Abstract
Rationale
Previous studies demonstrate the neuroprotective effects of progesterone in numerous animal injury models, but a systematic dose–response study in a transient ischemic stroke model is lacking.
Objectives
We investigated the effects of progesterone at different doses on post-stroke brain infarction and functional deficits in middle-aged rats.
Methods
Cerebral ischemia was induced in 13-month-old male Sprague–Dawley rats by right middle cerebral artery occlusion for 2 h followed by reperfusion. Rats received intraperitoneal injections of 8, 16, or 32 mg/kg of progesterone (P8, P16, P32) or vehicle at 2 h post-occlusion followed by subcutaneous injections at 6 h and every 24 h post-injury for 7 days. Functional recovery was evaluated at intervals over 22 days using motor, sensory, and cognitive tests. Infarct size was evaluated at 22 days post-stroke.
Results
Repeated-measures ANOVA showed significant group effects on grip strength, rotarod, and sensory neglect. All progesterone-treated groups had improved (p<0.05) spatial memory performance. The P8 and P16 groups showed maximum improvement in long-term memory compared to vehicle. Significant (p<0.05) gait impairments were observed in the vehicle group compared to shams. Animals receiving the P8 dose showed maximum gait improvement compared to vehicle. Post hoc analysis revealed that the P8 and P16 groups showed significant attenuation in infarct volume compared to vehicle. Animals receiving the P32 dose did not show any effect on infarct volume.
Conclusions
Although all doses were somewhat effective, progesterone given at 8 mg/kg led to the most consistent improvements across a panel of behavioral/functional tests and reduced the severity of ischemic infarct injury.
Keywords: Stroke, Progesterone, Dose response, Functional outcomes, Infarct
Introduction
Stroke occurs every 40 s in the USA and takes a life every 4 min (Roger et al. 2012). Ischemic stroke accounts for 87 % of all strokes (Muntner et al. 2006). Aging is a major non-modifiable risk factor, and an aged person with stroke is more likely to suffer cognitive impairments. One in six older people suffer a stroke, and 30 % of these individuals develop vascular dementia or vascular cognitive impairment (Savva and Stephan 2010).
A number of clinical trials seeking a safe and effective stroke treatment have failed in recent years and tissue plasminogen activator (tPA) remains one of the few pharmacologic treatment options. Unfortunately, tPA has a very narrow therapeutic time window and can have severe side effects including intracerebral hemorrhage. Mortality rates reach a striking 75 % at 3 months (The NINDS t-PA Stroke Study Group 1997; Strbian et al. 2011). Using tPA for stroke has been compared to using “gasoline to put out fire” (Millard 2013). Therefore, there is an urgent need to develop a safe and effective drug with a wide therapeutic window.
Progesterone (PROG), an ovarian and adrenal steroid, is also a potent neurosteroid (Schumacher et al. 1996; Baulieu et al. 2001) because it is also synthesized in the brain. Numerous pre-clinical studies have reported its efficacy in a variety of injury models (for review see Deutsch et al. 2013). PROG’s efficacy and safety have been demonstrated in two independent phase II clinical trials for moderate to severe traumatic brain injury (TBI) (Wright et al. 2007; Xiao et al. 2008). PROG effects are currently being studied under two independent phase III clinical trials for TBI (Protect III, at http://www.clinicaltrials.gov/NCT00822900; SyNAPSe, at http://www.synapse-trial.com). Increasing pre-clinical evidence from our and other laboratories around the world provides important corroboration that PROG treatment improves functional recovery and reduces brain infarction in different stroke models (Atif et al. 2013; Yousuf et al. 2013; De Nicola et al. 2013; Gibson et al. 2011; Wong et al. 2013).
The Stroke Treatment Academic Industry Roundtable (STAIR) committee, which seeks to advance the development of acute and restorative stroke therapies (Albers et al. 2011), recommends that preclinical stroke studies include dose–response relationships, extend therapeutic time windows, and test efficacy in multiple stroke models, aged animals, and animals with comorbidities. In the present study, we evaluated dose–response relationships for PROG treatment following ischemic stroke in older rats. We tested the therapeutic effects of three dosages of PROG (8, 16, and 32 mg/kg) using a transient middle cerebral artery occlusion (MCAO) model in middle-aged male rats, which are better models of the older human population more at risk for stroke than their younger counterparts. Because behavioral assessment after stroke is among the most important factors in evaluating the effectiveness of any neuroprotective drug (Stroke Treatment Academic Industry Roundtable 1999), we used a battery of behavioral tests to determine the most effective dose of PROG that could potentially be used for human ischemic stroke patients. We evaluated brain infarction and functional recovery following PROG treatment over 3 weeks.
Materials and methods
Delete
All behavioral testing, drug treatment, and histological assays were performed independently by a researcher double blinded to the experimental conditions.
Animals and treatment regimen
Male Sprague–Dawley rats (450–500 g; 13 months of age at the beginning of the experiments; Harlan) were obtained, quarantined for 7 days before the experiment, and housed in an AAALAC-approved Research Animal Facility with a temperature- (21–25 °C), humidity-controlled (45–50 %) and light-controlled environment under a 12-h reverse light/dark cycle with free access to food and water. Public Health Service Policy on Humane Care and Use of Laboratory Animals, the Guide for the Care and Use of Laboratory Animals, and all other applicable regulations, policies, and procedures, were followed and approved by Emory University Institutional Animal Use and Care Committee (Protocol #200-1517). Rats were randomized to the treatment conditions, and the identity of the groups was coded to avoid experimenter bias. Investigators were blinded to the allocation of treatment while doing surgeries or evaluating outcomes.
We calculated the starting sample sizes to be at least eight animals/group to reject the null hypothesis (of no differences among the treatment groups relative to untreated controls) at a power of 0.8 with a p value of <0.05. A total of 40 rats had tMCAO surgery and 35 survived. Three additional rats were excluded based on the criteria for LDF inclusion/exclusion of animals. Animals were assigned to tMCAO vehicle (n=8), PROG 8-mg/kg (P8, n=8), PROG 16-mg/kg (P16, n=8), or PROG 32-mg kg (P32, n=8) groups. A total of eight animals underwent sham surgery as a control but received no PROG (n=8).
PROG (P-0130; Sigma–Aldrich Co., St. Louis, MO) was dissolved in 22.5 % 2-hydroxypropyl-cyclodextrin (HBC). One hour post-tMCAO, the animals were given an i.p. injection (to ensure more rapid absorption) of 8, 16, or 32 mg/kg of PROG, followed by subcutaneous injections at 6 h post-injury and then every 24 h for the next 7 days. The dose was tapered over the final two treatments. The PROG dose was reduced by 50 % each day for the last 2 days of treatment to avoid PROG withdrawal effects, which compromise functional recovery and produce an inflammatory rebound effect (Cutler et al. 2005, 2006a, b). The tMCAO vehicle and sham-operated animals received only HBC.
Transient middle cerebral artery occlusion
Transient cerebral ischemia was induced by occlusion of the right middle cerebral artery as previously described (Longa et al. 1989). Our standard procedures are as follows: A midline incision is made on the ventral surface of the neck and the right common carotid arteries are isolated and ligated with 6.0 silk suture. The internal carotid and pterygopalatine artery are temporarily occluded with a microvascular clip. A 4-0 Doccol filament (Doccol Corporation, Redlands, CA) is introduced into the internal carotid artery through the incision in the external carotid artery. The filament is advanced approximately 20 mm distal to the carotid bifurcation. Relative cerebral blood flow (CBF) is monitored by laser Doppler flowmetry (LDF) for the entire 2 h of occlusion. In sham-operated rats, the external carotid artery is surgically prepared for insertion of the filament but the filament is not inserted.
Drug treatment was randomly assigned 5 min before onset of reperfusion. After 2 h of MCAO, the occluding filament was withdrawn back into the common carotid artery to allow for reperfusion. Relative CBF was monitored for 5 min before the wound was sutured and the rats were then permitted to recover from anesthesia. We monitored heartbeat and blood oxygen saturation levels (SpO2) using a SurgiVet™ pulse oximeter. CBF, heart rate, hemoglobin levels, blood glucose level, and other biochemical variables were monitored continuously during surgery. Body temperature was maintained at 37 °C using an automated heat lamp (Harvard Apparatus, South Natick, MA). All animals with LDF >40 % were excluded from the study to ensure uniform and consistent large ischemic damage. The animals’ baseline and post-surgery weights on days 2, 7, 14, and 21 were taken as an indicator of their general well-being.
Behavioral testing
Motor coordination
Motor impairment was assessed with the accelerating rotarod (Atif et al. 2013). Rats were pre-trained before surgery in two sessions 5 min apart. The animals were habituated to the stationary rod and then placed on the rotating rod. The rod was started at 2 rpm and accelerated linearly to 5 rpm within 180 s. Latency to fall off the rotarod was determined before ischemia and then post-surgery. The animals were evaluated at days 2, 6, 9, and 21 post-surgery.
Grip strength
A grip strength meter (Columbus Instruments, Columbus, OH) was used to measure the degree of force necessary to make the animal release a pull grid assembly with the forepaws. A digital reading (in Newtons) of two successive trials was obtained for each rat, and then averaged for analysis. Baseline values for forelimb grip strength were measured pre-surgery and at 2, 6, 9, and 21 days post-surgery.
Somatosensory-neglect test
This test measures the detection of tactile sensation of the affected limb to small pieces of adhesive tape placed on the forelimbs after MCAO (Esneault et al. 2008). Removable sticky tape was placed on the ventral side of the paw contralateral to the induced stroke. The time taken to contact/sense the tape and the time taken to remove it were recorded during a 180-s observation period. Two trials per animal were averaged for analysis. Baseline values were taken prior to surgery; post-surgical testing was repeated at 3, 7, 10, and 22 days.
Cognitive testing
The Morris water maze (MWM) apparatus consists of a 133-cm diameter circular tank filled with opaque water (20±1 °C; Artista™ nontoxic white paint) to a depth of 64 cm (23 cm from top of tank). A platform (11×11 cm) was submerged to a depth of 2 cm and placed approximately 28 cm from the wall of the pool in the center of the northeast quadrant. Each trial was videotaped by a ceiling-mounted video camera and the animals’ movement tracked using a computer-assisted tracking system. We administered two types of tests: (1) acquisition of spatial memory and (2) spatial probe trial performed after the acquisition phase. Testing began 13 days post-injury, and the rats were examined for 7 days with two trials each session. Parameters for the acquisition of spatial memory test were latency to reach the platform, length of path to platform, and swim strategy, i.e., percent of total time spent in the outer versus inner annulus. The eighth session was a spatial probe trial in which the platform was removed and the rats were placed into the core of the pool and allowed to swim freely for 90 s. This task measures swim strategies and working (short-term, trial-to-trial) and reference (longer-term, day-to-day) memory. As a measure of reference memory, time spent in the quadrant that previously contained the platform was recorded and calculated as percentage of total time spent in pool.
Gait analysis
Animals were tested on a Catwalk system (Noldus Information Technology, Wageningen, The Netherlands) which consists of an enclosed walkway set on a glass plate on which a rat can run from one side to the other. Green light enters at the long edge of the plate and can escape only where the animal’s paws make contact with the plate. When this happens the light is scattered and the paws’ images are captured by a high-speed video camera under the walkway, transformed into digital images, and transferred to a computer. Rats were pre-trained on the walkway 5 days before surgery and evaluated at post-surgical days 6 and 21. After each footprint was identified and labeled, a wide range of gait data was generated, including: (1) the spatial parameters related to individual paws (intensity, maximum area, print area); (2) relative spatial relationship between different paws (stride length); (3) inter-limb coordination (step pattern, regularity index, and phase lag); and (4) temporal parameters (swing, stance, cadence, and walk speed).
Analysis of infarct volume
Cerebral infarct size was evaluated using previously applied methods (Ishrat et al. 2010). On post-ischemia day 22, animals were deeply anesthetized using isoflurane. After transcardial perfusion with cold saline followed by 10 % buffered formalin, brains were extracted, fixed in gradient sucrose solution, and cut coronally into 20-μm sections for histological analysis. On average, a total of 14 brain sections were used from each animal to evaluate infarct size. Entire brain sections were stained in 0.1 % cresyl violet solution for 10 min at 45 °C, and then rinsed in distilled water. Stained sections were fixed by serial dehydration in alcohol and xylene and mounted with xylene-based cytoseal. Fixed sections were coded to hide group identity and then scanned. Infarct areas, defined as areas showing reduced Nissl staining under light microscopy, were traced and quantified with an image-analysis system (ImageJ, 1.38, NIH, Rockville, MD). Infarct size was calculated by multiplying the infarct area on each section by the distance between sections and represented as a percentage of the size of the contralateral hemisphere±the standard error of the mean (SEM).
Statistical analysis of data
As noted, based on a delta value of 1.5 we calculated the sample sizes and power needed to reject the null hypothesis (of no differences among the treatment groups relative to untreated controls) to achieve >80 % power to detect a 50 % difference. The number of rats per group at these criteria was determined to be at least eight. Calculations were obtained using SPSS 11.0 software. All behavioral data were analyzed by repeated-measures ANOVA, followed by a Tukey post hoc test. Other results were analyzed with one-way ANOVA followed by LSD and Tukey HSD post test for multiple comparisons. All results were expressed as mean± SEM and the criterion for statistical significance was set at p<0.05. To establish a correlation between brain infarction and functional deficits, the Pearson correlation coefficient was evaluated using Microsoft Excel 10.
Results
Delete
Physiological monitoring
We observed no significant differences among the groups in CBF during ischemia and early reperfusion, or in glucose or Hb, suggesting that the relative ischemic insult, glucose, and Hb were equivalent among all groups (Table 1).
Table 1.
Physiological parameters during tMCAO
| Weight | SpO2 | Temperature | Heart beats | Glucose | Hb | |
|---|---|---|---|---|---|---|
| Sham | 508.76±21.75 | 95.42±2.15 | 37.76±1.44 | 350.12±2.35 | 233±39.49 | 15.3±.954 |
| tMCAO | 505±21.97 | 95.12±1.21 | 37±1.54 | 350±3.21 | 221.11±16.62 | 15.22±2.08 |
| P8 | 505±33.70 | 94.10±2.10 | 37.76±1.11 | 351.22±2.54 | 230.8±54.37 | 15.68±1.21 |
| P16 | 501.9±24.61 | 95.41±1.45 | 37.11±1.47 | 352.54±1.54 | 216.5±32.31 | 16.2±1.05 |
| P32 | 504.62±27.02 | 95.41±2.21 | 37.14±1.23 | 350.65±2.34 | 245.6±45.64 | 14.62±1.20 |
Body weight
General well-being of the animals is shown by their body weight changes at different time-points after tMCAO. No significant differences were seen in the body weights of the animals between any of the groups at 21 days. Sham animals maintained their body weights consistently, whereas in the vehicle group a significant decrease (p<0.05) was seen compared with sham values. Baseline values were almost identical for all groups. Some improvement in weight was seen in the 8- and 16-mg/kg PROG groups compared with vehicle but it was not significant. No weight gains were seen in the P32 animals compared to the vehicle-treated group (data not shown).
Effect of PROG treatment on rotarod performance
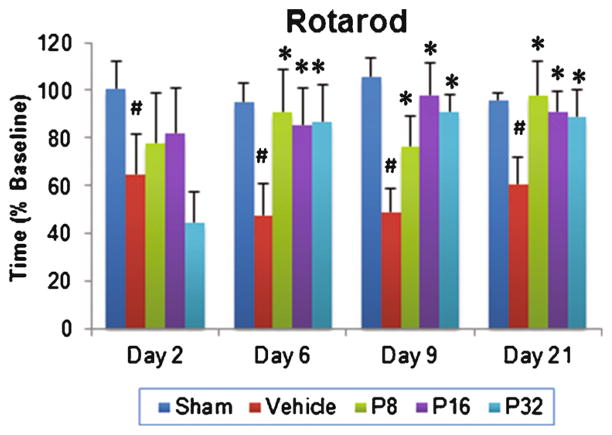
RM-ANOVA showed a significant group effect in the rotarod performance of PROG-treated animals (F(4, 35) =3.308; p<0.021). We observed a significant deficit in motor performance in vehicle-treated animals compared to their sham counterparts at 2, 6, 9, and 21 days post-injury (p<0.05; Fig. 1). Post hoc analyses showed that PROG treatment at 8-, 16-, and 32-mg/kg doses significantly improved the ability of animals to remain on the rotarod at 6, 9, and 21 days post-injury compared to their vehicle group (p<0.05; Fig. 1). At day 21 post-injury, the 8-mg/kg dose of PROG was found to be more neuroprotective, showing a 61.84 % improvement in rotarod performance compared to 50.93 and 46.54 % in the 16- and 32-mg/kg groups, respectively.
Fig. 1.
Dose–response effect of PROG on tMCAO-induced motor deficits in middle-aged rats. Rats were tested on the rotarod at 2, 6, 9, and 21 days following tMCAO. PROG at 8 mg showed maximum improvement compared to other doses at day 21. Values are expressed as means± SEM (n=8). Significant difference #p<0.05 compared to sham and *p<0.05 compared to vehicle
Effect of PROG treatment on grip strength
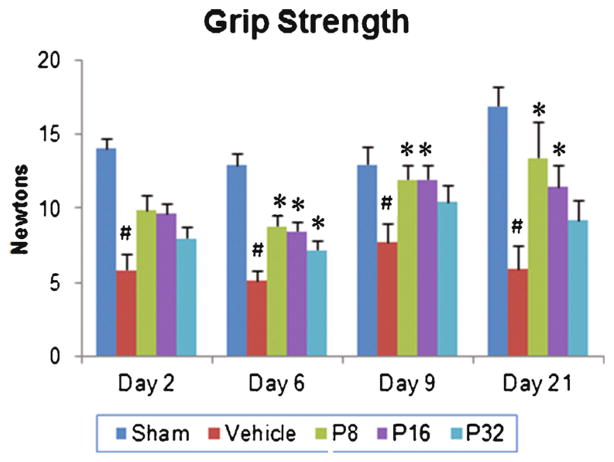
A significant group effect (F(4, 35)=17.89; p<0.001) in the grip strength of PROG-treated animals was observed. There was a decrease in grip strength in vehicle-treated animals compared to shams (p<0.05; Fig. 2). Post hoc analyses showed that PROG treatments at both 8- and 16-mg/kg doses significantly improved grip strength compared to vehicle group (p<0.05; Fig. 2). PROG at the 32-mg/kg dose was neuroprotective only at 6 days post-injury. At day 21, the 8-mg/kg dose was more effective in restoring grip strength (127.62 %) compared to the vehicle group. PROG at 16 mg/kg showed a 94.23 % improvement.
Fig. 2.
Dose–response effect of PROG on tMCAO-induced grip strength deficits in middle-aged rats. Rats were tested for grip strength at 2, 6, 9, and 21 days following tMCAO. At day 21, PROG at 8 mg showed maximum improvement. Values are expressed as means±SEM (n=8 each). Significant difference #p<0.05 compared to sham and *p<0.05 compared to vehicle
Effect of PROG treatment on sensory neglect
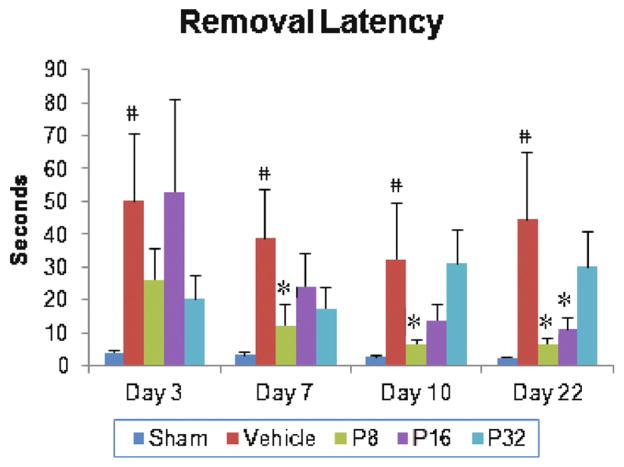
We observed a significant group effect on latency (F(4, 35)=3.13; p<0.026) to remove sticky tape from the contralateral forepaw. There was a significant increase in latency to remove the sticker in vehicle-treated animals compared to shams at 3, 7, 10, and 22 days post-injury (p<0.05; Fig. 3). Post hoc analyses showed that PROG treatments at 8 mg significantly decreased removal latency compared to vehicle-only values at 7, 10, and 22 days post-injury (p<0.05; Fig. 3). Rats given PROG at 16 mg/kg showed improvement in removal latency at day 22 only. Rats receiving 32 mg/kg PROG showed no significant effect on sensory neglect at any time point. At day 22 post-injury the most effective dose was found to be 8 mg/kg, which led to an 84.79 % reduction in removal latency; the 16-mg/kg dose led to 75.11 % reduced removal latency.
Fig. 3.
Dose–response effect of PROG on tMCAO-induced sensory-neglect deficits in middle- aged rats. Rats underwent a sticky tape removal test at 3, 7, 10, and 22 days following tMCAO. PROG at 8 mg showed maximum improvement in removal latency. Values are expressed as means±SEM (n=8). Significant difference #p<0.05 compared to sham and *p<0.05 compared to vehicle
Effect of PROG treatment on spatial learning and memory (MWM)
Learning
Figure 4a shows the effects of PROG treatment on mean latency (duration) to reach the hidden platform in the MWM. RM-ANOVA on swim duration showed a significant group effect (F(4, 35) =46.64; p <0.001) following PROG treatment. MCAO caused a significant (p<0.05) increase in time to reach the platform in vehicle-treated animals compared to shams. PROG treatment at 8, 16, and 32 mg/kg produced a significant decrease in swim-time to reach the hidden platform at all time-points compared to the vehicle-treated animals (p<0.05; Fig. 4a).
Fig. 4.
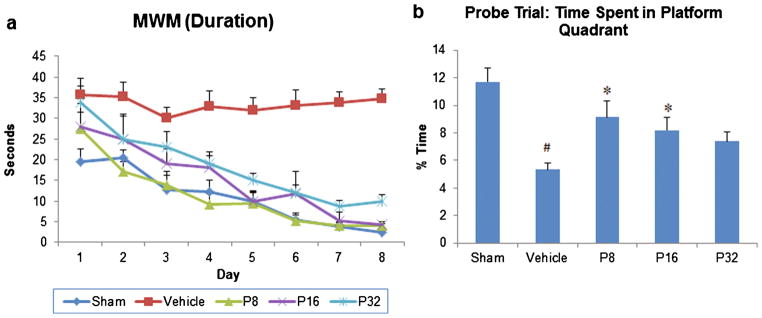
Dose–response effect of PROG on tMCAO-induced cognitive dysfunctions in middle-aged rats. Spatial learning (a) and memory deficits (probe trial) (b) following PROG treatment at different doses. Values are expressed as means±SEM (n=8). Significant difference #p<0.05 compared to sham and *p<0.05 compared to vehicle
Memory
The probe trial revealed that vehicle-treated animals spent significantly (p<0.05) less time in the platform quadrant compared to shams. Rats treated with PROG at 8 and 16 mg/kg spent significantly more time in the platform quadrant (136.25 and 96.53 %, respectively; p<0.05) compared to the vehicle-treated animals.
Delayed PROG treatment reduces gait impairment
Stand phase
We measured the duration in seconds of the contact of each rat’s paw with the glass floor of the apparatus. Stand time in the use of the contralateral forepaw (% baseline) showed a significant group effect (F(4, 35)=12.63; p<0.001) following PROG treatment. There was a significant (p<0.05) decrease in stand time in vehicle-treated rats compared to their sham group. Post hoc analyses showed that PROG treatment at 8 mg/kg significantly increased stand time compared to the vehicle groups at 6 and 21 days post-injury (p<0.05; Fig. 5a). PROG at 16 mg/kg was effective only at 21 days post-injury. No significant effect of 32-mg/kg PROG treatment on stand time was observed at any time point compared to vehicle (Fig. 5a).
Fig. 5.
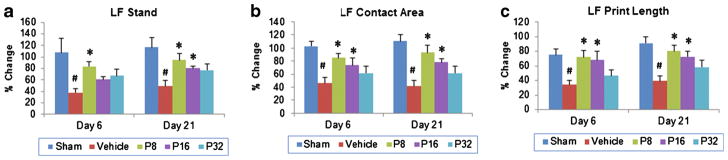
Dose–response effect of PROG on tMCAO-induced gait deficits in middle-aged rats. a Left foot (LF) stand, b LF print length, and c LF contact area following PROG treatment. PROG at 8 mg/kg showed maximum improvement in gait. Values are expressed as means±SEM (n=8). Significant difference #p<0.05 compared to sham and *p<0.05 compared to vehicle
Contact area
Contact area is a measure of spasticity. Transient MCAO led to a persistent reduction of maximal paw contact area. Contact area (percent baseline) of the contralateral fore-paw showed a significant group effect (F(4, 35) =13.61; p<0.001) following PROG treatment. There was a significant (p<0.05) decrease in contact area in vehicle-treated rats at 6 and 21 days post-injury compared to shams. Post hoc analyses showed that delayed PROG treatment at 8- and 16-mg/kg doses significantly increased the contact area compared to the vehicle group at 6 and 21 days post-injury (p<0.05; Fig. 5b). No significant effect of 32-mg/kg PROG treatment on contact area was observed at any time compared to vehicle (Fig. 5b). We observed 125 and 88.25 % increases in contact area following PROG treatment at 8- and 16-mg/kg doses, respectively, compared to vehicle at 21 days.
Print length
This is the length (horizontal direction) of the complete paw print, which is the sum of all contacts with the floor. The print length (% baseline) of the contralateral fore-paw showed a significant group effect (F(4, 35) =9.09; p<0.001) following PROG treatment. We observed a significant (p<0.05) decrease in the print length of vehicle-treated rats at different times post-injury compared to sham groups. Post hoc analyses showed that delayed PROG treatment at 8 and 16 mg/kg significantly improved print length compared to vehicle controls at 6 and 21 days post-injury (p<0.05; Fig. 5c). No significant effect of 32-mg/kg PROG treatment on print length was observed at any time compared to vehicle (Fig. 5c).
PROG treatment attenuates infarction volume
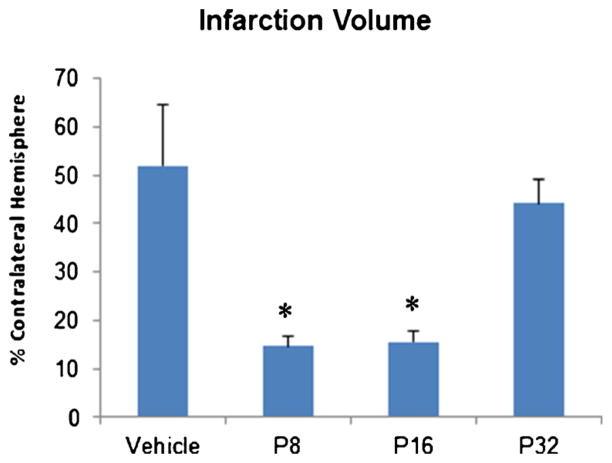
A significant group effect (F(3, 20)=7.529; p<0.001) was observed in infarction volume among the groups on day 21 post-surgery. CV staining revealed a significant increase in infarct volume in vehicle-treated animals (p<0.05; Fig. 6) compared with the PROG-treated animals. Post hoc analysis revealed that PROG treatment at 8 and 16 mg/kg resulted in a significant (p<0.05) reduction in infarct volume compared to vehicle-treated rats. However, there was no significant effect of P32 treatment on infarct volume.
Fig. 6.
Effect of PROG treatment on stroke-induced infarction volume in middle-aged rats. At day 22 following tMCAO, brains were removed, sectioned, and CV stained for calculating infarction volume. PROG at 8-and 16-mg/kg doses showed significant reduction in infarction compared to vehicle. Values are expressed as means±SEM. Significant difference *p<0.05 compared to vehicle
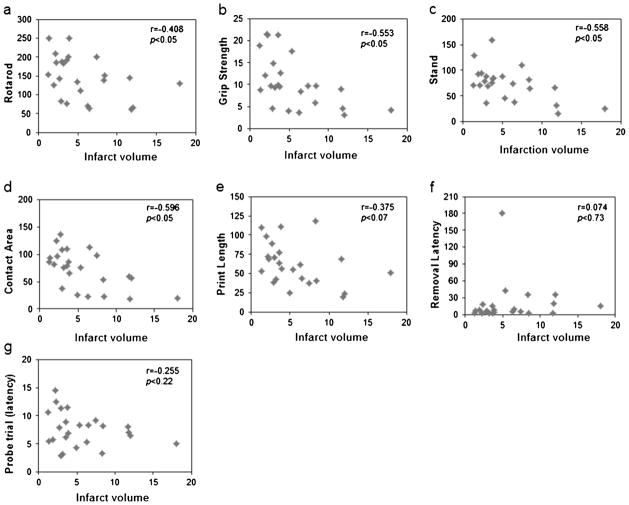
Correlations to brain infarction
We conducted a correlation analysis between brain infarction volume and functional deficits in different groups at post-stroke day 21 to determine whether infarct size affects functional outcomes (Fig. 7). We observed a significant correlation between infarct volume and motor activity on rotarod (r=−0.408; p<0.05) and grip strength (r=−0.553; p<0.05) at day 21. The analysis of infarction volume and gait deficits revealed a significant correlation with LF stand (r=−0.558; p<0.05) and contact area (r=−0.596; p<0.05), whereas no significant correlation was found with print length (r=−0.375; p<0.07). We found no significant correlation of infarction volume with the sensory-neglect test (r=−0.074; p<0.730) or cognitive function (MWM probe trial, r=−0.255; p<0.227).
Fig. 7.
Scatter plots showing the correlation between infarct volume and functional outcome at day 21 after transient MCAO. a Rotarod, b grip strength, c stand, d contact area, e print length, f sticky tape removal, and g MWM (probe trial). A Pearson correlation was evaluated for each behavioral measure. Significance was set at p<0.05
Discussion
We conducted our research in older animals which, like humans, are more prone to stroke than the younger population (Donnan et al. 2008). In most stroke research, young animals are preferred over aged, probably for two main reasons: aged animals are more expensive, and they present technical and methodological challenges during surgery and training on behavioral outcomes (Turner et al. 2013). In this study, we established a dose–response relationship of PROG against transient ischemic stroke-induced functional deficits and brain infarction in middle-aged male rats. We tested three PROG doses—8, 16, and 32 mg/kg—and found that the 8-mg/kg treatment was, overall, more neuroprotective in decreasing infarct volume and improving behavioral outcomes over 3 weeks of functional testing.
While considerable attention has been paid to treatment with PROG in the acute phases of ischemic injury, less is known about the changes that occur in the sub-acute phase. In this study, we tried to model a clinical setting by using middle-aged rats tested for 3 weeks after injuries to evaluate the efficacy of PROG over a longer period of time. Using behavioral tests that remain sensitive to deficits up to weeks after ischemic injury, we found that functional impairments were much more persistent in the vehicle group compared to the sham-operated animals. PROG treatment at 8, 16, and 32 mg/kg led to behavioral restoration and decreased infarct volumes. Additional support for the results reported here is provided by the neuroprotective effectiveness of PROG treatment demonstrated in different models of stroke (Ishrat et al. 2009, 2012; Yousuf et al. 2013; Atif et al. 2013; Gibson and Murphy 2004; Gibson et al. 2011).
Overall dose effects
We found that 8- and 16-mg/kg doses of PROG provided the best neuroprotection after tMCAO, while 32 mg/kg was not as effective. Goss et al. (2003) tested the same three doses of PROG after TBI and hypothesized an inverted U-shaped response curve, with 16 mg/kg the best dose. Drugs that mimic or enhance the activity of the inhibitory neurotransmitter γ-amino butyric acid (GABA) have been found to disrupt functional recovery due to their sedative–hypnotic effects after cortical injury (Jones and Schallert 1992). There is a time window for the process of recovery after brain injury which is vulnerable to the effects of GABAergic agents that may alter or permanently disrupt recovery. Thus 32 mg/kg of PROG may have been ineffective because the very high dose could have blocked levels of neural/metabolic activity needed to trigger an acute reparative response to the injury. High doses of PROG could possibly be beneficial if treatment is delayed by 2 h or more, but this question will require new experiments to test the hypothesis.
Behavioral impairments
Hemiparesis following stroke reduces the patient’s ability to walk, so improving extremity functions contributes to an important quantitative measure of recovery. Numerous studies have measured subtle gait impairments after stroke (Wang et al. 2008; Encarnacion et al. 2011). MCAO is associated with an increase in the number of contralateral foot faults or paw slips that occur when animals are subjected to a task that requires good motor coordination (DeVries et al. 2001). The computer-assisted gait measurement test we used for the present study is sensitive to the effects of different doses of PROG treatments and enabled us to track behavioral recovery over time. After tMCAO, the pattern of deficits and functional recovery can vary in severity and pattern in aged animals. Because tMCAO was induced on the right side of the brain, the contralateral or left foot was used to evaluate gait deficits. We observed significant impairments in the spatial parameters related to individual paws, the spatial relationship between paws, and in the temporal gait parameters in the vehicle-treated animals compared to shams. We tested all the animals at 6, 9, and 21 days post-stroke. Vehicle-treated animals were severely impaired in their gait outcomes, whereas the animals receiving P8, P16, and P32 doses showed significantly fewer deficits. The P8 group showed the best improvements in gait compared with the other two groups.
We used the motor and grip strength tasks to look for potential asymmetry in neuromuscular function and the sticky tape test for somatosensory neglect. We observed that the vehicle-treated animals showed the worst outcomes in rotarod, grip strength, and somatosensory neglect compared to sham animals. PROG at all three doses led to significant improvement in outcome measures, but again, the P8 group had the best performance overall. A number of studies have reported that somatosensory stimulation results in improved motor functions and motor cortex plasticity in stroke patients (Ward and Cohen 2004; Scalha et al. 2011). We found that PROG reduced somatosensory neglect, which in turn led to better motor recovery in the sub-acute post-stroke period. In contrast, vehicle-treated animals were not able to sense the sticky tape and had severe motor deficits.
The MWM assesses cognitive deficits associated with frontal cortex and hippocampal damage. MWM testing revealed severe deficits in learning and memory in vehicle-treated aged rats compared to sham animals. These deficits may be the result not only of spatial memory impairments but also of a combination of motor, sensory, and cognitive deficits caused by cortical and hippocampal damage. The vehicle-treated animals showed a much stronger thigmotaxic response: they swam along the walls of the pool and did not try to search for the platform by swimming out to the center or crossing through the pool. In contrast, rats given the P8 or P16 dose navigated by crisscrossing the pool to locate the platform, suggesting that they used other kinesthetic or extramaze cues to navigate to the platform. These groups also had significantly decreased latencies to find the hidden platform and performed significantly better than the vehicle group in the probe trial. Again, PROG at 8 mg/kg was most effective compared to the other doses. Our findings confirm earlier studies in young animals from our and other laboratories suggesting that PROG treatment effectively improves cognitive, motor, and sensory functions following stroke and TBI (Cai et al. 2008; Gibson and Murphy 2004, 2005, 2011; Hua et al. 2011 Djebaili et al. 2004; Goss et al. 2003).
Infarct volume correlation with behavior
In the present study, we found differential results in correlating infarct volume and functional recovery on several behavioral tests taken on day 21 post-stroke. We found a significant correlation between rotarod performance, grip strength, and infarction volume. Of three gait analysis parameters, only two (stand and contact area) showed a significant correlation with infarction volume, and no correlation was found between infarct volume and somatosensory and cognitive tests. Our data suggest that not all behavioral measures of functional recovery correlate with infarct volume. The literature reports inconsistent findings on the correlation between infarct size and functional recovery. Some studies show a strong correlation (Borlongon et al. 1999; Peeling et al. 2001) whereas others suggest no correlation (Wahl et al. 1992; Wallace et al. 1999). Several factors may account for this variability. For instance, there are reports suggesting that the timing of various behavioral tests and the end point at which the tissue was collected may influence the extent of correlation (DeVries et al. 2001). It is also possible that neurons surviving the ischemic insult may remain alive but highly abnormal, and thus could lead to long-term functional disruptions (Aronowski et al. 1996; DeVries et al. 2001). Therefore, in light of our findings and previous literature, we speculate that infarct volume may not always correlate with every type of functional outcome measure.
We have recently published a follow-up time-window study for PROG in ischemic stroke where the best neuroprotective dose of PROG (8 mg/kg) was administered at 3, 6, and 24 h post-occlusion (Yousuf et al. 2013). We reported that delayed PROG treatment restores functional recovery and reduces brain infarction when administered up to 6 h post-occlusion. However, 8-mg/kg PROG treatment delayed for 24 h did not significantly improve functional outcomes or reduce infarction volume. Such effects could be attributed, in part, to the functional recovery resulting from the inhibitory/modulatory effect of PROG on key cytotoxic events that occur during the acute phase of the ischemic brain injury cascade (within 6–12 h), e.g., neuronal depolarization due to energy depletion, excitotoxicity, apoptosis, etc. These are processes which typically tend to dissipate at 24 h and are therefore less amenable to the window of treatment known to be effective for PROG. As we noted earlier, it may be worthwhile to test high-dose PROG at delays up to 24 or more hours, but it is a very unlikely clinical model since epidemiological data shows that very few stroke patients wait that long to seek care. Nonetheless, it may be worthwhile from an experimental perspective to verify whether the treatment could show benefit after such a long delay.
What do we know about mechanisms of action?
There is still a gap in what is known about the mechanisms involved in progesterone PROG neuroprotection after stroke in older animals. Various mechanisms have been proposed for PROG’s pleiotropic actions in enhancing neuronal repair after various kinds of brain injury (Meffre et al. 2013; Stein 2013; Petersen et al. 2013; Schumacher et al. 1996). Recently Yousuf et al. (2013) reported that PROG treatment delayed up to 6 h after transient ischemic stroke can improve functional deficits and reduce brain infarction, possibly by modulating glial gibrillary acidic protein (GFAP), vascular endothelial growth factor (VEGF), and matrix metalloproteinase-9 (MMP-9) expression. PROG repairs the blood–brain barrier by reducing the expression of MMPs, thereby preventing degradation of tight junction proteins (Ishrat et al. 2010) and decreasing apoptosis through modulation of the P13K/Akt pathway (Ishrat et al. 2012). Atif et al. (2013) showed that PROG exerts its effects through a variety of intra-nuclear and membrane-bound molecular mechanisms after transient stroke. Wong et al. (2013) found that PROG inhibits the tPA-induced increase in MMP-9 and VEGF, thereby attenuating cerebral hemorrhage after ischemic stroke. Espinosa-Garcia et al. (2014) reported reduction of neurite growth inhibitory molecules Nogo-A, Ng-R, and Rho-A due to the restorative effects of PROG treatment, which in turn supported functional preservation of the hippocampus following global cerebral ischemia. It appears that PROG’s pleiotropic actions on the reduction of various complex molecular and genomic cascades of injury are responsible for the improvement in various injury markers and functional outcomes. Briefly stated, PROG offers neuroprotection through numerous pathways and this is why it can be considered a pleiotropic hormone.
In conclusion, stroke is a complex systemic disease more likely to affect the elderly, so stroke studies in older animals should be given consideration as a necessary first step in identifying neuroprotective agents and how they should be employed. Older animals clearly show a more profound and clinically comparable response to ischemic injury and treatment than “hardy” juveniles. We found that the optimized dose of PROG (8 mg/kg) most consistently and effectively reduced brain infarction and improved functional outcomes in middle-aged animals following tMCAO. These preclinical dose–response data provide a foundation for PROG as a potential test candidate for ischemic stroke clinical trial.
Acknowledgments
This research was supported by a NIH award U01 NS062676, BHR Pharma, and Allen and Company. The authors would like to thank Leslie McCann for her invaluable editorial assistance. SY is thankful to Jun Wang for his assistance in brain histology.
Footnotes
Conflict of interest DGS is entitled to royalties from products of BHR Pharma LLC (BHR) related to the use of PROG in TBI and stroke, and may receive research funding from BHR, which is developing products related to this research. In addition, he serves as a consultant to BHR and receives compensation for these services. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
References
- Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, Hurn P, Liebeskind DS, Nogueira RG, Saver JL STAIR VII Consortium. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42:2645–2650. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Samways E, Strong R, Rhoades H, Grotta J. An alternative method for the quantitation of neuronal damage after experimental middle cerebral artery occlusion in rats: analysis of behavioral deficit. J Cereb Blood Flow Metab. 1996;16:705–713. doi: 10.1097/00004647-199607000-00022. [DOI] [PubMed] [Google Scholar]
- Atif F, Yousuf S, Sayeed I, Ishrat T, Hua F, Stein DG. Combination treatment with progesterone and vitamin D hormone is more effective than monotherapy in ischemic stroke: the role of BDNF/TrkB/Erk1/2 signaling in neuroprotection. Neuropharmacol. 2013;67:78–87. doi: 10.1016/j.neuropharm.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE, Robel P, Schumacher M. Neurosteroids: beginning of the story. Int Rev Neurobiol. 2001;46:1–32. doi: 10.1016/s0074-7742(01)46057-0. [DOI] [PubMed] [Google Scholar]
- Borlongon CV, Hida H, Nishino H. Early assessment of motor dysfunctions aids in successful occlusion of the middle cerebral artery. Neuroreport. 1999;9:3615–3621. doi: 10.1097/00001756-199811160-00012. [DOI] [PubMed] [Google Scholar]
- Cai W, Zhu Y, Furuya K, Li Z, Sokabe M, Chen L. Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacol. 2008;55:127–138. doi: 10.1016/j.neuropharm.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Cutler SM, Pettus EH, Hoffman SW, Stein DG. Tapered progesterone withdrawal enhances behavioral and molecular recovery after traumatic brain injury. Exp Neurol. 2005;195(2):423–429. doi: 10.1016/j.expneurol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Cutler SM, VanLandingham JW, Murphy AZ, Stein DG. Slow-release and injected progesterone treatments enhance acute recovery after traumatic brain injury. Pharmacol Biochem Behav. 2006a;84(3):420–428. doi: 10.1016/j.pbb.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Cutler SM, Vanlandingham JW, Stein DG. Tapered progesterone withdrawal promotes long-term recovery following brain trauma. Exp Neurol. 2006b;200(2):378–385. doi: 10.1016/j.expneurol.2006.02.137. [DOI] [PubMed] [Google Scholar]
- De Nicola AF, Coronel F, Li G, Gargiulo-Monachelli G, McG Deniselle EY, Gonzalez SL, Labombarda F, Meyer M, Guennoun R, Schumacher M. Therapeutic effects of progesterone in animal models of neurological disorders. CNS Neurol Disord Drug Targets. 2013 Sep 4; [PubMed] [Google Scholar]
- Deutsch ER, Espinoza TR, Atif F, Woodall E, Kaylor J, Wright DW. Progesterone’s role in neuroprotection, a review of the evidence. Brain Res. 2013;1530:82–105. doi: 10.1016/j.brainres.2013.07.014. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Nelson RJ, Traystman RJ, Hurn PD. Cognitive and behavioral assessment in experimental stroke research: will it prove useful? Neurosci Biobehav Rev. 2001;25:325–342. doi: 10.1016/s0149-7634(01)00017-3. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Encarnacion A, Horie N, Keren-Gill H, Bliss TM, Steinberg GK, Shamloo M. Long-term behavioral assessment of function in an experimental model for ischemic stroke. J Neurosci Methods. 2011;196:247–257. doi: 10.1016/j.jneumeth.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esneault E, Castagne V, Moser P, Bonny C, Bernaudin M. D-JNKi, a peptide inhibitor of c-Jun N-terminal kinase, promotes functional recovery after transient focal cerebral ischemia in rats. Neuroscience. 2008;152:308–320. doi: 10.1016/j.neuroscience.2007.12.036. [DOI] [PubMed] [Google Scholar]
- Espinosa-Garcia C, Aguilar-Hernandez A, Cervantes M, Morali G. Effects of progesterone on neurite growth inhibitors in the hippocampus following global cerebral ischemia. Brain Res. 2014;1545:23–34. doi: 10.1016/j.brainres.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24:805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP. Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp Neurol. 2005;193:522–530. doi: 10.1016/j.expneurol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Coomber B, Murphy SP. Progesterone is neuroprotective following cerebral ischaemia in reproductively ageing female mice. Brain. 2011;134(Pt 7):2125–2133. doi: 10.1093/brain/awr132. [DOI] [PubMed] [Google Scholar]
- Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: a progesterone dose–response study. Pharmacol Biochem Behav. 2003;76:231–242. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Hua F, Wang J, Ishrat T, Wei W, Atif F, Sayeed I, Stein D. Genomic profile of Toll-like receptor pathways in traumatically brain-injured mice: effect of exogenous progesterone. J Neuroinflammation. 2011;8:42. doi: 10.1186/1742-2094-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone and allopregnanolone attenuate blood–brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol. 2010;226:183–190. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone is neuroprotective against ischemic brain injury through its effects on the phosphoinositide 3-kinase/protein kinase B signaling pathway. Neurosci. 2012;210:442–450. doi: 10.1016/j.neuroscience.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Subcortical deterioration after cortical damage: effects of diazepam and relation to recovery of function. Behav Brain Res. 1992;51:1–13. doi: 10.1016/s0166-4328(05)80306-7. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Meffre D, Labombarda F, Delespierre B, Chastre A, De Nicola AF, Stein DG, Schumacher M, Guennoun R. Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury. Neuroscience. 2013;231:111–124. doi: 10.1016/j.neuroscience.2012.11.039. [DOI] [PubMed] [Google Scholar]
- Millard WB. New guidelines on tPA in stroke: putting out fires with gasoline? Ann Emerg Med. 2013;62:A13–A18. doi: 10.1016/j.annemergmed.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Muntner P, DeSalvo KB, Wildman RP, Raggi P, He J, Whelton PK. Trends in the prevalence, awareness, treatment, and control of cardiovascular disease risk factors among noninstitutionalized patients with a history of myocardial infarction and stroke. Am J Epidemiol. 2006;163:913–920. doi: 10.1093/aje/kwj124. [DOI] [PubMed] [Google Scholar]
- Peeling J, Corbett D, Del Bigio MR, Hudzik TJ, Campbell TM, Palmer GC. Rat middle cerebral artery occlusion: correlations between histopathology, T2-weighted magnetic resonance imaging, and behavioral indices. J Stroke Cerebrovasc Dis. 2001;10:166–177. doi: 10.1053/jscd.2001.26865. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Intlekofer KA, Moura-Conlon PJ, Brewer DN, Del Pino SJ, Lopez JA. Non-classical progesterone signalling molecules in the nervous system. J Neuroendocrinol. 2013 doi: 10.1111/jne.12060. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva GM, Stephan BC. Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41:e41–e46. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- Scalha TB, Miyasaki E, Lima NM, Borges G. Correlations between motor and sensory functions in upper limb chronic hemiparetics after stroke. Arq Neuropsiquiatr. 2011;69(4):624–629. doi: 10.1590/s0004-282x2011000500010. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Robel P, Baulieu EE. Development and regeneration of the nervous system: a role for neurosteroids. Dev Neurosci. 1996;18(1–2):6–21. doi: 10.1159/000111391. [DOI] [PubMed] [Google Scholar]
- Stein DG. A clinical/translational perspective: can a developmental hormone play a role in the treatment of traumatic brain injury? Horm Behav. 2013;63:291–300. doi: 10.1016/j.yhbeh.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Strbian D, Sairanen T, Meretoja A, Pitkäniemi J, Putaala J, Salonen O Helsinki Stroke Thrombolysis Registry Group et al . Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology. 2011;77:341–348. doi: 10.1212/WNL.0b013e3182267b8c. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2578. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- Turner RC, Lucke-Wold B, Lucke-Wold N, Elliott AS, Logsdon AF, Rosen CL, Huber JD. Neuroprotection for ischemic stroke: moving past shortcomings and identifying promising directions. Int J Mol Sci. 2013;14(1):1890–1917. doi: 10.3390/ijms14011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl F, Allix M, Plotkin M, Boulu RG. Neurological and behavioural outcomes of focal cerebral ischemia in rats. Stroke. 1992;23:267–272. doi: 10.1161/01.str.23.2.267. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomoter activity, stereotypic behavior, and stimulated dopamine release in the rat. J Neurosci. 1999;19:9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bontempi B, Hong SM, Mehta K, Weinstein PR, Abrams GM, Liu J. A comprehensive analysis of gait impairment after experimental stroke and the therapeutic effect of environmental enrichment in rats. J Cereb Blood Flow Metab. 2008;28:1936–1950. doi: 10.1038/jcbfm.2008.82. [DOI] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Renton C, Gibson CL, Murphy SJ, Kendall DA, Bath PM. Progesterone treatment for experimental stroke: an individual animal meta-analysis. J Cereb Blood Flow Metab. 2013;33(9):1362–1372. doi: 10.1038/jcbfm.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, et al. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49(4):391–402. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12(2):R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf S, Atif F, Sayeed I, Wang J, Stein DG. Post-stroke infections exacerbate ischemic brain injury in middle-aged rats: immunomodulation and neuroprotection by progesterone. Neuroscience. 2013;239:92–102. doi: 10.1016/j.neuroscience.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]