Abstract
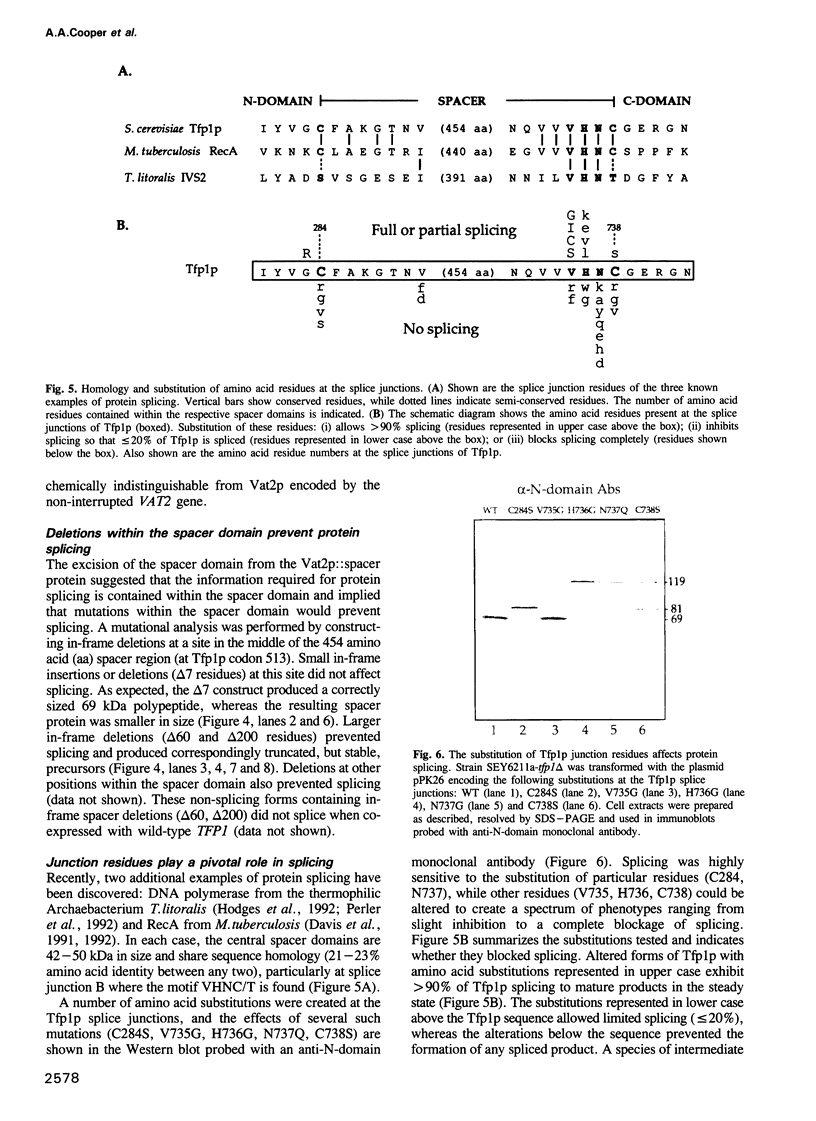
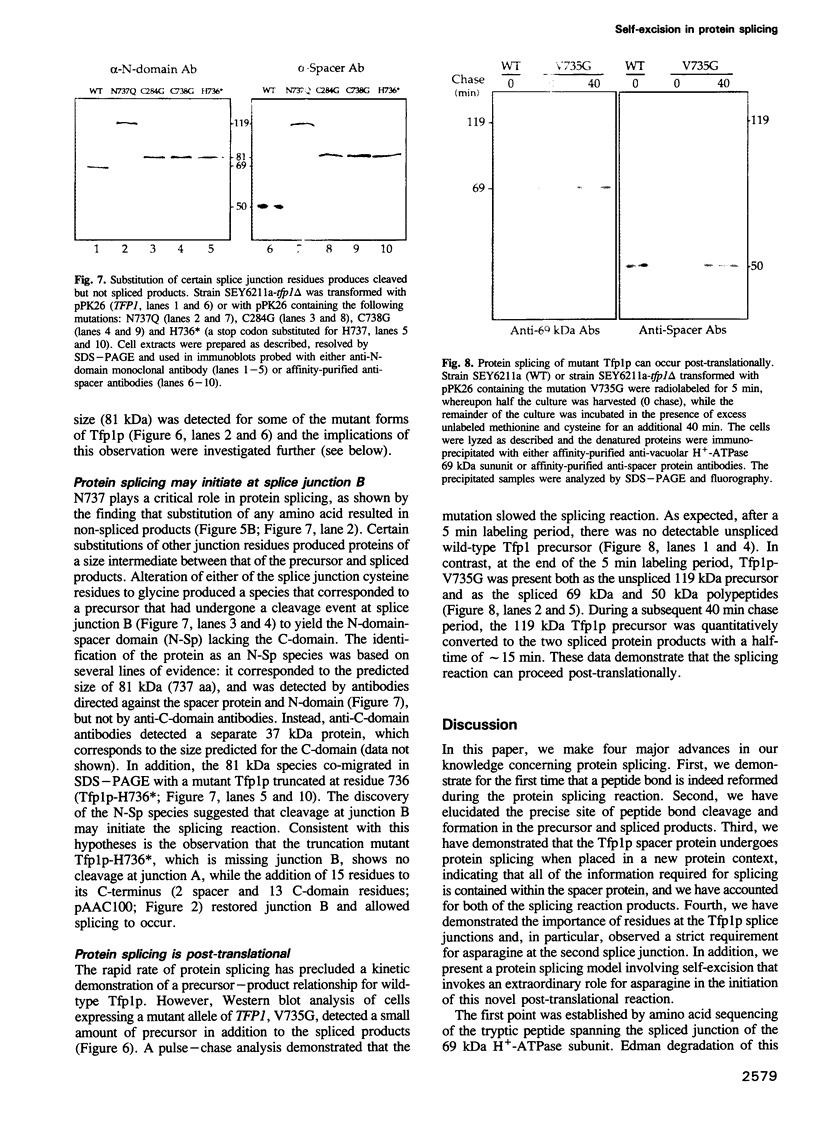
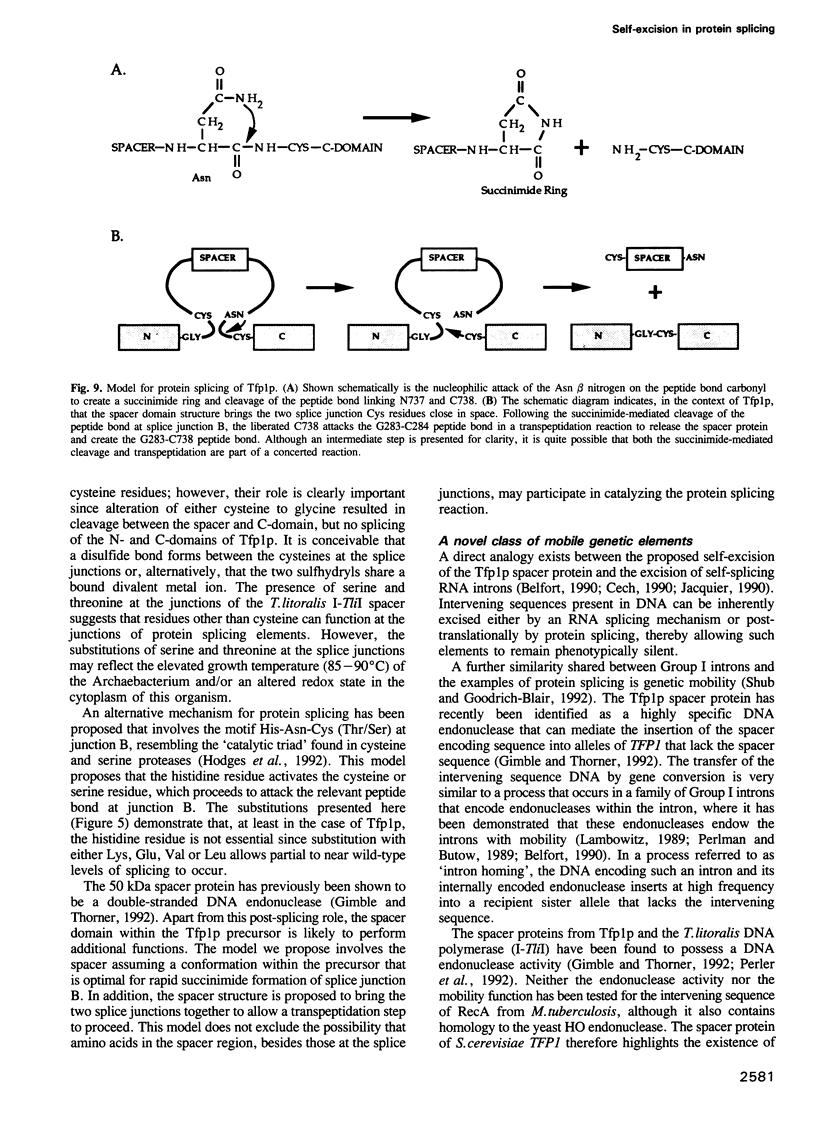
Protein splicing is the protein analogue of RNA splicing in which the central portion (spacer) of a protein precursor is excised and the amino- and carboxy-terminal portions of the precursor reconnected. The yeast Tfp1 protein undergoes a rapid protein splicing reaction to yield a spliced 69 kDa polypeptide and an excised 50 kDa spacer protein. We have demonstrated that the 69 kDa species arises by reformation of a bona fide peptide bond. Deletion analyses indicate that only sequences in the central spacer protein of the Tfp1 precursor are critical for the protein splicing reaction. A fusion protein in which only the Tfp1 spacer domain was inserted into an unrelated protein also underwent efficient splicing, demonstrating that all of the information required for protein splicing resides within the spacer domain. Alteration of Tfp1p splice junction residues blocked or kinetically impaired protein splicing. A protein splicing model is presented in which asparagine rearrangement initiates the self-excision of the spacer protein from the Tfp1 precursor. The Tfp1 spacer protein belongs to a new class of intervening sequences that are excised at the protein rather than the RNA level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort M. Phage T4 introns: self-splicing and mobility. Annu Rev Genet. 1990;24:363–385. doi: 10.1146/annurev.ge.24.120190.002051. [DOI] [PubMed] [Google Scholar]
- Bowles D. J., Marcus S. E., Pappin D. J., Findlay J. B., Eliopoulos E., Maycox P. R., Burgess J. Posttranslational processing of concanavalin A precursors in jackbean cotyledons. J Cell Biol. 1986 Apr;102(4):1284–1297. doi: 10.1083/jcb.102.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J., Pappin D. J. Traffic and assembly of concanavalin A. Trends Biochem Sci. 1988 Feb;13(2):60–64. doi: 10.1016/0968-0004(88)90030-8. [DOI] [PubMed] [Google Scholar]
- Bremer M. C., Gimble F. S., Thorner J., Smith C. L. VDE endonuclease cleaves Saccharomyces cerevisiae genomic DNA at a single site: physical mapping of the VMA1 gene. Nucleic Acids Res. 1992 Oct 25;20(20):5484–5484. doi: 10.1093/nar/20.20.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington D. M., Auffret A., Hanke D. E. Polypeptide ligation occurs during post-translational modification of concanavalin A. Nature. 1985 Jan 3;313(5997):64–67. doi: 10.1038/313064a0. [DOI] [PubMed] [Google Scholar]
- Cech T. R. Self-splicing of group I introns. Annu Rev Biochem. 1990;59:543–568. doi: 10.1146/annurev.bi.59.070190.002551. [DOI] [PubMed] [Google Scholar]
- Davis E. O., Jenner P. J., Brooks P. C., Colston M. J., Sedgwick S. G. Protein splicing in the maturation of M. tuberculosis recA protein: a mechanism for tolerating a novel class of intervening sequence. Cell. 1992 Oct 16;71(2):201–210. doi: 10.1016/0092-8674(92)90349-h. [DOI] [PubMed] [Google Scholar]
- Davis E. O., Sedgwick S. G., Colston M. J. Novel structure of the recA locus of Mycobacterium tuberculosis implies processing of the gene product. J Bacteriol. 1991 Sep;173(18):5653–5662. doi: 10.1128/jb.173.18.5653-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foury F. The 31-kDa polypeptide is an essential subunit of the vacuolar ATPase in Saccharomyces cerevisiae. J Biol Chem. 1990 Oct 25;265(30):18554–18560. [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- Geiger T., Clarke S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem. 1987 Jan 15;262(2):785–794. [PubMed] [Google Scholar]
- Gimble F. S., Thorner J. Homing of a DNA endonuclease gene by meiotic gene conversion in Saccharomyces cerevisiae. Nature. 1992 May 28;357(6376):301–306. doi: 10.1038/357301a0. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W. Protein carpentry. Curr Biol. 1991 Apr;1(2):71–73. doi: 10.1016/0960-9822(91)90280-a. [DOI] [PubMed] [Google Scholar]
- Hirata R., Anraku Y. Mutations at the putative junction sites of the yeast VMA1 protein, the catalytic subunit of the vacuolar membrane H(+)-ATPase, inhibit its processing by protein splicing. Biochem Biophys Res Commun. 1992 Oct 15;188(1):40–47. doi: 10.1016/0006-291x(92)92347-z. [DOI] [PubMed] [Google Scholar]
- Hirata R., Ohsumk Y., Nakano A., Kawasaki H., Suzuki K., Anraku Y. Molecular structure of a gene, VMA1, encoding the catalytic subunit of H(+)-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem. 1990 Apr 25;265(12):6726–6733. [PubMed] [Google Scholar]
- Ho M. N., Hill K. J., Lindorfer M. A., Stevens T. H. Isolation of vacuolar membrane H(+)-ATPase-deficient yeast mutants; the VMA5 and VMA4 genes are essential for assembly and activity of the vacuolar H(+)-ATPase. J Biol Chem. 1993 Jan 5;268(1):221–227. [PubMed] [Google Scholar]
- Hodges R. A., Perler F. B., Noren C. J., Jack W. E. Protein splicing removes intervening sequences in an archaea DNA polymerase. Nucleic Acids Res. 1992 Dec 11;20(23):6153–6157. doi: 10.1093/nar/20.23.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Hwang C., Sinskey A. J., Lodish H. F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992 Sep 11;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Jacquier A. Self-splicing group II and nuclear pre-mRNA introns: how similar are they? Trends Biochem Sci. 1990 Sep;15(9):351–354. doi: 10.1016/0968-0004(90)90075-m. [DOI] [PubMed] [Google Scholar]
- Kane P. M., Yamashiro C. T., Wolczyk D. F., Neff N., Goebl M., Stevens T. H. Protein splicing converts the yeast TFP1 gene product to the 69-kD subunit of the vacuolar H(+)-adenosine triphosphatase. Science. 1990 Nov 2;250(4981):651–657. doi: 10.1126/science.2146742. [DOI] [PubMed] [Google Scholar]
- Nelson H., Mandiyan S., Nelson N. A conserved gene encoding the 57-kDa subunit of the yeast vacuolar H+-ATPase. J Biol Chem. 1989 Jan 25;264(3):1775–1778. [PubMed] [Google Scholar]
- Perler F. B., Comb D. G., Jack W. E., Moran L. S., Qiang B., Kucera R. B., Benner J., Slatko B. E., Nwankwo D. O., Hempstead S. K. Intervening sequences in an Archaea DNA polymerase gene. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5577–5581. doi: 10.1073/pnas.89.12.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman P. S., Butow R. A. Mobile introns and intron-encoded proteins. Science. 1989 Dec 1;246(4934):1106–1109. doi: 10.1126/science.2479980. [DOI] [PubMed] [Google Scholar]
- Raymond C. K., O'Hara P. J., Eichinger G., Rothman J. H., Stevens T. H. Molecular analysis of the yeast VPS3 gene and the role of its product in vacuolar protein sorting and vacuolar segregation during the cell cycle. J Cell Biol. 1990 Sep;111(3):877–892. doi: 10.1083/jcb.111.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Nothwehr S. F., Stevens T. H. Membrane protein sorting in the yeast secretory pathway: evidence that the vacuole may be the default compartment. J Cell Biol. 1992 Oct;119(1):69–83. doi: 10.1083/jcb.119.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C., Stevens T. H., Schlesinger M. J. Inhibitory effects of HSP70 chaperones on nascent polypeptides. Protein Sci. 1992 Aug;1(8):980–985. doi: 10.1002/pro.5560010803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. K., Wagner R., Feinstein S., Kanik-Ennulat C., Neff N. A dominant trifluoperazine resistance gene from Saccharomyces cerevisiae has homology with F0F1 ATP synthase and confers calcium-sensitive growth. Mol Cell Biol. 1988 Aug;8(8):3094–3103. doi: 10.1128/mcb.8.8.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shub D. A., Goodrich-Blair H. Protein introns: a new home for endonucleases. Cell. 1992 Oct 16;71(2):183–186. doi: 10.1016/0092-8674(92)90345-d. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T. H., Rothman J. H., Payne G. S., Schekman R. Gene dosage-dependent secretion of yeast vacuolar carboxypeptidase Y. J Cell Biol. 1986 May;102(5):1551–1557. doi: 10.1083/jcb.102.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violand B. N., Schlittler M. R., Toren P. C., Siegel N. R. Formation of isoaspartate 99 in bovine and porcine somatotropins. J Protein Chem. 1990 Feb;9(1):109–117. doi: 10.1007/BF01024992. [DOI] [PubMed] [Google Scholar]
- Voorter C. E., de Haard-Hoekman W. A., van den Oetelaar P. J., Bloemendal H., de Jong W. W. Spontaneous peptide bond cleavage in aging alpha-crystallin through a succinimide intermediate. J Biol Chem. 1988 Dec 15;263(35):19020–19023. [PubMed] [Google Scholar]
- Yamashiro C. T., Kane P. M., Wolczyk D. F., Preston R. A., Stevens T. H. Role of vacuolar acidification in protein sorting and zymogen activation: a genetic analysis of the yeast vacuolar proton-translocating ATPase. Mol Cell Biol. 1990 Jul;10(7):3737–3749. doi: 10.1128/mcb.10.7.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]