INTRODUCTION
Heart Failure with Preserved Ejection Fraction: A Major Health Care Problem with No Proven Therapy
Heart failure with preserved ejection fraction (HFPEF) is a relatively recently recognized disorder and the fastest growing form of HF.1, 2 HFPEF is nearly exclusively found in older persons, particularly women, in whom 90% of new HF cases are HFPEF.3 HFPEF is associated with markedly increased morbidity, mortality, and health care expenditures.4–7 Despite its importance, the prognosis of HFPEF is worsening, its pathophysiology is poorly understood, and no medication trials have had positive effect on their primary endpoints.2 Consequently, there are no evidence-based guideline-recommendations for improving clinical outcomes in the growing population of elderly HFPEF patients.
Exercise Intolerance is the Primary Symptom in HFPEF Patients
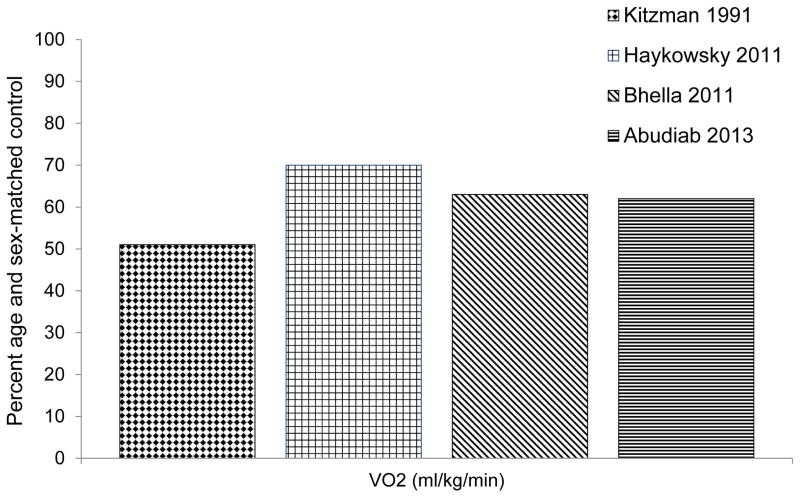
The primary chronic symptom in HFPEF patients, even when well compensated, is severe exercise intolerance, which can be measured objectively during whole body exercise as decreased peak exercise oxygen uptake (peak VO2).8–19 Specifically, peak VO2 in HFPEF patients is 40% lower than age and sex-matched controls (Figure 1). Reduced exercise tolerance is a strong determinant of prognosis and reduced quality of life.10, 20 A clear understanding of the pathophysiology of exercise intolerance is necessary to guide future therapies aimed at improving HFPEF patients’ symptoms.
Figure 1.
Peak oxygen uptake in HFFEF patients. Data from Refs 8,12,15,18.
Pathophysiology of Exercise Intolerance in HFPEF Patients
In accordance with the Fick principle, the amount of oxygen consumed per minute is equal to the product of cardiac output and arterial-venous oxygen content difference; therefore the reduced peak VO2 in HFPEF patients may be due to decreased oxygen delivery to and/or impaired oxygen extraction by the exercising skeletal muscles (Figure 2).10 Cross-sectional studies by Borlaug et al. suggest that the lower peak VO2 in HFPEF patients compared to age-matched healthy or co-morbidity matched controls without HF was associated with reduced peak cardiac output which was due primarily to blunted heart rate response, myocardial contractility, and peripheral vascular vasodilator reserve.16–19 A series of studies by Kitzman et al. extended these results by demonstrating that the lower peak VO2 in older HFPEF patients versus age-matched healthy controls was due not only to reduced peak exercise cardiac output but also due to an equal contribution of reduced systemic arterial-venous oxygen content difference.8, 12 Moreover, the change in arterial-venous oxygen content difference from rest to peak exercise was the strongest independent predictor of peak VO2 for both HFPEF patients and controls.12 Bhella et al. confirmed that non-cardiac ‘peripheral’ factors play an important role in limiting exercise tolerance as the reduced peak VO2 in older HFPEF patients compared age-matched healthy controls occurred despite no significant difference in peak exercise cardiac output between groups.15 Potential peripheral mechanisms that may limit exercise capacity include decreased skeletal muscle mass, reduced type I (oxidative-fatigue resistant) muscle fibers, and impaired blood flow to and/or extraction by the active skeletal muscles.10
Figure 2.
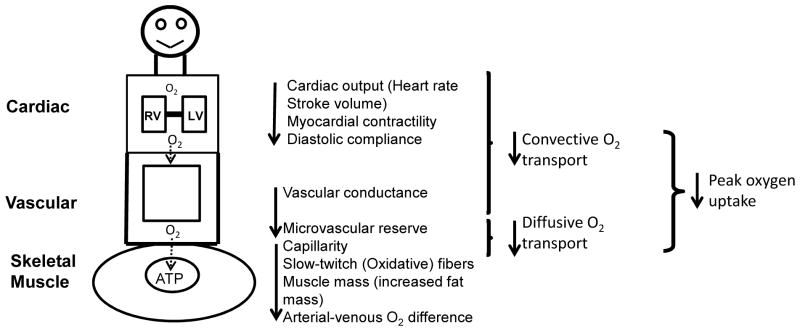
Determinants of exercise intolerance in HFPEF patients. ATP, adenosine triphosphate; RV, right ventricle; LV, left ventricle; O2, oxygen.
Skeletal Muscle Mass and Oxygen Utilization and Exercise Intolerance in HFPEF Patients
Most of the oxygen consumed during the transition from rest to peak cycle exercise occurs in the active muscles, therefore a loss in metabolically active tissue may contribute to exercise intolerance in HFPEF patients.14, 21 Our group tested this hypothesis and compared lean body mass and peak VO2 in 60 older HFPEF patients and 40 age-matched healthy controls.14 Three novel findings were reported. First, the percent total lean body mass and percent leg lean mass were significantly lower in HFPEF patients compared to healthy controls.14 Second, peak VO2 indexed to total lean body mass or leg lean mass was significantly lower in elderly HFPEF versus healthy controls.14 Third, the change in peak VO2 with increasing percent leg lean mass was markedly reduced in HFPEF patients compared to healthy controls (HFPEF mean slope: 11 ± 5 ml O2/min vs. healthy controls mean slope: 36 ± 5 ml O2/min, p<0.001). Taken together, these findings show that older HFPEF patients have decreased lean muscle mass, however they also have abnormal O2 utilization that is independent of and in addition to the reduced muscle mass.
Impaired Muscle Blood Flow and Exercise Intolerance in HFPEF Patients
In healthy older adults, the 11-fold increase in blood flow to the active muscles during the transition from rest to peak cycle exercise is due to sympathetic-mediated redistribution of blood from non-exercising regions to the working muscles coupled with metabolic-mediated vasodilation in the exercising muscles.22, 23 Importantly, changes in central and peripheral arterial function may result in inefficient distribution of cardiac output to the active muscles and contribute to exercise intolerance in HFPEF patients.10
Kitzman et al. compared carotid arterial and proximal thoracic aortic distensibility in healthy younger (≤30 years) and older individuals (≥60 years) and older HFPEF patients.24, 25 The novel finding of these studies was that carotid arterial distensibility and proximal thoracic aortic distensibility were reduced in older HFPEF patients beyond the changes that occur with normal aging alone and both were directly related to peak VO2. These findings suggest that increased central arterial stiffness contributes to exercise intolerance in older HFPEF patients and may be a potential therapeutic target.
Impaired peripheral arterial endothelial function may result in impaired exercise blood flow reserve in HFPEF patients. Haykowsky et al. assessed brachial artery flow mediated dilation in response to 5 minutes of cuff ischemia, a non-invasive measure of arterial endothelial function, in 47 younger and older healthy controls (mean age: 25 years and 70 years, respectively) and 66 older HFPEF patients (mean age: 70 years).11 The major finding of this study was that brachial artery flow mediated dilation was significantly reduced in healthy older compared with healthy young control participants, however brachial artery flow mediated dilation was not significantly different in HFPEF patients compared with healthy age-matched older controls.11 Hundley and associates, using phase contrast magnetic resonance imaging, measured the change in superficial femoral artery cross sectional area and velocity at baseline and in response to 5 minutes of thigh cuff occlusion, in elderly HFPEF patients and age-matched healthy controls.26 The change in superficial femoral artery cross sectional area and velocity were not significantly different between HFPEF patients compared to healthy controls.26 Taken together, large conduit arterial endothelial function is preserved and may not limit exercise tolerance in older HFPEF patients. An important feature of the above studies was exclusion of patients with any evidence of clinical atherosclerosis which is known to independently reduce endothelial function.
Although conduit arterial endothelial function appears to be preserved in HFPEF impaired microvascular function may limit exercise performance in older HFPEF patients. Borlaug et al, using automated finger-tip plethysmography in response to cuff ischemia and peak exercise as a measure of microvasculature reserve, reported that the change in finger blood flow in response to cuff occlusion or cycle exercise was reduced in elderly HFPEF patients compared with age-matched healthy control participants but was not different in HFPEF patients compared with hypertensive control participants without HF.16 A consequence of the blunted microvascular reserve is that it may be associated with decreased diffusive oxygen transport to the active muscle which would reduce exercise tolerance. Indeed, Borlaug et al. found that systemic vascular conductance and microvascular reserve were positively related to peak VO2 in HFPEF.16
Skeletal Muscle Composition, Fiber type, and Capillarity and Exercise Intolerance in HFPEF Patients
Increased intermuscular adipose has been reported in a number of conditions associated with severely reduced physical function, including heart failure with reduced ejection fraction and aging, and is potentially modifiable. Haykowsky et al. recently examined the composition of thigh muscle and its relationship to peak VO2 in 23 older HFpEF patients compared to 15 age-matched healthy controls.27 Despite no significant inter-group differences in total thigh area or subcutaneous adipose area, HFPEF patients had significantly increased intermuscular adipose area (35.6±11.5 vs. 22.3±7.6 cm2, p=0.01) and ratio of intermuscular adipose to skeletal muscle area (0.38±0.10 vs. 0.28±0.09, p=0.007).27 In multivariate analyses, intermuscular adipose area and intermuscular adipose to muscle area (partial r= −0.51 and r= −0.45 respectively, p<0.01 for both) were independent predictors of peak VO2.27 Thus abnormal skeletal muscle composition contributes to the severely reduced exercise capacity in older HFPEF patients and is a potential therapeutic target.
It is well established that patients with HF and reduced ejection fraction have multiple skeletal muscle abnormalities including reduced percent type I (oxidative) fibers and oxidative enzymes, reduced volume density of mitochondria, and surface density of mitochondrial cristae.28–31 Kitzman et al. recently reported that compared to healthy controls, older HFPEF patients exhibited a shift in skeletal muscle fiber type distribution with reduced percent slow-twitch type I fibers, type I/type II fiber ratio and capillary to fiber ratio, and these alterations are associated with their severely reduced peak exercise VO2.32 A consequence of the fiber type shift from oxidative to glycolytic fibers coupled with abnormal mitochondrial function is that it may impair oxidative metabolism during exercise. In a preliminary report, Bhella et al found reduced leg muscle oxidative metabolism by magnetic resonance imaging during exercise in HFPEF patients.15 Accordingly, interventions that reverse skeletal muscle oxidative dysfunction may result in a concomitant increase in peak VO2 in HFPEF patients.
THERAPEUTIC OPTIONS AND CLINICAL OUTCOMES
Effects of Physical Conditioning on Exercise Tolerance in HFPEF Patients
Kitzman et al. performed the first single-center, single-blind, medically supervised, randomized controlled trial comparing the effects of 4 months of endurance exercise training versus attention control in 46 clinically stable older (mean age = 70 years) HFPEF patients.33 The novel finding of this study was that 4 months of endurance exercise training increased peak VO2, ventilatory anaerobic threshold, distance walked in six minutes, improved physical quality of life without altering left ventricular mass, mass to volume ratio, ejection function, diastolic filling or neuroendocrine function.33
In a follow-up analysis, the determinants of the training related increase in peak VO2 were examined. There was a modest but significant increase in peak heart rate, but a modest decline in peak stroke volume, such that there was no significant change in peak cardiac output with training.13 However, there was a significant training-related increase in peak arterial venous oxygen content difference, and in multivariate analysis this accounted for nearly 100% of the improvement in peak VO2.13 This indicated that the endurance exercise training-related improvement in peak VO2 was due to peripheral adaptations.13
Kitzman et al. performed a second, separate, randomized, attention-controlled, single-blind trial of exercise training, to determine if improved arterial function, measured either as carotid arterial stiffness or brachial artery flow mediated dilation, improved with training and accounted for the training related improvement in peak VO2.34 The study included 54 older HFPEF patients (mean age = 70 years). The investigators found that 4 months of upper and lower extremity endurance exercise training resulted in a significant increase in peak VO2 without altering carotid arterial stiffness or brachial artery flow-mediated dilation.34 This finding suggested, by elimination, that skeletal muscle adaptations may account for the training related increase in peak VO2 in older HFPEF patients.34 Notably, in both trials the change exercise capacity was clinically meaningful as the baseline peak VO2 in HFPEF patients randomly assigned to endurance exercise training was at or below the VO2 threshold required for independent living and was above this value after the 4 month intervention.34
Edelmann et al. performed the first multicenter randomized controlled exercise trial comparing the effects of 3 months of combined endurance and strength training versus usual care on peak VO2, six minute walk distance, cardiac morphology, diastolic function, HF biomarkers, and quality of life in 64 clinically stable HFPEF patients (mean age = 65 years).35 The major novel finding was that 3 months of endurance and strength training significantly improved peak VO2, ventilatory anaerobic threshold, quality of life, and resting measures of left atrial volume, early diastolic mitral annulus velocity, early transmitral inflow velocity to early diastolic mitral annulus velocity (E/e′) ratio, and procollagen type I levels compared to usual care. Moreover, the increase in peak VO2 was correlated with the improvement in resting E/e′.35 In contrast, Smart et al. demonstrated that the increase in peak VO2 after 4 months of moderate-intensity endurance exercise was not associated with a change in resting systolic and diastolic function or quality of life in 30 HFPEF patients (mean age = 65 years).36
Taken together, the relatively small number of studies performed to date indicate that endurance exercise training with or without supplemental strength training is an effective non-pharmacological therapy that improves clinically stable HFPEF patients exercise tolerance. The improvement in peak VO2 appears to be primarily due to favorable microvascular or skeletal muscle adaptations that increase diffusive oxygen transport and/or oxygen utilization by the working muscles.10
COMPLICATIONS AND CONCERNS
Diagnostic utility of exercise stress testing in HFPEF
Cardiopulmonary exercise testing (CPX) with or without invasive hemodynamic monitoring, may enhance the diagnosis and treatment of HPFEF patients, particularly those with early stage disease in whom abnormalities in hemodynamic and cardiovascular reserve may only occur during incremental to peak exercise.37 Moreover, non-invasive CPX testing with expired gas analysis is the gold-standard measure of aerobic (cardiorespiratory) fitness and provides important clinical information regarding risk stratification and prognosis, as well as an assessment of functional performance. 20 CPX testing can also identify abnormalities in individual patients that can have therapeutic implications, such as severe chronotropic incompetence and exaggerated blood pressure response to exercise.20 Finally, CPX testing provides information (heart rate, power output, VO2 and rate of perceived exertion at the ventilation threshold and during peak exercise) that can be used by heart failure specialists and exercise specialists to prescribe endurance training programs for clinically stable HFPEF patients as part of a comprehensive cardiac rehabilitation program.20
Safety of Supervised Exercise Training in HFPEF Patients
The safety of supervised endurance training performed alone or combined with supplemental strength training was reported in three randomized controlled trails.33–35 In all studies, no major adverse cardiac events were reported during the 3 to 4 month training period, however 20% of the HFPEF patients in the combined endurance and strength training program conducted by Edelmann et al. reported mild skeletal muscle discomfort with exercise.35 Thus, it appears that in clinically stable older HFPEF patients who do not have any contraindications to exercise testing or training can safely participate in medically supervised 3 to 4 month physical conditioning training program. Although the efficacy of home based exercise training after completed supervised training has not been studied in HFPEF patients, findings from the HF-ACTION trial suggest that 40% of HF patients with reduced ejection fraction do not adhere to unsupervised exercise training.38 Adherence to home based exercise training, can be facilitated by a number of strategies during regular clinic visits to overcome barriers to exercise: provide information of the safety of exercise; discuss preferred modes of exercise and encourage activities the patient prefers; discuss how exercise can fit into HF patients lives; set realistic goals for increasing physical activity; provide information on how regular exercise improves symptoms; provide positive reinforcement for exercise adherence.39
FUTURE DIRECTIONS
Traditional exercise training programs for HFPEF patients have primarily focused on moderate-intensity endurance exercise training, however Tomczak and associates found that an acute bout of high-intensity treadmill exercise (4 minute interval performed on a treadmill at 95% peak heart rate interspersed by a 3 minute recovery period performed at 76% peak heart rate, repeated 4 times) was associated with an increase in post exercise left and right ventricular ejection fraction in clinically stable HF patients with reduced ejection fraction.40 A recent systematic review and meta-analysis extended these findings by reporting that vigorous-to-maximal aerobic interval exercise was superior to moderate-intensity continuous endurance training for improving peak VO2 in HF patients with reduced ejection fraction.41
The benefits and optimal intensity of strength training performed alone or in combination with endurance training to improve peak VO2, skeletal muscle morphology and function remains unknown. Given these uncertainties, the recent National Heart, Lung, and Blood Institute working group on ‘Exercise as Therapy for Heart Failure’ recommended future research regarding innovative exercise training modalities: What is the optimal training intensity (high-intensity versus moderate-intensity continuous exercise), mode (large versus small muscle mass training +/− resistance training) and duration of training (short-term: 2–3 months versus one year) to improve cardiovascular and skeletal muscle function, health status, physical functional performance and survival in HF patients.42
SUMMARY
The primary chronic symptom in HFPEF patients, even when well compensated, is severe exercise intolerance. Recent advances in the pathophysiology of exercise intolerance in HFPEF patients suggest that non-cardiac ‘peripheral’ factors contribute to the reduced peak VO2, and are the major contributor to its improvement following endurance exercise training. Although the peripheral adaptations responsible for the increase in peak VO2 after endurance training are unknown, they may be the result of favorable changes in microvascular and skeletal muscle function that result in increased diffusive O2 transport and/or greater oxygen utilization by the working muscles. Currently, there is no guideline recommended therapy that improves clinical outcomes in HFPEF patients. A greater understanding of the peripheral skeletal muscle vascular adaptations that occur with physical conditioning may allow for individually tailored exercise rehabilitation programs for HFPEF patients to improve their primary chronic symptom, exercise intolerance. Furthermore, the identification of specific mechanisms that improve whole body and peripheral skeletal muscle oxygen uptake could establish potential therapeutic targets for medical therapies and a means to follow therapeutic response.
KEY POINTS.
Heart failure with preserved ejection fraction (HFPEF) is the most common and fastest growing form of HF.
HFPEF is associated with markedly increased morbidity, mortality, and health care expenditures.
The prognosis of HFPEF is worsening, its pathophysiology is poorly understood, and no medications have been proven to be effective.
The primary chronic symptom in HFPEF patients, even when well compensated, is severe exercise intolerance, measured objectively as decreased peak oxygen uptake (peak VO2).
Recent advances in the pathophysiology of exercise intolerance in HFPEF suggest that non-cardiac ‘peripheral’ factors contribute to the reduced peak VO2, and are the major contributor to its improvement following supervised endurance exercise training.
Acknowledgments
Dr. Haykowsky is the Exercise Physiology team lead for the Alberta Heart study funded by Alberta Innovates Health Solutions (AIHS). Dr. Kitzman research was funded by NIH grants R37AG18917 and P30AG21332.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Redfield MM. Understanding “diastolic” heart failure. N Engl J Med. 2004;350:1930–1931. doi: 10.1056/NEJMp048064. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: The cardiovascular health study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 4.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: Influence of left ventricular systolic function. The cardiovascular health study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Liao L, Jollis JG, Anstrom KJ, Whellan DJ, Kitzman DW, Aurigemma GP, Mark DB, Schulman KA, Gottdiener JS. Costs for heart failure with normal vs reduced ejection fraction. Arch Intern Med. 2006;166:112–118. doi: 10.1001/archinte.166.1.112. [DOI] [PubMed] [Google Scholar]
- 6.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: Trends in incidence and survival in a 10-year period. Arch Intern Med. 1999;159:29–34. doi: 10.1001/archinte.159.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: Failure of the frank-starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 9.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 10.Haykowsky M, Brubaker P, Kitzman D. Role of physical training in heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2012;9:101–106. doi: 10.1007/s11897-012-0087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haykowsky MJ, Herrington DM, Brubaker PH, Morgan TM, Hundley WG, Kitzman DW. Relationship of flow-mediated arterial dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. J Gerontol A Biol Sci Med Sci. 2013;68:161–167. doi: 10.1093/gerona/gls099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: Role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–75. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 18.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borlaug BA. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circ J. 2013 doi: 10.1253/circj.cj-13-1103. [DOI] [PubMed] [Google Scholar]
- 20.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J. Eacpr/aha scientific statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–2274. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell JH, Blomqvist G. Maximal oxygen uptake. N Engl J Med. 1971;284:1018–1022. doi: 10.1056/NEJM197105062841809. [DOI] [PubMed] [Google Scholar]
- 22.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 23.Katz SD, Zheng H. Peripheral limitations of maximal aerobic capacity in patients with chronic heart failure. J Nucl Cardiol. 2002;9:215–225. doi: 10.1067/mnc.2002.123183. [DOI] [PubMed] [Google Scholar]
- 24.Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J, Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61:112–119. doi: 10.1161/HYPERTENSIONAHA.111.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 26.Hundley WG, Bayram E, Hamilton CA, Hamilton EA, Morgan TM, Darty SN, Stewart KP, Link KM, Herrington DM, Kitzman DW. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol. 2007;292:H1427–1434. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 27.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 30.Mancini DM, Coyle E, Coggan A, Beltz J, Ferraro N, Montain S, Wilson JR. Contribution of intrinsic skeletal muscle changes to 31p nmr skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation. 1989;80:1338–1346. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- 31.Schaufelberger M, Eriksson BO, Grimby G, Held P, Swedberg K. Skeletal muscle alterations in patients with chronic heart failure. Eur Heart J. 1997;18:971–980. doi: 10.1093/oxfordjournals.eurheartj.a015386. [DOI] [PubMed] [Google Scholar]
- 32.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014 doi: 10.1152/ajpheart.00004.2014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A, Haykowsky MJ. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: Results of the ex-dhf (exercise training in diastolic heart failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 36.Smart NA, Haluska B, Jeffriess L, Leung D. Exercise training in heart failure with preserved systolic function: A randomized controlled trial of the effects on cardiac function and functional capacity. Congest Heart Fail. 2012;18:295–301. doi: 10.1111/j.1751-7133.2012.00295.x. [DOI] [PubMed] [Google Scholar]
- 37.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keteyian SJ, Pina IL, Hibner BA, Fleg JL. Clinical role of exercise training in the management of patients with chronic heart failure. J Cardiopulm Rehabil Prev. 2010;30:67–76. doi: 10.1097/HCR.0b013e3181d0c1c1. [DOI] [PubMed] [Google Scholar]
- 39.Conraads VM, Deaton C, Piotrowicz E, Santaularia N, Tierney S, Piepoli MF, Pieske B, Schmid JP, Dickstein K, Ponikowski PP, Jaarsma T. Adherence of heart failure patients to exercise: Barriers and possible solutions: A position statement of the study group on exercise training in heart failure of the heart failure association of the european society of cardiology. Eur J Heart Fail. 2012;14:451–458. doi: 10.1093/eurjhf/hfs048. [DOI] [PubMed] [Google Scholar]
- 40.Tomczak CR, Thompson RB, Paterson I, Schulte F, Cheng-Baron J, Haennel RG, Haykowsky MJ. Effect of acute high-intensity interval exercise on postexercise biventricular function in mild heart failure. J Appl Physiol. 2011;110:398–406. doi: 10.1152/japplphysiol.01114.2010. [DOI] [PubMed] [Google Scholar]
- 41.Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: The benefit depends on the type of training performed. J Am Coll Cardiol. 2007;49:2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 42.Fleg J, Cooper LS, Borlaug BA, et al. Exercise training as therapy for heart failure: results from a national heart, lung, and blood working group. Submitted for publication. [Google Scholar]