Abstract
OBJECTIVE
Plasma homocysteine (tHcy) has been positively associated with carotid intima-media thickness (IMT) in non-diabetic populations and in a few cross-sectional studies of diabetic patients. We investigated cross-sectional and prospective associations of a single measure of tHcy with common and internal carotid IMT over a 6-year period in type 1 diabetes.
RESEARCH DESIGN AND METHODS
tHcy levels were measured once, in plasma obtained in 1997-1999 from patients (n=599) in the Epidemiology of Diabetes Interventions and Complications (EDIC) study, the observational follow-up of the Diabetes Control and Complications Trial (DCCT). Common and internal carotid IMT were determined twice, in EDIC “Year 6” (1998-2000) and “Year 12” (2004-2006), using B-mode ultra-sonography.
RESULTS
After adjustment, plasma tHcy [median (interquartile range): 6.2 (5.1, 7.5) μmol/L] was significantly correlated with age, diastolic blood pressure, renal dysfunction, and smoking (all p<0.05). In an unadjusted model only, increasing quartiles of tHcy correlated with common and internal carotid IMT, again at both EDIC time-points (p<0.01). However, multivariate logistic regression revealed no significant associations between increasing quartiles of tHcy and the 6-year change in common and internal carotid IMT (highest vs. lowest quintile) when adjusted for conventional risk factors.
CONCLUSIONS
In a type 1 diabetes cohort from the EDIC study, plasma tHcy measured in samples drawn in 1997-1999 was associated with measures of common and internal carotid IMT measured both one and seven years later, but not with IMT progression between the two time-points. The data do not support routine measurement of tHcy in people with Type 1 diabetes.
1. Introduction
Homocysteine (Hcy), a sulphur-containing amino acid, is the demethylated metabolite of the essential amino acid methionine. The metabolic pathways involved in Hcy formation rely on coenzymes derived from vitamins B6 and B12 and folic acid [1]. Plasma Hcy is extensively metabolized in the kidneys [2], and there is an independent inverse association between glomerular filtration rate (GFR) and plasma total Hcy (tHcy) in patients with type 1 diabetes (T1DM) [3] and [4]. The association between tHcy and atherosclerosis was first identified by McCully in 1969 [5], and since then evidence has accumulated on the role of elevated tHcy as a cardiovascular risk factor in T1DM [3], [6] and [7].
Carotid intima-media thickness (IMT) is a well-accepted non-invasive marker of subclinical atherosclerosis [8]. Increased IMT of the common carotid artery reflects diffuse arterial wall thickening [9], whereas increased IMT of the proximal internal carotid artery is considered a surrogate for focal atherosclerotic plaque [10]. The role of tHcy in endothelial dysfunction is thought to be mediated by mechanisms including oxidative stress, nuclear factor-κb (NF-κb) activation, inflammation [11], and inhibition of endothelial nitric oxide synthase (eNOS) [12]. While several observational studies have reported weak positive associations between tHcy and carotid IMT in the non-diabetic population [13], few cross-sectional studies address this association in the context of diabetes mellitus, and most involve type 2 diabetes (T2DM) [14], [15] and [16]. No prospective study has reported tHcy levels in relation to IMT progression in diabetic patients, a major goal of our current analyses.
Intensive diabetes management with a focus on glycemic control slows the progression of common carotid IMT in the Epidemiology of Diabetes Interventions and Complications (EDIC) trial, the on-going long-term observational phase of the Diabetes Control and Complications Trial (DCCT) [17]. Data from the same cohort have also shown significant associations of lipids [18], markers of oxidative stress, and inflammation [19] with IMT progression. We hypothesized that tHcy is associated with common and internal carotid IMT (cross-sectional study) and with subsequent IMT progression (longitudinal study). For this purpose, in a large subset of EDIC patients (n=599), we measured plasma tHcy in plasma samples obtained in 1997-1999, and related the findings to common and internal carotid IMT measured at EDIC “Year 6” (1998-2000) and “Year 12” (2004-2006), and to IMT progression between those time-points.
2. Methods
2.1. Study population
This study involved a subset of 599 patients from the DCCT/EDIC cohort. The original DCCT cohort comprised 1,441 patients with T1DM (age range: 13-39 years), who were without hypertension or dyslipidemia at study entry (1983-89) [20]. The patients were randomly assigned to intensive vs. conventional diabetes therapy and followed for an average of 6.5 years. In 1994, the observational phase of the study (EDIC) was initiated, and 1394 patients (97% of the original DCCT cohort) were enrolled. In 1997-99, blood and urine samples were collected from 905 EDIC participants who participated in our ancillary longitudinal study based at the Medical University of South Carolina, and plasma tHcy was measured in 769 of these. Of the 769, 599 had available common and internal carotid IMT measurements at both EDIC ‘Year 6’ (1998-2000) and ‘Year 12’ (2004-2006). Plasma samples for tHcy analysis were collected after an overnight fast and stored at −80°C. The DCCT and EDIC and our laboratory studies were approved by the Institutional Review Boards at all participating institutions, and all participants provided written informed consent.
2.2. Assessment of carotid IMT
IMT measurements by B-mode ultrasound, and their reporting in a Central Reading Facility, have previously been described in detail [21]. Reliability measures for IMT readers at EDIC years 1, 6 and 12 have also been reported previously: for common carotid IMT, the primary reader had an intra-reader coefficient of reliability of >0.93, and the inter-reader reliability was >0.81. The coefficients were similar for the internal carotid IMT measures (>0.93 and >0.90, respectively) [17]. A clinically elevated IMT as measured by ultrasound is defined as ≥0.75mm for common and ≥1.0mm for internal IMT [21].
2.3. Measurement of plasma total homocysteine (tHcy)
Assays were performed within three years of sample collection using never-thawed samples. tHcy was measured as described by Araki & Sako (1987) [22]. This procedure involved reduction of the sample with tri-n-butylphosphine followed by de-proteinization and reaction of sulfhydryl compounds with the fluorophore, ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate (SBD-F). The sample was then applied to the reversed phase high-performance liquid chromatography (HPLC) and detected fluorimetrically. The derivatized tHcy is readily separated from other sulfhydryl compounds in plasma under isocratic conditions. Elution time and standard curves for quantification were prepared using commercially available D, L-homocysteine (Sigma, St. Louis, MO, USA). The inter-assay coefficient of variation (CV) was 6.6%, and duplicate measures (n=92) revealed an intra-sample CV of 0.27%.
2.4. Other procedures
Standardized procedures for medical history, physical examination, and routine laboratory analyses, including HbA1c, lipids and urinary albumin excretion rate (AER), have been previously described [23] and [24].
2.5. Statistical analysis
Demographic and clinical measures collected at EDIC Year 6 were summarized for groups of participants who were categorized according to quartiles of tHcy. When comparisons of means or proportions were made among more than two ordered categories, including groups defined by quartiles of tHcy, a test for linear trend among means was implemented using an ANOVA model, and a test for trend among proportions was implemented using a Cochran-Armitage trend test. A Chi-square test of independence was used to investigate the association between two factors, each including more than 2 categories, and Fisher’s exact test was used to compare proportions when expected contingency table counts were small (more than 20% of the expected counts less than 5).
Linear regression models were used to identify factors independently associated with tHcy (dependent factor). Values of tHcy were log-transformed because of their non-normal distribution, and tHcy values greater than 50 μmol/L (n=3) were excluded from the final regression analyses. To aid in the interpretation of the regression coefficients, standardized coefficients were estimated for the continuous independent variables. All continuous measures, including the natural log of tHcy, were standardized to have a mean of 0 and a standard deviation of 1; therefore, regression coefficients for continuous measures reflect the number of standard deviations difference in the natural log transformed tHcy that are expected with a one standard deviation increase in the independent variable. Categorical covariates were not standardized; corresponding coefficients can be interpreted as the number of standard deviations difference in the natural log transformed tHcy that are expected when comparing groups, e.g., males, relative to the reference group, e.g., females. Factors associated with tHcy based on univariate analyses at the 0.10 alpha level were entered into a multivariate regression model. A backwards elimination modeling approach was used in which covariates were deleted one by one from the regression model until all coefficient estimates were significant at a 0.05 alpha level. Effect modification by gender and multivitamin use was investigated by including tests of interaction. The distributions of common and internal carotid IMT measures were compared among groups of participants defined by quartile of tHcy using the Kruskal-Wallis test, given the non-normal distribution of carotid IMT measures. The proportion of patients with clinically-elevated carotid IMT measures was compared among ordered categories of quartiles of tHcy using a Cochran-Armitage trend test.
Investigations of the association between tHcy and carotid IMT were based on categorical forms of both variables due to violations of linear modeling assumptions when continuous forms were considered. Logistic regression was used to examine our primary analysis of the odds of highest versus lowest quintile change between year 6 and 12 in common and internal carotid IMT measures (dependent factor) defined by quartiles of tHcy (independent factor), which were modeled using three dummy variables with reference to the lowest tHcy quartile. We also performed secondary analyses to compare the odds of 6-year highest quintile change versus all four lower quintiles combined, in common and internal carotid IMT measures, defined by quartiles of tHcy. Model fitting began by first exploring the significance of the gender by tHcy group interaction (to detect modification of the association between tHcy and elevated carotid IMT progression by gender). If a significant interaction was found, models were stratified by gender. The models were adjusted for the baseline common and internal carotid IMT at EDIC year 6. In addition, models included the following adjustment for confounding factors: Model A: unadjusted; Model B: adjusted for age, gender, randomized therapy, diabetes duration, obesity, IMT reader ID, time-averaged HbA1c (year 6 time-weighted average), LDL-cholesterol, HDL-cholesterol, natural log triglyceride, smoking status, alcohol consumption, multivitamin use (EDIC year 6); Model C: all factors included in Model B as well as hypertension, natural log albumin excretion rate (AER), estimated glomerular filtration rate (eGFR), angiotensin converting enzyme inhibitors (ACE)/angiotensin receptor blocker (ARB) use (number of years of use during EDIC years 1- 6); Model D: all factors included in Model C, as well as secondary intervention group and Early Treatment Diabetic Retinopathy Study [ETDRS] scores. A two-sided alpha level of 0.05 was used to define statistical significance.
3. Results
The baseline characteristics of the 599 participants at EDIC Year 6 included in the present study revealed significant differences in age; systolic and diastolic blood pressure; renal function assessed by AER, GFR and serum creatinine; HDL-cholesterol, LDL-cholesterol and triglyceride across increasing quartiles of tHcy (Table 1). Also, the proportions of participants from the DCCT secondary intervention group, of male gender, with hypertension, and reporting multivitamin or ACE/ARB user differed significantly across tHcy quartiles (p<0.05, with majority p<0.01; Table 1). Comparisons of demographic and clinical characteristics of EDIC subjects included in our study (n=599) vs. those not included (n=721) revealed that the analyzed cohort tended to have a shorter diabetes duration, higher diastolic blood pressure, lower AER, lower HbA1c, and lower ETDRS scores compared to those who were not included in the analysis. Furthermore, our participants were more likely to be male, less likely to be in the secondary intervention cohort, and were less likely to report using aspirin (p<0.05; Supplemental Table S1).
Table 1.
Demographics and clinical characteristics of EDIC participants at the time of the ‘Year 6’ IMT determination (1998-2000), stratified by quartiles of plasma total homocysteine (tHcy) measured in samples from 1997-99.
| Quartile of Homocysteine (μmol/L) EDIC Years 4-6 | ||||||
|---|---|---|---|---|---|---|
|
Demographic or Clinical
Measure at EDIC Year 6 |
All Patients
(n=599) |
Quartile 1:
2.7 – 5.05 μmol/L (n=149) |
Quartile 2:
5.06 – 6.15 μmol/L (n=150) |
Quartile 3:
6.16 – 7.46 μmol/L (n=147) |
Quartile 4:
≥ 7.47 μmol/L (n=153) |
p-value (trend test) |
| Mean ± SD | Mean ± SD | Mean ± SD |
Mean ± SD | Mean ± SD | ||
| Attained Age (years) | 40.3 ± 6.7 | 38.5 ± 6.6 | 39.8 ± 6.7 | 41.1 ± 6.6 | 41.7 ± 6.4 | <0.0001 |
| Attained Diabetes Duration (years) † |
17.0 ± 1.3 | 16.4 ± 1.3 | 17.0 ± 1.3 | 17.3 ± 1.3 | 17.2 ± 1.3 | 0.098 |
| BMI (kg/m2) | 26.9 ± 4.2 | 26.9 ± 4.7 | 26.3 ± 3.9 | 27.1 ± 3.7 | 27.5 ± 4.5 | 0.080 |
| Systolic Blood Pressure (mmHg) |
121 ± 14 | 119 ± 15 | 119 ± 12 | 122 ± 15 | 123 ± 13 | 0.0045 |
| Diastolic Blood Pressure (mmHg) |
76 ± 9 | 74 ± 9 | 76 ± 8 | 77 ± 7 | 76 ± 10 | 0.031 |
| AER (mg/24 hours) †# | 12.5 ± 3.4 | 10.1 ± 2.7 | 11.8 ± 3.3 | 12.3 ± 3.0 | 16.6 ± 4.3 | 0.0007 |
| GFR (mL/min) | 91.6 ± 17.8 | 94.4 ± 19.3 | 93.8 ± 16.7 | 92.3 ± 15.5 | 86.1 ± 18.3 | <0.0001 |
| Serum Creatinine (ng/mL) | 0.9 ± 0.3 | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.5 | <0.0001 |
| CRP (mg/L) †* | 1.5 ± 3.0 | 1.7 ± 3.5 | 1.3 ± 2.8 | 1.7 ± 3.0 | 1.5 ± 2.7 | 0.70 |
| HbA1c (%) (weighted mean from DCCT to EDIC Year 6) |
8.1 ± 1.1 | 8.1 ± 1.1 | 8.1 ± 1.1 | 8.1 ± 1.0 | 8.2 ± 1.1 | 0.20 |
| Total Cholesterol (mg/dl) # | 187 ± 33 | 184 ± 33 | 183 ± 31 | 190 ± 36 | 191 ± 32 | 0.074 |
| HDL Cholesterol (mg/dl) # | 57 ± 15 | 59 ± 16 | 58 ± 15 | 55 ± 12 | 56 ± 15 | 0.023 |
| LDL Cholesterol (mg/dl) # | 113 ± 30 | 109 ± 28 | 109 ± 27 | 117 ± 34 | 116 ± 29 | 0.0061 |
| Triglyceride (mg/dl) †# | 75 ± 2 | 70 ± 2 | 70 ± 2 | 79 ± 2 | 82 ± 2 | 0.0008 |
| Homocysteine (μmol/L) † | 6.3 ± 1.4 | 4.3 ± 1.1 | 5.6 ± 1.1 | 6.7 ± 1.1 | 9.5 ± 1.4 | NP |
|
Demographic or Clinical
Measure at EDIC Year 6 |
n (%) | n (%) | n (%) | n (%) | n (%) |
p-value
(trend test) |
| Randomized to Intensive Therapy Group |
311 (52) | 87 (58) | 69 (46) | 78 (53) | 77 (50) | 0.35 |
| Secondary Intervention Group | 276 (46) | 56 (38) | 71 (47) | 73 (50) | 76 (50) | 0.035 |
| Male | 346 (58) | 50 (34) | 73 (49) | 108 (73) | 115 (75) | <0.0001 |
| Smoker in the past 12 months | 95 (16) | 22 (15) | 15 (10) | 28 (19) | 30 (20) | 0.076 |
| Obesity (BMI > 30 kg/m2) | 120 (20) | 34 (23) | 22 (15) | 25 (17) | 39 (26) | 0.48 |
| Hypertension | 173 (29) | 37 (25) | 33 (22) | 42 (29) | 61 (40) | 0.0016 |
| ETDRS (EDIC Year 4) ## | 0.21** | |||||
| None-Minimal (Score 1-3) | 296 (53) | 84 (60) | 73 (51) | 77 (54) | 62 (46) | |
| Mild-Moderate (Score 4-9) | 231 (41) | 50 (36) | 59 (41) | 57 (40) | 65 (49) | |
| Preproliferative and Proliferative (Score 10-23) |
32 (6) | 5 (4) | 12 (8) | 8 (6) | 7 (5) | |
| Multivitamin Use (Year 6) | 271 (46) | 80 (54) | 70 (48) | 68 (46) | 53 (35) | 0.0014 |
| Any prior lipid lowering medication (Year 1 – Year 5) |
57 (10) | 9 (6) | 14 (9) | 18 (12) | 16 (10) | 0.13 |
| Any prior statin use (Year 1 – Year 5) |
0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ---- |
| Any prior ACE/ARB use (Year 1 – Year 5) |
103 (17) | 20 (13) | 22 (15) | 24 (16) | 37 (24) | 0.013 |
| Aspirin Use at least 14 days/month (Year 6) |
79 (13) | 18 (12) | 17 (11) | 27 (18) | 17 (11) | 0.75 |
Demographics and clinical characteristics at EDIC year 6 are represented as mean ± SD for continuous covariates and n (%) for categorical covariates. Data are stratified by quartiles of total plasma homocysteine (tHcy) measured at EDIC years 4-6.
geometric mean reported due to skewed distribution of duration of diabetes, AER, CRP, triglycerides, and homocysteine.
CRP measured at the same study visit as homocysteine (EDIC Years 4-6).
Overall (6 degrees of freedom) Chi-square test
Lipids (total-, HDL-, LDL- cholesterol and triglyceride) and AER measures were made at either EDIC Year 5 or EDIC Year 6 depending on the cycle of specimen collection.
ETDRS reflects EDIC Year 4 information, which was a common assessment time point among the patients.
Significant p values (<0.05) have been indicated in boldface.
BMI: body mass index, AER: albumin excretion rate, GFR: glomerular filtration rate, CRP: C-reactive protein, ETDRS: Early Treatment Diabetic Retinopathy Study, NP: not performed.
The median plasma tHcy level in our participants was 6.2μmol/L with minimum and maximum values of 2.7 and 101μmol/L, and interquartile range 5.1 - 7.5 μmol/L. The overall distribution of elevated tHcy in our subset, defined according to thresholds of macrovascualar risk in previously reported studies [25], [26] and [27], was as follows: 25% participants had tHcy > 7.5μmol/L, 7% had tHcy >10μmol/L, and 1% had tHcy > 15μmol/L.
Table 2 summarizes associations of demographic/clinical characteristics at EDIC Years 4-6 (i.e. contemporaneous with blood sample collection for tHcy) with tHcy as a continuous variable. In the univariate model, tHcy was positively associated with age, diabetes duration, BMI, systolic and diastolic blood pressure, AER, serum creatinine, total cholesterol, LDL-cholesterol, triglycerides, former membership of the DCCT secondary intervention group, male gender, hypertension, and ACE/ARB use (p<0.05). Significant negative associations were observed between tHcy levels and GFR, HDL-cholesterol, and multivitamin use (p<0.05; most p<0.01). In the multivariate model, tHcy remained significantly associated only with age, diastolic blood pressure, renal function (AER, GFR and serum creatinine) and smoking (p<0.05; again, most p<0.01; Table 2). The associations in the multivariate model were not significantly different between men and women, or between multivitamin users and non-users (data not shown).
Table 2.
Associations of the natural log of total plasma homocysteine (tHcy)† with demographic characteristics and cardiovascular risk factors at EDIC ‘Years 4-6’
| Univariate Model | Multivariate Model†† | |||||
|---|---|---|---|---|---|---|
| Demographic or Clinical Measure at EDIC Years 4-6 |
β- coefficient* |
P-value | R2 | β- coefficient* |
P-value | R2 |
| 0.20 (model) |
||||||
| Attained Age (years) | 0.15 | <0.0001 | 0.03 | 0.13 | 0.0004 | |
| Attained Diabetes Duration (years) | 0.08 | 0.029 | 0.008 | |||
| BMI (kg/m2) | 0.07 | 0.045 | 0.0068 | |||
| Diastolic Blood Pressure (mmHg) | 0.19 | <0.0001 | 0.048 | 0.09 | 0.0093 | |
| Systolic Blood Pressure (mmHg) | 0.16 | <0.0001 | 0.036 | |||
| Natural Log AER (mg/24 hours) # # | 0.14 | <0.0001 | 0.028 | 0.07 | 0.032 | |
| GFR (ml/min) | −0.14 | <0.0001 | 0.027 | 0.15 | 0.0016 | |
| Serum Creatinine (ng/mL) | 0.31 | <0.0001 | 0.13 | 0.38 | <0.0001 | |
| Natural Log CRP (mg/L)** | −0.02 | 0.66 | <0.001 | |||
| HbA1c (%) (weighted mean from DCCT to EDIC Year 6) |
0.06 | 0.071 | 0.0056 | |||
| Total Cholesterol (mg/dl) # | 0.10 | 0.0046 | 0.013 | |||
| HDL Cholesterol (mg/dl) # | −0.07 | 0.034 | 0.0075 | |||
| LDL Cholesterol (mg/dl) # | 0.10 | 0.0044 | 0.014a | |||
| Natural Log Triglyceride (mg/dl) # | 0.15 | <0.0001 | 0.029 | |||
| Randomized to Intensive Therapy Group |
−0.06 | 0.41 | 0.0011 | |||
| Secondary Intervention Group | 0.16 | 0.019 | 0.0093 | |||
| Male | 0.52 | <0.0001 | 0.092 | |||
| Smoker in the past 12 months | 0.18 | 0.058 | 0.0061 | 0.35 | <0.0001 | |
| Obesity (BMI > 30 kg/m2) | 0.09 | 0.32 | 0.0017 | |||
| Hypertension | 0.23 | 0.0031 | 0.015 | |||
| ETDRS # # | 0.0064 | |||||
| None-Minimal (Score 1-3) | Reference | |||||
| Mild-Moderate (Score 4-9) | 0.13 | 0.066 | ||||
| Preproliferative and Proliferative (Score 10-23) |
0.12 | 0.43 | ||||
| Multivitamin Use (Year 6) | −0.24 | 0.0006 | 0.020 | |||
| Any prior lipid lowering medication | 0.02 | 0.87 | <0.0001 | |||
| Any prior statin use | −0.002 | 0.99 | <0.0001 | |||
| Any prior ACE/ARB use | 0.25 | 0.0061 | 0.013 | |||
| Aspirin Use at least 14 days/month (Year 6) |
0.05 | 0.62 | 0.00041 | |||
All continuous measures have been standardized to have a mean of 0 and a standard deviation of 1; therefore, regression coefficients for continuous measures reflect the number of standard deviations difference in the natural log transformed homocysteine that are expected with a one standard deviation increase in the independent variable.
Categorical covariates were not standardized; corresponding coefficients can be interpreted as the number of standard deviations difference in the natural log transformed homocysteine that are expected when comparing groups, e.g., males, relative to the reference group, e.g., females.
CRP measured at the same study visit as homocysteine (EDIC Years 4-6).
Lipids (total-, HDL-, LDL- cholesterol and triglyceride) and AER measures were made at either EDIC Year 5 or EDIC Year 6 depending on the cycle of specimen collection.
ETDRS was measured at Year 4 because only a subset of patients had measures at subsequent years.
Extreme homocysteine measures (beyond 50 μmol/L) were removed prior to analysis (n=3).
Includes all terms that remained statistically significant in the multivariate model.
Significant p values (<0.05) have been indicated in boldface. BMI: body mass index, GFR: glomerular filtration rate, CRP: C-reactive protein, ETDRS: Early Treatment Diabetic Retinopathy Study.
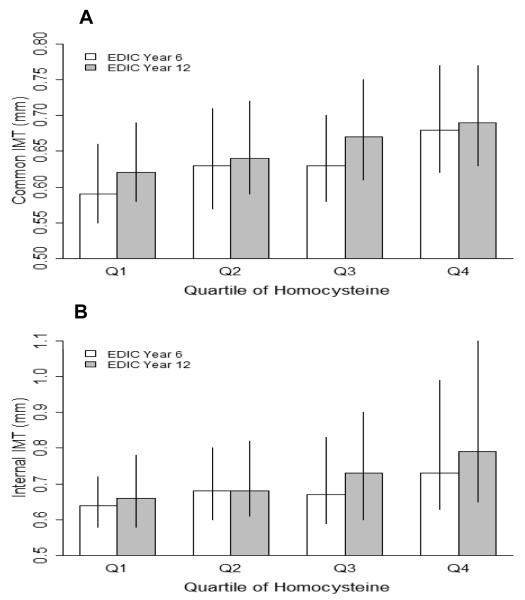
Figures 1A and 1B describe the distribution of common and internal carotid IMT in our cohort, summarized as medians and interquartile ranges of IMT and defined by quartiles of tHcy. Both common (Figure 1A) and internal (Figure 1B) carotid IMT measurements increased significantly with increasing quartiles of tHcy at both time points (i.e. EDIC Years 6 and 12; p<0.0001), but these associations were no longer significant when adjusted for conventional risk factors. In gender-specific analyses, only common carotid IMT in women remained significantly associated with quartiles of tHcy, again at both time points (p<0.05).
Figure 1.
(A) Common and (B) internal carotid IMT (mm) at EDIC Years 6 and 12 defined by quartiles of total plasma homocysteine (μmol/L) (n=599). Data for IMT are summarized as medians and interquartile ranges. The origin of the vertical axes is 0.5 mm. For homocysteine: Q1: 2.7-5.05 μmol/L; Q2: 5.06-6.15 μmol/L; Q3: 6.16-7.46 μmol/L; Q4: >7.46 μmol/L. There were significant overall differences (p≤0.001) among tHcy quartiles at EDIC years 6 and 12 using Kruskal-Wallis Test.
We observed no significant differences in the percentage of patients with clinically elevated common (≥0.75 mm) and internal (≥ 1.0 mm) carotid IMT, based on tHcy levels below and above 10μmol/L at EDIC Years 6 and 12 (Supplemental Table S2).
In Table 3, multivariate logistic regression models were used to examine the ability of increasing quartiles of tHcy to predict the highest vs. lowest changes in common and internal carotid IMT between Year 6 and 12, defined as top and bottom quintiles of change. tHcy was categorized into quartiles based on an even distribution of participants within each clinically defined quartile, while the carotid IMT changes were stratified into quintiles based on the magnitude of 6-year changes observed in our cohort, and what would be considered a clinically meaningful change in IMT for predicting CVD events. For common carotid IMT, the range of values for the highest quintile of change (year 12 minus Year 6 measures) was +0.11 to +1.04 mm (n=120) and for the lowest quintile, −0.021 to −0.41 mm (n=119). For internal carotid IMT, corresponding ranges were +0.24 to +2.27mm (n=118) and −0.047 to −1.20 mm (n=116) respectively. Overall, no significant associations were noted among increasing quartiles of tHcy and the highest versus lowest 6-year changes in common and internal carotid IMT, when adjusted for conventional risk factors. In the case of internal carotid IMT, in the unadjusted model only, the highest quartile of tHcy was associated with a significantly higher odds of 6-year change in carotid IMT compared to the lowest tHcy quartile. This, however, did not persist in adjusted analyses (Table 3). These results were largely unchanged when stratified by primary prevention/secondary intervention group or by conventional/intensive treatment. Also, our exploratory analysis revealed no significant associations of tHcy with highest vs. all four lower quintiles of changes in common and internal carotid IMT. Furthermore, there were no significant differences in 6-year longitudinal changes among those with clinically elevated common and internal carotid IMT, based on tHcy levels below and above 10μmol/L (Supplemental Table S3).
Table 3.
Odds ratios and 95% confidence intervals: highest versus lowest quintile of 6-year changes in common and internal carotid IMT according to quartiles of homocysteine (tHcy)
| 6-year Changes in Common CIMT (odds of highest versus lowest quintile of change)* |
6-year Changes in Internal CIMT (odds of highest versus lowest quintile of change)** |
|||||||
|---|---|---|---|---|---|---|---|---|
| Homocysteine† | Odds Ratio | 95% CI | p-value | Odds Ratio | 95% CI | p-value | ||
| Model A | P=0.74 (overall) |
P=0.11 (overall) |
||||||
| Lowest Quartile | 1 (reference) |
1 (reference) |
||||||
| Quartile 2 | 1.01 | 0.46 | 2.20 | 0.99 | 1.73 | 0.80 | 3.76 | 0.16 |
| Quartile 3 | 1.42 | 0.65 | 3.12 | 0.38 | 1.53 | 0.71 | 3.30 | 0.28 |
| Quartile 4 | 1.33 | 0.64 | 2.76 | 0.45 | 2.59 | 1.20 | 5.56 | 0.015 |
| Model B | P=0.98 (overall) |
P=0.59 (overall) |
||||||
| Lowest Quartile | 1 (reference) |
1 (reference) |
||||||
| Quartile 2 | 0.93 | 0.36 | 2.41 | 0.88 | 1.43 | 0.59 | 3.47 | 0.43 |
| Quartile 3 | 1.11 | 0.42 | 2.92 | 0.83 | 0.88 | 0.35 | 2.22 | 0.78 |
| Quartile 4 | 0.92 | 0.37 | 2.27 | 0.86 | 1.39 | 0.55 | 3.53 | 0.48 |
| Model C | P=0.96 (overall) |
P=0.54 (overall) |
||||||
| Lowest Quartile | 1 (reference) |
1 (reference) |
||||||
| Quartile 2 | 1.11 | 0.41 | 3.02 | 0.83 | 1.39 | 0.56 | 3.48 | 0.48 |
| Quartile 3 | 1.32 | 0.47 | 3.73 | 0.60 | 0.75 | 0.29 | 1.97 | 0.56 |
| Quartile 4 | 1.08 | 0.41 | 2.81 | 0.88 | 1.19 | 0.45 | 3.15 | 0.73 |
| Model D | P=0.73 (overall) |
P=0.63 (overall) |
||||||
| Lowest Quartile | 1 (reference) |
1 (reference) |
||||||
| Quartile 2 | 1.60 | 0.54 | 4.77 | 0.40 | 1.46 | 0.56 | 3.80 | 0.44 |
| Quartile 3 | 1.42 | 0.48 | 4.24 | 0.53 | 0.79 | 0.30 | 2.14 | 0.65 |
| Quartile 4 | 1.79 | 0.62 | 5.13 | 0.28 | 1.03 | 0.37 | 2.85 | 0.95 |
All models were adjusted for EDIC year 6 common or internal CIMT and all covariates were assessed at EDIC year 6 unless otherwise indicated. Model A: unadjusted; Model B: adjusted for age, gender, randomized therapy, obesity, IMT reader, diabetes duration, HbA1c (EDIC year 6 time-weighted average), LDL-, HDL-cholesterol, triglycerides, smoking status, alcohol consumption and multivitamin use (EDIC year 6); Model C: all factors in Model B + eGFR, AER, hypertension and ACE/ARB use (number of years of use for EDIC years 1-6); Model D: all factors in Model C + secondary intervention group (at randomization) and ETDRS scores.
The numerical cut points for quartiles of homocysteine were 2.7 – 5.05 μmol/L (n=149), 5.06 – 6.15 μmol/L (n=150), 6.16 – 7.46 μmol/L (n=147), and ≥ 7.47 μmol/L (n=153).
The numerical cut point values for the highest and lowest quintiles of common carotid IMT is 1.04 to 0.113 mm (n=120) and −0.412 to −0.0205mm (n=119).
The numerical cut point values for the highest and lowest quintiles of internal carotid IMT is 2.27 to 0.24mm (n=118) and −1.199 to −0.0467mm (n=116). Significant p values (<0.05) have been indicated in boldface.
Sensitivity analyses were performed to examine the effects of six subjects with extreme tHcy levels (>15 μmol/L) on the predictive ability of tHcy for the ‘highest versus lowest 6-year changes’ in common and internal carotid IMT. Excluding these subjects from the logistic regression analyses had no notable effect on the results described in Table 3.
4. Discussion
This is the first report of a prospective investigation of associations between circulating tHcy levels and carotid IMT in a large, well-characterized cohort of T1DM patients. We found significant associations between tHcy measured in 1997-1999 and common and internal carotid IMT at EDIC Years 6 (1998-2000) and 12 (2004-2006). Despite this, the overall 6-year progression of IMT was largely similar when examined by increasing quartiles of tHcy. Furthermore, the logistic regression analyses revealed no significant associations between increasing quartiles of tHcy and ‘highest vs. lowest 6-year changes’ in common and internal carotid IMT (including clinically elevated IMT), when adjusted for age, gender, multivitamin use and traditional cardiovascular risk factors. Our observations may be explained by the fact that approximately 93% of our cohort had tHcy levels less than 10 μmol/L, and thus a low prevalence of hyperhomocysteinemia. Hyperhomocysteinemia is typically defined as levels >10μmol/L in reported studies [25], [26] and [27]. Levels of tHcy measured in our DCCT/EDIC cohort are comparable to observed tHcy concentrations in other studies of T1DM of similar time frame and/or participant characteristics [3], [6], [28] and [29]. While we lacked a non-diabetic control group, the observed tHcy levels in our cohort are also similar or lower than in other studies of apparently healthy non-diabetic subjects [30], and may reflect healthy dietary habits among our study participants, and for some (46%), the use of multivitamins (likely to contain tHcy-lowering B-group vitamins).
In univariate analyses, tHcy levels were significantly and positively associated with age, diabetes duration, BMI, blood pressure, hypertension, ACE/ARB use, renal dysfunction (AER and serum creatinine), LDL-cholesterol, total cholesterol, triglyceride, previous membership of the DCCT secondary intervention group, and male gender, and inversely associated with multivitamin use, GFR, and HDL-cholesterol. In the more rigorous multivariate model, tHcy remained positively associated only with age, diastolic blood pressure, renal dysfunction, and smoking. These findings are similar to those in other cross-sectional studies in diabetic and renal disease patients [3], [4] and [31].
Few previous studies have examined the association between tHcy levels and carotid IMT in diabetic populations, and the results of these studies are discordant. In patients with Type 2 diabetes (T2DM) with and without nephropathy, and in elderly men with cardiovascular risk factors including diabetes, mean tHcy levels were not significantly correlated with common carotid IMT [15], [32], [33] and [34]. On the other hand, significant cross-sectional associations of IMT with higher (> 9-12 μmol/L) versus lower levels of tHcy have been reported by The National Heart, Lung, and Blood Institute (NHLBI) Family Heart Study in older subjects (n=1467) with and without diabetes mellitus [14]. Similar findings were reported from the Hoorn Study of T2DM patients (n=231) following adjustment for potential confounding factors [16]. Cross-sectional data from the Homocysteine and Atherosclerosis Reduction Trial (HART) in patients with T2DM and established vascular disease (n=923) showed no significant association between IMT and increasing quartiles of tHcy when adjusted for traditional vascular risk factors [25]. However, carotid plaque calcification, representing an advanced form of vascular disease, was significantly associated with increasing tHcy levels in HART [25]. Discrepancies in these cross-sectional study findings might be explained by factors such as sample size, patient characteristics, type of diabetes, folate status, and the vascular outcomes measured. While prospective data on the association between IMT progression and tHcy in a diabetic population are lacking, a few follow-up studies address the role of tHcy in advanced cardiovascular disease and mortality in small samples of diabetic patients (n ~ 12-147): these studies show significant correlations between hyperhomocysteinemia (> 12 or 14 μmol/L) and incidence of stroke or overall mortality in patients with T2DM when compared to non-diabetic controls, but do not report on the association with IMT [26] and [27]. While we are not aware of any studies reporting prospective associations of tHcy with IMT in T1DM, tHcy revealed no significant correlations with markers of subclinical atherosclerosis [carotid IMT, coronary artery calcification (CAC)] in preadolescent children and adults with T1DM exhibiting mean tHcy levels ≤ 10μmol/L [35] and [36]. Thus, in the absence of hyperhomocysteinemia in uncomplicated T1DM, as represented by the original DCCT/EDIC cohort [20], tHcy may not be significantly associated with progression of subclinical atherosclerosis.
In our prospective study, the significant associations of tHcy with common and internal carotid IMT at EDIC Years 6 and 12, although not with interim progression, may be attributed to Hcy inducing vascular endothelial dysfunction via mechanisms such as oxidative stress and inflammation [11] and [12]. We, however, did not find any significant correlation between tHcy levels and inflammation as reflected by serum CRP levels. Multivitamin use was inversely associated with tHcy, supporting the role of folate and B-vitamins in lowering tHcy in T1DM, although in studies by others, such effects were not associated with changes in endothelial function in T1DM [37]. Other studies of participants with hyperhomocysteinemia, though none exclusively in T1DM, demonstrate that oral vitamin supplementation lowers Hcy levels leading to significant decreases (~ 0.03-0.05 mm) in carotid IMT over a period of 18 months [38], including in patients with chronic renal disease [39]. However, there were no positive outcomes of Hcy-lowering treatments on clinical cardiovascular events, including stroke, in prospective studies among patients with vascular disease [40] and [41]. Thus, tHcy may be an “innocent” bystander, and the tHcy-CVD association may be largely modulated by conventional risk factors and other biomarkers of oxidative stress and inflammation. To our knowledge, no specific intervention studies addressing the effects of lowering tHcy on hard clinical cardiovascular events (acute myocardial infarction (AMI), stroke, mortality) have been reported in T1DM.
Our study has specific limitations: the absence of non-diabetic control subjects, absence of data on plasma/serum levels or dietary intakes of B-vitamins including folic acid, the short follow-up period, and a relatively small number of T1DM patients in each quartile of tHcy. The latter could limit power to demonstrate differences in inter-quartile comparisons, especially in relation to 6-year IMT changes. Also, we obtained only a single measurement of tHcy (in 1997-1999), and lack measurements at periodic intervals between EDIC Years 6 and 12. However, long-term stability and low within-person variability of tHcy are well documented, supporting the reliability of the single “baseline tHcy” measurement in our study [42] and [43]. tHcy levels are influenced by genetic variants, and we have not included relevant genotypes in our study. Though our analyses were adjusted for significant demographic and clinical variables, including concurrent multivitamin use at the time of blood sampling, the possibility of confounding by unknown/undefined factors remains. Concerning ‘generalizability’, we consider that any bias caused by DCCT entry criteria (DCCT enrollees (1983-1987) were required to have normal blood pressure and cholesterol) is unlikely to have had a residual effect by 1997-1999 when samples for the current study were collected. DCCT participants were residents of the US and Canada, and were 97% Caucasian, so generalizability to other racial or ethnic groups is unclear.
5. Conclusions
In 599 Type 1 diabetic patients from DCCT/EDIC cohort, plasma tHcy levels were similar to those established for the general population, and correlated with numerous demographic and clinical parameters. In multivariate analyses, significant correlations were maintained for age, diastolic blood pressure, and renal function. Plasma tHcy also correlated with common and internal carotid IMT measurements obtained approximately one and seven years later, but did not correlate with IMT progression as defined by the difference between these two determinations. A tHcy cut-point of 10 mol/L failed to delineate patients with higher IMT or higher IMT progression. In the future we will assess tHcy as a predictor of actual CVD events and mortality in the cohort, but the current evidence does not support the routine measurement of tHcy in people with T1DM.
Supplementary Material
Highlights.
Plasma total homocysteine (tHcy) measured in 599 participants with type 1 diabetes
tHcy positively correlated with carotid IMT in cross-sectional analyses
tHcy correlated with age, diastolic blood pressure, renal dysfunction and smoking
tHcy not correlated with 6-year IMT progression after adjustment for CVD risk factors
Acknowledgements
Funding was provided by the American Diabetes Association, Juvenile Diabetes Foundation International (41998272, 996001), National Institutes of Health (PO1 HL55782), and the Diabetes Research and Wellness Foundation (Fairfax, VA).
A complete list of participants in the DCCT/EDIC research group can be found in New England Journal of Medicine, 2011;365:2366-2376.
Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown NY )Becton Dickinson (Franklin Lakes, NJ), CanAm (Atlanta, GA) , Eli Lilly (Indianapolis, IN), Lifescan (Milpitas, CA) , Medtronic Diabetes Minneapolis, MI) , Omron (Shelton CT), OmniPod® Insulin Management System Bedford, MA , Roche Diabetes Care (Indianapolis, IN) , and Sanofi-Aventis (Bridgewater NJ)
DCCT/EDIC has been supported by U01 Cooperative Agreement grants (1982-93, 2011-2016), and contracts (1982-2011) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the Genetic Clinical Research Centers Program (1993- 2007), and Clinical Translational Science Center Program (2006-present), Bethesda, Maryland, USA. The authors thank DCCT/EDIC patients, study nurses and co-ordinators. Technical assistance of Karina Moller, Kevin Joyce, Yanis Bellil, Lyle Walton, Leslie Potter, Andrea Semler, Jenny Smith, Leslie Nichols, Hillarie Stecker and Azar Dashti is acknowledged.
Grants were provided by: the American Diabetes Association (7-12-CT-46), the National Institutes of Health NIH (PO1 HL55782 to WTG, and RO1DK080043 to TJL), Juvenile Diabetes Research Foundation (# 4-1998-272, 996001, and 197028), the Diabetes Research and Wellness Foundation, Inc. (Fairfax, Virginia), and the Presbyterian Health Foundation of Oklahoma City. No other potential conflicts of interest relevant to this article were reported. The authors would like to thank John W. Baynes, PhD, for helpful discussions.
A.B., A.J.J., and J.A.S. analyzed data, wrote, reviewed and edited the manuscript. S.R.T. analyzed samples and reviewed the manuscript. R.L.K., M.F. L-V. and W.T.G. reviewed and edited the manuscript. T.J.L. wrote, reviewed and edited the manuscript. T.J.L. is the guarantor of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–46. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- [2].Bostom A, Brosnan JT, Hall B, et al. Net uptake of plasma homocysteine by the rat kidney in vivo. Atherosclerosis. 1995;116:59–62. doi: 10.1016/0021-9150(95)05522-x. [DOI] [PubMed] [Google Scholar]
- [3].Soedamah-Muthu SS, Chaturvedi N, Teerlink T, et al. Plasma homocysteine and microvascular and macrovascular complications in type 1 diabetes: a cross-sectional nested case-control study. J Intern Med. 2005;258:450–9. doi: 10.1111/j.1365-2796.2005.01560.x. [DOI] [PubMed] [Google Scholar]
- [4].Wollesen F, Brattstrom L, Refsum H, et al. Plasma total homocysteine and cysteine in relation to glomerular filtration rate in diabetes mellitus. Kidney Int. 1999;55:1028–35. doi: 10.1046/j.1523-1755.1999.0550031028.x. [DOI] [PubMed] [Google Scholar]
- [5].McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–28. [PMC free article] [PubMed] [Google Scholar]
- [6].Neugebauer S, Tarnow L, Stehouwer C, et al. Total plasma homocysteine is associated with hypertension in Type I diabetic patients. Diabetologia. 2002;45:1315–24. doi: 10.1007/s00125-002-0908-4. [DOI] [PubMed] [Google Scholar]
- [7].Targher G, Zenari L, Bertolini L, et al. Plasma total homocysteine levels are associated with von Willebrand factor, soluble intercellular adhesion molecule-1, and soluble tumor necrosis factor-alpha receptors in young type 1 diabetic patients without clinical evidence of macrovascular complications. Diabetes Care. 2001;24:1496–97. doi: 10.2337/diacare.24.8.1496-a. [DOI] [PubMed] [Google Scholar]
- [8].Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- [9].Pignoli P, Tremoli E, Poli A, et al. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- [10].Dalager S, Paaske WP, Kristensen IB, et al. Artery-related differences in atherosclerosis expression: implications for atherogenesis and dynamics in intima-media thickness. Stroke. 2007;38:2698–2705. doi: 10.1161/STROKEAHA.107.486480. [DOI] [PubMed] [Google Scholar]
- [11].Hofmann MA, Lalla E, Lu Y, et al. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107:675–83. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bagi Z, Ungvari Z, Szollar L, et al. Flow-induced constriction in arterioles of hyperhomocysteinemic rats is due to impaired nitric oxide and enhanced thromboxane A(2) mediation. Arterioscler Thromb Vasc Biol. 2001;21:233–37. doi: 10.1161/01.atv.21.2.233. [DOI] [PubMed] [Google Scholar]
- [13].Durga J, Verhoef P, Bots ML, et al. Homocysteine and carotid intima-media thickness: a critical appraisal of the evidence. Atherosclerosis. 2004;176:1–19. doi: 10.1016/j.atherosclerosis.2003.11.022. [DOI] [PubMed] [Google Scholar]
- [14].Tsai MY, Arnett DK, Eckfeldt JH, et al. Plasma homocysteine and its association with carotid intimal-medial wall thickness and prevalent coronary heart disease: NHLBI Family Heart Study. Atherosclerosis. 2000;151:519–24. doi: 10.1016/s0021-9150(99)00409-8. [DOI] [PubMed] [Google Scholar]
- [15].Mazza A, Motti C, Nulli A, et al. Lack of association between carotid intima-media thickness and methylenetetrahydrofolate reductase gene polymorphism or serum homocysteine in non-insulin-dependent diabetes mellitus. Metabolism. 2000;49:718–23. doi: 10.1053/meta.2000.6254. [DOI] [PubMed] [Google Scholar]
- [16].Becker A, Henry RM, Kostense PJ, et al. Plasma homocysteine and S-adenosylmethionine in erythrocytes as determinants of carotid intima-media thickness: different effects in diabetic and non-diabetic individuals. The Hoorn Study. Atherosclerosis. 2003;169:323–30. doi: 10.1016/s0021-9150(03)00199-0. [DOI] [PubMed] [Google Scholar]
- [17].Polak JF, Backlund JY, Cleary PA, et al. Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes. 2011;60:607–13. doi: 10.2337/db10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lyons TJ, Jenkins AJ, Zheng D, et al. Nuclear magnetic resonance-determined lipoprotein subclass profile in the DCCT/EDIC cohort: associations with carotid intima-media thickness. Diabet Med. 2006;23:955–66. doi: 10.1111/j.1464-5491.2006.01905.x. [DOI] [PubMed] [Google Scholar]
- [19].Lopes-Virella MF, Carter RE, Gilbert GE, et al. Risk factors related to inflammation and endothelial dysfunction in the DCCT/EDIC cohort and their relationship with nephropathy and macrovascular complications. Diabetes Care. 2008;31:2006–12. doi: 10.2337/dc08-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- [21].Nathan DM, Lachin J, Cleary P, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348:2294–303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- [23].Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. The DCCT Research Group. Clin Chem. 1987;33:2267–71. [PubMed] [Google Scholar]
- [25].Held C, Sumner G, Sheridan P, et al. Correlations between plasma homocysteine and folate concentrations and carotid atherosclerosis in high-risk individuals: baseline data from the Homocysteine and Atherosclerosis Reduction Trial (HART) Vasc Med. 2008;13:245–53. doi: 10.1177/1358863X08092102. [DOI] [PubMed] [Google Scholar]
- [26].Hoogeveen EK, Kostense PJ, Jakobs C, et al. Hyperhomocysteinemia increases risk of death, especially in type 2 diabetes : 5-year follow-up of the Hoorn Study. Circulation. 2000;101:1506–11. doi: 10.1161/01.cir.101.13.1506. [DOI] [PubMed] [Google Scholar]
- [27].Perry IJ, Refsum H, Morris RW, et al. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet. 1995;346:1395–8. doi: 10.1016/s0140-6736(95)92407-8. [DOI] [PubMed] [Google Scholar]
- [28].Sahakyan K, Klein BE, Myers CE, et al. Novel risk factors in long-term hypertension incidence in type 1 diabetes mellitus. Am Heart J. 2010;159:1074–80. doi: 10.1016/j.ahj.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Meloni GF, Tonolo GC, Zuppi C, et al. Hyper-homocysteinemia is not a main feature of juvenile uncomplicated type 1 diabetes. J Atheroscler Thromb. 2005;12:14–9. doi: 10.5551/jat.12.14. [DOI] [PubMed] [Google Scholar]
- [30].Matteucci E, Rossi L, Mariani S, et al. Blood levels of total homocysteine in patients with type 1 diabetes (with no complications, diabetic nephropathy and/or retinopathy) and in their non-diabetic relatives. Nutr Metab Cardiovasc Dis. 2002;12:184–9. [PubMed] [Google Scholar]
- [31].Kurella Tamura M, Xie D, Yaffe K, et al. Vascular risk factors and cognitive impairment in chronic kidney disease: the Chronic Renal Insufficiency Cohort (CRIC) study. Clin J Am Soc Nephrol. 2011;6:248–56. doi: 10.2215/CJN.02660310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Scaglione L, Gambino R, Rolfo E, et al. Plasma homocysteine, methylenetetrahydrofolate reductase gene polymorphism and carotid intima-media thickness in Italian type 2 diabetic patients. Eur J Clin Invest. 2002;32:24–8. doi: 10.1046/j.1365-2362.2002.00936.x. [DOI] [PubMed] [Google Scholar]
- [33].Linnebank M, Moskau S, Farmand S, et al. Homocysteine and carotid intima-media thickness in a german population: lack of clinical relevance. Stroke. 2006;37:2840–2. doi: 10.1161/01.STR.0000244764.02851.d3. [DOI] [PubMed] [Google Scholar]
- [34].Nakhai-Pour HR, Grobbee DE, Bots ML, et al. Circulating homocysteine and large arterial stiffness and thickness in a population-based sample of middle-aged and elderly men. J Hum Hypertens. 2007;21:942–8. doi: 10.1038/sj.jhh.1002247. [DOI] [PubMed] [Google Scholar]
- [35].Babar GS, Zidan H, Widlansky ME. Impaired endothelial function in preadolescent children wth type 1 diabetes. Diabetes Care. 2011;34:681–5. doi: 10.2337/dc10-2134. e al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wadwa RP, Kinney GL, Ogden L, et al. Soluble interleukin-2 receptor as a marker for progression of coronary artery calcification in type 1 diabetes. Int J Biochem Cell Biol. 2006;38:996–1003. doi: 10.1016/j.biocel.2005.09.015. [DOI] [PubMed] [Google Scholar]
- [37].Wotherspoon F, Laight DW, Turner C, et al. The effect of oral folic acid upon plasma homocysteine, endothelial function and oxidative stress in patients with type 1 diabetes and microalbuminuria. Int J Clin Pract. 2008;62:569–74. doi: 10.1111/j.1742-1241.2007.01658.x. [DOI] [PubMed] [Google Scholar]
- [38].Ntaios G, Savopoulos C, Karamitsos D, et al. The effect of folic acid supplementation on carotid intima-media thickness in patients with cardiovascular risk: a randomized, placebo-controlled trial. Int J Cardiol. 2010;143:16–9. doi: 10.1016/j.ijcard.2009.01.023. [DOI] [PubMed] [Google Scholar]
- [39].Nanayakkara PW, van Guldener C, ter Wee PM, et al. Effect of a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on carotid intima-media thickness, endothelial function, and renal function in patients with mild to moderate chronic kidney disease: results from the Anti-Oxidant Therapy in Chronic Renal Insufficiency (ATIC) Study. Arch Intern Med. 2007;167:1262–70. doi: 10.1001/archinte.167.12.1262. [DOI] [PubMed] [Google Scholar]
- [40].Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- [41].Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- [42].McKinley MC, Strain JJ, McPartlin J, et al. Plasma homocysteine is not subject to seasonal variation. Clin Chem. 2001;47:1430–6. [PubMed] [Google Scholar]
- [43].Rasmussen K, Moller J. Total homocysteine measurement in clinical practice. Ann Clin Biochem. 2000;37:627–48. doi: 10.1258/0004563001899915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.