 10.1096/fj.05-4501fje. [DOI] [PubMed] [Google Scholar]
- 27.Dolnikov K, Shilkrut M, Zeevi-Levin N, et al. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006;24:236–45. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- 28.James D, Nam HS, Seandel M, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–6. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ge X, Ren Y, Bartulos O, et al. Modeling supravalvular aortic stenosis syndrome with human induced pluripotent stem cells. Circulation. 2012;126:1695–704. doi: 10.1161/CIRCULATIONAHA.112.116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Narsinh KH, Lan F, et al. Early stem cell engraftment predicts late cardiac functional recovery: preclinical insights from molecular imaging. Circ Cardiovasc Imaging. 2012;5:481–90. doi: 10.1161/CIRCIMAGING.111.969329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bar-Nur O, Russ HA, Efrat S, et al. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Polo JM, Liu S, Figueroa ME, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–55. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Fu JD, Siu CW, et al. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 2007;25:3038–44. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- 34.Itzhaki I, Rapoport S, Huber I, et al. Calcium handling in human induced pluripotent stem cell derived cardiomyocytes. PLoS One. 2011;6:e18037. doi: 10.1371/journal.pone.0018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Lee A, Huang M, et al. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009;53:1229–40. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu M, Nguyen PK, Lee AS, et al. Microfluidic single-cell analysis shows that porcine induced pluripotent stem cell-derived endothelial cells improve myocardial function by paracrine activation. Circ Res. 2012;111:882–93. doi: 10.1161/CIRCRESAHA.112.269001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.