Abstract
Adult neurogenesis is now widely accepted as an important contributor to hippocampal integrity and function but also dysfunction when adult neurogenesis is affected in neuropsychiatric diseases such as alcohol use disorders. Excessive alcohol consumption, the defining characteristic of alcohol use disorders, results in a variety of cognitive and behavioral impairments related wholly or in part to hippocampal structure and function. Recent preclinical work has shown that adult neurogenesis may be one route by which alcohol produces hippocampal neuropathology. Alcohol is a pharmacologically promiscuous drug capable of interfering with adult neurogenesis through multiple mechanisms. This review will discuss the primary mechanisms underlying alcohol-induced changes in adult hippocampal neurogenesis including alcohol's effects on neurotransmitters, CREB and its downstream effectors, and the neurogenic niche.
1. Introduction
Alcohol use disorders (AUDs), more commonly referred to as alcoholism, include alcohol abuse or alcohol dependence, the diagnostic terms for uncontrollable, excessive alcohol intake despite negative consequences. AUDs are major social and economic problems. With nearly 8.5% of the U.S. population meeting the diagnostic criteria for an AUD on any given day, it is not surprising that the economic burden was estimated to be $223.5 billion dollars in 2006 alone (Bouchery et al., 2011; Grant et al., 2004). Further, excessive alcohol consumption is the third leading cause of preventable death in the United States (Mokdad et al., 2004). Despite these tolls, both the incidence of AUDs and the number of new users have increased in past decades (Grant et al., 2004). AUDs are a significant problem across much of the lifespan as experimentation with alcohol begins in adolescence. Indeed, disturbingly similar rates of AUDs exist between adolescents and adults (Clark et al., 2002). Although there are many theories about the development of AUDs, all involve the fact that repeated bouts of excessive alcohol intake change the brain in a way that drives a loss of control over consumption (Koob and Le Moal, 1997). This loss of control may be driven by hijacked learning processes, impairments in behavioral control, and/or impaired decision-making (Crews, 1999; Noel et al., 2013). All of these processes involve, at least partially, the contribution of an intact hippocampus, and a wide variety of studies have shown that excessive alcohol intake impacts the structure and function of hippocampal circuitry.
Alcohol (ethanol) is a small, highly lipid soluble and pharmacologically promiscuous drug capable of penetrating virtually every organ system including the brain. Excessive alcohol consumption has widespread deleterious effects on many of these organ systems, but central nervous system injury is a critical consequence as 50 to 75% of alcohol-dependent adults show permanent cognitive impairment (Eckardt and Martin, 1986). These impairments are thought to be due to both structural and functional changes resulting from excessive alcohol consumption (Crews, 1999; Sullivan and Pfefferbaum, 2005). Alcohol appears to target some brain regions more than others with a critical cluster of alcohol-induced impairments in behavioral control, learning, memory, mood, and decision-making attributed, at least in part, to the integrity of the hippocampus (Mechtcheriakov et al., 2007). Because ethanol is so pharmacologically promiscuous, there are many reported and potential mechanisms of alcohol-induced effects on the hippocampus. This review focuses on the emerging role of adult hippocampal neurogenesis in alcoholic neuropathology and the various potential mechanisms involved in alcohol's effects on neural progenitor cells (NPCs) and the neurogenic niche.
2. The hippocampus and alcohol use disorders
Excessive alcohol consumption results in extensive deficits in neuropsychological functions, many of which are subserved by the hippocampus (Chanraud et al., 2007; Ozsoy et al., 2013; Parsons, 1993). As has been reviewed extensively elsewhere (Belujon and Grace, 2011; Eichenbaum, 2001; Gilbert and Kesner, 2006; Johnson et al., 2007), the hippocampus is especially critical for aspects of learning and memory and as such is implicated in the acquisition, consolidation, and expression of context-dependent drug memories (reviewed in Hyman et al., 2006; Nixon et al., 2011). However, many now postulate a role for the hippocampus in relapse and drug seeking as well (Belujon and Grace, 2011; Vorel et al., 2001). This expanded role has emerged from data demonstrating its broader role in cognitive functions, specifically through its interconnections with frontal cortices and reward systems. For example, glutamatergic efferents projecting from the hippocampus and terminating in the prefrontal cortex (PFC) are implicated in the proper processing of executive functions, working memory, and contextual information (Godsil et al., 2013). Therefore, disruptions in the structural integrity of the hippocampus may be an underling substrate for impairments in these functions. Certainly, long-lasting deficits in executive function and working memory are observed following excessive alcohol consumption (O'Daly et al., 2012; Stavro et al., 2012; Stephens and Duka, 2008) and it is hypothesized that compromised hippocampal integrity in alcoholics may contribute to these impairments. In support of this hypothesis, a correlation between deficits in executive function and hippocampal gray matter volume has been reported (Chanraud et al., 2007). Although correlation does not imply causation, increasing new evidence supports that hippocampal integrity influences a broader range of cognitive functions than classically considered.
With newer technology, more consistent reports of hippocampal pathology have emerged in the last several years (Beresford et al., 2006; Ozsoy et al., 2013) including grey matter loss (Mechtcheriakov et al., 2007). Historically, ongoing debate is evident in the literature over whether the hippocampus was impacted by excessive alcohol: whether the left, right, or both hippocampi are affected, whether degeneration is due to effects in white or gray matter, and/or the magnitude of this effect (Agartz et al., 1999; Laakso et al., 2000; Sullivan et al., 1995). Importantly, one commonality that has emerged is that differences in the study population may underlie these discrepancies. For example, early onset drinking has been shown to be associated with greater hippocampal volumetric deficits (Ozsoy et al., 2013) and adolescent AUDs consistently result in hippocampal volume loss (De Bellis et al., 2000; Nagel et al., 2005). However, on the other end of the age spectrum, greater anterior volume loss was reported in the hippocampi of older alcoholics (Sullivan et al., 1995).
Imaging studies thus far, however, lack sufficient resolution to identify specific subregions of the hippocampus or cellular populations. Therefore, post-mortem studies in humans and experimental evidence in animal models have been necessary to offer insight into dentate gyrus specific effects and/or alcohol-induced neuronal loss. One study observed significant reductions in neuron number in all hippocampal subfields, including the dentate gyrus, in alcoholics less than 45 years of age (Bengochea and Gonzalo, 1990). However, a more rigorous stereological estimation failed to observe neuronal loss in any hippocampal subfield, though these subjects were older averaging 55 years of age (Harding et al., 1997). Although Harding et al. (1997) associated hippocampal volume deficits in alcoholic cases with white matter loss, others have proposed that astroglial loss underlies hippocampal neurodegeneration (Korbo, 1999). Nonetheless, animal models of chronic alcohol exposure have shown consistently that alcohol is toxic to hippocampal neurons, including the dentate gyrus granule cells (Cadete-Leite et al., 1988b; Lukoyanov et al., 2000; Walker et al., 1980). Furthermore, animal models allow researchers to examine the effect of dose, duration and/or pattern of exposure which led to the discovery that subtle evidence of damage in the hippocampus is apparent after as little as 24-48 hours of high dose, binge-like ethanol exposure (Hayes et al., 2013; Obernier et al., 2002b). In the four-day binge model of alcohol dependence, neurons are lost throughout the corticolimbic pathway with degenerating cells particularly evident in the entorhinal cortex and ventral dentate gyrus (Collins et al., 1996; Crews et al., 2000; Kelso et al., 2011; Obernier et al., 2002a; Obernier et al., 2002b). Importantly, these binge models mimic the high blood ethanol concentrations (BECs) experienced by binge drinking alcoholics, which is estimated to be 60% or more of the alcoholic population (Robin et al., 1998; Zeigler et al., 2005). Additionally, alcoholics who drink in a binge pattern are much more likely to have neurodegeneration (Hunt, 1993).
Some portion of alcohol-induced neurodegeneration and impairments in cognitive function can recover with abstinence from alcohol (Bartels et al., 2007; Carlen et al., 1978; Gazdzinski et al., 2005; Mann et al., 1999; Pfefferbaum et al., 1995). For the hippocampus, a host of plastic changes were originally thought to underlie this recovery, such as, dendritic expansion (Cadete-Leite et al., 1989; Cadete-Leite et al., 1988b) and spine density recovery (King et al., 1988); however, alcohol withdrawal may compromise some of these effects (Durand et al., 1989). Although it has long been hypothesized that this plasticity underlies recovery in hippocampal volume and/or function observed in animals (Lukoyanov et al., 2000) and humans (Bartels et al., 2007), the contribution of these mechanisms seems insufficient to overcome such a significant volume loss. Many of these theories, however, did not take in to consideration the newly accepted phenomena of adult neurogenesis in the dentate gyrus (Armstrong and Barker, 2001; Nixon, 2006).
3. Adult neurogenesis – a critical component of hippocampal integrity
Adult neurogenesis, the process by which new neurons are created in the postnatal brain, occurs constitutively in two brain regions, the subventricular zone of the walls of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus (Altman and Das, 1965; Doetsch et al., 1999). Recent discoveries about the lack of newborn neurons in the adult human olfactory bulb (Bergmann et al., 2012) however, suggest a more critical role for adult neurogenesis in hippocampal structure and function. Although Altman and Das's (1965) seminal discovery of adult neurogenesis was not widely accepted until the late-1990s (Eriksson et al., 1998; Gross, 2000; Kaplan and Hinds, 1977; Palmer et al., 1997), the exponential increase in published work on adult neural stem cells and adult neurogenesis has fundamentally changed thinking about the hippocampus; specifically, how ongoing neuron generation and potential for regeneration impacts its function and dysfunction in neuropsychiatric disorders. In the hippocampus, new neurons are generated constitutively throughout life in a multi-step process from radial glia-like stem cells that reside along the SGZ of the dentate gyrus (described in Figure 1; see Zhao et al., 2008 for a detailed review of this process). Approximately 6% of the GCL is generated each month in the adult rodent (Cameron and McKay, 2001), even though not every cell that divides survives through incorporation into the GCL. Furthermore, baseline rates of proliferation, survival, and incorporation vary across species, strain, age, and sex that are additionally impacted by environment and experience (Kempermann, 2012; Kempermann and Gage, 2002; Zhao et al., 2008). Although this phenomenon is now accepted as a contributor to the structural integrity of the hippocampus (Imayoshi et al., 2008), the functional role of newborn neurons remains unclear. Early work relied upon correlation to implicate adult neurogenesis in many of the same functions of the hippocampus (van Praag et al., 1999). However, conditional and inducible transgenic animals have provided the most compelling evidence for the role of adult neurogenesis. These tools have allowed for the discovery that adult neurogenesis is critical for pattern separation, a process that acts to enhance differences between similar experiences so they can be distinguished from one another (Clelland et al., 2009; Nakashiba et al., 2012). These more specific knock-out methods have also helped resolve contradictory results across the hallmark of hippocampal function in rodents, spatial learning and memory. Well-controlled, genetic manipulations by inducible knockouts or lentiviral transgene delivery show, fairly consistently, impairments in spatial memory (reviewed in Marin-Burgin and Schinder, 2012). More importantly, of those that did not detect deficits with the classic test of spatial memory, the Morris water maze, several did report impairments in other hippocampus-dependent behavioral tasks such as, contextual fear conditioning (Saxe et al., 2006), trace fear conditioning (Shors et al., 2002), or place recognition (Madsen et al., 2003). Therefore, although there are some contradictory results, new technologies are helping to resolve these issues to strengthen the conclusion that decreases in adult neurogenesis result in some kind of deficit in hippocampal-dependent behavior (Marin-Burgin and Schinder, 2012; Zhao et al., 2008).
Figure 1.
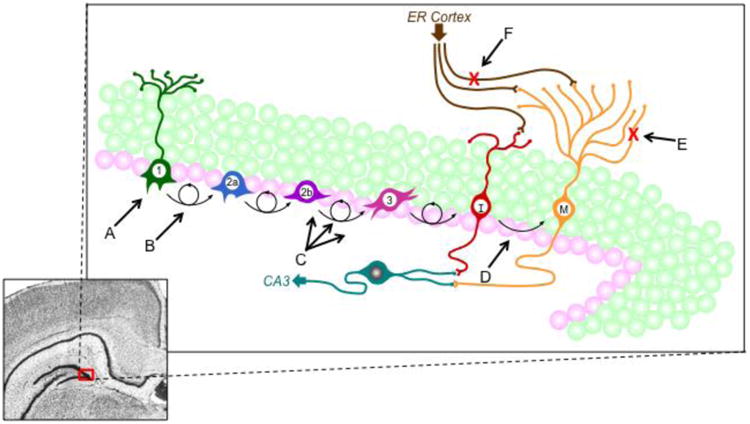
- Alcohol dependence may kill progenitor cells with long duration, chronic exposure (Richardson et al., 2009; Taffe et al., 2010).
- Alcohol intoxication reduces NPC proliferation whereas reactive increases in neurogenesis occur at one week in abstinence (Nixon and Crews, 2002; 2004). Alcohol also alters the cell cycle of NPCs (McClain et al., 2011a).
- Multiple ways in which alcohol may directly affect the neurogenic niche, notably alcohol produces reactive astrocytes and reactive microglia (Kelso et al., 2011; Marshall et al., 2013; McClain et al., 2011b).
- High peak blood ethanol concentrations are likely required to impact survival of new born cells (Herrera et al., 2003; Nixon and Crews, 2002).
- Chronic alcohol exposure blunted dendritic arborization in newborn cells (He et al., 2005), an effect that would have functional implications for new cells incorporation into hippocampal circuitry.
- Binge alcohol exposure kills entorhinal cortex cells that project to the hippocampal dentate gyrus (Collins et al., 1996; Kelso et al., 2011).
4. Alcohol and hippocampal neurogenesis
For over 30 years, scientists have known that a direct relationship exists between alcohol intake and disruptions in hippocampal integrity (Walker et al., 1980). Preliminary conjecture coupled with extensive literature revealing overt cell death following alcohol exposure in vivo led to the original assumption that cell loss in the hippocampus was due to dying neurons (Bengochea and Gonzalo, 1990; Collins et al., 1996; Walker et al., 1980). However, this theory failed to incorporate the then controversial idea that adult neurogenesis was an essential component of hippocampal structure and function (Zhao et al., 2008). In fact, more recent data utilizing adult and adolescent alcohol exposure models strongly suggest that alcoholic neuropathology is at least partially due to an attenuation of adult neurogenesis (Crews et al., 2006c; Morris et al., 2010; Nixon and Crews, 2002). In this same model, hippocampal integrity is impacted, resulting in an 8% loss of granule cells despite a lack of equivalent levels of cell death (Leasure and Nixon, 2010). Therefore, alcohol likely impairs hippocampal integrity, in part, through its effects on adult neurogenesis (Morris et al., 2010).
As described in figure 1, adult neurogenesis is a process comprised of four separate components: cell proliferation, differentiation, migration, and survival. Alcohol exposure can affect neurogenesis at any one of these individual phases or through a combination of effects at multiple stages (Figure 1). Indeed, the various phases are differentially affected by alcohol intoxication, alcohol dependence, and other sequelae that result from chronic alcohol exposure such as seizures and/or neurodegeneration. Therefore, the effects of alcohol intoxication versus alcohol withdrawal and abstinence are discussed separately.
The vast majority of in vivo studies have shown that alcohol intoxication leads to an overall decrease in neurogenesis through alcohol's effects on cell proliferation and cell survival (Crews and Nixon, 2009; Herrera et al., 2003; Nixon, 2006; Nixon and Crews, 2002; Richardson et al., 2009), while increased neurogenesis has been observed in abstinence following alcohol dependence (Nixon and Crews, 2004). Although a few studies showed no effect or an increase in proliferation resulting from 10 days (Rice et al., 2004), 2 weeks (Aberg et al., 2005), 6 weeks (Herrera et al., 2003), or 10 weeks (Aberg et al., 2005) of chronic alcohol exposure, these differences could result from the use of different animal models, the animal's BEC at time of sacrifice/analysis, the timing of analysis with respect to alcohol exposure, and methods used to label proliferating cells (Nixon, 2006). Despite all of these explanations, an interesting trend can be gleaned from these reports: ethanol intoxication appears to dose-dependently inhibit neural stem cell proliferation, an effect which has been shown in vivo with acute doses in adolescent rats (Crews et al., 2006b) and can be extrapolated from a similar correlation in mice (Contet et al., 2013). Determining or estimating the BEC at the time of sacrifice and labeling of the proliferating population continues to support the view that some level of significant intoxication, perhaps above 80 mg/dl, is necessary to inhibit proliferation (Aberg et al., 2005; Crews et al., 2006b; Nixon, 2006). Recent publications continue to support this point. For example, rats that were fed an ethanol containing liquid diet had an average BEC of 86.4 mg/dl (0.08%) and showed a 40% decrease in proliferation (Anderson et al., 2012). It is important to note that these BECs were obtained from animals at sacrifice, which is typically done during lights on, at the start of the sleeping period for rodents, and thus 12+ hours past when they would likely have had a drinking bout and BECs would have peaked. Therefore, alcohol may have reduced proliferation in some of these divergent studies, but the sacrifice time point missed the time of peak intoxication and therefore peak inhibition of cell proliferation. Alternatively, it is of note that the two studies that have observed an increase in proliferation have been in mice (Aberg et al., 2005; Pawlak et al., 2002). Mice metabolize ethanol much faster than rats (Livy et al., 2003), meaning withdrawal events will occur relatively earlier. Therefore, the timeline of these alcohol-induced neurogenesis events may be shifted in mice compared to rats.
A handful of studies have observed persistent decreases in proliferation despite animals being days to weeks past their last exposure and therefore no longer significantly intoxicated. Bromodeoxyuridine-labeled cells and other endogenous measures remain decreased in these reports, but it is of note that the majority of these reports were from long-term chronic exposure models with weeks or months of ethanol exposure (Contet et al., 2013; Hansson et al., 2010; Richardson et al., 2009; Taffe et al., 2010). Therefore, the duration (i.e. number of days, whether repeated binge-like or chronic) may impact the number of proliferating progenitors.
Although hippocampal NPC proliferation is decreased during alcohol intoxication, the percentage of newly born cells that differentiate into neurons or glia appears unchanged. No studies have examined differentiation specifically. Several intriguing reports on alcohol-induced effects in human neural stem cells (NSCs) within the context of Fetal Alcohol Spectrum Disorders (Vangipuram and Lyman, 2010; Zhou et al., 2011) suggest that this might be a potential area for discovery in AUD models. NPC/neuroblast survival is generally decreased in alcohol exposure models, but again, this effect is heavily dose-dependent, i.e., higher doses of alcohol produce greater decreases in new cell survival (Nixon and Crews, 2002; Richardson et al., 2009). Alcohol also potentially kills NSCs and/or NPCs according to long-term alcohol dependence models, again highlighting that dose and duration of exposure may be critical factors in understanding the mechanism of alcohol's effects on NSCs, NPCs, and adult neurogenesis (Contet et al., 2013; Hansson et al., 2010; Nixon and Crews, 2002; Richardson et al., 2009; Taffe et al., 2010). This dose and duration dependency is not surprising as alcohol-induced neuronal cell death, in general, is also hypothesized to be dose and duration dependent. Several groups, including ours, speculate that sustained BECs greater than 200 mg/dl (0.20%) are necessary to produce neurodegeneration (Collins et al., 1996). In support of this idea, a single 5 g/kg dose of alcohol, which only transiently produces >0.20% BEC, does not inhibit survival of hippocampal NPCs (Nixon and Crews, 2002).
Although chronic alcohol intoxication inhibits multiple aspects of adult neurogenesis, a different set of effects on adult neurogenesis are observed in abstinence after intoxication. The most distinct change involves a large, transient increase in NPC proliferation well after the cessation of alcohol exposure. Specifically, in a four-day binge model of alcohol dependence, a burst in NPC proliferation occurs after one week of abstinence, leading to an increased number of newly formed mature neurons four weeks later (Nixon and Crews, 2004). Notably, proliferation returns to control levels by at least two weeks following the last ethanol dose, which emphasizes the short-lived nature of the reactive neurogenesis response (Nixon et al., 2008). Similar increases in NPC proliferation and neurogenesis in abstinence have been described in alcohol-preferring rats subjected to a seven-week two-bottle choice paradigm and in rats exposed chronically to ethanol via vapor chambers (Hansson et al., 2010; He et al., 2009). In contrast, Taffe and colleagues reported a decrease in NPC proliferation and neurogenesis after two months of abstinence in adolescent rhesus macaques given alcohol chronically for 11 months (Taffe et al., 2010). This discrepancy may involve several factors related to the vastly different models: binge versus chronic, species of animals, the time in abstinence analyzed, and possibly even the age of the animals. In adolescents with AUDs, hippocampal neurodegeneration is more consistently reported than it is in adults (Harding et al., 1997; Nagel et al., 2005). Plus, the adolescent brain is more susceptible to negative consequences of ethanol, including degeneration in some cortical regions (Crews et al., 2000). This suggests that the NPC population in the adolescent brain may be more susceptible to alcohol-induced neurotoxicity. NPCs in the adolescent brain have shown different reactions to alcohol versus adults, such as a shortened cell cycle during alcohol-dependence (McClain et al., 2011a). In addition, given the transient nature of reactive neurogenesis observed early in abstinence in rodent models of alcohol exposure, it is possible that a similar transient change occurred but was missed due to the timing of analysis (which was months into abstinence). Importantly, if a short-lived increase in neurogenesis was missed, the subsequent return to an overall decrease in neurogenesis represents a potential barrier to full recovery of hippocampal structure and function. For this reason, understanding the full timeline of alcohol's effects on the progenitor population and the mechanisms responsible for reactive neurogenesis may be important for designing strategies aimed at sustaining neurogenesis at normal levels after long-term abstinence (Mandyam and Koob, 2012).
The specific components and the degree to which each stage of adult neurogenesis is affected by alcohol depend upon the dose, duration, and pattern of alcohol exposure as well as the age of the organism (Goodlett et al., 2005; Nixon, 2006). The mechanisms of alcohol-induced effects on neurogenesis have yet to be described and remain a critical gap in understanding the role of alcohol-induced brain damage in AUDs. In the following section, select plausible interactions are discussed through which alcohol may exert its effects on adult neurogenesis.
5. Potential mechanisms of alcohol's effects on adult neurogenesis
Excessive consumption of alcohol results in behavioral and neurochemical changes that are difficult to isolate due to the promiscuous pharmacology of ethanol on the brain. Ethanol's effects range from direct modulation of many neurotransmitter systems to indirect outcomes that are caused by or are comorbid with alcohol dependence. Alcohol's effects also vary depending on dose and duration of exposure, especially noting that a variety of neuroadaptations occur with the development of dependence (Vengeliene et al., 2008). Here, we focus on direct effects of ethanol on neurotransmission, cell signaling, and ethanol's cellular effects on the neurogenic niche. However, all of these events likely interact with each other as well as the host of indirect effects/comorbidities to generate the net effect alcohol has on the components of hippocampal adult neurogenesis.
5.1. Altered neurotransmission
Ethanol affects virtually every neurotransmitter system in the central nervous system (CNS; reviewed in Vengeliene et al., 2008). For example, concentrations of alcohol that reflect those seen in human consumption, may directly interact with a host of neurotransmitters, receptors and ion channels including acetylcholine, endocannabinoids, neuropeptide Y (NPY), gamma-aminobutyric acid (GABA) receptors, n-methyl-d-aspartate (NMDA) type glutamate receptors, and serotonin receptors along with G-protein-activated inwardly rectifying K+ channels (Arnone et al., 1997; Fadda and Rossetti, 1998; Grobin et al., 2001; Lewohl et al., 1999; Lovinger, 1999; Lovinger et al., 1989; Vengeliene et al., 2008). This list notably overlaps with the neurotransmitter systems that regulate adult hippocampal neurogenesis, including acetylcholine, endocannabinoids, GABA, glutamate, norepinephrine, NPY, and serotonin to name a few (Aguado et al., 2005; Bruel-Jungerman et al., 2011; Cameron et al., 1998; Conover and Notti, 2008; Gray, 2008). As many of these have been reviewed previously (Crews and Nixon, 2003; Nixon et al., 2010; Nixon et al., 2011), only primary targets GABA and glutamate as well as new insights into endocannabinoid signaling are discussed below.
The process of adult neurogenesis and maturation of newly born neurons involves two neurotransmitter systems that are also considered the primary pharmacodynamic targets of ethanol, GABA and glutamate. GABAA receptors are present on newly born neurons in the SGZ and GABA acts as an excitatory neurotransmitter during the first two-four weeks of new neuron development (Esposito et al., 2005; Ge et al., 2006; Overstreet Wadiche et al., 2005). Around this time, the neuron begins to express NMDA receptors and undergoes a switch from GABA being excitatory to inhibitory and NMDA being excitatory, recapitulating that seen in development (Nacher et al., 2007; Zhao et al., 2008). Previous studies show that treatment with a GABAA agonist results in decreased SGZ NPC proliferation while antagonist treatment yielded the opposite effect (Tozuka et al., 2005). However, treatment with a GABAA agonist was shown to enhance activity-dependent neuronal differentiation (Tozuka et al., 2005). Since ethanol is a positive allosteric modulator of the GABAA receptor it is possible that this receptor could be one avenue through which alcohol acts to alter neurogenesis. These findings also suggest that alcohol could have very specific effects on NPCs and developing neuroblasts depending on their state of ontogeny, especially when GABA is initially excitatory. However, no one has explored this potential relationship or whether alcohol affects the maturation of neuroblasts in the dentate gyrus.
The role of NMDA receptors in adult neurogenesis is complex. Several studies have shown that NMDA receptor activation inhibits adult hippocampal neurogenesis (Cameron et al., 1995; Maekawa et al., 2009; Nacher et al., 2001; Pava and Woodward, 2012); whereas NMDA receptor antagonism increases adult neurogenesis (Cameron et al., 1995; Petrus et al., 2009). Acutely, ethanol intoxication inhibits NMDA receptors (Lovinger et al., 1989) which indicates that NMDA receptors are not likely the mechanism by which ethanol intoxication decreases adult neurogenesis (Nixon and Crews, 2002). Prolonged ethanol exposure and the development of dependence, however, augments NMDA function and enhances glutamate release, both of which contribute to neuronal hyperexcitability associated with the ethanol withdrawal syndrome (Hoffman, 2003). Increased neurogenesis is observed after four days of ethanol inhibition of NMDA receptors, which is consistent with an NMDA antagonist similarly increasing neurogenesis (Cameron et al., 1995). Furthermore, ethanol withdrawal-induced hyperexcitability increases activation of granule cell neurons as measured by c-fos immunoreactivity (Knapp et al., 1998; Matsumoto et al., 1993). Although hyperexcitability associated with ethanol withdrawal typically subsides within 24 hours (Matsumoto et al., 1993), previous studies demonstrate that the effects of granule cell excitation on neurogenesis can be delayed by several days (Bruel-Jungerman et al., 2006; Stone et al., 2011). Additional studies are needed, but the delayed neurogenic effects associated with activity-dependent neurogenesis fits the temporal profile for reactive neurogenesis that occurs after a four-day binge ethanol exposure (Nixon and Crews, 2004). Altogether, these findings suggest that altered glutamatergic neurotransmission could serve a central role regulating the reactive adult neurogenesis response observed during early abstinence.
Hippocampal NPCs express a functional endocannabinoid system, including plasma membrane expression of CB1 and CB2 receptors and cytoplasmic expression of fatty acid amide hydrolase (FAAH) which catabolizes the endocannabinoid anandamide (Aguado et al., 2005). Deficits in adult neurogenesis occur in CB1−/− and CB2−/− mice, to confirm that engagement of the endocannabinoid system helps to maintain basal neurogenesis (Jin et al., 2004; Kim et al., 2006; Palazuelos et al., 2006). In addition, in vitro cell culture experiments demonstrate that cannabinoid receptor activation or FAAH inhibition promote NPC proliferation and neurosphere formation, while receptor blockade has the opposite effect (Aguado et al., 2005; Palazuelos et al., 2006). Activation of CB1 receptors reverses diminished neurogenesis in aged rodents and mediates exercise-induced increases in NPC proliferation (Hill et al., 2010; Marchalant et al., 2009). Furthermore, genetic ablation of CB1 or CB2 receptors blunts the increase in NPC proliferation and neurogenesis that follows kainic acid-induced excitotoxicity, directly implicating the endocannabinoid system in reactive hippocampal neurogenesis (Aguado et al., 2007; Palazuelos et al., 2012). Although direct evidence connecting the endocannabinoid system with ethanol-induced reactive neurogenesis is lacking, the well-characterized increase in NMDA receptor expression associated with chronic ethanol consumption depends on CB1 receptor activity (Warnault et al., 2007). This indicates that the endocannabinoid system regulates ethanol-induced neuroadaptations in the hippocampus. In addition, hippocampal CB1 and endocannabinoid levels are increased during abstinence in models of alcohol dependence (Mitrirattanakul et al., 2007), suggesting an increase in endocannabinoid signaling may occur during reactive neurogenesis.
5.2. CREB and its downstream effectors
The cyclic adenosine monophosphate (cAMP) signal transduction cascade and its most notorious transcription factor, the cAMP-responsive element binding (CREB) protein, are involved in a diverse array of physiological functions that include adult neurogenesis. Specifically, the activated, phosphorylated form of CREB (pCREB) has been shown consistently to be present in the SGZ with more recent evidence indicating that pCREB is predominantly localized in immature neurons (Mantamadiotis et al., 2012; Meshi et al., 2006; Nakagawa et al., 2002a). Further, systemic delivery of Rolipram, an agent known to increase CREB signaling, led to enhanced proliferation, survival, and dendritic branching of neuroblasts in the SGZ while retroviral inhibition of CREB signaling reduced cell survival and dendrite extension (Buffo et al., 2008; Crews, 2012; Nakagawa et al., 2002b). Importantly, alcohol is a known modulator of this pathway and some of its target effectors such as NPY and brain-derived neurotrophic factor (BDNF) allow for the possibility that alcohol-induced inhibition of neurogenesis may result from these interactions (Moonat et al., 2010; Pava and Woodward, 2012; Tateno and Saito, 2008).
Although both NPY and BDNF have been shown consistently to play a role in alcohol-related behaviors (Davis, 2008; Miller et al., 1996; Thiele et al., 1998), more recent data has identified NPY and BDNF as promoters of adult hippocampal neurogenesis (Koo and Duman, 2008; Malva et al., 2012). Specifically, in the dentate gyrus, a subpopulation of GABAergic hilar interneurons express NPY, while SGZ NPCs express the Y1 receptor (Sperk et al., 2007). Mice lacking NPY exhibit a significant reduction in cell proliferation while exogenous NPY administration both stimulates cell proliferation and promotes differentiation toward a neuronal phenotype (Hauser et al., 2011; Hensler et al., 2003). Furthermore, modulation of the NPY system could be involved in regulation of alcohol-induced reactive neurogenesis. For example, neurotoxin-induced reactive neurogenesis can be enhanced with exogenous NPY treatment (Corvino et al., 2012), while seizure-induced neurogenesis is attenuated in NPY knockout mice (Howell et al., 2007). Importantly, alcohols effects on NPY mirror its effects on adult neurogenesis observed following alcohol dependence. In the four-day binge ethanol model, hilar NPY expression decreases during intoxication but rebounds and increases more than 30-fold compared to controls after three days of abstinence (Bison and Crews, 2003). Ethanol's effects on hippocampal NPY expression closely parallel effects on NPC proliferation, which also decreases during ethanol intoxication and transiently increases during abstinence (He et al., 2009; Jang et al., 2002; Nixon and Crews, 2002; 2004). The increase in NPY during abstinence precedes the start of reactive neurogenesis by four days, similar to the delayed neurogenic response following exogenous NPY administration (Decressac et al., 2011).
In addition to NPY, reports from a variety of disease states and models demonstrate a relationship between BDNF levels and adult hippocampal neurogenesis (Kalkman, 2009; Martinotti et al., 2012; Post, 2007; Schmidt and Duman, 2007). BDNF augments neurogenesis via an enhancement of cell birth, survival, and maturation within the hippocampal SGZ (Koo and Duman, 2008; Lee et al., 2002). Heterozygous BDNF (knockdown) mice show decreases in adult neurogenesis (Lee et al., 2002). Indeed, alcohol-dependent humans have decreased serum BDNF concentrations; however, once alcohol consumption ceased there was a general increase in BDNF levels (Costa et al., 2011; Köhler et al., 2013). Several rodent studies have shown a similar pattern: chronic alcohol administration reduces BDNF expression in the rat hippocampus, cortex, and hypothalamus (MacLennan et al., 1995; Pandey et al., 1999; TapiaArancibia et al., 2001) whereas BDNF mRNA increases post-withdrawal (Tapia-Arancibia et al., 2001; see also Davis, 2008 for review). Decreases in hippocampal BDNF have also been linked with greater depression-like behavior following alcohol exposure (Briones and Woods, 2013; Hauser et al., 2011). Studies show that administration of antidepressants following alcohol exposure can help mitigate depression-like behavior seen in rodents as well as alcohol-induced effects on some components of adult neurogenesis (Hauser et al., 2011; Stevenson et al., 2009). Furthermore, an agonist for the BDNF receptor, the tropomyosin-related kinase receptor (trkB), reduced depression-like symptoms associated with alcohol withdrawal/abstinence and also restored neurogenesis during this period (Briones and Woods, 2013). Therefore, since alcohol exposure leads to deficits in NPY and BDNF in the brain and decreases in these factors attenuate measures of neurogenesis, it is increasingly possible that alcohol could be altering neurogenesis via its effect on NPY and BDNF and/or CREB. These findings are particularly noteworthy because of the significant comorbidity between AUDs and mood disorders coupled with this striking overlap in the pathophysiology of AUDs and mood (Kalkman, 2009; Martinotti et al., 2012; Post, 2007; Schmidt and Duman, 2007; Schuckit and Monteiro, 1988). However, results of BDNF studies vary widely, some have found no change in hippocampal BDNF (Okamoto et al., 2006; Miller at al., 2002) or an increase in BDNF in the cortex (Baek et al., 1996). These disparities may be related to the animal model, age, or the time course of neurobiological events with regard to whether the animal is intoxicated, dependent, and/or dependent but in abstinence. For example, examining BDNF and adult neurogenesis after alcohol withdrawal suggests a very different role from what may happen during alcohol intoxication. Based on the role of BDNF in ectopic adult neurogenesis in seizures and the development of epilepsy, we examined BDNF expression during abstinence following a four-day model of alcohol dependence in adolescent rats. In this short-term model of alcohol dependence, which results in withdrawal seizure-like behavior in some animals, no changes were observed in BDNF protein expression and BDNF expression did not correlate to withdrawal severity (McClain et al., in press). Importantly, this same study reported ectopic or aberrant neurogenesis, an effect that has been linked to hyper-BDNF signaling in models of epilepsy (e.g. Scharfman et al., 2005) and schizophrenia (Kalkman, 2009). Remarkable inconsistencies in the BDNF literature, detailed in a thorough review by Davis (2008), coupled with what appears to be very diverse roles of BDNF on the different aspects of alcohol intoxication and/or dependence make it difficult to definitively conclude the role of BDNF in alcohol-induced effects on adult neurogenesis, let alone alcohol use disorders. However, BDNF plays a critical and well-established role in the pathophysiology of other psychiatric disorders and notably ones with significant comorbidity to AUDs (Martinotti et al., 2012; Post, 2007; Schuckit and Monteiro, 1988). Thus, this is an area of work that is ripe for discovery but needs a critical eye for the peculiarities of not only BDNF's role in the different aspects of adult neurogenesis but also the various phases of alcohol dependence.
5.3. Direct effects on the neurogenic niche
The neurogenic niche is the neurogenesis-permissive zone where stem cells have been retained from embryonic pools (for review see Taupin, 2006). The SGZ of the hippocampal dentate gyrus is one such region in the adult brain where adult neurogenesis from NSCs continues because of the permissiveness or instructiveness of the microenvironment of the SGZ. The important role of the niche in adult neurogenesis emerged from data that stem cells can be isolated from multiple locations of the brain (Morshead et al., 1994; Reynolds and Weiss, 1992), but adult neurogenesis is restricted to these two specific locations under normal, non-pathological conditions. This permissiveness, however, can be induced by damage (Magavi et al., 2000) though aspects of damage and disease may further impair the production of new neurons (Monje et al., 2003). The cytoarchitectural organization of the niche is thought to be the critical factor for maintaining stem cell populations, as niche cells regulate self-renewal, stem cell activation, and cell fate choice (reviewed in Conover and Notti, 2008). Critical components of the hippocampal niche include the vasculature (Palmer et al., 2000), astrocytes (Seri et al., 2001; Song et al., 2002), and their host of secreted factors and signals (Notch, bone morphogenic proteins, ephrins, sonic hedgehog; Alvarez-Buylla and Lim, 2004). Importantly, this means that astrocytes may not only be acting as the stem cells themselves, but that they also constitute a significant portion of the microenvironment that makes up the niche (Taupin, 2006).
Excessive alcohol exposure is detrimental to astrocytes. In the least, astrocytes are activated by alcohol and at worst astrocytes may be damaged by excessive alcohol exposure (Crews and Nixon, 2009; Kelso et al., 2011; Korbo, 1999). Therefore, understanding the effect of alcohol on astrocytes is critical to understanding how alcohol impacts the neurogenic niche. When astrocytes react, termed astrogliosis, various changes in morphology and gene expression coincide with changes in their functional role (Sofroniew, 2009). Astrogliosis occurs in response to a host of damaging, neurodegenerative, and inflammatory conditions, including several models of AUDs (Baydas and Tuzcu, 2005; Dalcik et al., 2009; Evrard et al., 2006; Franke, 1995; Kelso et al., 2011; Robel et al., 2011; Sofroniew, 2009; Tagliaferro et al., 2002; Udomuksorn et al., 2011). A hallmark of astrogliosis is the up-regulation of intermediate filament proteins such as glial fibrillary acidic protein (GFAP), nestin, and vimentin, with the latter two also widely used to identify progenitor cells (Pekny et al., 2007). Indeed, reactive astrocytes may be a potential source of stem cells (Buffo et al., 2008; Robel et al., 2011). Unfortunately, due to the similarity in markers used to identify stem cells and reactive astrocytes, it remains a challenge to tease apart effects on niche astrocytes versus niche stem cells in conditions with astrogliosis such as the AUDs (Kelso et al., 2011). Regardless, normal astrocytic functions would clearly be impacted in the AUDs, such as providing metabolic and trophic support, buffering ions, taking up excessive extracellular neurotransmitter (especially glutamate), and eliminating free radicals, all of which would have major effects on the niche and the production of new neurons.
Several studies include a role for microglia in the niche, especially activated microglia where their role as either being pro-neurogenic (Battista et al., 2006) or anti-neurogenic (Monje et al., 2003) is hotly debated (Whitney et al., 2009). Their role is intertwined within the discussion of neuroinflammation, as they are the macrophages of the CNS. Although astrocytes play a role in neuroinflammation, as they are a component of the blood brain barrier (BBB), we focus on the role of microglia as the primary cells of the brain's innate immune system.
Much like the discovery of adult neurogenesis, inflammation in the CNS originally was not believed (Murphy and Sturm, 1923). This historical belief grew from the idea that the CNS was immune privileged and segregated from systemic inflammation by the BBB (Pachter et al., 2003). However, with the discovery that microglia could act as macrophages within the CNS came the concept of an innate immune system within the brain (Neuwelt and Clark, 1978). Classical inflammation in the CNS has three cardinal hallmarks: microglial activation, BBB disruption, and lymphocytic infiltration (Colton and Wilcock, 2010; Graeber et al., 2011). Although “activated” microglia have been reported to be anything from beneficial to detrimental to the processes of adult neurogenesis, in retrospect it appears that only the pro-inflammatory, classically-activated phenotype of microglia inhibit or impair adult neurogenesis (Russo et al., 2011). Microglia exist in a continuum of activation states, or phenotypes (Town et al., 2005), such that it is now clear that it is the microglial phenotype, whether anti-inflammatory or pro-inflammatory, that predicts which cytokines and chemokines are secreted and therefore whether adult neurogenesis is promoted or inhibited (Ekdahl et al., 2009; Whitney et al., 2009). Thus, it is the fully or classically activated microglia that secrete pro-inflammatory cytokines that are associated with reductions in neurogenesis (Monje et al., 2003). Inflammation can affect various phases of adult neurogenesis. For example, pro-inflammatory cytokine interleukin 6 (IL-6), results in decreased proliferation (Vallieres et al., 2002); whereas other pro-inflammatory cytokines such as interferon-gamma (IFN-γ) can alter fate choices from neuronal to astrocytic (Walter et al., 2011).
Whether inflammation occurs in AUDs and the extent to which it influences alcohol-induced neurodegeneration and the development of AUDs is a matter of current debate (Crews, 2012). That excessive alcohol consumption produces neuroinflammation has been inferred from observations of increased expression of pro-inflammatory genes and cytokines following alcohol exposure (Crews et al., 2006c; He and Crews, 2008; Knapp and Crews, 1999; Qin et al., 2008), while other groups have shown that peripheral inflammation promotes increased ethanol consumption (Agrawal et al., 2011; Blednov et al., 2011). Furthermore, in animal models of chronic alcohol intake, innate immune signaling cascades are induced by ethanol activation of the pro-inflammatory transcription factor, nuclear factor-kappa-B (Crews et al., 2006a; Crews et al., 2011; Valles et al., 2004). However, classical definitions of inflammation state that it is a “multicellular process characterized by changes in the vasculature and infiltration of mobile cells” (p. 3800, Graeber et al., 2011). Although studies have shown microglial activation in alcoholic brain (He and Crews, 2008), no studies to date in human tissue have shown inflammation according to these classical definitions. Moreover, in the four-day binge model of an AUD, the most damaging of the AUD models, microglia are activated but only towards a low grade or beneficial phenotype (Marshall et al., 2013). That is not to say that alcohol abuse is not producing a pro-inflammatory environment, but true inflammation according to definition, may not be occurring.
A few studies have attempted to show direct links between alcohol, pro-inflammatory factors, and adult neurogenesis. In theory, pro-inflammatory cytokines produced by activated microglia in models of AUDs could be involved in the inhibition of neurogenesis (Ekdahl et al., 2009). For example, interleukin 1β (IL-1β), which is enhanced in LPS and ethanol-treated mice (Qin et al., 2008), has been shown repeatedly in other models to inhibit various stages of neurogenesis including proliferation and maturation (Koo and Duman, 2008; Zunszain et al., 2012). A recent in vitro study, however, showed a more direct relationship between alcohol-induced pro-inflammatory events and alcohol-induced inhibition of neurogenesis. Blocking the alcohol-induced increase in IL-1β synthesis ameliorated the alcohol-induced decrease in both NPC proliferation and new cell survival (Zou and Crews, 2012). This finding further indicates that the other cytokines typical of classical microglial activation and seen during intoxication may play a role in alcohol-induced decreased neurogenesis.
Microglial activation may also be involved in reactive neurogenesis after damage (Deboy et al., 2006; Wainwright et al., 2009). In other neurodegenerative conditions, cytokines are key to promoting reactive neurogenesis. For example, in an adrenalectomy model of reactive neurogenesis, blocking transforming growth factor beta (TGF-β) receptors reduced neurogenesis (Battista et al., 2006) whereas increases in interleukin-10 (IL-10) enhanced neurogenesis (Kiyota et al., 2012). Similar trends have been observed in the four-day binge model: a significant increase in the anti-inflammatory cytokine IL-10 and low-grade activation of microglia (Marshall et al., 2013) precedes a reactive burst in NSC proliferation (Nixon and Crews, 2004). Many studies have reported that alcohol exposure induces a microglial response, however no reports to date have reported on the potential for anti-inflammatory microglia (Marshall et al., 2013; McClain et al., 2011b; Nixon et al., 2008). Although alcohol-induced changes in anti-inflammatory cytokines have not been directly linked to the reported burst in NPC proliferation, the coincidental timing of this striking, anti-inflammatory microglia response and reactive neurogenesis suggests that alcohol-activated microglia directly influence the neurogenic niche.
6. Conclusions
Countless studies have illustrated that AUDs result in damage to the central nervous system with the hippocampus as a central target of its neurotoxic effects. Recent preclinical studies have highlighted that alcohol's effects on the various components of adult neurogenesis are one way in which excessive alcohol intake produces hippocampal pathology. By impacting the structure and function of the dentate gyrus through inhibition of adult neurogenesis, alcohol has downstream effects on hippocampal circuitry and subsequently the host of behaviors controlled or influenced by the hippocampus. Ethanol is a pharmacologically promiscuous drug that acts on many targets, several of which are involved in adult neurogenesis. Alcohol pharmacodynamically alters several neurotransmitter systems such as glutamate and GABA that are also critically involved in the process of adult neurogenesis. Additionally, alcohol modulates the transcription factor CREB and its downstream effectors (including NPY and BDNF) which also have roles in hippocampal neurogenesis and new cell survival. Alcohol-induced changes in neurogenesis could result from any combination of these targets. In addition to these direct, pharmacological effects, alcohol has significant cellular effects that impact the hippocampal neurogenic niche. Specifically, excessive alcohol consumption results in activation and/or damage to astrocytes, which are the primary components of the niche, if not the stem cells themselves. Microglia are also activated by excessive alcohol consumption although their contribution to adult neurogenesis depends upon their phenotype and inflammatory state. New data emerging on alcohol's effects on glia strongly imply that excessive alcohol consumption may impact the neurogenic niche through these cellular effects.
Importantly, research has shown that some recovery is possible when alcoholics abstain from alcohol use (e.g. Carlen et al., 1978; Pfefferbaum et al., 1995). Although there are a host of plastic changes that occur with abstinence, one way that the hippocampus may recover in abstinence is through the repopulation of the dentate gyrus by adult neurogenesis. Although it has been reported that a compensatory increase in neurogenesis occurs in abstinence, it is still not known whether the new cells are functionally integrated correctly or the extent to which this process influences cognitive recovery. Despite observation of this potential mechanism of recovery in animal models, detectable cognitive impairments remain in over 50% of AUD cases (Eckardt and Martin, 1986; Parsons, 1993). Therefore important future questions should include understanding the contribution of these phenomena – alcohol-induced decreases to pathology and reactive neurogenesis to recovery – to hippocampal integrity and function in AUDs.
Highlights.
Alcoholism may result in hippocampal pathology through effects on adult neurogenesis.
Alcohol's pharmacological promiscuity impacts neurogenesis via multiple mechanisms.
Alcohol affects neuronal signaling and/or the neurogenic niche to alter neurogenesis.
Acknowledgments
The authors thank M. Ayumi Deeny for producing Figure 1 and the support of National Institute of Alcohol Abuse and Alcoholism grants R01AA016959, R21AA16307 and National Institute of Drug Abuse training grants T32DA016176 and T32DA007304 in conducting the work described herein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Aberg E, Hofstetter CP, Olson L, Brene S. Moderate ethanol consumption increases hippocampal cell proliferation and neurogenesis in the adult mouse. Int J Neuropsychopharmacol. 2005;8:557–67. doi: 10.1017/S1461145705005286. [DOI] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–63. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE. Minocycline reduces ethanol drinking. Brain Behav Immun. 2011;25(Suppl 1):S165–9. doi: 10.1016/j.bbi.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;12:1704–6. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- Aguado T, Romero E, Monory K, Palazuelos J, Sendtner M, Marsicano G, et al. The CB1 Cannabinoid Receptor Mediates Excitotoxicity-induced Neural Progenitor Proliferation and Neurogenesis. J Biol Chem. 2007;282:23892–8. doi: 10.1074/jbc.M700678200. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–35. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–6. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Anderson ML, Nokia MS, Govindaraju KP, Shors TJ. Moderate drinking? Alcohol consumption significantly decreases neurogenesis in the adult hippocampus. Neuroscience. 2012;224:202–9. doi: 10.1016/j.neuroscience.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RJ, Barker RA. Neurodegeneration: a failure of neuroregeneration. Lancet. 2001;358:1174–6. doi: 10.1016/S0140-6736(01)06260-2. [DOI] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–6. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Baek JK, Heaton MB, Walker DW. Up-regulation of high-affinity neurotrophin receptor, TrkB-like protein on western blots of rat cortex after chronic ethanol treatment. Brain Res Mol Brain Res. 1996;40:161–164. doi: 10.1016/0169-328x(96)00109-x. [DOI] [PubMed] [Google Scholar]
- Bartels C, Kunert HJ, Stawicki S, Kroner-Herwig B, Ehrenreich H, Krampe H. Recovery of hippocampus-related functions in chronic alcoholics during monitored long-term abstinence. Alcohol Alcohol. 2007;42:92–102. doi: 10.1093/alcalc/agl104. [DOI] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Baydas G, Tuzcu M. Protective effects of melatonin against ethanol-induced reactive gliosis in hippocampus and cortex of young and aged rats. Exp Neurol. 2005;194:175–81. doi: 10.1016/j.expneurol.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci. 2011;1216:114–21. doi: 10.1111/j.1749-6632.2010.05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengochea O, Gonzalo LM. Effect of chronic alcoholism on the human hippocampus. Histol Histopathol. 1990;5:349–57. [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, et al. Hippocampus volume loss due to chronic heavy drinking. Alcohol Clin Exp Res. 2006;30:1866–70. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MS, Steier P, et al. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634–9. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Bison S, Crews F. Alcohol Withdrawal Increases Neuropeptide Y Immunoreactivity in Rat Brain. Alcohol Clin Exp Res. 2003;27:1173–83. doi: 10.1097/01.ALC.0000075827.74538.FE. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25(Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Song J, Ming GL, Song H. A unifying hypothesis on mammalian neural stem cell properties in the adult hippocampus. Curr Opin Neurobiol. 2012;22:754–61. doi: 10.1016/j.conb.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–55. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–24. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Briones TL, Woods J. Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience. 2013;254:324–34. doi: 10.1016/j.neuroscience.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-Term Potentiation Enhances Neurogenesis in the Adult Dentate Gyrus. J Neurosci. 2006;26:5888–93. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Lucassen PJ, Francis F. Cholinergic influences on cortical development and adult neurogenesis. Behav Brain Res. 2011;221:379–88. doi: 10.1016/j.bbr.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, et al. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–6. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadete-Leite A, Tavares MA, Alves MC, Uylings HB, Paula-Barbosa MM. Metric analysis of hippocampal granule cell dendritic trees after alcohol withdrawal in rats. Alcohol Clin Exp Res. 1989;13:837–40. doi: 10.1111/j.1530-0277.1989.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Cadete-Leite A, Tavares MA, Paula-Barbosa MM. Alcohol withdrawal does not impede hippocampal granule cell progressive loss in chronic alcohol-fed rats. Neurosci Lett. 1988a;86:45–50. doi: 10.1016/0304-3940(88)90180-2. [DOI] [PubMed] [Google Scholar]
- Cadete-Leite A, Tavares MA, Uylings HB, Paula-Barbosa M. Granule cell loss and dendritic regrowth in the hippocampal dentate gyrus of the rat after chronic alcohol consumption. Brain Res. 1988b;473:1–14. doi: 10.1016/0006-8993(88)90309-5. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–92. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Wortzman G, Holgate RC, Wilkinson DA, Rankin JC. Reversible cerebral atrophy in recently abstinent chronic alcoholics measured by computed tomography scans. Science. 1978;200:1076–8. doi: 10.1126/science.653357. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–38. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Clark DB, Bukstein O, Cornelius J. Alcohol use disorders in adolescents: epidemiology, diagnosis, psychosocial interventions, and pharmacological treatment. Paediatr Drugs. 2002;4:493–502. doi: 10.2165/00128072-200204080-00002. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–3. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Corse TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20:284–92. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Colton C, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174–91. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Conover JC, Notti RQ. The neural stem cell niche. Cell Tissue Res. 2008;331:211–24. doi: 10.1007/s00441-007-0503-6. [DOI] [PubMed] [Google Scholar]
- Contet C, Kim A, Le D, Iyengar SK, Kotzebue RW, Yuan CJ, et al. mu-Opioid receptors mediate the effects of chronic ethanol binge drinking on the hippocampal neurogenic niche. Addict Biol. 2013 doi: 10.1111/adb.12040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvino V, Marchese E, Giannetti S, Lattanzi W, Bonvissuto D, Biamonte F, et al. The neuroprotective and neurogenic effects of neuropeptide Y administration in an animal model of hippocampal neurodegeneration and temporal lobe epilepsy induced by trimethyltin. J Neurochem. 2012;122:415–26. doi: 10.1111/j.1471-4159.2012.07770.x. [DOI] [PubMed] [Google Scholar]
- Costa M, Girard M, Dalmay F, Malauzat D. Brain-derived neurotrophic factor serum levels in alcohol-dependent subjects 6 months after alcohol withdrawal. Alcohol Clin Exp Res. 2011;35:1966–1973. doi: 10.1111/j.1530-0277.2011.01548.x. [DOI] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, et al. BHT blocks NFkappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006a;30:1938–49. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT. Alcohol and neurodegeneration. CNS Drug Reviews. 1999;5:379–94. [Google Scholar]
- Crews FT. Immune function genes, genetics, and the neurobiology of addiction. Alcohol Res. 2012;34(3):355–61. doi: 10.35946/arcr.v34.3.11. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–23. [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006b;137:437–45. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Alcohol, neural stem cells, and adult neurogenesis. Alcohol Res Health. 2003;27:197–204. [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–27. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Kim DH, Qin L, Shukitt-Hale B, Josephs JA. BHT blocks NFkB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006c;30:1938–49. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalcik H, Yardimoglu M, Filiz S, Gonca S, Dalcik C, Erden BF. Chronic ethanol-induced glial fibrillary acidic protein (GFAP) immunoreactivity: an immunocytochemical observation in various regions of adult rat brain. Int J Neurosci. 2009;119:1303–18. doi: 10.1080/00207450802333672. [DOI] [PubMed] [Google Scholar]
- Davis MI. Ethanol-BDNF interactions: still more questions than answers. Pharmacol Ther. 2008;118:36–57. doi: 10.1016/j.pharmthera.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–44. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Deboy CA, Xin J, Byram SC, Serpe CJ, Sanders VM, Jones KJ. Immune-mediated neuroprotection of axotomized mouse facial motoneurons is dependent on the IL-4/STAT6 signaling pathway in CD4(+) T cells. Exp Neurol. 2006;201:212–24. doi: 10.1016/j.expneurol.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Decressac M, Wright B, David B, Tyers P, Jaber M, Barker RA, et al. Exogenous neuropeptide Y promotes in vivo hippocampal neurogenesis. Hippocampus. 2011;21:233–8. doi: 10.1002/hipo.20765. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Picchini AM, Moadel T, Sisti AC, Yamada A, Kimura S, et al. Experience dictates stem cell fate in the adult hippocampus. Neuron. 2011;70:908–23. doi: 10.1016/j.neuron.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D, Saint-Cyr JA, Gurevich N, Carlen PL. Ethanol-induced dendritic alterations in hippocampal granule cells. Brain Res. 1989;477:373–7. doi: 10.1016/0006-8993(89)91430-3. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, Martin PR. Clinical assessment of cognition in alcoholism. Alcohol Clin Exp Res. 1986;10:123–7. doi: 10.1111/j.1530-0277.1986.tb05058.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–9. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–79. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–86. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard SG, Duhalde-Vega M, Tagliaferro P, Mirochnic S, Caltana LR, Brusco A. A low chronic ethanol exposure induces morphological changes in the adolescent rat brain that are not fully recovered even after a long abstinence: an immunohistochemical study. Exp Neurol. 2006;200:438–59. doi: 10.1016/j.expneurol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Fadda F, Rossetti Z. Chronic ethanol consumption: From neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Franke H. Influence of chronic alcohol treatment on the GFAP-immunoreactivity in astrocytes of the hippocampus in rats. Acta Histochem. 1995;97:263–71. doi: 10.1016/S0065-1281(11)80187-X. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend. 2005;78:263–73. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behav Brain Res. 2006;169:142–9. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: The weak link in psychiatric disorders? Eur Neuropsychopharmacol. 2013;23:1165–81. doi: 10.1016/j.euroneuro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood) 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Li W, Rodriguez ML. Role of microglia in CNS inflammation. FEBS Lett. 2011;585:3798–805. doi: 10.1016/j.febslet.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74:223–34. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Gray WP. Neuropeptide Y signalling on hippocampal stem cells in health and disease. Mol Cell Endocrinol. 2008;288:52–62. doi: 10.1016/j.mce.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Montoya D, Wilson WA, Morrow AL, Swartzwelder HS. Age-related differences in neurosteroid potentiation of muscimol-stimulated 36Cl(-) flux following chronic ethanol treatment. Neuroscience. 2001;105:547–52. doi: 10.1016/s0306-4522(01)00232-9. [DOI] [PubMed] [Google Scholar]
- Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Nixon K, Rimondini R, Damadzic R, Sommer WH, Eskay R, et al. Long-term suppression of forebrain neurogenesis and loss of neuronal progenitor cells following prolonged alcohol dependence in rats. Int J Neuropsychopharmacol. 2010;13:583–93. doi: 10.1017/S1461145710000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding AJ, Wong A, Svoboda M, Kril JJ, Halliday GM. Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus. 1997;7:78–87. doi: 10.1002/(SICI)1098-1063(1997)7:1<78::AID-HIPO8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Taylor RE, Tizabi Y. Alcohol induced depressive-like behavior is associated with a reduction in hippocampal BDNF. Pharmacol Biochem Behav. 2011;100:253–8. doi: 10.1016/j.pbb.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DM, Deeny MA, Shaner CA, Nixon K. Determining the threshold for tlcohol-induced brain damage: New evidence with gliosis markers. Alcohol Clin Exp Res. 2013;37:425–34. doi: 10.1111/j.1530-0277.2012.01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–58. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur J Neurosci. 2005;21:2711–20. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- He J, Overstreet DH, Crews FT. Abstinence from moderate alcohol self-administration alters progenitor cell proliferation and differentiation in multiple brain regions of male and female P rats. Alcohol Clin Exp Res. 2009;33:129–38. doi: 10.1111/j.1530-0277.2008.00823.x. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Ladenheim EE, Lyons WE. Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/−) mice. J Neurochem. 2003;85:1139–47. doi: 10.1046/j.1471-4159.2003.01748.x. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, et al. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci U S A. 2003;100:7919–24. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TTY, Gil-Mohapel J, et al. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20:513–23. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PL. International Review of Neurobiology. Academic Press; 2003. NMDA receptors in alcoholism; pp. 35–82. [DOI] [PubMed] [Google Scholar]
- Howell OW, Silva S, Scharfman HE, Sosunov AA, Zaben M, Shatya A, et al. Neuropeptide Y is important for basal and seizure-induced precursor cell proliferation in the hippocampus. Neurobiol Dis. 2007;26:174–88. doi: 10.1016/j.nbd.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Hunt WA. Are binge drinkers more at risk of developing brain damage? Alcohol. 1993;10:559–561. doi: 10.1016/0741-8329(93)90083-z. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jang MH, Shin MC, Jung SB, Lee TH, Bahn GH, Kwon YK, et al. Alcohol and nicotine reduce cell proliferation and enhance apoptosis in dentate gyrus. Neuroreport. 2002;13:1509–13. doi: 10.1097/00001756-200208270-00004. [DOI] [PubMed] [Google Scholar]
- Jin K, Xie L, Kim SH, Parmentier-Batteur S, Sun Y, Mao XO, et al. Defective adult neurogenesis in CB1 cannabinoid receptor knockout Mice. Mol Pharmacol. 2004;66:204–8. doi: 10.1124/mol.66.2.204. [DOI] [PubMed] [Google Scholar]
- Johnson A, van der Meer MA, Redish AD. Integrating hippocampus and striatum in decision-making. Curr Opin Neurobiol. 2007;17:692–7. doi: 10.1016/j.conb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkman HO. Altered growth factor signaling pathways as the basis of aberrant stem cell maturation in schizophrenia. Pharmacol Ther. 2009;121:115–22. doi: 10.1016/j.pharmthera.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–4. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kelso ML, Liput DJ, Eaves DW, Nixon K. Upregulated vimentin suggests new areas of neurodegeneration in a model of an alcohol use disorder. Neuroscience. 2011;197:381–93. doi: 10.1016/j.neuroscience.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. New neurons for ‘survival of the fittest’. Nat Rev Neurosci. 2012;13:727–36. doi: 10.1038/nrn3319. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Genetic influence on phenotypic differentiation in adult hippocampal neurogenesis. Brain Res Dev Brain Res. 2002;134:1–12. doi: 10.1016/s0165-3806(01)00224-3. [DOI] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Mao XO, Ledent C, Jin K, Greenberg DA. Role for neuronal nitric-oxide synthase in cannabinoid-induced neurogenesis. J Pharmacol Exp Ther. 2006;319:150–4. doi: 10.1124/jpet.106.107698. [DOI] [PubMed] [Google Scholar]
- King MA, Hunter BE, Walker DW. Alterations and recovery of dendritic spine density in rat hippocampus following long-term ethanol ingestion. Brain Res. 1988;459:381–5. doi: 10.1016/0006-8993(88)90656-7. [DOI] [PubMed] [Google Scholar]
- Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2012;19:724–33. doi: 10.1038/gt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res. 1999;23:633–43. [PubMed] [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like rroteins and ultrasonic vocalizations during ethanol withdrawal: Further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998;22:481–93. [PubMed] [Google Scholar]
- Köhler S, Klimke S, Hellweg R, Lang UE. Serum brain-derived neurotrophic factor and nerve growth factor concentrations change after alcohol withdrawal: preliminary data of a case-control comparison. Eur Addict Res. 2013;19:98–104. doi: 10.1159/000342334. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–6. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Korbo L. Glial cell loss in the hippocampus of alcoholics. Alcohol Clin Exp Res. 1999;23:164–8. [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, et al. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res. 2000;109:177–86. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Nixon K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcohol Clin Exp Res. 2010;34:404–14. doi: 10.1111/j.1530-0277.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–75. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci. 1999;2:1084–90. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Parnell SE, West JR. Blood ethanol concentration profiles: a comparison between rats and mice. Alcohol. 2003;29:165–71. doi: 10.1016/s0741-8329(03)00025-9. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. 5-HT3 receptors and the neural actions of alcohols: an increasingly exciting topic. Neurochem Int. 1999;35:125–30. doi: 10.1016/s0197-0186(99)00054-6. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–4. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Brandao F, Cadete-Leite A, Madeira MD, Paula-Barbosa MM. Synaptic reorganization in the hippocampal formation of alcohol-fed rats may compensate for functional deficits related to neuronal loss. Alcohol. 2000;20:139–48. doi: 10.1016/s0741-8329(99)00069-5. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Madeira MD, Paula-Barbosa MM. Behavioral and neuroanatomical consequences of chronic ethanol intake and withdrawal. Physiol Behav. 1999;66:337–46. doi: 10.1016/s0031-9384(98)00301-1. [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, Lee N, Walker DW. Chronic ethanol administration decreases brain-derived neurotrophic factor gene expression in the rat hippocampus. Neurosci Lett. 1995;197:105–8. doi: 10.1016/0304-3940(95)11922-j. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–42. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Namba T, Suzuki E, Yuasa S, Kohsaka S, Uchino S. NMDA receptor antagonist memantine promotes cell proliferation and production of mature granule neurons in the adult hippocampus. Neurosci Res. 2009;63:259–66. doi: 10.1016/j.neures.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–5. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Malva JO, Xapelli S, Baptista S, Valero J, Agasse F, Ferreira R, et al. Multifaces of neuropeptide Y in the brain--neuroprotection, neurogenesis and neuroinflammation. Neuropeptides. 2012;46:299–308. doi: 10.1016/j.npep.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012;35:250–60. doi: 10.1016/j.tins.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Gunther A, Stetter F, Ackermann K. Rapid recovery from cognitive deficits in abstinent alcoholics: a controlled test-retest study. Alcohol Alcohol. 1999;34:567–74. doi: 10.1093/alcalc/34.4.567. [DOI] [PubMed] [Google Scholar]
- Mantamadiotis T, Papalexis N, Dworkin S. CREB signalling in neural stem/progenitor cells: recent developments and the implications for brain tumour biology. Bioessays. 2012;34:293–300. doi: 10.1002/bies.201100133. [DOI] [PubMed] [Google Scholar]
- Marchalant Y, Brothers HM, Norman GJ, Karelina K, DeVries AC, Wenk GL. Cannabinoids attenuate the effects of aging upon neuroinflammation and neurogenesis. Neurobiol of Dis. 2009;34:300–7. doi: 10.1016/j.nbd.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Marin-Burgin A, Schinder AF. Requirement of adult-born neurons for hippocampus-dependent learning. Behav Brain Res. 2012;227:391–9. doi: 10.1016/j.bbr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol Dis. 2013;8:00007–7. doi: 10.1016/j.nbd.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti G, Di Iorio G, Marini S, Ricci V, De Berardis D, Di Giannantonio M. Nerve growth factor and brain-derived neurotrophic factor concentrations in schizophrenia: a review. J Biol Regul Homeost Agents. 2012;3:347–56. [PubMed] [Google Scholar]
- Matsumoto I, Leah J, Shanley B, Wilce P. Immediate Early Gene Expression in the Rat Brain during Ethanol Withdrawal. Mol Cell Neurosci. 1993;4:485–91. doi: 10.1006/mcne.1993.1060. [DOI] [PubMed] [Google Scholar]
- McClain JA, Hayes DM, Morris SA, Nixon K. Adolescent binge alcohol exposure alters hippocampal progenitor cell proliferation in rats: effects on cell cycle kinetics. J Comp Neurol. 2011a;519:2697–710. doi: 10.1002/cne.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, et al. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun. 2011b;25(Suppl 1):S120–8. doi: 10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:610–4. doi: 10.1136/jnnp.2006.095869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–31. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Miller HL, Delgado PL, Salomon RM, Berman R, Krystal JH, Heninger GR, et al. Clinical and biochemical effects of catecholamine depletion on antidepressant-induced remission of depression. Arch Gen Psychiatry. 1996;53:117–28. doi: 10.1001/archpsyc.1996.01830020031005. [DOI] [PubMed] [Google Scholar]
- Miller R, King MA, Heaton MB, Walker DW. The effects of chronic ethanol consumption on neurotrophins and their receptors in the rat hippocampus and basal forebrain. Brain Res. 2002;950:137–47. doi: 10.1016/s0006-8993(02)03014-7. [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, López-Valdés HE, Liang J, Matsuka Y, Mackie K, Faull KF, et al. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin Exp Res. 2007;31:855–67. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Moonat S, Starkman BG, Sakharkar A, Pandey SC. Neuroscience of alcoholism: molecular and cellular mechanisms. Cell Mol Life Sci. 2010;67:73–88. doi: 10.1007/s00018-009-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–82. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Murphy JB, Sturm E. Conditions Determining the Transplantability of Tissues in the Brain. J Exp Med. 1923;38:183–97. doi: 10.1084/jem.38.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J, Rosell DR, Alonso-Llosa G, McEwen BS. NMDA receptor antagonist treatment induces a long-lasting increase in the number of proliferating cells, PSA-NCAMimmunoreactive granule neurons and radial glia in the adult rat dentate gyrus. Eur J Neurosci. 2001;13:512–20. doi: 10.1046/j.0953-816x.2000.01424.x. [DOI] [PubMed] [Google Scholar]
- Nacher J, Varea E, Miguel Blasco-Ibanez J, Gomez-Climent MA, Castillo-Gomez E, Crespo C, et al. N-methyl-d-aspartate receptor expression during adult neurogenesis in the rat dentate gyrus. Neuroscience. 2007;144:855–64. doi: 10.1016/j.neuroscience.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–90. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, et al. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci. 2002a;22:9868–76. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, et al. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002b;22:3673–82. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt EA, Clark WK. Unique aspects of central nervous system immunology. Neurosurgery. 1978;3:419–30. doi: 10.1227/00006123-197811000-00018. [DOI] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–95. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–93. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24:9714–22. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31:218–29. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Morris SA, Liput DJ, Kelso ML. Roles of neural stem cells and adult neurogenesis in adolescent alcohol use disorders. Alcohol. 2010;44:39–56. doi: 10.1016/j.alcohol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Pandya JD, Butler TR, Liput DJ, Morris SA, Prendergast MA, et al. Binge ethanol impairs neuronal mitochondrial bioenergetics - reversal by antioxidants and uncouplers. Alcohol Clin Exp Res. 2009;33:27A. [Google Scholar]
- Nixon K, Pauly JR, Hayes DM. Alcohol, nicotine, and adult neurogenesis. In: Olive MF, editor. Drug Addiction and Adult Neurogenesis. Kerala, India: Research Signpost; 2011. pp. 95–119. [Google Scholar]
- Noel X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol. 2013;23:632–8. doi: 10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, et al. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology. 2012;37:2267–76. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002a;26:547–57. [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol Biochem Behav. 2002b;72:521–32. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Miki T, Lee KY, Yokoyama T, Kuma H, Gu H, et al. Effects of chronic ethanol administration on the expression levels of neurotrophic factors in the rat hippocampus. Okajimas Folia Anat Jpn. 2006;83:1–6. doi: 10.2535/ofaj.83.1. [DOI] [PubMed] [Google Scholar]
- Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–32. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- Ozsoy S, Durak AC, Esel E. Hippocampal volumes and cognitive functions in adult alcoholic patients with adolescent-onset. Alcohol. 2013;47:9–14. doi: 10.1016/j.alcohol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Pachter JS, de Vries HE, Fabry Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol. 2003;62:593–604. doi: 10.1093/jnen/62.6.593. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzmán M, Galve-Roperh I. Nonpsychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20:2405–7. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Ortega Z, Díaz-Alonso J, Guzmán M, Galve-Roperh I. CB2 Cannabinoid Receptors Promote Neural Progenitor Cell Proliferation via mTORC1 Signaling. J Biol Chem. 2012;287:1198–209. doi: 10.1074/jbc.M111.291294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang D, Mittal N, Nayyar D. Potential role of the gene transcription factor cyclic AMP-responsive element binding protein in ethanol withdrawal-related anxiety. J Pharmacol Exp Ther. 1999;288:866–78. [PubMed] [Google Scholar]
- Parsons OA. Impaired neuropsychological cognitive functioning in sober alcoholics. In: Hunt WA, Nixon SJ, editors. Alcohol-Induced Brain Damage (NIAAA Research Monograph No 22. Rockville, MD: National Institutes of Health; 1993. pp. 173–94. [Google Scholar]
- Paula-Barbosa MM, Brandao F, Madeira MD, Cadete-Leite A. Structural changes in the hippocampal formation after long-term alcohol consumption and withdrawal in the rat. Addiction. 1993;88:237–47. doi: 10.1111/j.1360-0443.1993.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol. 2012;46:185–204. doi: 10.1016/j.alcohol.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak R, Skrzypiec A, Sulkowski S, Buczko W. Ethanol-induced neurotoxicity is counterbalanced by increased cell proliferation in mouse dentate gyrus. Neurosci Lett. 2002;327:83–6. doi: 10.1016/s0304-3940(02)00369-5. [DOI] [PubMed] [Google Scholar]
- Pekny M, Wilhelmsson U, Bogestal YR, Pekna M. The role of astrocytes and complement system in neural plasticity. Int Rev Neurobiol. 2007;82:95–111. doi: 10.1016/S0074-7742(07)82005-8. [DOI] [PubMed] [Google Scholar]
- Petrus DS, Fabel K, Kronenberg G, Winter C, Steiner B, Kempermann G. NMDA and benzodiazepine receptors have synergistic and antagonistic effects on precursor cells in adult hippocampal neurogenesis. Eur J Neurosci. 2009;29:244–52. doi: 10.1111/j.1460-9568.2008.06579.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–91. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Post RM. Role of BDNF in bipolar and unipolar disorder: clinical and theoretical implications. J Psychiatr Res. 2007;41:979–90. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Blanchard JA, 2nd, Mayer S, Gibson DA, Littleton JM. In vitro effects of ethanol withdrawal and spermidine on viability of hippocampus from male and female rat. Alcohol Clin Exp Res. 2000;24:1855–61. [PubMed] [Google Scholar]
- Prendergast MA, Mulholland PJ. Glucocorticoid and polyamine interactions in the plasticity of glutamatergic synapses that contribute to ethanol-associated dependence and neuronal injury. Addict Biol. 2012;17:209–23. doi: 10.1111/j.1369-1600.2011.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rice AC, Bullock MR, Shelton KL. Chronic ethanol consumption transiently reduces adult neural progenitor cell proliferation. Brain Res. 2004;1011:94–8. doi: 10.1016/j.brainres.2004.01.091. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, et al. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- Robin RW, Long JC, Rasmussen JK, Albaugh B, Goldman D. Relationship of binge drinking to alcohol dependence, other psychiatric disorders, and behavioral problems in an american indian tribe. Alcohol Clin Exp Res. 1998;22:518–23. [PubMed] [Google Scholar]
- Russo I, Barlati S, Bosetti F. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J Neurochem. 2011;116:947–56. doi: 10.1111/j.1471-4159.2010.07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–84. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Monteiro MG. Alcoholism, anxiety and depression. Brit J Addict. 1988;83:1373–1380. doi: 10.1111/j.1360-0443.1988.tb02551.x. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–47. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Sperk G, Hamilton T, Colmers WF. Neuropeptide Y in the dentate gyrus. In: Helen ES, editor. Progress in Brain Research. Elsevier; 2007. pp. 285–97. [DOI] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2012;18:203–13. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Duka T. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 2008;363:3169–79. doi: 10.1098/rstb.2008.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, Hodge CW. Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology. 2009;34:1209–22. doi: 10.1038/npp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SSD, Teixeira CM, DeVito LM, Zaslavsky K, Josselyn SA, Lozano AM, et al. Stimulation of Entorhinal Cortex Promotes Adult Neurogenesis and Facilitates Spatial Memory. J Neurosci. 2011;31:13469–84. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19:110–22. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology. 2005;180:583–94. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci U S A. 2010;107:11104–9. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferro P, Vega MD, Evrard SG, Ramos AJ, Brusco A. Alcohol exposure during adulthood induces neuronal and astroglial alterations in the hippocampal CA-1 area. Ann N Y Acad Sci. 2002;965:334–42. doi: 10.1111/j.1749-6632.2002.tb04175.x. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Rage F, Givalois L, Dingeon P, Arancibia S, Beauge F. Effects of alcohol on brain-derived neurotrophic factor mRNA expression in discrete regions of the rat hippocampus and hypothalamus. J Neurosci Res. 2001;63:200–208. doi: 10.1002/1097-4547(20010115)63:2<200::AID-JNR1012>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Tateno M, Saito T. Biological studies on alcohol-induced neuronal damage. Psychiatry Investig. 2008;5:21–7. doi: 10.4306/pi.2008.5.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. Adult neural stem cells, neurogenic niches, and cellular therapy. Stem Cell Rev. 2006;2:213–9. doi: 10.1007/s12015-006-0049-0. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–9. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–15. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Udomuksorn W, Mukem S, Kumarnsit E, Vongvatcharanon S, Vongvatcharanon U. Effects of alcohol administration during adulthood on parvalbumin and glial fibrillary acidic protein immunoreactivity in the rat cerebral cortex. Acta Histochem. 2011;113:283–9. doi: 10.1016/j.acthis.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004;14:365–71. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–92. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangipuram SD, Lyman WD. Ethanol alters cell fate of fetal human brain-derived stem and progenitor cells. Alcohol Clin Exp Res. 2010;34:1574–83. doi: 10.1111/j.1530-0277.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–8. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Wainwright DA, Xin J, Mesnard NA, Beahrs TR, Politis CM, Sanders VM, et al. Exacerbation of facial motoneuron loss after facial nerve axotomy in CCR3-deficient mice. ASN Neuro. 2009;1:e00024. doi: 10.1042/AN20090017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DW, Barnes DE, Zornetzer SF, Hunter BE, Kubanis P. Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science. 1980;209:711–3. doi: 10.1126/science.7394532. [DOI] [PubMed] [Google Scholar]
- Walter J, Honsek SD, Illes S, Wellen JM, Hartung HP, Rose CR, et al. A new role for interferon gamma in neural stem/precursor cell dysregulation. Mol Neurodegener. 2011;6:18. doi: 10.1186/1750-1326-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Houchi H, Barbier E, Pierrefiche O, Vilpoux C, Ledent C, et al. The lack of CB1 receptors prevents neuroadapatations of both NMDA and GABAA receptors after chronic ethanol exposure. J Neurochem. 2007;102:741–52. doi: 10.1111/j.1471-4159.2007.04577.x. [DOI] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–59. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, et al. The neurocognitive effects of alcohol on adolescents and college students. Prev Med. 2005;40:23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and Functional Implications of Adult Neurogenesis. Cell. 2008;132:645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Balaraman Y, Teng M, Liu Y, Singh RP, Nephew KP. Alcohol alters DNA methylation patterns and inhibits neural stem cell differentiation. Alcohol Clin Exp Res. 2011;35:735–46. doi: 10.1111/j.1530-0277.2010.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Crews FT. Inflammasome-IL-1beta Signaling Mediates Ethanol Inhibition of Hippocampal Neurogenesis. Front Neurosci. 2012;6:30. doi: 10.3389/fnins.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, et al. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37:939–49. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]


