Abstract
Background
The role of oxidative stress and systemic inflammation on the association between personal exposures to ambient fine particulate matter ≤ 2.5 μm in diameter (PM2.5) and cardiac autonomic dysfunction, indicated by reduction in heart rate variability (HRV), has not been examined.
Methods
We performed a repeated measures study on community adults in a densely populated inner city neighborhood in Boston, Massachusetts. Continuous ambulatory electrocardiogram (ECG) monitoring and personal exposure to PM2.5 were measured for up to two consecutive days. Peripheral blood and spot urine samples were collected at 12-hour intervals for the measurements of markers of inflammation including C-reactive protein (CRP), fibrinogen, white blood cell (WBC) and platelet counts as well as for the analysis of urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG), a marker of oxidative DNA damage.
Results
After adjusting for confounders, we found a pronounced decrease in nighttime standard deviation of normal-to normal intervals (SDNN): an interquartile range (IQR) increase in PM2.5 (13.6 μg/m3) was associated with an 8.4% decrease in SDNN (95% CI: −11.3 to −5.5). Compared with the lower eightieth percentile, significantly greater PM2.5 associated nighttime SDNN reductions were observed among subjects in the upper twentieth percentile of 8-OHdG by −25.3%, CRP by −24.9%, fibrinogen by −28.7%, WBC by −23.4%, and platelet counts by −24.0% (all P < 0.0001; all Pinteraction <0.01).
Conclusions
These data suggest that oxidative stress and systemic inflammation exacerbate the adverse effects of PM2.5 on the cardiac autonomic function even at ambient levels of exposure.
Keywords: particulate air pollution, oxidative stress, systemic inflammation, effect modifier, heart rate variability, environmental health
1. INTRODUCTION
Associations between ambient PM2.5 and cardiovascular health outcomes have been widely reported in the epidemiological and clinical literature [1]. Possible mechanisms for the cardiovascular effects of PM2.5 exposure include oxidative stress-induced endothelial dysfunction and/or systemic inflammation [2].
Heart rate variability (HRV), a non-invasive marker of cardiac autonomic control and cardiovascular events and mortality [3], reflects the influence of both sympathetic and parasympathetic systems. Nighttime may be a better time to investigate the cardiovascular effects of environmental pollutants because HRV is less influenced by potential confounders such as physical activity and mental stress. Several epidemiological studies have observed larger effects of environmental toxic pollutants including PM2.5 and polycyclic aromatic hydrocarbons (PAHs) on HRV, especially during the night [4, 5]. Increased risk of stroke is associated with nighttime HRV, but not with 24-hr HRV in healthy individuals [6]. Further, a recent study found that elevated nighttime heart rate (HR) is a stronger risk factor in predicting risk of all-cause mortality as well as cardiovascular events [7].
Reactive oxygen species (ROS) may be potential modifiers for particle effects on HRV and other cardiovascular events in experimental studies [8, 9, 10]. Although stronger particle effects on HRV were found in subjects with elevated systemic inflammation, as defined by the upper 20th percentile of C-reactive protein (CRP), the reported role of other markers of inflammation is inconsistent [11]. Moreover, to our knowledge, there is no epidemiologic study that has addressed the role of oxidative stress and inflammation in the association between personal exposures to particulates and nocturnal HRV in a community population.
Therefore, we aimed to assess whether oxidative stress and inflammation modify the association between continuous personal exposure to PM2.5 and HRV measured up to two consecutive days in a community adult population.
2. METHODS
2.1. Study Design and Population
This study is a repeated-measures panel study design for assessing cardiovascular responses to particulates in community members from an inner city neighborhood of Boston, Massachusetts, consisting of twenty one individuals. Detailed information regarding our study population has been reported previously [12]. Briefly, the study population was recruited from a neighborhood health center during the period of March to August 2004. Each participant completed a modified American Thoracic Society questionnaire [13], which also included information on socio-demographic factors (age, gender and smoking status) and medication use. Information on medication use included statins, non-steroidal anti-inflammatory drugs (NSAIDs), antihypertensive medications (beta blocker, calcium channel blocker or ACE inhibitors/angiotensin receptor blockers), or aspirin that have been taken at least once during the study period. Participants completed blood and urine collections at approximately twelve hour intervals and continuous ECG monitoring up to two consecutive days. This study was approved by the Institutional Review Board of the Harvard School of Public Health. All subjects gave written informed consent prior to the study.
2.2 Personal PM2.5 Exposure
Continuous real-time personal PM2.5 concentrations were obtained for each individual with the TSI SidePak Model AM510 Personal Aerosol Monitor, which uses light-scattering technology to determine mass concentration (TSI Inc., Shoreview, MN). Each SidePak was fit with a PM2.5 inlet impactor. The air sample was drawn through a 1.2 meter long Tygon tube into the impaction inlet at a flow rate of 1.7 L/min, adjusted using a DryCal DC-Lite Primary Air Flow Meter (BIOS, Butler, New Jersey, USA). The PM2.5 monitor was placed in a padded pouch, with the inlet tubing secured in the participant's breathing zone, and attached to each participant. Participants were instructed to wear their monitors throughout the day and to place the monitor by their bed when sleeping at night. The monitor took PM2.5 concentrations every 10 seconds and reported 5-min averages.
2.3. ECG Monitoring
We used HRV and HR as measures of autonomic cardiac response to PM2.5 exposure. The ECG of each individual was measured continuously up to two 24-hr periods using a five-lead ECG Holter monitor, Dynacord 3-Channel Model 423 (Raytel Cardiac Services, Windsor, Connecticut). A detailed description for the ECG monitoring protocol has been provided previously [5]. Briefly, the holter monitor was calibrated 15 minutes before placing electrodes. Separate electrodes were placed on the participant's skin, and if necessary, the area was shaved for proper adhesion, and the leads were periodically checked by study staff. Each 24 hr recording was sent to Raytel Cardiac Services for processing and analysis using a StrataScan 563 (DelMar Avionics, Irvine, California) and then screened to correct data artifacts. A trained professional with no exposure information performed all analyses and edited all normal or abnormal findings based on standard criteria. The mean of SD of normal-to-normal intervals (SDNN, in milliseconds), as a time-domain HRV measure, and the mean heart rate (HR, in beats per minute) were calculated in standard 5-min segments throughout the entire recording and matched with the corresponding personal 5-min PM2.5 intervals.
2.4. Markers of Oxidative Stress and Systemic Inflammation
Blood and urine samples were collected at the start of monitoring and at twelve hour intervals throughout the duration of the monitoring period, for up to 4 measurements per person. Analysis of blood samples was performed by Path Labs, Inc. (Portsmouth, New Hampshire). Quantitative determination of CRP in serum was performed with a Hitachi 747–200 analyzer using the latex particle-enhanced immunoturbidimetric assay (ITA). The concentration of fibrinogen in plasma was determined using the Sysmex CA5000 coagulation analyzer using the Clauss clotting method. Blood samples for complete blood count (CBC) including WBC and platelet count was collected in ethylenediaminetetraacetic acid (EDTA) and analyzed using an Advia 120 automatic cell counter. 8-OHdG, one of the major DNA base modified products due to attack by hydroxyl radicals at the C8 of guanine, is a widely used biomarker of oxidative DNA stress [14]. A detailed description for the analysis of urinary 8-OHdG has been provided previously [12]. Spot urine samples were collected in 120mL sterile urine cups at 12-hour intervals during the monitoring periods with the collection of up to four urine samples from each individual. All urine samples were aliquotted and frozen at −20°C until laboratory analysis. The analysis of urinary 8-OHdG was performed by Genox Corporation (Baltimore, MD). Urinary 8-OHdG was determined by competitive enzyme-linked immunosorbent assay. The limit of detection (LOD) for urinary 8-OHdG was 0.64 ng/mL. Urine creatinine concentrations were used to adjust the urinary concentrations of 8-OHdG. These creatinine-corrected urinary concentrations of 8-OHdG were calculated as μg/g creatinine.
2.5. Statistical Analysis
Dependent variables, SDNN and HR, were log-transformed to improve normality and stabilize the variance. Linear mixed effects models with random intercepts and unconstructed covariance were used to estimate the changes in 5-min SDNN and 5-min HR per interquartile range (IQR) increase in PM2.5 concentrations. Each outcome measure (SDNN and HR) were split into three time periods: the 24-hr recording, the 14-hr day period (07:30–21:30 hours) and the 7-hr night period (00:00 to 07:00 hours) [4]. This allowed us to analyze three exposure-response models for each outcome. We adjusted each model for age, gender, smoking status (current smoker and non-smoker), use of statin, NSAIDs, antihypertensive medications (beta blocker, calcium channel blocker or ACE inhibitors/angiotensin receptor blockers), and aspirin. Indicators for time of day (divided by 14-hr day, 7-hr night, and the remaining time of day) were additionally included to account for the circadian variation of HRV in the analysis of 24-hr HRV and 24-hr HR [15]. For analysis of effect modifiers, we used measures of oxidative stress and inflammation which were collected at the most proximal time point of 5-min SDNN. The oxidative stress and inflammatory markers were considered as dichotomous variables determined by the value at the upper 20% of each marker [11]. Results are given as estimated percent changes and 95% confidence intervals (CIs) in HRV and HR per IQR increase in PM2.5 exposure (13.6 μg/m3). For statistical efficiency, we included multiplicative interaction terms along with the main effects in the models to assess effect modification by urinary 8-OHdG and blood markers of inflammation, rather than stratified analyses. The estimated percent changes per IQR increase in PM2.5 are reported by the level of each urine and blood marker based on dichotomized criteria (<80th percentile and ≥20th percentile). We also conducted a sensitivity analyses after additionally adjusting for medical history of hypertension and asthma, and analyses excluding one subject with the maximum value of CRP (34.3 mg/L). Significance for statistical tests was evaluated at the p = 0.05 level. All statistical analyses were performed using PROC MIXED in SAS version 9.2 (SAS Institute Inc, Cary, North Carolina, USA).
3. RESULTS
The demographic and clinical characteristics of the study population are shown in Table 1. Subjects were on average 44 years of age; 81% were female, 29% were current smoker, and 48% took regular medications including any of statin (10%), NSAIDs (29%), antihypertensive medications (43%), or aspirin (14%). Of the 21 participants, 15 participants (71%) had one or more disease including diabetes (9.5%), chronic bronchitis (9.5%), asthma (33.3%) or hypertension (57.1%).
Table 1.
Demographic and clinical characteristics of study population (n = 21)
| N (%) or Mean ± SD | |
|---|---|
| Age, years (range) | 44 (21–69) |
| Gender | |
| Men | 4 (19) |
| Women | 17 (81) |
| Race/ethnicity | |
| Non-Hispanic white | 1 (5) |
| Non-Hispanic black | 15 (71) |
| Hispanic | 5 (24) |
| Current smoker | 6 (29) |
| Use of medication† | |
| Statin | 2 (10) |
| NSAIDs | 6 (29) |
| Antihypertensive medication‡ | 9 (43) |
| Aspirin | 3 (14) |
| Medical history | |
| Diabetes | 2 (9.5) |
| Chronic bronchitis | 2 (9.5) |
| Asthma | 7 (33.3) |
| Hypertension | 12 (57.1) |
| SDNN (5-min mean, msec) | 49.6 ± 27.2 |
| Heart rate (5-min mean, bpm) | 82.8 ± 14.8 |
| PM2.5 (5-min mean, μg/m3) | 29.8 ± 77.7 |
At least once during the study period.
Antihypertensive medication includes any of beta blocker, calcium channel blocker or ACE inhibitors/angiotensin receptor blockers.
Table 2 summarizes the baseline levels of markers of urine and blood. The median values of urine and blood markers were 14.3 μg/g creatinine for urinary 8-OHdG, 4.9 mg/L for CRP, 3.1 g/L for fibrinogen, 6.1×109/L for WBC count, and 242×109/L for platelet count.
Table 2.
Baseline levels of markers of urine and blood (n = 21)
| Urine and blood markers | Percentile | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean | Min | 20th | 50th | 80th | Max | |
| 8-OHdG (μg/g creatinine) | 16.4 | 1.8 | 6.2 | 14.3 | 23.8 | 46.5 |
| CRP (mg/L) | 7.3 | 0.6 | 1.7 | 4.9 | 7.5 | 34.3 |
| Fibrinogen (g/L) | 3.1 | 2.2 | 2.4 | 3.1 | 4.0 | 4.6 |
| WBC count (109/L) | 6.2 | 3.0 | 3.9 | 6.1 | 8.1 | 10.1 |
| Platelet count (109/L) | 250 | 151 | 190 | 242 | 293 | 420 |
8-OHdG, 8-hydroxy-2'-deoxyguanosine; CRP, C-reactive protein; WBC, white blood cell
Table 3 presents adjusted associations of HRV and HR with PM2.5 over the three time periods of 24 hours, day and night. Associations between PM2.5 and both HRV and HR were most pronounced at night. After adjusting for age, gender, smoking status, use of statin, NSAIDs, hypertensive medications, and aspirin, one IQR increment in PM2.5 (13.6 μg/m3) was associated with decreases of 8.4% (95% CI: −11.3% to −5.5%) in nighttime SDNN and increases of 1.9% (95% CI: 1.1% to 2.7%) in nighttime HR. Subjects with the highest tertile of PM2.5 had 34.1% (95% CI, −41.3% to −26.0%) lower nocturnal SDNN than individuals with the lowest tertile and a dose-response relation was observed (P for trend < 0.0001). Similar patterns of association were found between PM2.5 exposure and daytime and 24-hr HRV, though effect sizes were lower than those observed during night time. When we additionally adjusted for hypertension and asthma, the results were similar. Excluding the maximum value of CRP (34.3 mg/L) did not change our observed results.
Table 3.
Adjusted percent change (95% CIs) in HRV and HR associated with PM2.5 (μg/m3)
| Main effect of PM2.5 | 24-hr† | Day‡ | Night‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| SDNN | Power¶ | HR | Power¶ | SDNN | Power¶ | HR | Power¶ | SDNN | Power¶ | HR | Power¶ | |
| PM2.5 (μg/m3)§ | 0.07 (−0.15 to 0.29) | 0.9066 | −0.13 (−0.20 to −0.06) | 0.9999 | −0.18 (−0.43 to 0.06) | 0.9997 | 0.16 (0.08 to 0.24) | 0.9671 | −8.42 (−11.36 to −5.49) | 0.9999 | 1.88 (1.12 to 2.65) | 0.9982 |
| PM2.5 (tertile, μg/m3) | ||||||||||||
| Low (<5.97) | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Medium (5.97–14.5) | −3.78 (−7.00 to −0.44) | 0.9999 | 1.83 (0.92 to 2.74) | 0.9778 | −6.00 (−9.08 to −2.87) | 0.9999 | 1.85 (0.72 to 3.00) | 0.8944 | −9.96 (−16.39 to −3.04) | 0.9999 | 3.69 (1.89 to 5.52) | 0.9817 |
| High (>14.5) | −5.92 (−8.55 to −3.22) | 0.9999 | 1.50 (0.41 to 2.59) | 0.7734 | −7.53 (−10.97 to −3.96) | 0.9999 | 2.49 (1.18 to 3.82) | 0.9634 | −34.10 (−41.30 to −26.02) | 0.9999 | 8.98 (5.99 to 12.06) | 0.9999 |
| P for trend | 0.0129 | 0.7019 | 0.0034 | 0.9999 | <0.0001 | 0.9838 | 0.0002 | 0.9999 | <0.0001 | 0.9999 | <0.0001 | 0.9999 |
Adjusted for age, gender, smoking status, use of statin, NSAIDs, hypertensive medication (beta blocker, calcium channel blocker or ACE inhibitors/angiotensin receptor blockers), aspirin, and time of day.
Adjusted for age, gender, smoking status, use of statin, NSAIDs, hypertensive medication (beta blocker, calcium channel blocker or ACE inhibitors/angiotensin receptor blockers), and aspirin.
Per IQR (13.6 μg/m3) increase in PM2.5.
, where β and SE are the estimated regression coefficient and its standard error from mixed analysis [25].
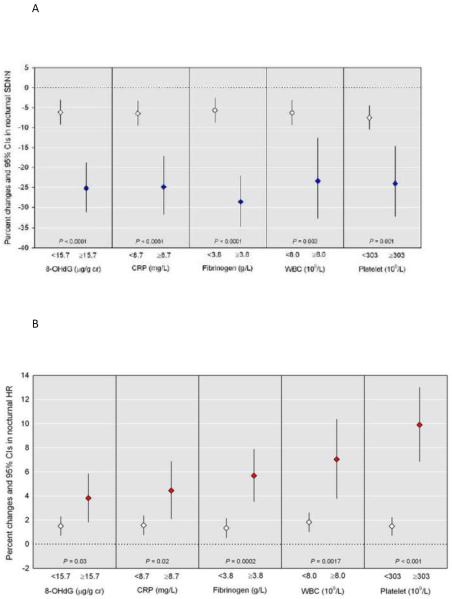
Figure 1 shows effect modification by oxidative stress and systemic inflammation in the association between PM2.5 and nocturnal HRV and nocturnal HR. In this analysis, the estimated percent changes in nocturnal HRV and HR per IQR increase in PM2.5 are presented by the values of each marker in the upper 20th percentile (8-OHdG: 15.7 μg/g creatinine, CRP: 8.7 mg/L, fibrinogen: 3.8 g/L, WBC: 8.0 109/L, platelet: 303 109/L). We found statistically significant effect modification: PM2.5-associated SDNN declines at night were greater among individuals in the upper 20th percentile of 8-OHdG by −25.3% (95% CI: −31.2% to −18.7%, Pinteraction < 0.0001), CRP by −24.9% (95% CI: −31.9% to −17.2%, Pinteraction < 0.0001), fibrinogen by −28.7% (95% CI: −34.7% to −22.1%, Pinteraction < 0.0001), WBC by −23.4% (95% CI: −32.9% to −12.7%, Pinteraction = 0.003) and platelet by −24.0% (95% CI, −32.4% to −14.7%, Pinteraction = 0.001) (Figure 1-A). PM2.5-associated HR increases at night were greater among individuals in the upper 20th percentile of 8-OHdG by 3.8% (95% CI: 1.8% to 5.8%, Pinteraction = 0.03), CRP by 4.5% (95% CI: 2.1% to 6.9%, Pinteraction = 0.023), fibrinogen by 5.7% (95% CI: 3.5% to 7.9%, Pinteraction = 0.0002), WBC by 7.0% (95% CI: 3.8% to 10.4%, Pinteraction = 0.002) and platelet by 9.9% (95% CI: 6.9% to 13.0%, Pinteraction < 0.0001) (Figure 1-B). Neither additionally adjusting for hypertension and asthma nor excluding one subject with the maximum value of CRP did not essentially change the results.
Figure 1.
Adjusted percent changes and 95% CIs in (A) nocturnal SDNN and (B) nocturnal HR for an IQR increase in PM2.5 (13.6 μg/m3) by level of oxidative stress and systemic inflammation. Models were adjusted for age, gender, smoking status, use of statin, NSAIDs, hypertensive medication (beta blocker, calcium channel blocker or ACE inhibitors/angiotensin receptor blockers), and aspirin. Diamond blue/red symbols indicate the effect estimate for the upper 20th percentile; diamond white symbols indicate the effect estimate for the lower 80th percentile. P values for interaction effect.
4. DISCUSSION
To our knowledge, this is the first study to examine the modifying role of oxidative stress and inflammation on the association between personal exposure to PM2.5 and changes in cardiac autonomic function in a general adult population. In this repeated measures panel study among community adults, we found that PM2.5 exposure was associated with reductions in HRV and increases in HR. More pronounced declines in HRV and increases in HR were found during the night. Significantly stronger effects of PM2.5 exposure on HRV and HR were found among the subjects with elevated biomarkers of oxidative stress and systemic inflammation, implying that individuals with excessive oxidative damage and systemic inflammation are more susceptible to adverse cardiac effects by particulate air pollution, as mediated at least in part by adverse effects on cardiac autonomic function.
Epidemiologic evidence suggests that particulate air pollution has been associated with oxidative damage, systemic inflammation and/or autonomic dysfunction [4, 11, 12, 15, 16, 17, 18]. Limited evidence exists for the modifying role of oxidative stress and/or systemic inflammation on particle-induced autonomic dysfunction. In a study of non-smoking CVD patients in China, greater HRV declines by acute exposure to PM2.5 were found among subjects with high-sensitivity CRP values (> 1.76 mg/L) in the upper 10th percentile [16]. In an occupational cohort study of 25 male boilermaker construction workers, greater SDNN declines were observed among subjects with CRP values in the upper 25% of CRP (4.7 mg/L) [15]. In a community-based study conducted in twenty five elderly in Steubenville, Ohio, individuals with elevated systemic inflammation, defined by the upper 20% of the CRP (> 9 mg/L), had a 6.1% decrease in SDNN associated with an IQR (13.4 μg/m2) increase in PM2.5, whereas no association was found among subjects with the 80 percentile of the CRP or lower (≤ 9 mg/L) [11]. We found a similar pattern of a modifying role of systemic inflammation showing significantly greater effect of PM2.5 among the subjects with elevated systemic inflammation both defined by the highest tertile and upper 20 percentile of CRP, and the magnitude of the association is much greater than reported in previous studies. In the present study, oxidative stress and excessive systemic inflammation measured by other indicators including fibrinogen, platelet and WBC counts showed similar modifying roles. In contrast, no effect modification by higher systemic inflammation using other biomarkers on HRV was found in both previous studies [11, 16]. These inconsistent findings may have resulted from exposure misclassification using ambient air monitoring data in previous studies, rather than the personal monitoring of particulate pollution that we used.
Although biological mechanisms linking particulate air pollution exposure to heart rate variability, a noninvasive marker of autonomic nervous system (ANS) dysfunction characterized by increased sympathetic activity and decreased parasympathetic activity, are still not fully understood, oxidative stress and systemic inflammation may be involved [8, 9, 10, 19, 20]. Experimental studies in animal models indicate the role of reactive oxygen species (ROS) as potential mediators of particle effects on HRV and other cardiovascular endpoints [10, 20]. Excessive generation of ROS and/or deficiency in antioxidant capacity by inhaled airborne particles can lead to oxidative stress in the lungs, which can induce proinflammatory cytokines such as tumor necrosis factor-a (TNF-a), interleukin (IL)-1 and IL-8 [19] that can enter into the peripheral circulatory system, affecting the heart and cardiovascular disease [21]. In addition, preexisting oxidative stress and inflammation enhance the production of inflammatory cytokines following particle exposure [19, 22]. These products may play a role in autonomic imbalance with an increased sympathetic tone and a reduced parasympathetic tone [23]. Subsequently, impaired antioxidant defense due to preexisting oxidative stress and inflammation may exacerbate the cardiac toxicity of PM exposure.
While this is a first study of this issue and the use of repeated measurements increases the study power to detect significant interaction effects [24] in a relatively small sample size, as in other epidemiologic study [11], our results must be interpreted with caution. Further studies of larger populations are needed to generalize our findings. Another limitation of our study is that we are unable to provide information on which constituents of PM2.5 result in detrimental cardiac autonomic effects.
5. Conclusion
In conclusion, our findings suggest that oxidative stress and systemic inflammation may exacerbate particle-induced cardiac autonomic dysfunction.
Highlights.
Few epidemiologic studies have examined the role of oxidative stress and inflammation in the association between personal exposures to ambient PM2.5 and nocturnal HRV in a general adult population. We found stronger effects of PM2.5 exposure on nocturnal SDNN and HR in subjects with elevated biomarkers of oxidative stress and systemic inflammation, implying that individuals with excessive oxidative damage and inflammation are more susceptible to particle-induced autonomic dysfunction. Our results underline the need for continued efforts in ambient particulate pollution control and the surveillance of cardiac autonomic health, in particular among individuals more susceptible to air pollution.
Acknowledgements
The authors would like to thank Dr. Jee Young Kim and Ms. Li Su for their help in primary data collection and analyses. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [26].
Funding This study was supported by grants from the U.S. EPA STAR grant (RD-83083801) and NIH (ES00002).
Abbreviations
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- CI
confidence interval
- CRP
C-reactive protein
- CVD
cardiovascular disease
- ECG
electrocardiogram
- HR
heart rate
- HRV
heart rate variability
- IQR
interquartile range
- OR
odds ratio
- PM2.5
particulate matter less than 2.5 micrometer in aerodynamic diameter
- ROS
reactive oxygen species
- SDNN
standard deviation of normal-to-normal intervals
- WBC
white blood cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Brook RD. Cardiovascular effects of air pollution. Clin Sci (Lond) 2008;115:175–87. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- [2].Utell MJ, Frampton MW, Zareba W, et al. Cardiovascular effects associated with air pollution: potential mechanisms and methods of testing. Inhal Toxicol. 2002;14:1231–47. doi: 10.1080/08958370290084881. [DOI] [PubMed] [Google Scholar]
- [3].Stein PK, Kleiger RE. Insights from the study of heart rate variability. Annu Rev Med. 1999;50:249–61. doi: 10.1146/annurev.med.50.1.249. [DOI] [PubMed] [Google Scholar]
- [4].Cavallari JM, Eisen EA, Chen JC, et al. Night heart rate variability and particulate exposures among boilermaker construction workers. Environ Health Perspect. 2007;115:1046–51. doi: 10.1289/ehp.10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee MS, Magari S, Christiani DC. Cardiac autonomic dysfunction from occupational exposure to polycyclic aromatic hydrocarbons. Occup Environ Med. 2011;68:474–8. doi: 10.1136/oem.2010.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Binici Z, Mouridsen MR, Kober L, et al. Decreased nighttime heart rate variability is associated with increased stroke risk. Stroke. 2011;42:3196–201. doi: 10.1161/STROKEAHA.110.607697. [DOI] [PubMed] [Google Scholar]
- [7].Johansen CD, Olsen RH, Pedersen LR, et al. Resting, night-time, and 24 h heart rate as markers of cardiovascular risk in middle-aged and elderly men and women with no apparent heart disease. Eur Heart J. 2013 doi: 10.1093/eurheartj/ehs449. [DOI] [PubMed] [Google Scholar]
- [8].Nel A. Atmosphere. Air pollution-related illness: effects of particles. Science. 2005;308:804–6. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- [9].Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–71. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- [10].Gurgueira SA, Lawrence J, Coull B, et al. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110:749–55. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Luttmann-Gibson H, Suh HH, Coull BA, et al. Systemic inflammation, heart rate variability and air pollution in a cohort of senior adults. Occup Environ Med. 2010;67:625–30. doi: 10.1136/oem.2009.050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim JY, Prouty LA, Fang SC, et al. Association between fine particulate matter and oxidative DNA damage may be modified in individuals with hypertension. J Occup Environ Med. 2009;51:1158–66. doi: 10.1097/JOM.0b013e3181b967aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- [14].Lai CH, Liou SH, Lin HC, et al. Exposure to traffic exhausts and oxidative DNA damage. Occup Environ Med. 2005;62:216–22. doi: 10.1136/oem.2004.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fang SC, Cavallari JM, Eisen EA, et al. Vascular function, inflammation, and variations in cardiac autonomic responses to particulate matter among welders. Am J Epidemiol. 2009;169:848–56. doi: 10.1093/aje/kwn405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang W, Zhu T, Pan X, et al. Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: interactions of systemic inflammation, overweight, and gender. Am J Epidemiol. 2012;176:117–26. doi: 10.1093/aje/kwr511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Park SK, Auchincloss AH, O'Neill MS, et al. Particulate air pollution, metabolic syndrome, and heart rate variability: the multi-ethnic study of atherosclerosis (MESA) Environ Health Perspect. 2010;118:1406–11. doi: 10.1289/ehp.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chuang KJ, Chan CC, Su TC, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–6. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- [19].Donaldson K, Stone V, Seaton A, et al. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect. 2001;109(Suppl 4):523–7. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rhoden CR, Lawrence J, Godleski JJ, et al. N-acetylcysteine prevents lung inflammation after short-term inhalation exposure to concentrated ambient particles. Toxicol Sci. 2004;79:296–303. doi: 10.1093/toxsci/kfh122. [DOI] [PubMed] [Google Scholar]
- [21].Nelin TD, Joseph AM, Gorr MW, et al. Direct and indirect effects of particulate matter on the cardiovascular system. Toxicol Lett. 2012;208:293–9. doi: 10.1016/j.toxlet.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stringer B, Kobzik L. Environmental particulate-mediated cytokine production in lung epithelial cells (A549): role of preexisting inflammation and oxidant stress. J Toxicol Environ Health A. 1998;55:31–44. doi: 10.1080/009841098158601. [DOI] [PubMed] [Google Scholar]
- [23].Aronson D, Mittleman MA, Burger AJ. Interleukin-6 levels are inversely correlated with heart rate variability in patients with decompensated heart failure. J Cardiovasc Electrophysiol. 2001;12:294–300. doi: 10.1046/j.1540-8167.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- [24].Guo Y, Logan HL, Glueck DH, et al. Selecting a sample size for studies with repeated measures. BMC Med Res Methodol. 2013;13:100. doi: 10.1186/1471-2288-13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hoenig JM, Heisey DM. The abuse of power: the pervasive fallacy of power calculations for data analysis. The American Statistician. 2001;55:19–24. [Google Scholar]
- [26].Coats AJ. Ethical authorship and publishing. Int J Cardiol. 2009;131:149–50. doi: 10.1016/j.ijcard.2008.11.048. [DOI] [PubMed] [Google Scholar]