Abstract
Developmental exposure to BPA adversely affects reproductive function. In sheep, prenatal BPA treatment induces reproductive neuroendocrine defects, manifested as LH excess and dampened LH surge and perturbs early ovarian gene expression. In this study we hypothesized that prenatal BPA treatment will also disrupt ovarian follicular dynamics. Pregnant sheep were treated from days 30 to 90 of gestation with 3 different BPA doses (0.05, 0.5, or 5 mg/kg BW/day). All female offspring were estrus synchronized and transrectal ultrasonography was performed daily for 22 days to monitor ovarian follicular and corpora lutea dynamics. Blood samples were collected to assess hormonal preovulatory changes and luteal progesterone dynamics. Statistical analysis revealed that the time interval between the estradiol rise and the preovulatory LH surge was shortened in the BPA-treated females. None of the three BPA doses had an effect on corpora lutea, progestogenic cycles, and mean or duration of ovulatory and non-ovulatory follicles. However, differences in follicular count trajectories were evident in all three follicular size classes (2–3 mm, 4–5 mm, and ≥ 6 mm) of prenatal BPA-treated animals compared to controls. Number of follicular waves tended also to be more variable in the prenatal BPA-treated groups ranging from 2 to 5 follicular waves per cycle, while this was restricted to 3 to 4 waves in control females. These changes in ovarian follicular dynamics coupled with defects in time interval between estradiol rise and preovulatory LH release are likely to lead to subfertility in prenatal BPA-treated females.
Keywords: Infertility, follicular dynamics, bisphenol A
Introduction
Inappropriate exposure to native or environmental steroids during early development can induce long term deleterious effects at the reproductive level. Common sources of such abnormal exposures, in addition to excess native steroid production from disease states, are the use of anabolic steroids (Hartgens and Kuipers, 2004), contraceptive pills (Waller et al., 2010), or unintended exposure to environmental steroid mimics through food sources or industrial pollutants (Hotchkiss et al., 2008). As such, endocrine disrupting chemicals (EDCs) from the environment are a looming threat to proper differentiation of organ systems. One of the most controversial EDC is bisphenol A (BPA), a component of polycarbonate plastic and epoxy resins, which is used in the manufacture of many consumer products (vom Saal et al., 2005). Humans are exposed ubiquitously to BPA not only via diet, but also due to its presence in dust, air, and water (Vandenberg et al., 2013). BPA has been found to signal through estrogen receptors (Nagel et al., 1999). Potential health risks from exposure to BPA are evident from studies reporting the presence of BPA in human maternal circulation (Ranjit et al., 2010), amniotic and placental fluids (Ikezuki et al., 2002), breast milk (Sun et al., 2004), and neonates (Calafat et al., 2009), representing exposures during critical early developmental windows.
Experimentally, pre-and perinatal BPA exposures below the lowest-observed-adverse-effect level (LOAEL) dose have demonstrated estrogen-like effects on reproductive function in rodents (Durando et al., 2007, Newbold et al., 2007, Howdeshell et al., 1999). Information regarding the effects of prenatal exposure to BPA on reproductive function comes mostly from altricial species (Markey et al., 2003, Ryan et al., 2010, Lawson et al., 2011, Hunt et al., 2012a, 2012b, Peretz et al., 2014, EFSA 2014)requiring validation from precocial models to enable human translation. Our studies with sheep found that prenatal exposure to BPA leads to LH surge disruption (Savabieafsahani et al., 2006) and early ovarian disruption, manifested as changes in expression of genes encoding key steroidogenic enzymes and microRNAs, regulators of gene function (Veiga-Lopez et al., 2013). The consequences of these early ovarian changes in terms of adult ovarian and cyclic function remain to be determined. Rodent studies carried out in vivo(Molina et al., 2013) and in vitro(Peretz et al., 2011) have reported that BPA can lead to follicular atresia.
Interestingly, the changes in early ovarian gene expression parallel disruptions seen with prenatal testosterone (T)-treated sheep (Luense et al., 2011). Several gene disruptions seen in prenatal T-treated animals appear to be estrogen-mediated stemming conceivably from aromatization of T to estrogen. Similar to the prenatal T-treated model, prenatal BPA-treated sheep females are born underweight and manifest LH excess (Savabiesfahani et al., 2006, Manikkam et al., 2004, Padmanahban and Veiga -Lopez, 2011). Because prenatal BPA-treated animals manifested dampened LH surges similar to prenatal T-treated sheep (Savabiesfahani et al., 2006), we predicted that prenatal BPA-treated animals will also manifest similar periovulatory disruptions. Considering that they share several phenotypic attributes and the early ovarian changes are similar, we also predicted that the adult ovarian phenotype of prenatal BPA-treated sheep will be similar to the prenatal T-treated sheep, which manifest in creased follicular recruitment, follicular persistence, and luteal dysfunction (Padmanabhan and Veiga -Lopez, 2011). Therefore, the aim of this study was to gain a comprehensive understanding of the impact of prenatal BPA treatment on adult follicular and periovulatory hormonal dynamics in sheep, a precocial species.
Material and Methods
Animals and prenatal treatment
Prenatal treatments
The study was conducted at the University of Michigan Sheep Research Facility (Ann Arbor, MI; 42º 18’N). All procedures were approved by the Institutional Animal Care and Use Committee of the University of Michigan and are consistent with the National Institutes of Health Guide for Use and Care of Animals. Animals were housed in an open free-living area with access to shelter at all times. Adult Suffolk breed sheep, 3 to 5 years old and blocked by maternal weight and body score, were bred to generate control and BPA-treated animals. Aureomycin crumbles (chlortetracycline: 250 mg/ewe/day) were given to prevent abortion. The day of mating was determined by visual confirmation of paint markings left on the rumps of ewes by the raddled rams. Gestational BPA treatment consisted of daily scinjections of one of the following three BPA doses:0.05, 0.5, or 5 mg/kg/day (B low, Bmed, Bhigh, respectively) of BPA (purity ≥99%, cat# 239658; Aldrich Chemical Co., Milwaukee, WI) in corn oil from days 30 through 90 of gestation (term: ~ 147 days). The chosen window of exposure corresponds with key events in the hypothalamic and ovarian developmental trajectory of the female sheep fetus. The developmental trajectory of these systems in this precocial species is similar to both hypothalamic and ovarian developmental trajectories of humans (for review see Padmanabhan and Veiga -Lopez, 2013), thus enhancing the translational relevance of the current study. In brief, gestational day 30 in sheep coincides with the initiation of gonadal differentiation and gestational day 90 with the completion of primordial follicle formation. Appearance of GnRH neurons in the hypothalamus and detection of circulating LH also occurs during this window of exposure. Control (C) mothers received vehicle. Internal dose of BPA achieved in umbilical arterial samples using the 0.5 mg/kg/day dose has been already published (Veiga-Lopez et al., 2013) and targeted to produce maternal blood levels of BPA approaching the median level of BPA measured in maternal circulation of US women (Padmanabhan et al., 2008) and urine (Calafat et al., 2009). Husbandry and nutrition of pregnant sheep and newborn lambs have been published (Manikkam et al., 2004). Only one female offspring from each dam was utilized, when twin pregnancies were involved. Number of female lambs included in the C, Blow, Bmed, Bhigh groups was 7, 11, 12, and 6, respectively. Lambs were weaned at ~8 weeks of age and fed a pelleted diet (Shur-Gain, Elma, NY) comprised of 3.6 MCal/kg digestible energy and 18% crude protein. They were fed ad libitum until they attained ~40 kg of body weight, when they were switched to a diet with 15% crude protein. Adult sheep were fed a ration consisting of 2.3 MCal/kg digestible energy and 11.3% crude protein.
Experimental design
The study was conducted during the second breeding season when females were ~19 months old. All females were administered two prostaglandin F2α(PGF 2α, 5mg/ml; Lutalyse®, Pfizer Animal Health, MI) injections, im, 11 days apart to synchronize estrus. Beginning with the second PGF2α injection (0 h) blood samples were obtained every 2 hours for 5 consecutive days to assess preovulatory changes in LH and daily through day 28 to monitor plasma progesterone (P4) concentrations, which were used to confirm the presence of a corpus luteum (CL) and its function. Samples were also assayed for estradiol every 4 hours until 4 hours after the preovulatory LH surge. Plasma samples were stored at −20 °C until assayed.
Ultrasonographic assessment of ovarian status
Starting the day of the second PGF2α injection, a subset of females (7, 9, 10, and 6 for groups C, Blow, Bmed, and Bhigh, respectively) daily ultrasonographic examinations of the ovaries were carried out for 21 days, to monitor follicular dynamics. Ultrasonography was carried out using a scanner (Aloka SSD-900V, Aloka Co. Ltd., Wallington, CT) fitted to a 7.5 MHz linear array transducer adapted for transrectal examinations. Details of ultrasound scanning have been previously described (Veiga-Lopez et al., 2014). In brief, sheep were placed in dorsal recumbence and the probe inserted in the rectum with the transducer orientated perpendicularly to the abdomen wall to locate both ovaries. Follicles with an antral diameter of ≥ 2 mm and CL were identified and measured. The sizes and relative positions of the follicles and CL were sketched on ovarian charts and were used to assess daily changes in follicular dynamics. Digital video images of the ovarian scans were recorded (DCR-TRV33; Sony Corp. of America, New York, NY) to document follicular and luteal changes. Follicles were tracked across successive days using the CL and/or the largest follicles as landmarks.
Hormone assays
Plasma LH was measured in duplicate in all samples using a validated assay (Niswender et al., 1969) and expressed in terms of NIH-LH-S12. The assay sensitivity was 0.09 ± 0.01 ng/ml (mean ± SEM; n =14 assays) and intra -assay CV, based on the three quality control pools measuring 23.2 ± 0.3, 13.1 ± 0.2, and 8.9 ± 0.2 ng/ml were 5.7, 5.8, and 5.1%, respectively. The inter-assay CVs for the same quality control pools were 4.8, 4.9, and 8.8%, respectively. Plasma estradiol concentrations were assayed in duplicate diethylether extracts of 200μl plasma using a commercially available estradiol radioimmunoassay (Estradiol MAIA, Radim, Rome, Italy) modified and validated for use in sheep as previously described (Evans et al., 1994). The assay sensitivity was 0.24 ± 0.07 pg/ml (mean ± SEM, n =7 assays) and intra -assay CV, based on the two quality control pools measuring 6.7 ± 0.3 and 23.2 ± 1.1 ng/ml were 11.2 and 3.5%, respectively. The inter-assay CVs for the same quality control pools were 12.6 and 12.8%, respectively. Plasma P4 concentrations were measured in daily samples using a solid phase radioimmunoassay kit (Coat-A-Count P® Diagnostic Products Corp., Los Angeles, CA) as previously described (Padmanabhan et al., 1995). All the samples were assayed in duplicate 100μl aliquots. The assay sensitivity was 0.002 ± 0.001 ng/ml (mean ± SEM of 5 assays) and intra-assay coefficient of variation (CV), based on the two quality control pools measuring 1.6 ± 0.03 and 13.4 ± 0.4 ng/ml were 6.6 and 5.0%, respectively. The inter-assay CVs for the same quality control pools were 4.0 and 7.5%, respectively.
Statistical analysis
Hormonal analysis
Temporal changes in LH and estradiol were modeled using previously published smoothing splines (Veiga-Lopez et al., 2008), which allows capture of various shapes of follicular developmental trajectories. It also takes into account correlation between repeated measurements within subjects. For estradiol, where alternate samples were used and data then aligned to LH peak for analyses, smoothing splines allows comparing trajectories with such sample offsets. LH peak and estradiol concentrations were defined as the time points at which the smooth curves were at their maximum values.
Corpora lutea and P4 analysis
Data from CL were collected using three consecutive reproductive cycles: presence of CL when scanning began (regressing CLs due to PGF2α), CL formed from ovulatory follicles during the first follicular phase (1st cycle), and CL detected a week after cessation of daily ultrasonography (2nd cycle). Therefore, information of a complete luteal phase was solely available for the 1st cycle CLs. The difference in counts of CLs in all three cycles was assessed using Fisher’s exact test. The difference in size and duration of the CL among groups was tested using analysis of variance of repeated measures. Pearson correlation between circulating P4 levels and CL size for each group was calculated. Fisher z statistics was used to compare whether the correlation between P4 level and CL differed among groups. Time lag between CL detection and P4 was tested using the Fisher exact test. All data are presented as mean ± SE. Significance was defined as P < 0.05. All analyses were performed using SAS for Windows (release 9.1.3; SAS Institute Inc., Cary, NC) and SPSS for Windows release 17.0.0.
Number, diameter, and duration of follicles
Follicles were classified into three categories: ≤2–3 mm, ≥4–5 mm, and ≥6 mm in diameter. Mantel-Haenszel Chi-square test was used to compare follicle size distribution among groups. To test the difference in follicular counts among all groups a linear mixed effect model was used. Bonferroni adjustment was used to adjust for multiple comparisons. Assessment of follicular survival was evaluated in follicles that grew to ≥ 3 mm in diameter and were present on the ovary for ≥ 2 days. The duration was calculated as the difference between the day that was first observed at 3 mm size until its larger size was achieved and then back to the 3 mm size. For larger follicles detected during the first day of scanning, duration was set as right censored and infered based on the assumption that follicles grow and regress at a constant rate of approximately 1 mm/day (Ginther et al., 1995). A linear mixed effect model was used to test differences in the duration of follicles. To compare follicular count changes throughout the scanning period a mixed effects model with B-splines was used to fit a non-linear trajectory of follicle counts for each of the three size categories (2–3 mm, 4–5 mm, ≥6 mm). For all three models, B-splines of third degree functions and six equally spaced internal knots were used. Likelihood ratio tests were conducted to first examine whether there are significant global differences between the four treatment groups. When a statistical significance was evident from the global test, post-hoc likelihood ratio tests were conducted to compare each of the three BPA treated group with the control group..
Ovulatory follicles and follicular wave analysis
The percentage of females that ovulated and the diameter and duration of the ovulatory follicles were calculated as described above for non-ovulatory follicles. The duration of ovulatory follicles was calculated from the time they were 3 mm until ovulation, which was confirmed by presence of a new CL with a corresponding increase in plasma concentrations of P4. A follicular wave was defined as a single follicle or a group of follicles that grew from 2–3 mm to 5 mm in size. Follicular emergence was limited to a 24 h period. Days of follicular emergence were procured relative to the day of ovulation. For each of the identified waves, all follicular data were aligned to the day of emergence. A linear mixed effect model was used to assess differences in the number, timing, and duration of the waves and the number of follicles per wave. Bonferroni adjustment was used to adjust for multiple comparisons. For the number of waves, a power analysis was carried out using the effect size test (Nakagawa and Cuthill, 2007), which allows comparison of the means with respect to the magnitude of difference. Statistical results are reported as a Cohen’s d value, and 0.2, 0.5, and 0.8 are considered as small, medium, and large effect sizes, respectively.
Results
Hormonal dynamics
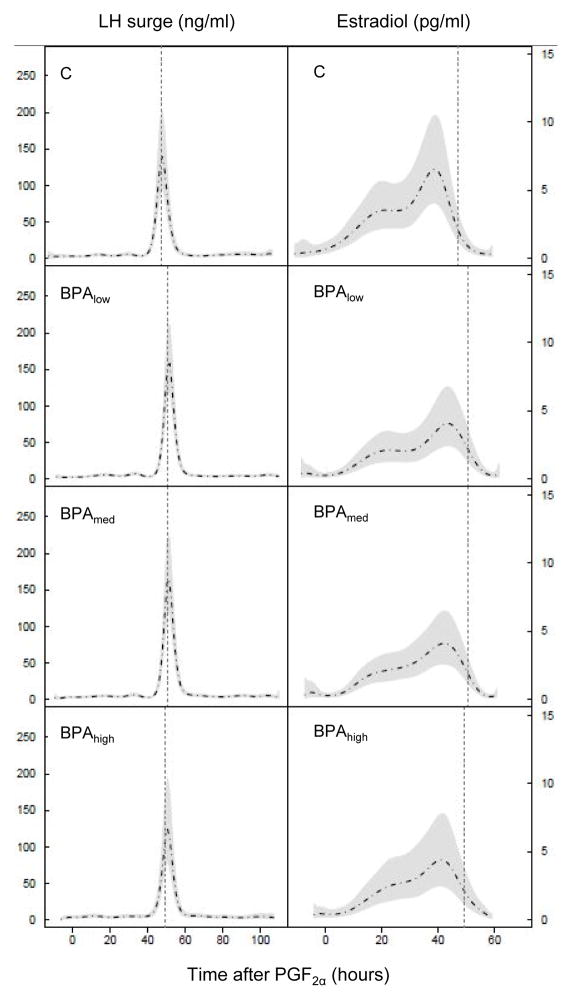
All control and BPA-treated females had the expected cyclic hormonal follicular changes after the estrous synchronization protocol, with a rise in circulating estradiol and subsequent preovulatory LH surge. Changes in circulating estradiol in control and BPA-treated females are shown in Figure 1 and summarized in Table 1. Preovulatory estradiol rise occurred ~4 hours later in BPA-treated females compared to the control group, but this did not reach statistical significance. The time of the preovulatory LH surge was also slightly delayed in BPA-treated females, but not significantly. The time interval between the estradiol rise and the preovulatory LH surge was shortened in the BPA-treated groups compared to the control females and reached significance when all BPA-treated groups were merged (Control: 6.9 ± 1.2 and all BPA: 5.1 ± 0.6 h ; P<0.05).
Figure 1.
Mean circulating patterns of two-hourly samples of LH (ng/ml, left panels) and estradiol (pg/ml, right panels) in control and BPA-treated females (all three doses) relative to PGF2α administration. The vertical dotted line represents mean time of the preovulatory LH surge, Shaded area in panels represents 95% confidence interval. For LH the mean patterns and SE are from observations at each time point. For estradiol, both the mean and SE are based on the fit of the semiparametric mixed model with regression splines. B low, Bmed, B high represent prenatal BPA doses at 0.05, 0.5, and 5 mg/kg/day, respectively.
Table 1.
Summary characteristics of preovulatory hormonal dynamics. Mean (± SE) time and amplitude of the preovulatory estradiol rise (pg/ml) and LH surge (ng/ml) relative to the second PGF2α injection. Data for all three BPA groups combined is shown on the far right column.
| Control | BPAlow (0.05 mg/kg/day) | BPAmed (0.5 mg/kg/day) | BPAhigh (5 mg/kg/day) | All BPA | |
|---|---|---|---|---|---|
| N | 7 | 11 | 12 | 6 | 29 |
| Time of estradiol peak (h after PGF2α) | 40.3 ± 1.9 | 44.4 ± 1.8 | 45.3 ± 1.5 | 44.7 ± 1.6 | 44.8 ± 1.9 |
| Estradiol peak (pg/ml) | 6.3 ± 1.1 | 5.9 ± 0.8 | 5.2 ± 0.7 | 5.3 ± 0.9 | 5.5 ± 0.4 |
| Time of LH surge (h after PGF2α) | 47.1 ± 2.5 | 50.0 ± 1.6 | 50.5 ± 1.3 | 48.7 ± 1.5 | 49.9 ± 0.8 |
| LH surge peak (ng/ml) | 273.9 ± 43.7 | 293.4 ± 20.4 | 305.5 ± 19.4 | 304.7 ± 33.9 | 300.7 ± 12.7 |
| Time interval between peak estradiol and LH surge peak (h after PGF2α) | 6.9 ± 1.2 | 5.6 ± 1.0 | 5.2 ± 0.8 | 4.0 ± 1.7 | 5.1 ± 0.6* |
Asterisk denotes significant difference from the control group (P < 0.05).
Luteal P4 dynamics
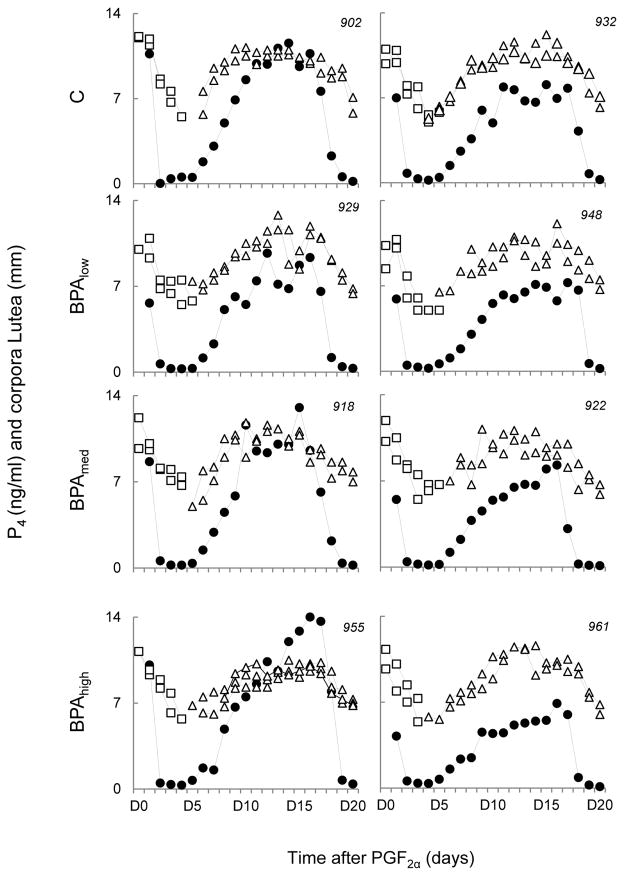
Representative profiles of P4 and CLs during the period of ultrasonographic evaluation are shown in Figure 2. No statistical differences were found in the number and size of CLs among all 4 groups in all three cycles evaluated; namely, regressing CLs after PGF2α administration, CLs for med from ovulatory follicles during the first follicular phase (first cycle), and CLs detected a week after cessation of daily ultrasonography (second cycle). As expected, P4 concentrations were positively correlated with CL size (r range: 0.73–0.8; P < 0.0001, for all groups), but Fisher z statistics did not find a significant difference among the correlation coefficients. Time lag (C: 0.29 ± 0.47, Blow: 0.0 ± 0.33, Bmed: 0.22 ± 0.4, and Bhigh: 0.67 ± 0.49 days) between CL detection by ultrasonography and P4 detection over 0.5 ng/ml was also similar among all four groups.
Figure 2.
Circulating P4 concentrations (filled circles), follicular size profiles (mm) of regressing (white squares) and first cycle (white triangles) corpora lutea in two representative females from each of control, Blow, Bmed, and Bhigh group relative to PGF2α administration. Each graph represents a single female (animal identification in top right corner). Multiple white square-or white triangle connected lines depict CLs from multiple ovulations. Blow, Bmed, Bhigh represent prenatal BPA doses at 0.05, 0.5, and 5 mg/kg/day, respectively.
Follicular number and follicular wave analysis
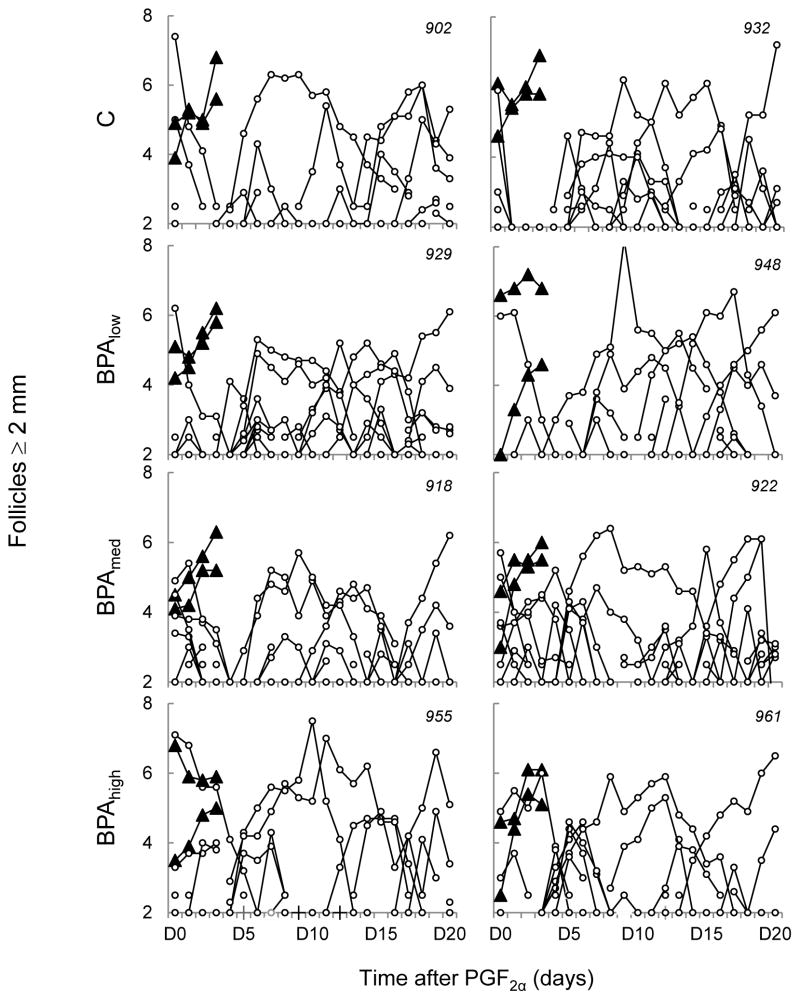
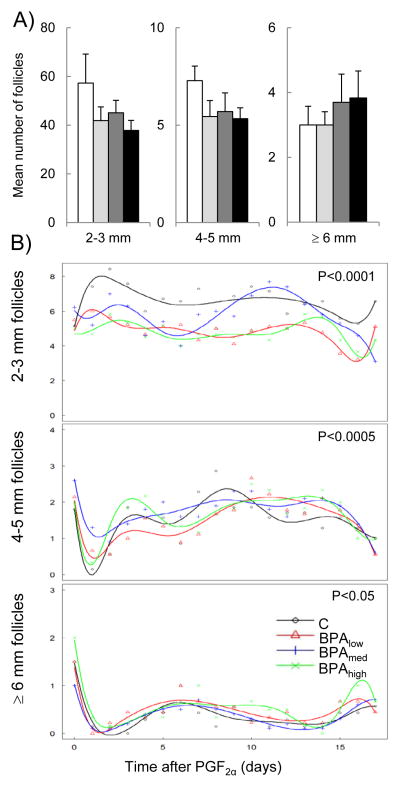
Representative follicular profiles of all ovulatory and non-ovulatory follicles monitored via transrectal ultrasonography are shown in Figure 3. Mean number or duration of ovulatory follicles (C: 3.9 ± 0.1, Blow: 3.9 ± 0.1, Bmed: 4.1 ± 0.3, and Bhigh: 4.0 ± 0.0 days, n.s.) was also similar among all four groups. No differences were found in the mean number or duration of non-ovulatory follicles in any follicular size category among all four groups studied (Figure 4, panel A). However, significant differences in follicular count trajectories were found during the course of the scanning period among all 4 groups in all three follicular size classes (2–3 mm, P < 0.0001; 4–5 mm, P < 0.001; and ≥6 mm, P < 0.05 ; Figure 4, panel B). Specifically, post hoc analyses of the 2–3 mm follicular count trajectory found significant differences between C and each of the 3 BPA dose groups (C vs. Blow[P = 0. 01], C vs. Bmed [P = 0.001], and C vs. Bhigh [P = 0.006]. Similarly, the trajectory of the 4–5 mm follicular count was also significantly different in C vs. Blow(P = 0.006), C vs. Bmed (P = 0.002), and C vs. Bhigh (P = 0.04). However, the effect on the trajectory of ≥6 mm follicular count was restricted to the highest BPA dose (C vs. Bhigh; P = 0.04).
Figure 3.
Ovarian follicular dynamics determined by transrectal ultrasonography for 20 days from two representative females in control, Blow, Bmed, and Bhigh females. Each graph represents a single female (animal identification in top right corner). Trajectory of ovulatory follicles (filled triangles) and non-ovulatory follicles (white circles) are plotted relative to PGF2α administration. Each line, whether triangle or circle, represents the trajectory of a single follicle. Blow, Bmed, and Bhigh represent prenatal BPA doses at 0.05, 0.5, and 5 mg/kg/day, respectively.
Figure 4.
Panel A. Mean (± SE) number of 2–3 mm (left panel), 4–5 mm (middle panel), and ≥ 6 mm (right panel) non-ovulatory follicles per cycle in control (white bars), Blow (light gray bars), Bmed (dark gray bars, and Bhigh (filled bars) females. Panel B. Follicular count trajectory of 2–3 mm (top panel), 4–5 mm (middle panel), and 6 mm (bottom panel) follicles in control (black), Blow (red), Bmed (blue), and Bhigh (green) groups plotted relative to the PGF2α administration throughout the scanning period. A mixed effect model with B-splines was used to fit non-linear trayectories of follicle counts. P value in top right corner of each graph represents overall ANOVA comparing all four groups. Blow, Bmed, B high represent prenatal BPA doses at 0.05, 0.5, and 5 mg/kg/day, respectively.
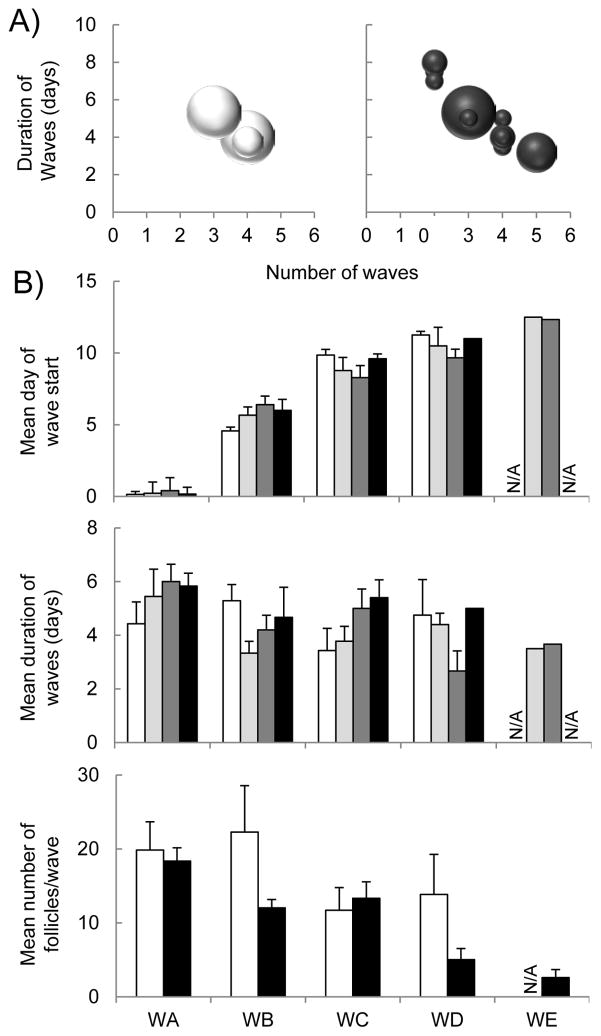
Number of follicular waves per cycle was restricted to 3 to 4 in C females, while it tended to be more variable in the BPA groups ranging from 2 to 5 follicular waves per cycle (P = 0.076; Figure 5, panel A). The variability of the number of waves was evident by a medium to a large effect size analysis when comparing C vs. Blow, Bmed and, Bhigh-treated groups with the C group (d= 0.46, 0.33, and 1.09, respectively). Due to the high variability in the BPA groups, detailed follicular wave analysis revealed no differences in the mean day of start or duration of each follicular wave or mean number of follicles per wave of the BPA group vs. controls (Figure 5, top and middle panel B).). When all BPA groups were merged as a single group, the total number of follicles (sum of all ≥2 mm follicles) within each follicular wave was numerically lower in BPA compared to the C group in both, 2ndand 4th follicular waves (Figure 5, bottom panel B).
Figure 5.
Panel A. Scatter plot representing duration of follicular waves against number of waves in control (left panel) and all BPA groups merged (right panel), where the size of each circle represents the sample size (number of females that fall under each category). Panel B. Mean (± SE) day of start (top panel) and duration (middle panel) of the follicular waves (WA through WE) in control (white), Blow (light gray), Bmed (dark gray), Bhigh (black). Mean (± SE) number of follicles per follicular wave (bottom panel) in control (white) and all BPA groups merged (black). Blow, Bmed, Bhigh represent prenatal BPA doses at 0.05, 0.5, and 5 mg/kg/day, respectively.
Discussion
The current study is the first in vivo dose-response study carried out in a non-rodent species investigating the effects of prenatal BPA treatment, at doses relevant to human exposure, on adult reproductive function. Findings from this study demonstrated that prenatal exposure to BPA (composite of all 3 BPA doses), shortened the timing between the preovulatory estradiol rise and the preovulatory LH surge with no dose response relationship evident. In addition, prenatal BPA treatment also had an impact on ovarian follicular dynamics evident as increased variability in follicular wave occurrence and altered follicular count trajectories during the interovulatory period. The relevance of these disruptions is discussed below.
Impact of prenatal BPA on periovulatory hormonal changes
A mid-cycle increase in estradiol serves as the positive feedback trigger for the generation of the LH surge. An appropriate response to estradiol positive feedback is dependent on the levels, as well as duration of estradiol release. The finding of a shortened interval between estradiol increase and the generation of LH surge indicates that this regulation is perturbed in prenatal BPA-treated animals. The finding that preovulatory estradiol rise in prenatal BPA -treated females is similar to that of controls indicates ovarian signal is normal and the defect involves the neuroendocrine sequence of events leading up to the LH surge generation. This likely stems from increased sensitivity of the neuroendocrine system (hypothalamic/pituitary) to respond early to the estradiol trigger. At the hypothalamic level, prenatal BPA treatment was found to increase ESR1 mRNA expression and decrease ESR2 expression in sheep (Mahoney and Padmanabhan, 2010). Other studies (Cao et al., 2013) have found that neonatal BPA treatment reduces ESR2 expression in hypothalamic areas relevant to behavior; but similar information is not available regarding hypothalamic areas implicated in the generation of the preovulatory LH surge. Considering that evidence supports estrogen receptor 1 (ESR1), but not ESR2 as the key player in the generation of the surge (Wintermantel et al., 2006), the increased ESR1 in concert with decreased ESR2 may provide an improper early trigger for the generation of the LH surge; ESR2 has been shown to oppose ESR1 actions (Matthews and Gustafsson, 2003). Another possibility is that the pituitary gonadotropin-releasing hormone receptor (GnRHR) is upregulated earlier relative to the estradiol signal in prenatal BPA-treated females, thus facilitating generation of an earlier surge. Studies in female mice have indeed shown that prenatal BPA treatment increases pituitary GnRHR expression (Brannick et al., 2012). Detailed time course studies spanning the time interval between the estradiol rise and LH surge are required to narrow down these possibilities.
Unfortunately, knowledge is limiting regarding specific factors that may affect the temporal relationship between the preovulatory estradiol rise and the generation of the subsequent preovulatory LH surge, such as food withdrawal. In sheep, 5 days off asting during the prior luteal phase has been shown to prolong this interval (Kiyma et al., 2004), which has been suggested to be due to a reduction of GnRH pulse frequency and amplitude (I’anson et al., 2000). Ultimately, an LH surge induced prematurely, as is the case in prenatal BPA-treated females may impact the final follicular maturation process. In humans, with approaching menopause, there is shortening of the follicular phase (Burger et al., 2005), impaired folliculogenesis with potential for ovulating an immature follicle (Santoro et al., 2009), and subsequent reduction inoocyte competence (Battaglia et al., 1996). To what extent the perturbed estradiol to LH interval contributes to the embryonic development delay in prenatal BPA -treated (Xiao et al., 2011) and/or the reduced implantational capability of the embryo in prenatal/postnatal BPA-treated mice and rats (Berger et al., 2008; Varayoud et al., 2011)remains to be elucidated.
Despite shortening of the interval between preovulatory estradiol rise and LH surge generation, contrary to our previous findings (Savabiesfahani et al., 2006) of reduced LH surge amplitude in prenatal BPA-treated (5 mg/kg/day) sheep, a decrease in amplitude of the preovulatory LH surge was not evident in the present study with any of the three doses studied. Given that the source of BPA was the same in both studies, the lack of effect of the 5 mg/kg/day dose on LH surge amplitude in the present study may be due to the age difference at which the study was carried out (~6 vs. ~19 months, Savabiesfahani et al., 2006 and this study, respectively). This may be a function of an organizational delay in the maturation of the LH surge system in prenatal BPA-treated females. That LH surge responses normalize with reproductive maturity is also supported by our finding in ovariectomized prenatally BPA-treated females at 21 months of age, which showed a normal LH response to an exogenous estradiol administration (Abi Salloum et al., 2013). A similar outcome was achieved with prenatal estradiol treated sheep, namely reduction in LH surge amplitude when tested at 3.5 months of age, but not at 12 months of age (Malcolm et al., 2006).
Alternatively, differences between both studies could be due to genetics. Gene-by-environment interactions in sheep was evidenced in a study in which pregnant sheep from two breeds subjected to the same nutrient restriction resulted in offspring with different metabolic outcomes (Vonnahme et al., 2006). Similarly, studies in mice that tested gene-by-environment interactions found two strains of mice to have different metabolic outcomes to exposures during prenatal life (Knight et al., 2007). Because homogeneity of the genetic background of the Suffolk breed used in our studies was not controlled for, the genetic background is likely to differ within this breed between our earlier (Savabiesfahani et al., 2006) and this study; hence, this may account for the differences seen in preovulatory LH surge amplitude observed in the current study.
Another possibility is that maternal condition may have played a role in the LH surge outcome differences. Studies have shown that maternal body condition and weight can program postnatal outcomes of the litter. Comparison of maternal weight between both studies (Savabiesfahani et al., 2006 and this study)found that body weight of mothers included in the current study was significantly lower (81.6 ± 1.1 kg) compared to mothers from the previous study (86.9 ±2.2; P = 0.017; Savabiesfahani et al., 2006). Whether the 5 kg body weight increase in the previous study amplified the response to BPA treatment remains to be determined.
BPA impact on follicular changes
Consistent with earlier findings of adult sheep having 3 to 4 waves of follicular emergence (Bartlewski et al., 2011), control sheep in this study had 3 or 4 follicular waves during the interovulatory period. The occurrence of such follicular waves has been found to be associated with systemic FSH concentrations (Baird et al., 1991)and follicular FSH threshold (Barrett et al., 2006). Our finding of a highly variable number of emerging follicular waves in BPA-treated females (2 to 5) suggests that either the circulating FSH concentrations or the FSH threshold to induce a new follicular wave may be highly variable in prenatal BPA-treated animals. Unfortunately, FSH measures during the interovulatory period are not available in this study. The possibility that changes in progesterone can contribute to differing FSH levels during the interovulatory period, as suggested by other studies (Bartlewski et al., 2011) and consequently follicular waves, has no credence in the absence of differences in progesterone levels/patterns during the interovulatory period. Other modulators implicated in follicular recruitment and wave emergence in cattle are IGF-1 and follistatin (Gong et al., 1991, Rouillier et al., 1997); high circulating IGF -1 are linked to increased numbers of recruited follicle per wave (Gong et al., 1991). Since BPA can directly reduce insulin-like growth factors (IGF-1 and IGF-2) expression (Aluru et al., 2010), changes in IGF-1 may have contributed to the changes seen in wave emergence. Irrespective of the mediary, the wide variability in follicular waves between animals is suggestive of individual susceptibility to BPA treatment.
The observed change in the count of 2–3 mm and 4–5 mm follicles that accompanied the follicular wave disruption in BPA -treated females was not BPA dose dependent. This effect was observed for each BPA dose and was highly pronounced in 2–3 mm follicles. Because 2 mm-sized follicles are recognized as the threshold for initiation of FSH-dependency and 4 mm-sized follicles as the threshold for initiation of the LH-dependency (Findlay et al., 2002), the lower 2–3 mm follicular count seen in BPA-treated animals is indicative of a defect in the irability to undergo timely follicular wave recruitment (follicles selected to grow within each wave). Specifically, these findings support a defect in responsiveness of FSH-dependent follicles (2–3 mm). Such defect may arise from either an intrinsic follicular defect or a defect in FSH release pattern. Our previous study conducted in BPA-treated sheep did not provide support for a defect in periovulatory FSH secretion (Savabiesfahani et al., 2006). An intrinsic follicular defect may be indicative of a reduction in FSH receptor expression in these follicles and/or a reduction in FSH responsiveness per se via modulating factors, such as androgens (Harlow et al., 1986).
Another possibility is that the intrinsic ovarian defect may relate to defects in steroidogenesis, as BPA has been shown to interfere with (Craig et al., 2011) and specifically reduce Cyp19a1 mRNA expression in granulosa cells of antral follicles (Kwintkiewicz et al., 2010). Because dynamic changes in aromatase mRNA expression have been associated with follicular recruitment within a follicular wave (Bao et al., 1997) and aromatase expression can be detected in antral follicles smaller than 2.5 mm (Ying et al., 2013), it is possible that an intrafollicular defect in steroidogenesis (e.g.: reduction in aromatase expression) occurred before the follicular recruitment stage (FSH-independent). Such a disruption would contribute to the reduction in 2–3 mm follicles observed in BPA -treated females, as aromatase is crucial for follicular growth and intrafollicular estradiol production. Prenatal BPA treatment was found to disrupt aromatase mRNA expression in sheep ovaries during fetal life (Veiga-Lopez et al., 2013). While evidence supports downregulation of aromatase expression in the ovary by postnatal BPA administration (Lee et al., 2013), similar studies have not been undertaken with prenatal BPA treatment.
Interestingly, the disruption in follicular wave emergence was observed in both ends of the spectrum, either too few (2 waves) or too many (5 waves) follicular waves, but was dose independent. The variability associated with this variable in this study is similar to variability associated with other outcomes in other BPA-treated animal models (Markey et al., 2003, Newbold et al., 2007, Delclos et al., 2014, Zhang et al., 2012). These include depletion of antral follicles and corpora lutea in mice (Delclos et al., 2014), alteration of estrous cycle and ovarian bursa defects in mice (Markey et al., 2003), development of uterine tumors and ovarian cysts in mice (Newbold et al., 2007), and inhibition of meiosis affecting primordial follicle formation in mice (Zhang et al., 2012). The variability in response to prenatal BPA treatment appears to be a common finding with only a subset of BPA-treated females showing defect and thus supportive of gene – environment interaction contributing to individual susceptibility.
Relative to the ovulatory process, the one to one parity in the number of ovulatory follicles and corpora lutea found in this study demonstrates that prenatal BPA-treatment does not compromise ovulatory function. Thus far, there is only one study (Declos et al., 2014) that found a reduction in corpora lutea formation following pre-and postnatal exposure to BPA (gestational day 6 through postnatal day 90). The dose employed in that study (300 mg/kg BW/day) is 60 times higher than the current no-observed-adverse- effect level (NOAEL) dose for BPA (5 mg/kg BW), the highest dose used in the present study.
Lack of disruption in corpora lutea of prenatal BPA -treated sheep contrasts with findings observed in sheep prenatally treated with testosterone and thought to be programmed via estrogenic actions (Steckler et al., 2007, Veiga-Lopez et al., 2014). Similarly, follicular persistence (longer than normal presence of follicles in the ovary) evidenced in prenatal testosterone -treated females shown to be programmed via estrogenic actions of testosterone (Steckler et al., 2007), was not a feature of prenatal BPA-treated females. Differences in ovarian outcomes of prenatal BPA and T-treated animals may relate to BPA being a weak estrogen (Declos et al., 2014)and/or mediation through other signaling pathways; BPA has been shown to act through estrogen-related receptor-γ, an orphan receptor that is not activated by estradiol, bind to a G-protein-coupled receptor, act as an androgen antagonist, and act as an agonist for the glucocorticoid receptor (Thayer and Belcher, 2010).
Together with other studies investigating the effects of prenatal BPA exposure in rats, mice, and rhesus monkeys on ovarian function our findings suggest that BPA might induce programming effects at the level of oocytes/follicles (Markey et al., 2003, Newbold et al., 2007, Delclos et al., 2014, Hunt et al., 2012b, Zhang et al., 2012, this study) potentially impairing their ability to respond to neuroendocrine cues in a physiological manner required for oocyte competence and embryo formation. Absence of ovulatory dysfunctions in the prenatal BPA-treated females in the face of follicular disruptions is suggestive of potential for subfertility requiring further investigation. Since the two lower doses used in the current study are well within the range of common human exposure (Vandenberg et al., 2013), findings of this study may be of translational relevance in understanding the threat prenatal exposure to BPA poses to human reproductive health.
Acknowledgments
We are grateful to Mr. Douglas Doop for his expert animal care, facility management and help with generation of the experimental lambs and Ms. Carol Herkimer and Mr. James Lee for assistance with prenatal steroid treatment. This work was supported by United States Public Health Service Grant R01 ES016541.
Abbreviation list
- BPA
bisphenol A
- Blow
bisphenol A low dose
- Bmed
bisphenol A medium dose
- Bhigh
bisphenol A high dose
- C
control
- CL
corpora lutea
- CV
coefficient of variation
- GnRHR
gonadotropin-releasing hormone receptor
- LH
luteinizing hormone
- LOAEL
lowest-observed-adverse-effect level
- NOAEL
no-observed-adverse-effect level
- P4
progesterone
- PGF2α
prostaglandin 2α
Footnotes
Disclosure statement: Authors have nothing to disclose.
References
- Abi Salloum B, Steckler TL, Herkimer C, Lee JS, Padmanabhan V. Developmental programming: impact of prenatal exposure to bisphenol-A and methoxychlor on steroid feedbacks in sheep. Toxicol Appl Pharmacol. 2013;268:300–308. doi: 10.1016/j.taap.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluru N, Leatherland JF, Vijayan MM. Bisphenol A in oocytes leads to growth suppression and altered stress performance in juvenile rainbow trout. PLoS One. 2010;5:e10741. doi: 10.1371/journal.pone.0010741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird DT, Campbell BK, Mann GE, McNeilly AS. Inhibin and oestradiol in the control of FSH secretion in the sheep. J Reprod Fertil Suppl. 1991;43:125–138. [PubMed] [Google Scholar]
- Bao B, Garverick HA, Smith GW, Smith MF, Salfen BE, Youngquist RS. Changes in messenger ribonucleic acid encoding luteinizing hormone receptor, cytochrome P450-side chain cleavage, and aromatase are associated with recruitment and selection of bovine ovarian follicles. Biol Reprod. 1997;56:1158–1168. doi: 10.1095/biolreprod56.5.1158. [DOI] [PubMed] [Google Scholar]
- Barrett DM, Bartlewski PM, Duggavathi R, Davies KL, Rawlings NC. Suppression of follicle wave emergence in cyclic ewes by supraphysiologic concentrations of estradiol-17beta and induction with a physiologic dose of exogenous ovine follicle-stimulating hormone. Biol Reprod. 2006;75:633–641. doi: 10.1095/biolreprod.105.048702. [DOI] [PubMed] [Google Scholar]
- Bartlewski PM, Baby TE, Giffin JL. Reproductive cycles in sheep. Anim Reprod Sci. 2011;124:259–268. doi: 10.1016/j.anireprosci.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–2222. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- Berger RG, Shaw J, deCatanzaro D. Impact of acute bisphenol-A exposure upon intrauterine implantation of fertilized ova and urinary levels of progesterone and 17beta-estradiol. Reprod Toxicol. 2008;26:94–99. doi: 10.1016/j.reprotox.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Brannick KE, Craig ZR, Himes AD, Peretz JR, Wang W, Flaws JA, Raetzman LT. Prenatal exposure to low doses of bisphenol A increases pituitary proliferation and gonadotroph number in female mice offspring at birth. Biol Reprod. 2012;87:82. doi: 10.1095/biolreprod.112.100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger HG, Robertson DM, Baksheev L, Collins A, Csemiczky G, Landgren BM. The relationship between the endocrine characteristics and the regularity of menstrual cycles in the approach to menopause. Menopause. 2005;12:267–274. doi: 10.1097/01.gme.0000147172.21183.86. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, Huttner K, Hauser R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117:639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Joyner L, Mickens JA, Leyrer SM, Patisaul HB. Sex-specific Esr2 mRNA expression in the rat hypothalamus and amygdala is altered by neonatal bisphenol A exposure. Reproduction. 2014;147:537–554. doi: 10.1530/REP-13-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig ZR, Wang W, Flaws JA. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. 2011;142:633–646. doi: 10.1530/REP-11-0136. [DOI] [PubMed] [Google Scholar]
- Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, Davis KJ, Patton RE, da Costa GG, Woodling KA, Bryant MS, Chidambaram M, et al. Toxicity evaluation of bisphenol a administered by gavage to sprague dawley rats from gestation day 6 through postnatal day 90. Toxicol Sci. 2014;139:174–197. doi: 10.1093/toxsci/kfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, Munoz-de-Toro M. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect. 2007;115:80–86. doi: 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority. [Last accessed: 04/16/2014];Opinion on bisphenol A (BPA) -assessment of human health risks. [Google Scholar]
- Evans NP, Dahl GE, Glover BH, Karsch FJ. Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology. 1994;134:1806–1811. doi: 10.1210/endo.134.4.8137746. [DOI] [PubMed] [Google Scholar]
- Findlay JK, Drummond AE, Dyson ML, Baillie AJ, Robertson DM, Ethier JF. Recruitment and development of the follicle; the roles of the transforming growth factor-beta superfamily. Mol Cell Endocrinol. 2002;191:35–43. doi: 10.1016/s0303-7207(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Ginther OJ, Kot K, Wiltbank MC. Associations between emergence of follicular waves and fluctuations in FSH concentrations during the estrous cycle in ewes. Theriogenology. 1995;43:689–703. doi: 10.1016/0093-691x(94)00074-5. [DOI] [PubMed] [Google Scholar]
- Gong JG, Bramley T, Webb R. The effect of recombinant bovine somatotropin on ovarian function in heifers: follicular populations and peripheral hormones. Biol Reprod. 1991;45:941–949. doi: 10.1095/biolreprod45.6.941. [DOI] [PubMed] [Google Scholar]
- Harlow CR, Hillier SG, Hodges JK. Androgen modulation of follicle-stimulating hormone-induced granulosa cell steroidogenesis in the primate ovary. Endocrinology. 1986;119:1403–1405. doi: 10.1210/endo-119-3-1403. [DOI] [PubMed] [Google Scholar]
- Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports medicine. 2004;34:513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. Fifteen years after “Wingspread”--environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicological sciences: an official journal of the Society of Toxicology. 2008;105:235–259. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, Hassold T, VandeVoort CA. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A. 2012a;109:17525–17530. doi: 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, Hassold T, VandeVoort CA. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A. 2012b;109:17525–17530. doi: 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I’Anson H, Manning JM, Herbosa CG, Pelt J, Friedman CR, Wood RI, Bucholtz DC, Foster DL. Central inhibition of gonadotropin-releasing hormone secretion in the growth-restricted hypogonadotropic female sheep. Endocrinology. 2000;141:520–527. doi: 10.1210/endo.141.2.7308. [DOI] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Kiyma Z, Alexander BM, Van Kirk EA, Murdoch WJ, Hallford DM, Moss GE. Effects of feed restriction on reproductive and metabolic hormones in ewes. J Anim Sci. 2004;82:2548–2557. doi: 10.2527/2004.8292548x. [DOI] [PubMed] [Google Scholar]
- Knight BS, Pennell CE, Adamson SL, Lye SJ. The impact of murine strain and sex on postnatal development after maternal dietary restriction during pregnancy. J Physiol. 2007;581:873–881. doi: 10.1113/jphysiol.2006.126573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwintkiewicz J, Nishi Y, Yanase T, Giudice LC. Peroxisome proliferator-activated receptor-gamma mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ Health Perspect. 2010;118:400–406. doi: 10.1289/ehp.0901161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C, Gieske M, Murdoch B, Ye P, Li Y, Hassold T, Hunt PA. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol Reprod. 2011;84:79–86. doi: 10.1095/biolreprod.110.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Kim JY, Chung JY, Kim YJ, Park JE, Oh S, Yoon YD, Yoo KS, Yoo YH, Kim JM. Bisphenol A exposure during adulthood causes augmentation of follicular atresia and luteal regression by decreasing 17beta-estradiol synthesis via downregulation of aromatase in rat ovary. Environ Health Perspect. 2013;121:663–669. doi: 10.1289/ehp.1205823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luense LJ, Veiga-Lopez A, Padmanabhan V, Christenson LK. Developmental programming: gestational testosterone treatment alters fetal ovarian gene expression. Endocrinology. 2011;152:4974–4983. doi: 10.1210/en.2011-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney MM, Padmanabhan V. Developmental programming: impact of fetal exposure to endocrine-disrupting chemicals on gonadotropin-releasing hormone and estrogen receptor mRNA in sheep hypothalamus. Toxicol Appl Pharmacol. 2010;247:98–104. doi: 10.1016/j.taap.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm KD, Jackson LM, Bergeon C, Lee TM, Padmanabhan V, Foster DL. Long-term exposure of female sheep to physiologic concentrations of estradiol: effects on the onset and maintenance of reproductive function, pregnancy, and social development in female offspring. Biol Reprod. 2006;75:844–852. doi: 10.1095/biolreprod.106.053264. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev. 2003;5:67–75. doi: 10.1046/j.1525-142x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Molina AM, Lora AJ, Blanco A, Monterde JG, Ayala N, Moyano R. Endocrine-active compound evaluation: qualitative and quantitative histomorphological assessment of zebrafish gonads after bisphenol-A exposure. Ecotoxicol Environ Saf. 2013;88:155–162. doi: 10.1016/j.ecoenv.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Nagel SC, vom Saal FS, Welshons WV. Developmental effects of estrogenic chemicals are predicted by an in vitro assay incorporating modification of cell uptake by serum. J Steroid Biochem Mol Biol. 1999;69:343–357. doi: 10.1016/s0960-0760(99)00078-3. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol. 2007;24:253–258. doi: 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender GD, Reichert LE, Jr, Midgley AR, Jr, Nalbandov AV. Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology. 1969;84:1166–1173. doi: 10.1210/endo-84-5-1166. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ. Evidence for short or ultrashort loop negative feedback of gonadotropin-releasing hormone secretion. Neuroendocrinology. 1995;62:248–258. doi: 10.1159/000127011. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, Tao L, Kannan K. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol. 2008;28:258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Developmental origin of reproductive and metabolic dysfunctions: androgenic versus estrogenic reprogramming. Semin Reprod Med. 2011;29:173–186. doi: 10.1055/s-0031-1275519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373:8–20. doi: 10.1016/j.mce.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicological sciences : an official journal of the Society of Toxicology. 2011;119:209–217. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz JR, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, Padmanabhan V, Taylor HS, Swan SH, VandeVoort CA, Flaws JA. Bisphenol A and Reproductive Health: Update of Experimental and Human Evidence. Environ Health Perspect. doi: 10.1289/ehp.1307728. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2010;30:2–9. doi: 10.1038/jp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouillier P, Sirard MA, Matton P, Guilbault LA. Immunoneutralization of transforming growth factor alpha present in bovine follicular fluid prevents the suppression of the follicle-stimulating hormone-induced production of estradiol by bovine granulosa cells cultured in vitro. Biol Reprod. 1997;57:341–346. doi: 10.1095/biolreprod57.2.341. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Hotchkiss AK, Crofton KM, Gray LE., Jr In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol Sci. 2010;114:133–148. doi: 10.1093/toxsci/kfp266. [DOI] [PubMed] [Google Scholar]
- Santoro N, Isaac B, Neal-Perry G, Adel T, Weingart L, Nussbaum A, Thakur S, Jinnai H, Khosla N, Barad D. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88:5502–5509. doi: 10.1210/jc.2002-021839. [DOI] [PubMed] [Google Scholar]
- Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147:5956–5966. doi: 10.1210/en.2006-0805. [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007;148:3532–3540. doi: 10.1210/en.2007-0339. [DOI] [PubMed] [Google Scholar]
- Sun Y, Irie M, Kishikawa N, Wada M, Kuroda N, Nakashima K. Determination of bisphenol A in human breast milk by HPLC with column-switching and fluorescence detection. Biomed Chromatogr. 2004;18:501–507. doi: 10.1002/bmc.345. [DOI] [PubMed] [Google Scholar]
- Thayer K, Belcher S. Mechanisms of action of bisphenol A and other biochemical/molecular interactions. FAO/WHO Expert Meeting on Bisphenol A (BPA); Ottawa, Canada. 2–5 November 2010; pp. 1–21. [Google Scholar]
- Vandenberg LN, Hunt PA, Myers JP, Vom Saal FS. Human exposures to bisphenol A: mismatches between data and assumptions. Rev Environ Health. 2013;28:37–58. doi: 10.1515/reveh-2012-0034. [DOI] [PubMed] [Google Scholar]
- Varayoud J, Ramos JG, Bosquiazzo VL, Lower M, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A alters rat uterine implantation-associated gene expression and reduces the number of implantation sites. Endocrinology. 2011;152:1101–1111. doi: 10.1210/en.2009-1037. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V. Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol Reprod. 2008;78:636–647. doi: 10.1095/biolreprod.107.065904. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Luense LJ, Christenson LK, Padmanabhan V. Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology. 2013;154:1873–1884. doi: 10.1210/en.2012-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Wurst AK, Steckler TL, Ye W, Padmanabhan V. developmental programming: postnatal estradiol amplifies ovarian follicular defects induced by fetal exposure to excess testosterone and dihydrotestosterone in sheep. Reprod Sci. 2014;21:444–455. doi: 10.1177/1933719113503412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonnahme KA, Hess BW, Nijland MJ, Nathanielsz PW, Ford SP. Placentomal differentiation may compensate for maternal nutrient restriction in ewes adapted to harsh range conditions. J Anim Sci. 2006;84:3451–3459. doi: 10.2527/jas.2006-132. [DOI] [PubMed] [Google Scholar]
- Waller DK, Gallaway MS, Taylor LG, Ramadhani TA, Canfield MA, Scheuerle A, Hernandez-Diaz S, Louik C, Correa A. Use of oral contraceptives in pregnancy and major structural birth defects in offspring. Epidemiology. 2010;21:232–239. doi: 10.1097/EDE.0b013e3181c9fbb3. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Diao H, Smith MA, Song X, Ye X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod Toxicol. 2011;32:434–441. doi: 10.1016/j.reprotox.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SJ, Xiao SH, Wang CL, Zhong BS, Zhang GM, Wang ZY, He DY, Ding XL, Xing HJ, Wang F. Effect of nutrition on plasma lipid profile and mRNA levels of ovarian genes involved in steroid hormone synthesis in Hu sheep during luteal phase. J Anim Sci. 2013;91:5229–5239. doi: 10.2527/jas.2013-6450. [DOI] [PubMed] [Google Scholar]
- Zhang HQ, Zhang XF, Zhang LJ, Chao HH, Pan B, Feng YM, Li L, Sun XF, Shen W. Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Mol Biol Rep. 2012;39:5651–5657. doi: 10.1007/s11033-011-1372-3. [DOI] [PubMed] [Google Scholar]