Abstract
Inflammasomes are central mediators of host defense to a wide range of microbial pathogens. The NLRP3 inflammasome plays a key role in triggering caspase-1 dependent IL-1β maturation and resistance to fungal dissemination in Candida albicans infection. β-glucans are major components of fungal cell walls that trigger IL-1β secretion in both murine and human immune cells. In this study, we sought to determine the contribution of β-glucans to C. albicans-induced inflammasome responses in mouse dendritic cells. We show that the NLRP3-ASC-caspase-1 inflammasome is absolutely critical for IL-1β production in response to β-glucans. Interestingly, we also found that both Complement Receptor 3 (CR3/Mac-1) and dectin-1 play a crucial role in coordinating β-glucan-induced IL-1β processing as well as a cell death response. In addition to the essential role of caspase-1, we identify an important role for the pro-apoptotic protease caspase-8 in promoting β-glucan-induced cell death and NLRP3 inflammasome-dependent IL-1β maturation. A strong requirement for Complement Receptor 3 and caspase-8 was also found for NLRP3 dependent IL-1β production in response to heat killed Candida albicans. Together, these results define the importance of dectin-1, CR3 and caspase-8, in addition to the canonical NLRP3 inflammasome, in mediating β-glucan and C. albicans induced innate responses in dendritic cells. Collectively, these findings establish a novel link between β-glucan recognition receptors and the inflammatory proteases caspase-8 and caspase-1 in coordinating cytokine secretion and cell death in response to immunostimulatory fungal components.
Introduction
Normally part of the human commensal flora, certain fungal species can become opportunistic pathogens when antibiotic treatment, immune suppressants or pathogenic microorganisms perturb normal homeostatic balance. Candida spp., especially Candida albicans, can lead to invasive, mucosal and systemic infections (1). The host innate immune system maintains a tolerant interaction with commensal microbial flora and in healthy individuals is normally able to protect the host against invasive fungal infection. Understanding how these immune responses are elicited and controlled has important therapeutic implications for the treatment of fungal disease in immune-compromised conditions. In this context, the pro-inflammatory cytokines IL-1β and IL-18 have been identified as integral components of anti-fungal immune defenses. Mouse models of candidiasis and human exvivo studies have uncovered a critical role for IL-1β and IL-18 in protecting the host against fungal dissemination, via their ability to trigger T-cell mediated production of IL-17 and IFNγ respectively (2-9).
In most cell types, secretion of mature IL-1β (Uniprot: P10749) requires at least two signals: synthesis of the precursor pro-IL-1β (and sometimes the NLR2, NLRP3) through NF-κB activation (signal 1) and the subsequent activation of caspase-1 through the formation of an inflammasome (signal 2). This inflammasome complex is responsible for cleaving pro-IL-1β and triggering the release of mature IL-1β. Inflammasomes are assembled and activated in the cytosol in response to both microbial and non-microbial danger signals. NLRs as well as DNA binding PYHIN proteins are capable of forming caspase-1 activating inflammasomes. ASC (UniProt: Q9EPB4), an adaptor protein that comprises a PYRIN and a CARD domain bridges the homotypic interaction between NLR/PYHIN proteins and CARD-domain containing caspase-1 (UniProt: P29452). Seminal studies have established the importance of NLR and PYHIN proteins such as NLRP3 (UniProt: Q8R4B8), NLRC4 (UniProt: Q3UP24) and AIM2 (UniProt: Q91VJ1) in the maturation of the IL-1 family of pro-inflammatory cytokines in response to pathogenic and sterile assaults (reviewed in (10)). We and others have shown important roles for NLRP3, NLRC4 and NLRP10 (UniProt: Q8CCN1) in different aspects of anti-fungal immune responses (2, 11-13).
Our interest in understanding the molecular mechanisms underlying inflammasome activation during infection with Candida albicans was particularly focused on defining the contribution of cell wall polysaccharide structures, which constitute 90% of the yeast cell wall and are the primary mechanism by which the innate system senses fungal infection (14, 15). One major component of fungal cell walls are the β(1,3) and β(1,6) glucans that are important for its structural framework, along with mannans, proteins and chitin (14). β-glucans are highly immunostimulatory and have also been considered effective as immune supplements and as well as possible vaccine adjuvants. Detection of β(1,3) glucan and antibodies to β(1,3) glucan in the plasma are considered biomarkers of candidiasis, and many diagnostic tests that exploit this finding are under clinical development (16-18). Studies on inflammasome responses to β-glucans are key to advancing our understanding of host fungal sensing pathways and their mode of action as immunomodulators.
In this study, we investigated the molecular and mechanistic details of inflammasome responses to C. albicans by employing β-glucans and heat-killed C. albicans in conjunction with live C. albicans. While previous studies have yielded contrasting observations regarding the role of NLRP3 in β-glucan triggered inflammasome responses (19-21), we found the canonical NLRP3-ASC-caspase-1 inflammasome to be essential in mediating IL-1β production by both β-glucans and C. albicans (live or heat-killed) in mouse dendritic cells. The receptors linking β-glucan to inflammasome activation were dectin-1 (UniProt: Q6QLQ4) and Complement Receptor 3 (CR3/Mac-1), another receptor implicated in β-glucan sensing. We also uncovered an essential role for CR3 in mediating IL-1β production to heat-killed (HK) and to a lesser extent, to live C. albicans. Moreover, Dectin-1 and CR3 appear to also function non-redundantly in a less-well characterized cellular death response to β-glucans and HK C. albicans.
A growing body of literature points to the existence of diverse and complex molecular platforms for inflammasomes including other caspases such as caspase-11 and -8 (19, 22-25). Gringhuis, S. I. et al. (2012) showed that IL-1β triggered by β-glucan and some heat-killed strains of C. albicans was caspase-8 (UniProt: O89110) dependent, but independent of caspase-1 in human cells. Further, recent studies show that caspase-8 localizes to ASC specks (26). Caspase-8 has also been found to be required for inflammasome responses during Salmonella infection or upon treatment of cells with pro-apoptotic, chemotherapeutic drugs and in Fas signaling (22, 26, 27). Casp8−/− mice are embryonic lethal due to RIP3-dependent necrosis (28). This lethality is rescued by the genetic deletion of RIP3 (UniProt: Q9QZL0) (29, 30). Using the viable Casp8−/− Rip3−/− double knockout and the Rip3−/− single knockouts as a control, we present evidence for caspase-8 involvement in β-glucan-triggered IL-1β production as well as cell death in mouse dendritic cells. Comparing the molecular requirements of inflammasome response between β-glucan and heat-killed (HK) C. albicans (which has exposed β-glucans), with that of live C. albicans (which has most of its β-glucans masked by mannans), we identify a differential requirement for CR3, dectin-1 and caspase-8 in IL-1β and cell death responses.
Our findings suggest a β-glucan-specific dectin-1/CR3/caspase-8 pathway that synergizes with the canonical NLRP3-ASC-caspase-1 pathway for optimal inflammasome responses. This study gives rise to new insights into the differences in host immune sensing of fungal molecular patterns such as β-glucans in contrast to live C. albicans infection.
Materials and Methods
β-glucans
Curdlan was purchased from Sigma, Invivogen or Wako Chemicals. Carboxy-methylated curdlan was also from Wako chemicals. Curdlan was resuspended in sterile PBS at 10mg/ml and used at 100μg/ml to stimulate BMDC or 1mg/ml for BMDM, unless otherwise indicated. Fungal whole glucan particle agonist or antagonist (WGP dispersible or WGP soluble) was purchased from Invivogen. WGP (dispersible/soluble) was used at 100μg/ml, unless otherwise indicated.
Mice
Mice were maintained and bred at UMASS Medical School in accordance with the Institutional Animal Care and Use Committee (IACUC). Casp8+/−Rip3−/− mice were kindly provided by Dr. Douglas Green (St. Jude Children's Research Hospital) and in some cases Casp8−/− Rip3−/− femurs were from Dr. Bill Kaiser and Dr. Edward S. Mocarski (Emory University School of Medicine), Card9−/− femurs were from Dr. Ramnik Xavier (Massachusetts General Hospital) (31) and Itgam−/− femurs from Dr. Tanya N. Mayadas (Brigham and Women's Hospital) (32). Casp1−/− and casp11−/− mice were kindly provided by Dr. Vishva Dixit (Genentech) (24), Clec7a−/− (Dectin-1−/−) mice were from Gordon Brown (University of Aberdeen) (33). Nlrp3−/− and Asc−/− mice were from Millennium Pharmaceuticals. Casp8+/−Rip3−/− mice were on a mixed C57/BL6-129 background and were intercrossed to generate Casp8−/−Rip3−/− and Casp8+/+Rip3−/− mice, which were used as littermate controls in all of our experiments. All other mice were on a C57/BL6 background.
Candida albicans and EHEC culture conditions
C. albicans UC820 (ATCC MYA-3573) strain, obtained from Dr. Mihai Netea (Nijmegen Institute for Infection, Inflammation and Immunity), was used in all experiments (34). C. albicans was maintained as glycerol stocks and cultured in the yeast-phase in Sabouraud Dextrose (SBD) broth at 30°C, 250 rpm. Overnight cultures were re-inoculated 1:20 in SBD broth for 4h to obtain yeast forms. Live log phase yeast cultures were washed twice and resuspended in PBS, counted and used directly or heat-killed (95°C for 30’) and used for BMDC stimulation. For differentiation into hyphal forms, overnight cultures were re-inoculated in serum free, antibiotic free RPMI, with 10% heat-inactivated fetal bovine serum for 4h at 37°C, 250 rpm. Hyphae were washed twice and resuspended in PBS, counted and used. EHEC (Enterohemorrhagic Escherichia coli, strain O157:H7, EDL 933) was maintained as glycerol stocks and cultured overnight in LB media, at 37°C, 250 rpm and used in stationary phase for experiments as previously described (25).
BMDC and BMDM culture
For bone marrow derived macrophages, progenitor cells were cultured in DMEM with 10% heat inactivated serum, Pen-strep and 20% L929 supernatant as the source of MCSF. Media was changed on day 3, 6 and 8 and cells were used for experiments between day 8-10. For dendritic cells, bone marrow progenitor cells were counted and plated at 8 x 106 cells/ 25cm2 dish in RPMI with 10% heat inactivated serum, Pen-strep, 20ng/ml recombinant GM-CSF and 50μM β-mercaptoethanol and cultured for 9-10 days as previously described (35).
For ELISAs, BMDC or BMDM were plated in 96 well plates at 1 x 106 cells/ml. Cells were unprimed or primed with Pam2CSK4 at 100ng/ml for 3h and stimulated with curdlan, WGP agonist or EHEC for 6h or 24h. Media was replaced with fresh gentamicin containing media for EHEC infected cells after 1h of infection. For controls, cells were stimulated with canonical inflammasome activators such as pdAdT or silica for 6h, nigericin or ATP for 1h after priming. For experiments with the WGP antagonist, unprimed or primed cells were pretreated with 100μg/ml antagonist for 1h, before stimulation with β-glucan ligands. For C. albicans infection, cells were infected with heat-killed or live log phase cultures at 1:1, 10:1 for 6h post priming. Cytokine levels were measured in triplicate and data are representative of three independent experiments.
For western blot analysis, cells were plated at 2 × 106 cells/ well in 12 well plates in serum free, antibiotic free medium, primed and stimulated as described above. Supernatant proteins were precipitated with methanol and chloroform. Cells were lysed with 1% NP-40 lysis buffer and Bradford assay was performed to normalize the amount of protein. Samples were run on 12% SDS-PAGE and transferred to nitrocellulose membrane. Western blotting was performed with the antibodies to caspase-1 p10 (sc-514, Santa Cruz Biotechnology), caspase-1 p20 (Casper1 clone; Adipogen), IL-1β (AF-401-NA, R&D Systems), caspase-8 (1G12, Enzo Life Sciences), RIP3 (2283, Pro-Sci), α-tubulin (2125, Cell signaling), β-glucan (400-2, Biosupplies), CF488A goat anti-mouse IgG (20010, Biotium), Fluorescein conjugated goat IgG to mouse complement C3 (0855500, MP Biomedicals).
Confocal Microscopy
Silica, WGP agonist, live or HK C. albicans were fixed with 4% paraformaldehyde and stained with 1:250 dilution of the mouse monoclonal β-glucan antibody, washed, followed by staining with CF488A goat anti mouse secondary antibody. The stained preparations were then visualized by confocal microscopy for glucan staining.
Serum opsonization and C3 staining
Blood was harvested from C57BL/6 mice by cardiac puncture and serum isolated by centrifugation. Live and HK C. albicans were left untreated or opsonized with serum (20%) and incubated at 37°C for 30’. The cells were then spun down, washed twice with PBS and resuspended in RPMI. Pam2CSK4 primed BMDC were then stimulated with opsonized/unopsonized live or HK C. albicans for 6h. C3 deposition was verified by fixing these cells with 1% paraformaldehyde and staining them with goat anti- mouse C3 FITC antibody or isotype control and analyzing them by flow cytometer.
ELISA
The amount of IL-1β present in cell culture supernatants was measured by ELISA (BD Biosciences or eBioscience) according to manufacturers’ instructions.
Quantitative RT-PCR
RNA was extracted from cells using RNeasy kit (QIAGEN). iScript Select cDNA synthesis kit (Bio-Rad) was used to synthesize cDNA from 1μg total RNA from each sample. Quantitative RT-PCR for pro-IL-1β and β-actin was performed using iQ SYBR green supermix (Bio-Rad). Primers used for pro-IL-1β were 5’-TCCCCAGCCCTTTTGTTGA-3’ (F), 5’-TTAGAACCAAATGTGGCCGTG-3’ (R) and β-actin were 5’-TTGAACATGGCATTGTTACCAA-3’ (F), 5’-TGGCATAGAGGTCTTTACGGA-3’ (R). Levels of pro-IL-1β mRNA were normalized to that of β-actin.
Caspase-8 Activity and Cell death Assay
Caspase-8 activity in cell lysates was assayed using Caspase-Glo 8 Assay kit (Promega). Cell viability was assessed using Cell-Titer Glo kit (Promega) according to manufacturers’ instructions.
Statistics
: Data were analyzed by two-way analysis of variance followed by Bonferroni test using PRISM software. P value less than 0.05 were considered significant. For cytokine measurements, experiments were performed in triplicate from one mouse and the graphs depicted are representative of atleast two and in most cases, three independent experiments. The triplicates of the representative experiment shown were analyzed using two-way ANOVA. The significant comparisons are marked by an asterisk (p value < 0.05) and the unmarked groups in the same graph are not. In the case of experiments with Casp8+/+ Rip3+/+, Casp8+/+ Rip3−/− and Casp8−/− Rip3−/− dendritic cells, the groups Casp8+/+ Rip3−/− and Casp8−/− Rip3−/− were compared to assess significance.
Results
NLRP3-ASC-caspase-1 Inflammasome is essential for C. albicans and β-glucan induced IL-1β
We and others have previously demonstrated that C. albicans induced IL-1β is mediated by the NLRP3-ASC-caspase-1 inflammasome (2, 11). In this study, we first examined if our strain of live C. albicans triggers IL-1β production in BMDC. As shown in figure 1A, we observed that both live yeast as well as hyphal forms induce IL-1β. In particular, the yeast form is highly immunostimulatory (as seen with both the MOI), has high colonizing and disseminating potential and can transition into the hyphal form during infection. Using the yeast form of C. albicans, we set out to characterize the mechanism of inflammasome activation and the immunostimulatory component(s) of C. albicans responsible for this activity. Interestingly, heat-killed C. albicans (HK C. albicans) triggers levels of IL-1β production similar to that seen with live fungi, and this response is also completely dependent on the NLRP3-ASC-caspase-1 inflammasome in mouse dendritic cells (Figure 1B and 1C). β-glucan, which is part of fungal cell walls, is a highly immune-stimulatory Pathogen Associated Molecular Pattern (PAMP) that is exposed in heat-killed fungi (36). Hence we sought to understand the underlying mechanism of inflammasome activation by β-glucans. The composition of microbial glucans may vary depending on the length, molecular weight, frequency of branching and the type of glycosidic linkage. Two representative β-glucans employed in this study are: (1) curdlan3, a β(1,3) glucan derived from the bacterium Alcaligenes faecalis; and (2) dispersible Whole fungal Glucan Particles derived from the cell walls of the baker's yeast Saccharomyces cerevisiae (WGP agonist). Curdlans as well as WGP are high molecular weight, insoluble particles. Glucan particles derived from S. cerevisiae (WGP agonist) as well as C. albicans contain a linear 1,3 linked glucan backbone with occasional 1,6 branches. Although the cell wall of S. cerevisiae and C. albicans are considered qualitatively similar (37, 38), they exhibit quantitative differences in the wall components (39). Previous studies showed that the alkali-insoluble glucan of C. albicans contains about 43-53% β(1,6) linkages, 32-39% β(1,3) linkages and is highly branched. In sharp contrast, the alkali-insoluble glucan of S. cerevisiae are primarily composed of linear β(1,3) glucose with about 3% β(1,6) linkages (39, 40). However, curdlan from A. faecalis is just linear 1,3 glucan with no branching. Curdlan and WGP agonist are considered to be good models for studying the innate immune responses to fungal β(1,3) glucans. We observed that, curdlan as well as WGP agonist induced IL-1β production in dendritic cells, in the absence or presence of Pam2CSK4 (TLR2) priming (Figure 1D). Pam2CSK4 priming greatly boosted this response. All remaining experiments in this study were performed in Pam2CSK4 primed dendritic cells, unless otherwise indicated. Further, we tested increasing doses of curdlan and WGP agonist and determined 100μg/ml to be optimal for inducing IL-1β production in Pam2CSK4 primed BMDC (Figure 1E).
Figure 1. NLRP3-ASC-caspase-1 inflammasome is essential for Candida albicans and β-glucan induced IL-1β.
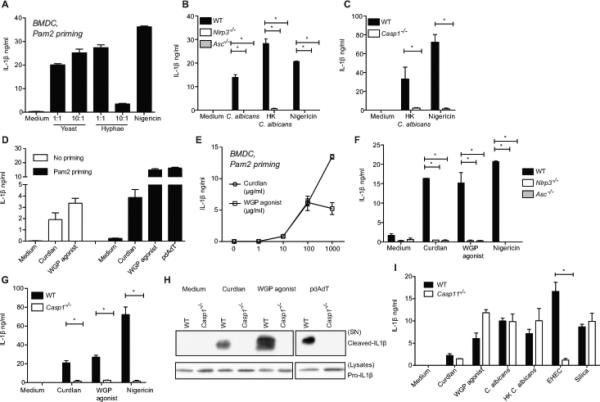
Pam2CSK4 primed BMDC from C57BL/6 mice were infected with live C. albicans yeast or hyphal forms at 1:1 or 10:1 for 6h or nigericin for 1h (A). Pam2CSK4 primed BMDC from Nlrp3−/−, Asc−/− (B, F) Casp1−/− (C, G, H) or Casp11−/− (I) along with C57BL/6 mice were stimulated with live or heat-killed C. albicans yeast at 10:1 for 6h, pdAdT or silica for 6h, nigericin for 1h, β-glucans for 24h (D, F, G) or 6h (E, H, I) or EHEC for 24h. IL-1β released in the supernatants was measured by ELISA (A-G, I). Precipitated supernatants or lysates of cells stimulated as indicated were probed for IL-1β (H). Statistical analysis was performed on results in panels B, C, F, G and I as described in the Materials and Methods section.
To determine the role of the NLRP3-ASC-caspase-1 inflammasome in β-glucan induced IL-1β production, Pam2CSK4-primed wild-type (WT), Nlrp3−/−, Asc−/−, Casp1−/− BMDC were stimulated with curdlan and WGP agonist. IL-1β secretion was completely abrogated in Nlrp3−/−, Asc−/−, Casp1−/− dendritic cells (Figure 1F and 1G). IL-1β processing detected by immunoblotting of BMDC culture supernatants, was severely impaired in the absence of caspase-1 (Figure 1H). Of note, our previous studies utilized the Casp1−/− mice that carried the Casp11 passenger mutation associated with the 129 mouse strain (24, 41, 42). Caspase-11 is essential for inflammasome activation by gram-negative bacteria (24, 25). To carefully examine the role of caspase-1, the experiments in Figure 1 used Casp1−/− mice expressing caspase-11 (UniProt: P70343) from a bacterial artificial chromosome (24). Moreover, caspase-11 was not required for IL-1β production in response to curdlan, live or heat-killed (HK) forms of the fungus in contrast to EHEC (Figure 1I). These results show a critical and specific role for the canonical NLRP3-ASC-caspase-1 axis in not only live, but also HK C. albicans and β-glucan induced IL-1β responses.
Sensing and phagocytosis of particulate β-glucan triggers IL-1β production
In order to ensure that the responses elicited by the curdlan preparations were specifically due to β-glucans, cells were pre-treated with a well-established antagonist, soluble β-glucan (WGP antagonist), to competitively bind and block signaling through host β-glucan receptors. WGP antagonist-treated cells were then stimulated with curdlan from two independent sources (Invivogen or Wako Chemicals) and IL-1β production was assayed. Curdlan is capable of triggering the synthesis (signal 1) and maturation (signal 2) of pro-IL-1β in dendritic cells, as observed in the experiments with unprimed cells shown in Figure 1D. In the unprimed condition, WGP antagonist treatment led to an attenuated curdlan-induced IL-1β response (Figure 2A). Since the WGP antagonist could be blocking either of the two signals, the effect of WGP antagonist specifically on signal 2 was addressed by priming the cells with a non-β-glucan ligand, Pam2CSK4, to induce signal 1. WGP antagonist also attenuated IL-1β production in the primed BMDCs specifically in response to curdlan, but not pdAdT, (an activator of the AIM2 inflammasome) (Figure 2B), indicating that WGP antagonist inhibits β-glucan induced inflammasome activation and IL-1β release (signal 2). Together, these results highlight that receptor mediated sensing of β-glucan is important for inflammasome activation.
Figure 2. Sensing and phagocytosis of particulate β-glucan triggers IL-1β production.
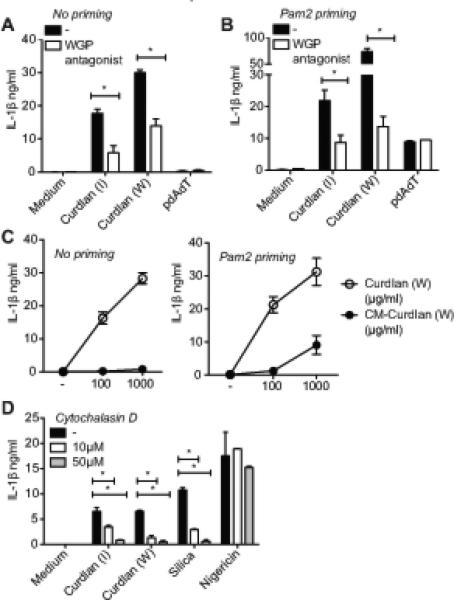
Unprimed or Pam2CSK4 primed BMDC from C57BL/6 mice were stimulated with curdlan (I or W) for 24h (A-C) or 6h (D), pdAdT or silica for 6h, nigericin for 1h in the presence of the soluble WGP antagonist (A-B) or Cytochalasin D (D). Curdlan or Carboxymethylated curdlan from Wako Chemicals (W) were used at increasing concentrations (C). IL-1β released in the supernatants was measured by ELISA. Statistical analysis was performed on results in panels A, B and D as described in the Materials and Methods section.
Considerable research is devoted to water-soluble chemical derivatives of β-glucans to improve the ease of their use in clinical applications. One such water-soluble derivative of curdlan, carboxy-methylated-curdlan (or CM-curdlan) was tested for its inflammasome triggering activity in BMDC. CM-curdlan induced IL-1β production was very weak compared to the same concentration of unmodified curdlan in unprimed as well as Pam2CSK4-primed cells (Figure 2C), indicating water soluble CM-curdlan is a poor activator of inflammasomes. Poor immune-stimulatory activity of CM-curdlan has also been observed in vivo, where pre-injection of curdlan, but not CM-curdlan protects mice from lethal E. coli infection (43). The weak ability of CM-curdlan to trigger IL-1β production indicates that the particulate properties of β-glucans are important for triggering inflammasome activation. In line with that idea, chitosan, a deacetylated derivative of chitin, induces higher IL-1β in macrophages in particulate form compared to its soluble counterpart (44).
Since the particulate form of curdlan was more immunostimulatory and dendritic cells are highly phagocytic, we determined whether inflammasome responses to β-glucans require their uptake. Cells were pretreated with cytochalasin D, an inhibitor of actin re-arrangement and phagocytosis. Cytochalasin D severely reduced β-glucan triggered IL-1β production (Figure 2D), showing that ingestion of curdlan is required for the triggering of IL-1β release, similar to other particulate inflammasome activators. Interestingly, β-glucans have been known to cause apoptosis of cancer cells (45). However the mechanism of β-glucan mediated cell death has not yet been characterized. Using a luciferase-based assay to measure intracellular ATP as an indicator of cell viability, we observed that β-glucans triggered a moderate decrease in the viability of dendritic cells (Figure S1A). Phagocytosis is essential for this process similar to that of the particulate stimulus silica (Figure S1A). Of note, when we measured lactate dehydrogenase (LDH) in the supernatants, minimal to no increase was observed in β-glucan stimulated cells, unlike nigericin treated cells (data not shown), suggesting that either the LDH assay was not as sensitive as the ATP based assay or that LDH may not be released in the cell death process observed here.
Dectin-1 and CARD9 signaling are important for β-glucan triggered IL-1β processing and release
The C-type lectin receptor family of proteins is considered the primary recognition and phagocytic receptors for fungal cell wall components, and dectin-1, in particular, plays an important role in anti-fungal immunity (46, 47). To identify innate immune receptors that couple β-glucan recognition to inflammasome activation, we tested the role of dectin-1 in IL-1β maturation and secretion. Curdlan (I) induced IL-1β production was markedly decreased in Pam2CSK4-primed Clec7a−/−4 (dectin-1-deficient) BMDC (Figure 3A). Western blotting of precipitated supernatants from curdlan (I) stimulated cells reflected this decrease in the amount of processed IL-1β in Clec7a−/− BMDC (Figure 3B). Curdlan induced caspase-1 processing was also markedly reduced in Clec7a−/− BMDC, indicating that dectin-1 signaling is important for β-glucan induced caspase-1 activation (Figure 3B). CARD9 is an adaptor molecule that mediates NF-κB activation by many ITAM associated receptors including dectin-1 and is an indispensable component of anti-fungal innate immune responses in myeloid cells (48). Consistent with a role for dectin-1 in inflammasome activation by curdlan, IL-1β production by Pam2CSK4-primed Card9−/− BMDC was also decreased in response to curdlan (I) (Figure 3C). Card9−/− BMDC exhibited reduced IL-1β processing in response to curdlan (Figure 3D), indicating that the dectin-1-CARD9 signaling axis is critical for curdlan (I) induced IL-1β maturation. Dectin-1 deficiency also protected cells from β-glucan induced cell death (Figure S1B), indicating that dectin-1 not only triggers IL-1β processing, but also cell death.
Figure 3. Dectin-1 and CARD9 signaling are important for β-glucan triggered IL-1β processing and release.
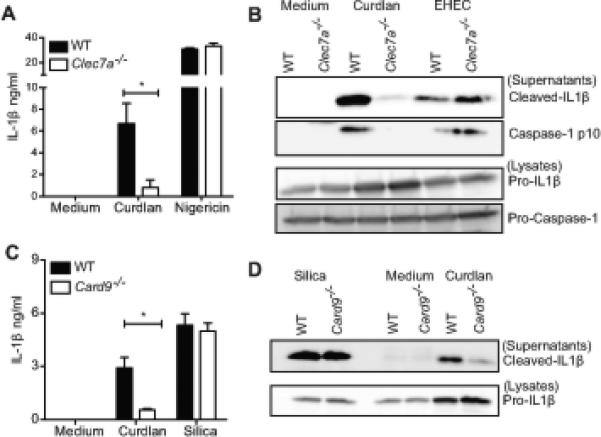
Pam2CSK4 primed BMDC from C57BL/6, Clec7a−/−, Card9−/− mice were stimulated with curdlan (I), EHEC or silica for 6h. IL-1β released in the supernatants was measured by ELISA (A, C). Cells were stimulated as indicated, precipitated supernatants and lysates were probed for IL-1β or caspase-1 p10 (B, D). Statistical analysis was performed on results in panels A and C as described in the Materials and Methods section.
Complement Receptor 3 mediates β-glucan induced inflammasome activation
Another receptor that has been implicated in β-glucan recognition is Complement Receptor 3 (CR3), an integrin composed of CD11b/CD18 (Uniprot: P05555/P11835). In addition to binding the complement component iC3b, CR3 has a distinct lectin site for binding β-glucan (49-51). Similar to other leukocyte integrins, upon receiving intracellular signals, CR3 undergoes conformational changes required for it to optimally bind its ligand. Recently, it was shown that dectin-1 activates inside-out signaling of CR3 that in turn promotes CR3 binding of C. albicans and subsequent neutrophil anti-fungal effector functions (52). The role of CR3 in inflammasome activation generally and particularly in response to β-glucans or C. albicans has not been investigated to date. To test the role of CR3 in this pathway, Pam2CSK4-primed Itgam−/− (CR3-deficient) BMDC were stimulated with curdlan (I), and IL-1β release was measured. CR3-deficient BMDC (Itgam−/− BMDC) displayed severely impaired IL-1β production in response to curdlan (Figure 4A). Curdlan induced IL-1β processing was also drastically reduced in the Itgam−/− BMDC, while that induced by silica was unaffected (Figure 4B). Remarkably, curdlan triggered caspase-1 processing was also impaired in CR3 deficient cells, suggesting that CR3 is important for inflammasome mediated caspase-1 activation in response to β-glucans. The role of CR3 observed in caspase-1 activation and IL-1β production appears to be complement-independent, since all of these experiments were performed in serum-free media. Interestingly, CR3-deficiency also protected dendritic cells from β-glucan triggered cell death (Figure S1C). Together, these results indicate an essential role for CR3 in innate immune responses to β-glucans.
Figure 4. Complement Receptor 3 is critical for β-glucan induced inflammasome activation and IL-1β production.
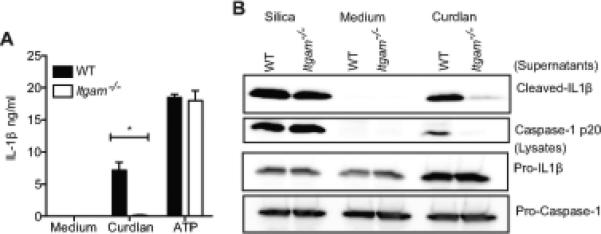
Pam2CSK4 primed BMDC from C57BL/6, Itgam−/− mice were stimulated with curdlan (I) for 6h or ATP for 1h. IL-1β released in the supernatants was measured by ELISA (A). Cells were stimulated as indicated, precipitated supernatants and lysates were probed for IL-1β or caspase-1 p20 (B). Statistical analysis was performed on results in panel A as described in the Materials and Methods section.
Caspase-8 promotes β-glucan-induced IL-1β production
We have shown that dectin-1 is required not only for β-glucan induced IL-1β maturation, but also for dendritic cell death (Figures 3A and S1B). A recent study reported that dectin-1 mediated caspase-8 activation is essential for β-glucan induced IL-1β responses in human dendritic cells (19). We have also linked caspase-8 to Fas ligand-induced IL-1β responses in mouse macrophages (22). Thus, we investigated if caspase-8 is involved in β-glucan triggered IL-1β production as well as cell death in mouse BMDCs. To define the role of caspase-8 in this pathway, we utilized Casp8−/−Rip3−/− mice which are viable (29, 30), in contrast to the embryonically lethal Casp8−/− (28). First, we stimulated BMDC from C57BL/6, Casp8−/−Rip3−/− and littermate Rip3−/− mice with curdlan, WGP agonist, pdAdT and measured both the release and processing of IL-1β. Additionally, we treated the cells with Fas ligand-containing vesicles, as a positive control for caspase-8 dependent IL-1β production. We observed that caspase-8 deficiency reduced curdlan and WGP agonist mediated IL-1β production in Pam2CSK4 primed BMDC (Figure 5A). This effect was observed across various time points (Figure 5B). Curdlan from other sources such as Sigma or Wako chemicals also showed a similar IL-1β phenotype in Casp8−/−Rip3−/− BMDC (Figure S2A). In contrast, the activation of the AIM2 inflammasome by pdAdT or NLRP3 inflammasome by silica was intact in Casp8−/−Rip3−/− BMDC (Figure 5B and S2A). Casp8−/−Rip3−/− BMDC exhibited reduced levels of cleaved IL-1β in the supernatants of stimulated cells compared to the control Rip3−/− BMDC (Figure 5C). Notably, even though BMDM do not respond to β-glucan as robustly as BMDC, we also observed a similar reduction of IL-1β release in BMDM (Figure S2B-C). IL-1β processing detected in the supernatants of stimulated macrophages was also decreased in Casp8−/−Rip3−/− compared to the littermate Rip3−/− cells (Figure S2D). Surprisingly, caspase-1 processing was intact in Casp8−/−Rip3−/− BMDCs, suggesting that caspase-8 regulates IL-1β processing independent of caspase-1 activation (Figure 5D). We also examined the effect of caspase-8 deficiency on cell death triggered by β-glucans. The moderate cell death induced by curdlan or WGP agonist was protected in Casp8−/−Rip3−/− cells compared to the controls (Figure S2E). These data indicate that caspase-8 and caspase-1 together coordinate β-glucan-induced IL-1β maturation in dendritic cells and caspase-8 promotes a β-glucan induced cell death pathway.
Figure 5. Caspase-8 promotes β-glucan induced IL-1β maturation.
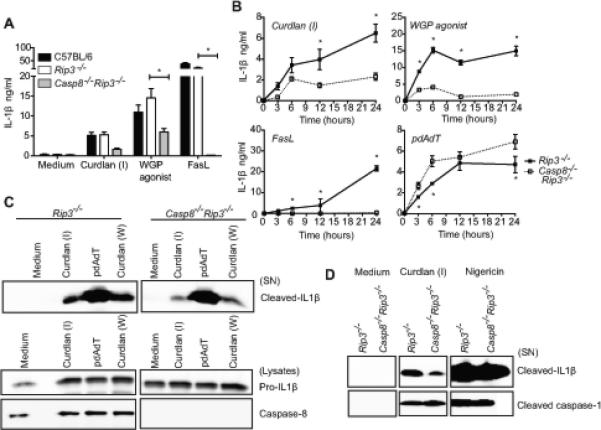
Pam2CSK4 primed BMDC from C57BL/6, littermate Rip3−/−, Casp8−/−Rip3−/− mice were stimulated with β-glucans for 24h, FasL, pdAdT for 6h (A) or as indicated (B). IL-1β released in the supernatants was measured by ELISA (A, B). Cells were stimulated as indicated and precipitated supernatants and lysates were probed for IL-1β, caspase-1 or caspase-8 (C, D). Statistical analysis was performed on results in panels A and B as described in the Materials and Methods section.
Many previous studies have suggested a role for caspase-8 in NF-κB activation in T cells, B cells and MEFs (53-55), yet this remains controversial (56, 57). Pro-IL1β is a transcriptional target of NF-κB and hence, we examined the impact of caspase-8 deficiency on pro-IL-1β synthesis in response to β-glucans. In the absence of Pam2CSK4 priming, pro-IL1β mRNA levels were similar in WT, Rip3−/−, Casp8−/−Rip3−/− BMDC stimulated with curdlan (Figure S3A), suggesting that caspase-8 deficiency does not affect curdlan-induced signal 1. Curdlan has been found to synergize with TLR2 and TLR4 agonists for TNF induction (58), and hence we also investigated the role of caspase-8 in TLR2 and TLR4 induced pro-IL-1β synthesis. When cells were primed with the TLR2 agonist Pam2CSK4, prior to curdlan (I) stimulation, (similar to the experimental conditions used in most of this study) pro-IL-1β protein levels in the Casp8−/−Rip3−/− DC were comparable to that in WT or Rip3−/− cells (Figure S3B), as is also seen in figures 5C and S2D. These results indicate that caspase-8 does not play a role in β-glucan or lipopeptide triggered signal 1 (NF-κB mediated pro-IL-1β induction) and confirm the effect of caspase-8 on signal 2 (inflammasome mediated pro-IL-1β processing). However, LPS (a TLR4 agonist) induced pro-IL-1β was markedly decreased in Casp8−/−Rip3−/− DC compared to WT or Rip3−/− cells (Figure S3C). Consistent with a defect in TLR4 induced signal 1, LPS induced TNF, another well-characterized NF-κB target gene, was also decreased in Casp8−/−Rip3−/− DC (Figure S3D). Other recently studies published have also demonstrated the critical role of caspase-8 in the pro-inflammatory cytokine (TNF, IL-6, pro-IL-1β) production as well as in the maturation of IL-1β in response to LPS and gram-negative bacteria such as Yersinia pestis and Citrobacter rodentium (59, 60) or in the case of ER stress (61). In stark contrast to TNF, LPS induced IFNβ levels were elevated in Casp8−/−Rip3−/− DC, compared to the controls (Figure S3E). This latter observation is consistent with a previous finding that revealed negative regulation of IFNβ production by caspase-8 (62). Prior studies have indicated that IFNβ blocks pro-IL1β transcription (63). It is possible that priming of Casp8−/−Rip3−/− cells via TLR4 may severely impact the synthesis of pro-IL1β, by both reducing signal 1 and inducing higher IFNβ, which collectively could contribute to reduced pro-IL-1β expression. We verified that TLR4 deficiency does not have any effect on curdlan induced IL-1β in BMDM, both in unprimed and primed conditions (Figure S3F). This rules out any possible role of caspase-8 on β-glucan induced IL-1β indirectly through its role in TLR4 signaling or LPS contaminants in curdlan. Importantly, the use of Pam2CSK4 priming throughout our study provided an ideal tool to avoid any potential impairment of pro-IL1β that could result from LPS priming.
Differential requirement of caspase-8, dectin-1 and CR3 for HK versus live C. albicans induced IL-1β production and cell death
Having identified the innate immune pathways involved in the execution of IL-1β and cell death responses to β-glucan, we sought to explore the relevance of these signaling pathways during infection with C. albicans. C57BL/6, Casp8−/−Rip3−/− and littermate Rip3−/− BMDC were infected with C. albicans at 1:1 or 10:1. To facilitate higher β-glucan presentation by C. albicans, we also used heat-killed (HK) fungus. As expected, IL-1β production by Casp8−/−Rip3−/− cells was significantly decreased in response to HK C. albicans (Figure 6A). However, live log phase C. albicans, did not require caspase-8 for IL-1β production (Figure 6B). Consistent with the IL-1β phenotype, HK C. albicans triggered cell death was protected to an extent, in Casp8−/−Rip3−/− cells, whereas the response to live C. albicans was unaffected (Figure 6C, D). Since HK C. albicans required caspase-8 for IL-1β production similar to curdlan and WGP agonist, we checked if the β-glucans exposure is higher in HK C. albicans compared to that of the live fungus. A monoclonal β(1,3) glucan specific antibody which robustly stained WGP agonist but not the negative control, silica, was used (Figure S4A and B). We observed relatively higher staining of β-glucan in HK C. albicans compared to that of its live counterpart (Figure S4C and D). These results link the requirement of caspase-8 to the β-glucans exposed by specific stimuli. To further validate that the exposed β-glucans are the main immunostimulatory components of HK C. albicans, we employed the WGP antagonist to test whether HK C. albicans induced responses are more β-glucan dependent. Cells treated with HK C. albicans, but not live C. albicans exhibited reduced IL-1β and protection from cell death in the presence of the antagonist (Figure 6E, F).
Figure 6. Differential requirement of caspase-8 for HK versus live C. albicans induced IL-1β and cell death.
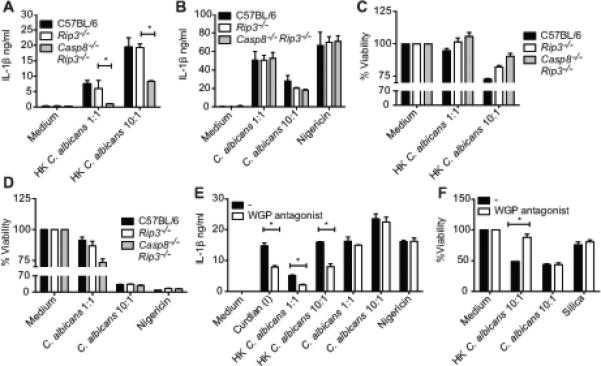
Pam2CSK4 primed BMDC from C57BL/6, littermate Rip3−/−, Casp8−/−Rip3−/− (A-D) or C57BL/6 mice alone were stimulated with live or HK C. albicans at 1:1, 10:1 or nigericin. IL-1β released in the supernatants was measured by ELISA (A, B, E). Cell Viability was measured by the cell titer-glo assay (C, D, F). Statistical analysis was performed on results in panels A, B, E and F as described in the Materials and Methods section.
Although β-glucans and HK C. albicans show an additional requirement for caspase-8, the canonical NLRP3 inflammasome was essential for all for these stimuli (Figure 1). To understand the position of caspase-8 in these pathways, we monitored caspase-8 activity in cells exposed to HK C. albicans and assessed the same in Asc−/− BMDC, which are deficient in inflammasome functions. Although caspase-8 activity was weak in the case of HK C. albicans compared to that of FasL-vesicles, this response was independent of ASC (Figure S4E). The assay was specific for caspase-8, since FasL-vesicles induced activity was undetectable in the Casp8−/−Rip3−/− cells (Figure S4E). Moreover, NLRP3 or ASC deficiency did not protect cells from HK C. albicans induced cell death (Figure S4F), indicating that caspase-8 is engaged independently of the NLRP3 inflammasome to trigger dendritic cell death.
To determine if the distinct phenotypes observed with live and HK candida are due to the difference in the upstream receptor engaged, we tested if dectin-1 or CR3 was required for C. albicans induced IL-1β in our system. No significant reduction in IL-1β production was noticed in Clec7a−/− BMDC treated with HK or live C. albicans (Figure 7A). In contrast, HK C. albicans showed a near complete requirement for CR3, while the live fungus exhibited reduced IL-1β production (Figure 7B), suggesting that CR3 may in fact be the dominant receptor involved in C. albicans induced IL-1β. Interestingly, Clec7a−/− BMDC were protected from heat-killed, but not live C. albicans induced cell death (Figure 7C). Similarly, cell death triggered by HK, but not live C. albicans, was CR3 dependent (Figure 7D). Thus, both CR3 and dectin-1 appear to be involved in the cell death response to heat-killed fungi.
Figure 7. HK C. albicans requires CR3 for IL-1β, CR3 as well as dectin-1 for cell death, while live C. albicans partially requires CR3 for IL-1β production.
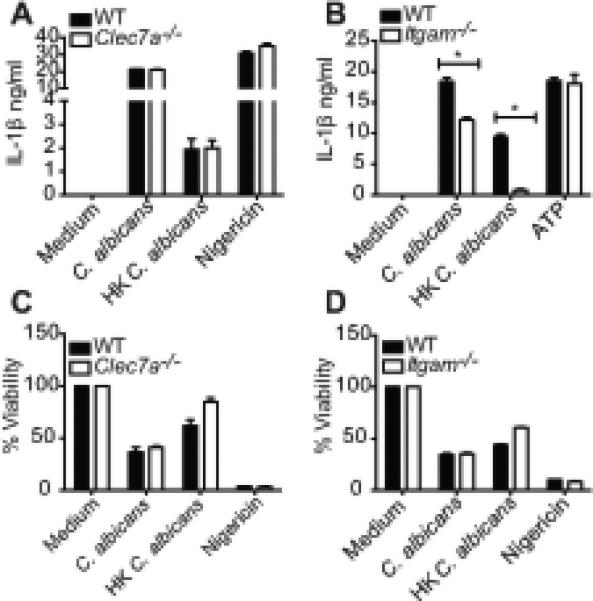
Pam2CSK4 primed BMDC from C57BL/6, Clec7a−/−, Itgam−/− mice were stimulated with HK C. albicans or live C. albicans at 10:1 for 6h, ATP or nigericin for 1h. IL-1β released in the supernatants was measured by ELISA (A, B) and cell viability by cell-titer glo assay (C, D). Statistical analysis was performed on results in panels A and B as described in the Materials and Methods section.
During the course of infection in humans and mice, C. albicans is often opsonized by serum and antibodies and the myeloid cells may encounter the opsonized fungus. Hence, we assessed the IL-1β production and cell death responses of BMDC, when exposed to opsonized yeast. As shown in figure S4G, IL-1β production and cell death observed with opsonized live and HK C. albicans was very similar in magnitude to that of their unopsonized counterparts. We also ascertained the opsonization of C. albicans by staining for C3 deposition on its surface (Figure S4H).
Discussion
The data presented here demonstrate that the canonical NLRP3 inflammasome (NLRP3, ASC and caspase-1), dectin-1, CARD9, CR3 and caspase-8 all contribute to β-glucan-induced inflammasome responses (Figure 8). These molecular requirements are highly specific to β-glucans, since dectin-1, CARD9, CR3 and caspase-8 were dispensable for IL-1β induced by canonical inflammasome triggers such as silica or pdAdT. Our data, for the first time, implicates CR3 in addition to dectin-1 as a critical sensor coupling β-glucan recognition to inflammasome activation. Strikingly, dectin-1 and CR3 were both essential, and appeared to function in a non-redundant manner in the IL-1β and cell death response to β-glucans. We used an ATP-based luminescence assay to measure the metabolic activity of dendritic cells, as surrogate for cell viability. Intracellular ATP can fluctuate in response to other physiological and mitochondrial perturbations and hence, an alternate interpretation of this assay, in the case of β-glucans in particular, is that ATP levels are decreased due to reduced metabolic activity, rather then cell death. Dectin-1 has previously been shown to activate the integrin CR3 through inside-out signaling, to recognize C. albicans PAMPs (52), indicating a potential functional connection between these 2 receptors. Thus, dectin-1 and CR3 could be acting sequentially for sensing and signaling in response to β-glucans.
Figure 8. Inflammasome Responses to β-glucans and Candida albicans.
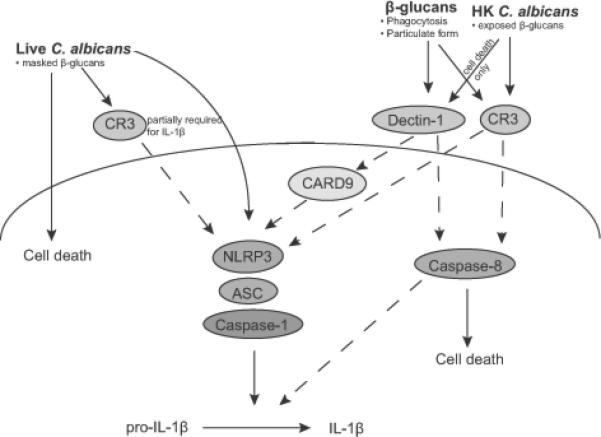
β-glucans, heat-killed (HK) as well as live C. albicans trigger IL-1β maturation and cell death in dendritic cells. All these stimuli absolutely require NLRP3-ASC-caspase-1 inflammasome for IL-1β processing and release. Both dectin-1 and CR3 are essential for β-glucan triggered inflammasome activation, IL-1β production and cell death, while CARD9 is required for β-glucan induced IL-1β. CR3 is essential for HK C. albicans induced IL-1β production and cell death, and is partially required for live C. albicans induced IL-1β. Dectin-1 is required for HK C. albicans induced cell death, but not for HK or live C. albicans triggered IL-1β. Caspase-8 promotes IL-1β maturation and mediates inflammasome independent cell death specifically in response to β-glucans and HK C. albicans. Thus, caspases-1 and -8, dectin-1, CR3 and the NLRP3 inflammasome co-ordinate to mount innate immune responses against the fungal PAMP, β-glucan and the pathogen C. albicans. Bullet points indicate the attributes of the stimuli that contribute to the responses studied. Dotted lines indicate inferred functional associations from our findings.
C. albicans induced IL-1β and cell death profiles showed some similarities and some differences from that of β-glucans (Figure 8). Live or HK C. albicans triggered IL-1β production didn’t require dectin-1. Instead, CR3 appeared to the critical receptor for C. albicans induced IL-1β production, with heat-killed and live fungus showing a complete or partial requirement respectively. Both dectin-1 and CR3 were important for heat-killed C. albicans induced cell death. We observed higher staining of β-glucan in HK C. albicans and the recognition of β-glucan is important for HK C. albicans triggered IL-1β and cell death, in contrast to live C. albicans. This is consistent with many published studies that have indicated that β-glucan is masked within the cell wall of live fungus (65), which might impede dectin-1/CR-3 recognition of live C. albicans. The differential requirement of these receptors for heat-killed and live C. albicans may represent their independent and redundant function or alternatively the involvement of receptors for other PAMPs, such as chitin and mannans, in triggering inflammasome activation. In vivo studies suggest that β-glucan that is masked by live C. albicans, especially in the yeast form, gets exposed later during the course of infection in organs such as the kidneys (65). Varied degree of β-glucan unmasking that occurs with disseminated infection, might elicit a qualitatively different cytokine response. In line with our findings with unopsonized yeast, a previous study has reported that CR3 drives caspase-8 activation and neutrophil apoptosis in response to phagocytosis of serum-opsonized yeast (64). Since serum opsonized live and HK C. albicans yeast also trigger IL-1β production, it will be very interesting to examine the role of caspase-8 and CR3 in this response in future.
Our findings present striking similarities and differences with that of Gringhuis et al. 2012, where IL-1β maturation in human dendritic cells, triggered by curdlan and some strains of HK and live C. albicans was dependent on dectin-1, ASC and caspase-8, but independent of NLPR3, caspase-1 and phagocytosis. Our studies in mouse dendritic cells, using genetic models, similarly show key roles for dectin-1 and caspase-8, but also a critical requirement for NLPR3, ASC and caspase-1 for optimal IL-1β maturation following β-glucan stimulation. Other studies have also linked the NLRP3 inflammasome to β-glucan induced IL-1β production in mouse and human macrophages and dendritic cells (20, 21). In contrast to β-glucans, dectin-1 and caspase-8 were not required for live C. albicans induced IL-1β production in mouse dendritic cells.
How caspase-8 is engaged by β-glucan-sensing pathways is currently unclear. Caspase-8 deficiency did not have a profound impact on curdlan-induced caspase-1 processing, suggesting that NLRP3 inflammasome assembly and caspase-1 activation are intact in Casp8−/−Rip3−/− cells. This is consistent with a recent study which linked caspase-8 to Salmonella induced pro-IL-1β synthesis and processing in a manner that is independent of caspase-1 (26). Further, caspase-8 mediated cell death in response to HK C. albicans is not dependent on NLRP3 or ASC, even though all of these molecules are important for IL-1β production. These results position caspase-8 on a parallel β-glucan activated pathway that is independent of inflammasome assembly but converges with the assembled NLRP3 inflammasome complex to promote IL-1β maturation (Figure 8).
Future studies will dissect the mode of action of caspase-8 to determine if caspase-8 promotes the activation and processing of IL-1β through direct or indirect mechanisms. Interestingly, recombinant caspase-8 has been shown to cleave pro-IL-1β at the same site as caspase-1, in an in vitro system (23), suggesting that caspase-8 may directly cleave pro-IL-1β. Caspase-1 and caspase-8 have CARD and DED domains respectively, which are related protein-protein interaction domains involved in a number of distinct homotypic interactions. Thus, one intriguing possibility would be direct interaction between these two caspases. It would also be interesting if caspase-8 regulates caspase-1 through activating cellular inhibitors of apoptosis proteins, cIAPs, and K63 ubiquitination (66). In our own studies, we have shown that the Drosophila caspase-8-like factor DREDD triggers IAP-dependent K63-ubiqutination (67) and perhaps some aspects of these pathways are conserved. Overall, the role of caspase-8 in bridging β-glucan sensing by dectin-1, CR3 and potentiating cell death and NLRP3 inflammasome dependent IL-1β processing is reminiscent of the role of caspase-11 in gram-negative bacterial infection, where caspase-11 integrates LPS sensing with cell death and NLRP3 dependent IL-1β maturation (25).
Extrinsic cell death pathways show significant overlap with innate immune signaling mechanisms and share molecular components including RIP1 and cIAPs. Ripoptosomes are dynamic cytosolic multi-protein complexes, which determine cell fate along the survival/apoptosis/necrosis axes (68, 69). Recent studies have elucidated their role in regulation of IL-1β processing in addition to their well-characterized roles in cell death. Two recent reports indicated that RIP3 triggers NLRP3-ASC dependent caspase-1 activation and IL-1β processing when caspase-8 is deficient or when cIAPs are depleted (70, 71). Surprisingly, IL-1β production observed in these scenarios required just a priming signal by TLRs. This suggests a tight quality control of the ripoptosome complex where removal of caspase-8 or cIAP proteins skews the ripoptosome to feed into the NLRP3-ASC-caspase-1 pathway for IL-1β processing. Although any potential role for RIP3 in caspase-8 deficient conditions is not evident in our assays, which use RIP3 and caspase-8 double deficient cells, we noticed that Rip3−/− DC exhibit IL-1β and cell death responses comparable to WT, in response to all stimuli examined (Figures 5A, S2A, S2E, 6A-D).
Our efforts to characterize the activation of the enzyme caspase-8 by HK C. albicans revealed that induction of caspase-8 activity, albeit detectable, was very weak as compared to that elicited by FasL-vesicles (Figure S4E). However, consistent with our data, caspase-8 activation has been observed when dectin-1 is activated by HK C. albicans or CR3 phagocytoses opsonized yeast in other cell types (19, 64). Catalytic functions of caspase-8 are regulated by a non-catalytic homologue and partner, cFLIP, which is also involved in NLRP3 and AIM2 inflammasome responses (72). Recent evidence that hemizygotic cFLIP deficient cells exhibit higher caspase-8 mediated IL-1β production specifically in response to HK C. albicans supports the important role of caspase-8 activity in the β-glucan signaling pathway (72).
Currently, no anti-fungal vaccines are available for humans, but β-glucan conjugates have shown promise as vaccines in rodent experimental models (73). Laminarin, a β-glucan derived from the alga, Laminaria digitata conjugated with a diphtheria toxoid conferred protection to mice against systemic C. albicans and A. fumigatus infection that was attributed to β-glucan specific antibodies (74). β-glucan, as a glucose polymer, finds applications in many therapeutic and adjuvant preparations and is considered to have anti-cancer potential (75, 76). Moreover, β-glucans also act as potent adjuvants and delivery systems for antigens and in eliciting Th1 and Th17 responses against protein antigens (77, 78). It would be exciting to determine if the anti-cancer and/or adjuvant properties of β- glucans results from engagement of caspase-8 and/or caspase-1 pathways.
Altogether, the findings presented here uncover new roles for CR3, dectin-1 as well as caspase-8 in coordinating cell death and inflammasome responses to β-glucans. These studies significantly enhance our understanding of β-glucan elicited inflammatory responses; provide improved understanding of anti-fungal immune responses and mechanistic understanding of the immune-modulating properties of β-glucans.
Supplementary Material
Acknowledgements
The authors thank Anna M. Cerny, Zhaozhao Jiang for animal husbandry; Kara L. Conway, Ramnik J. Xavier for Card9−/− femurs, Elizabeth Thayer for Itgam−/− femurs; Chrono K. Lee, Charles A. Specht, Gary R. Ostroff for reagents and suggestions on the use of C. albicans and β-glucans; Sivapriya Kailasan Vanaja for confocal microscopy; Dan Weng, Egil Lien, Shubhendu Ghosh, Gary R. Ostroff and all Fitzgerald and Silverman lab members for helpful technical advice and discussions.
This work was supported by grants from the NIH (AI083713 to K.A.F., AI60025 to N.S and AR050800 to T.M.).
Footnotes
NLR Nucleotide binding and oligomerization domain (NOD) and Leucine Rich Repeat (LRR) containing protein, TLR Toll Like Receptor, CLR C-Lectin Receptor, PYHIN PYRIN and HIN domain containing protein, NLRP3 NOD like receptor family, pyrin domain containing 3, NLRC4 NOD like receptor family, CARD domain containing 3, ASC Apoptosis associated Speck like protein containing CARD
curdlan (I) curdlan (Invivogen), curdlan (S) curdlan (Sigma), curdlan (W) curdlan (Wako Chemicals), WGP Whole Glucan Particles, CM-curdlan Carboxy-methylated curdlan
CLEC7A C-type Lectin domain family 7 member A, aka Dectin-1, CARD9 Caspase Recruitment Domain- protein 9, CR3 Complement Receptor 3, (Mac-1)
References
- 1.Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv13–165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An Essential Role for the NLRP3 Inflammasome in Host Defense against the Human Fungal Pathogen Candida albicans. Cell Host & Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Role of interleukin-18 in host defense against disseminated Candida albicans infection. 2002;70:3284–3286. doi: 10.1128/IAI.70.6.3284-3286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stuyt RJLR, Netea MGM, van Krieken JHJ, van der Meer JWMJ, Kullberg BJB. Recombinant interleukin-18 protects against disseminated Candida albicans infection in mice. J INFECT DIS. 2004;189:1524–1527. doi: 10.1086/382955. [DOI] [PubMed] [Google Scholar]
- 5.Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JWM, Kullberg BJ. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J INFECT DIS. 2006;193:1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- 6.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical Regulation of Early Th17 Cell Differentiation by Interleukin-1 Signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lasigliè DD, Traggiai EE, Federici SS, Alessio MM, Buoncompagni AA, Accogli AA, Chiesa SS, Penco FF, Martini AA, Gattorno MM. Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS ONE. 2011;6:e20014–e20014. doi: 10.1371/journal.pone.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. Journal of Experimental Medicine. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netea MG, Stuyt RJL, Kim S-H, van der Meer JWM, Kullberg BJ, Dinarello CA. The Role of Endogenous Interleukin (IL)–18, IL-12, IL-1β, and Tumor Necrosis Factor–α in the Production of Interferon-γ Induced by Candida albicansin Human Whole Blood Cultures. J INFECT DIS. 2002;185:963–970. doi: 10.1086/339410. [DOI] [PubMed] [Google Scholar]
- 10.Rathinam VAK, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nature Publishing Group. 2012;13:333–332. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 12.A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;7:e1002379. doi: 10.1371/journal.ppat.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joly S, Eisenbarth SC, Olivier AK, Williams A, Kaplan DH, Cassel SL, Flavell RA, Sutterwala FS. Cutting Edge: Nlrp10 Is Essential for Protective Antifungal Adaptive Immunity against Candida albicans. The Journal of Immunology. 2012;189:4713–4717. doi: 10.4049/jimmunol.1201715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levitz SM. Innate Recognition of Fungal Cell Walls. PLoS Pathog. 2010;6:e1000758. doi: 10.1371/journal.ppat.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gow NA, Hube B. Importance of the Candida albicans cell wall during commensalism and infection. Current Opinion in Microbiology. 2012;15:406–412. doi: 10.1016/j.mib.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Mori T, Ikemoto H, Matsumura M, Yoshida M, Inada K, Endo S, Ito A, Watanabe S, Yamaguchi H, Mitsuya M, Kodama M, Tani T, Yokota T, Kobayashi T, Kambayashi J, Nakamura T, Masaoka T, Teshima H, Yoshinaga T, Kohno S, Hara K, Miyazaki S. Evaluation of plasma (1-->3)-beta-D-glucan measurement by the kinetic turbidimetric Limulus test, for the clinical diagnosis of mycotic infections. Eur J Clin Chem Clin Biochem. 1997;35:553–560. [PubMed] [Google Scholar]
- 17.Miyazaki T, Kohno S, Mitsutake K, Maesaki S, Tanaka K, Ishikawa N, Hara K. Plasma (1-->3)-beta-D-glucan and fungal antigenemia in patients with candidemia, aspergillosis, and cryptococcosis. J. Clin. Microbiol. 1995;33:3115–3118. doi: 10.1128/jcm.33.12.3115-3118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. β-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin. Infect. Dis. 2011;52:750–770. doi: 10.1093/cid/ciq206. [DOI] [PubMed] [Google Scholar]
- 19.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TBH. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1b via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012:1–10. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 20.(1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. 2010;184:6335–6342. doi: 10.4049/jimmunol.0903019. [DOI] [PubMed] [Google Scholar]
- 21.Kumar H, Kumagai Y, Tsuchida T, Koenig PA, Satoh T, Guo Z, Jang MH, Saitoh T, Akira S, Kawai T. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. The Journal of Immunology. 2009;183:8061–8067. doi: 10.4049/jimmunol.0902477. [DOI] [PubMed] [Google Scholar]
- 22.Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. Journal of Experimental Medicine. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 25.Rathinam VAK, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Man SM, Man SM, Tourlomousis P, Tourlomousis P, Hopkins L, Hopkins L, Monie TP, Monie TP, Fitzgerald KA, Fitzgerald KA, Bryant CE, Bryant CE. Salmonella Infection Induces Recruitment of Caspase-8 to the Inflammasome To Modulate IL-1 Production. The Journal of Immunology. 2013;191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonopoulos C, El Sanadi C, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic Chemotherapeutic Drugs Induce Noncanonical Processing and Release of IL-1 via Caspase-8 in Dendritic Cells. The Journal of Immunology. 2013;191:4789–4803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VARFOLOMEEV E. Targeted Disruption of the Mouse Caspase 8 Gene Ablates Cell Death Induction by the TNF Receptors, Fas/Apo1, and DR3 and Is Lethal Prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 29.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8–FLIPL complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hara H, Ishihara C, Takeuchi A, Imanishi T, Xue L, Morris SW, Inui M, Takai T, Shibuya A, Saijo S, Iwakura Y, Ohno N, Koseki H, Yoshida H, Penninger JM, Saito T. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat Immunol. 2007;8:619–629. doi: 10.1038/ni1466. [DOI] [PubMed] [Google Scholar]
- 32.Coxon A, Coxon A, Rieu P, Rieu P, Barkalow FJ, Barkalow FJ, Askari S, Askari S, Sharpe AH, Sharpe AH, von Andrian UH, von Andrian UH, Arnaout MA, Arnaout MA, Mayadas TN, Mayadas TN. A Novel Role for the β2 Integrin CD11b/CD18 in Neutrophil Apoptosis: A Homeostatic Mechanism in Inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 33.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat Immunol. 2006;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. Journal of Leukocyte Biology. 2011;90:357–366. doi: 10.1189/jlb.1210702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathinam VAK, Hoag KA, Mansfield LS. Dendritic cells from C57BL/6 mice undergo activation and induce Th1-effector cell responses against Campylobacter jejuni. Microbes and Infection. 2008;10:1316–1324. doi: 10.1016/j.micinf.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gow NAR, Netea MG, Munro CA, Ferwerda G, Bates S, Mora Montes HM, Walker L, Jansen T, Jacobs L, Tsoni V, Brown GD, Odds FC, van der Meer JWM, Brown AJP, Kullberg BJ. Immune Recognition of Candida albicansβ glucan by Dectin 1. J INFECT DIS. 2007;196:1565–1571. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molecular organization of the cell wall of Candida albicans. Med Mycol. 2001;39(Suppl 1):1–8. [PubMed] [Google Scholar]
- 38.Klis FM, Mol P, Hellingwerf K, Brul S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS microbiology reviews. 2002;26:239–256. doi: 10.1111/j.1574-6976.2002.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 39.Gopal PK, Shepherd MG, Sullivan PA. Analysis of wall glucans from yeast, hyphal and germ-tube forming cells of Candida albicans. J. Gen. Microbiol. 1984;130:3295–3301. doi: 10.1099/00221287-130-12-3295. [DOI] [PubMed] [Google Scholar]
- 40.J MD, J MA, P. J. C. The structure of a beta-(1->3)-d-glucan from yeast cell walls. Biochem. J. 1973 doi: 10.1042/bj1350031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 42.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 43.CM-curdlan activity 2. 2010:1–7. [Google Scholar]
- 44.Bueter CL, Bueter CLC, Lee CK, Lee CKC, Rathinam VAKV, Rathinam VAK, Healy GJ, Healy GJG, Taron CH, Taron CHC, Specht CA, Specht CAC, Levitz SM, Levitz SMS. Chitosan but Not Chitin Activates the Inflammasome by a Mechanism Dependent upon Phagocytosis. Journal of Biological Chemistry. 2011;286:35447–35455. doi: 10.1074/jbc.M111.274936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim M-J, Hong S-Y, Kim S-K, Cheong C, Park H-J, Chun H-K, Jang K-H, Yoon B-D, Kim C-H, Kang SA. β-Glucan enhanced apoptosis in human colon cancer cells SNU-C4. Nutr Res Pract. 2009;3:180. doi: 10.4162/nrp.2009.3.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jouault T, El Abed-El Behi M, Martinez-Esparza M, Breuilh L, Trinel P-A, Chamaillard M, Trottein F, Poulain D. Specific Recognition of Candida albicans by Macrophages Requires Galectin-3 to Discriminate Saccharomyces cerevisiae and Needs Association with TLR2 for Signaling. The Journal of Immunology. 2006;177:4679–4687. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- 47.Esteban A, Popp MW, Vyas VK, Strijbis K, Ploegh HL, Fink GR. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. In vol. 108. National Acad Sciences. 2011:14270–14275. doi: 10.1073/pnas.1111415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The Macrophage-Inducible C-Type Lectin,Mincle, Is an Essential Component of the Innate Immune Response to. 2008:1–11. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 49.Wileman TE, Lennartz MR, Stahl PD. Identification of the macrophage mannose receptor as a 175-kDa membrane protein. Proc. Natl. Acad. Sci. U.S.A. 1986;83:2501–2505. doi: 10.1073/pnas.83.8.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephenson JD, Shepherd VL. Purification of the human alveolar macrophage mannose receptor. Biochemical and Biophysical Research Communications. 1987;148:883–889. doi: 10.1016/0006-291x(87)90958-2. [DOI] [PubMed] [Google Scholar]
- 51.Stahl PD, Rodman JS, Miller MJ, Schlesinger PH. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc. Natl. Acad. Sci. U.S.A. 1978;75:1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Utomo A, Cullere X, Choi MM, Milner DA, Jr, Venkatesh D, Yun S-H, Mayadas TN. The b-Glucan Receptor Dectin-1 Activatesthe Integrin Mac-1 in Neutrophils via Vav Protein Signaling to Promote Candida albicans Clearance. Cell Host & Microbe. 2011;10:603–615. doi: 10.1016/j.chom.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemmers B, Salmena L, Bidere N, Su H, Matysiak-Zablocki E, Murakami K, Ohashi PS, Jurisicova A, Lenardo M, Hakem R, Hakem A. Essential Role for Caspase-8 in Toll-like Receptors and NF B Signaling. Journal of Biological Chemistry. 2006;282:7416–7423. doi: 10.1074/jbc.M606721200. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi K, Kawai T, Kumar H, Sato S, Yonehara S, Akira S. Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J Immunol. 2006;176:4520–4524. doi: 10.4049/jimmunol.176.8.4520. [DOI] [PubMed] [Google Scholar]
- 55.Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 56.Ch'en IL, Beisner DR, Degterev A, Lynch C, Yuan J, Hoffmann A, Hedrick SM. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proceedings of the National Academy of Sciences. 2008;105:17463–17468. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol. 2005;175:3469–3473. doi: 10.4049/jimmunol.175.6.3469. [DOI] [PubMed] [Google Scholar]
- 58.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cellular Microbiology. 2008;10:2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 59.Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, Kaiser WJ, Mocarski ES, Pouliot K, Chan FKM, Kelliher MA, Harris PA, Bertin J, Gough PJ, Shayakhmetov DM, Goguen JD, Fitzgerald KA, Silverman N, Lien E. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proceedings of the National Academy of Sciences. 2014;111:7391–7396. doi: 10.1073/pnas.1403477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gurung P, Anand PK, Malireddi RKS, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. FADD and Caspase-8 Mediate Priming and Activation of the Canonical and Noncanonical Nlrp3 Inflammasomes. The Journal of Immunology. 2014:1302839. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shenderov K, Riteau N, Yip R, Mayer-Barber KD, Oland S, Hieny S, Fitzgerald P, Oberst A, Dillon CP, Green DR, Cerundolo V, Sher A. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1β in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. The Journal of Immunology. 2014;192:2029–2033. doi: 10.4049/jimmunol.1302549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajput A, Kovalenko A, Bogdanov K, Yang S-H, Kang T-B, Kim J-C, Du J, Wallach D. RIG-I RNA Helicase Activation of IRF3Transcription Factor Is Negatively Regulatedby Caspase-8-Mediated Cleavage of the RIP1 Protein. Immunity. 2011;34:340–351. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 63.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Förster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Zhang B, Zhang B, Hirahashi J, Cullere X, Mayadas TN. Elucidation of Molecular Events Leading to Neutrophil Apoptosis following Phagocytosis: CROSS-TALK BETWEEN CASPASE 8, REACTIVE OXYGEN SPECIES, AND MAPK/ERK ACTIVATION. Journal of Biological Chemistry. 2003;278:28443–28454. doi: 10.1074/jbc.M210727200. [DOI] [PubMed] [Google Scholar]
- 65.Wheeler RT, Kombe D, Agarwala SD, Fink GR. Dynamic, morphotype-specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog. 2008;4:e1000227. doi: 10.1371/journal.ppat.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Labbé K, McIntire CR, Doiron K, Leblanc PM, Saleh M. Cellular Inhibitors of Apoptosis Proteins cIAP1 and cIAP2 Are Required for Efficient Caspase-1 Activation by the Inflammasome. Immunity. 2011;35:897–907. doi: 10.1016/j.immuni.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Paquette N, Broemer M, Aggarwal K, Chen L, Husson M, Ertürk-Hasdemir D, Reichhart J-M, Meier P, Silverman N. Caspase-Mediated Cleavage, IAP Binding, and Ubiquitination: Linking Three Mechanisms Crucial for Drosophila NF-κB Signaling. Molecular Cell. 2010;37:172–182. doi: 10.1016/j.molcel.2009.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Häcker G, Leverkus M. cIAPs Block Ripoptosome Formation, a RIP1/Caspase-8 Containing Intracellular Cell Death Complex Differentially Regulated by cFLIP Isoforms. Molecular Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, Meier P. The Ripoptosome, a Signaling Platform that Assembles in Responseto Genotoxic Stress and Loss of IAPs. Molecular Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 70.Vince JE, Wong WW-L, Gentle I, Lawlor KE, Allam R, O'Reilly L, Mason K, Gross O, Ma S, Guarda G, Anderton H, Castillo R, Häcker G, Silke J, Tschopp J. Inhibitor of Apoptosis Proteins LimitRIP3 Kinase-Dependent Interleukin-1 Activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 71.Kang T-B, Yang S-H, Toth B, Kovalenko A, Wallach D. Caspase-8 Blocks Kinase RIPK3-Mediated Activation of the NLRP3 Inflammasome. Immunity. 2012:1–14. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 72.Wu Y-H, Kuo W-C, Wu Y-J, Yang K-T, Chen S-T, Jiang S-T, Gordy C, He Y-W, Lai M-Z. Participation of c-FLIP in NLRP3 and AIM2 inflammasome activation. 2013:1–11. doi: 10.1038/cdd.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bromuro CC, Bromuro C, Romano MM, Romano M, Chiani P, Chiani PP, Berti FF, Berti F, Tontini M, Tontini MM, Proietti D, Proietti DD, Mori EE, Mori E, Torosantucci AA, Torosantucci A, Costantino PP, Costantino P, Rappuoli RR, Rappuoli R, Cassone A, Cassone AA. Beta-glucan-CRM197 conjugates as candidates antifungal vaccines. Vaccine. 2010;28:2615–2623. doi: 10.1016/j.vaccine.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 74.Torosantucci A, Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A. A novel glyco-conjugate vaccine against fungal pathogens. Journal of Experimental Medicine. 2005;202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aleem E. β -Glucans and their Applications in Cancer Therapy: Focus on human studies. Anti-cancer agents in medicinal chemistry. 2013;13:709–719. doi: 10.2174/1871520611313050005. [DOI] [PubMed] [Google Scholar]
- 76.Chan G, Chan W, Sze D. The effects of β-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soto ER, Caras AC, Kut LC, Castle MK, Ostroff GR. Glucan Particles for Macrophage Targeted Delivery of Nanoparticles. Journal of Drug Delivery. 2012;2012:1–13. doi: 10.1155/2012/143524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.LeibundGut-Landmann S, LeibundGut-Landmann SS, Gross O, Gross OO, Robinson MJM, Robinson MJ, Osorio F, Osorio FF, Slack ECE, Slack EC, Tsoni SVS, Tsoni SV, Schweighoffer E, Schweighoffer EE, Tybulewicz V, Tybulewicz VV, Brown GD, Brown GDG, Ruland JJ, Ruland J, Reis e Sousa C, Sousa CCRE. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


