Abstract
Rationale
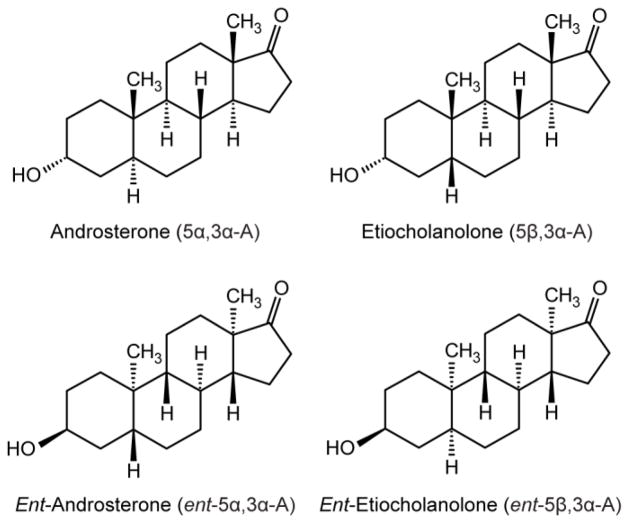
Androsterone [(3α,5α)-3-hydroxyandrostan-17-one; 5α,3α-A] and its 5β-epimer etiocholanolone [(3α,5β)-3-hydroxyandrostan-17-one; 5β,3α-A)], the major excreted metabolites of testosterone, are neurosteroid positive modulators of GABAA receptors. Such neurosteroids typically show enantioselectivity in which the natural form is more potent than the corresponding unnatural enantiomer. For 5α,3α-A and 5β,3α-A, the unnatural enantiomers are more potent at GABAA receptors than the natural forms.
Objectives
The aim of this study was to compare the anticonvulsant potencies and time courses of 5α,3α-A and 5β,3α-A with their enantiomers in mouse seizure models.
Methods
Steroids were administered intraperitoneally to male NIH Swiss mice 15 min (or up to 6 h in time course experiments) prior to administration of an electrical stimulus in the 6-Hz or maximal electroshock (MES) seizure tests or the convulsant pentylenetetrazol (PTZ).
Results
In the 6-Hz test, the ED50 values of ent-5α,3α-A was 5.0 mg/kg whereas the value for 5α,3α-A was 12.1 mg/kg; the corresponding values in the PTZ seizure test were 22.8 and 51.8 mg/kg. Neurosteroid GABAA receptor positive allosteric modulators are generally weak in the MES test and this was confirmed in the present study. However, the atypical relative potency relationship was maintained with ED50 values of 140 and 223 mg/kg for ent-5α,3α- A and 5α,3α-A, respectively. Similar relationships were obtained for the 5β-isomers, except that the enantioselectivity was accentuated. In the 6-Hz and PTZ tests, the ED50 values of ent-5β,3α-A were 11.8 and 20.4 mg/kg whereas the values for 5β,3α-A were 57.6 and 109.1 mg/kg. Protective activity in the 6-Hz test of ent-5α,3α-A persisted for somewhat longer (~5 h) than for 5α,3α-A (~4 h); protection by ent-5β,3α-A also persisted longer (~3 h) than for 5β,3α-A (~2 h).
Conclusions
The unnatural enantiomers of 17-keto androgen class neurosteroids have greater in vivo potency and a longer duration of action than their natural counterparts. The more prolonged duration of action of the unnatural enantiomers could reflect reduced susceptibility to metabolism. Unnatural enantiomers of androgen class neurosteroids could have therapeutic utility and may provide advantages over the corresponding natural isomers due to enhanced potency and improved pharmacokinetic characteristics.
Keywords: androsterone, etiocholanolone, enantiomer, 6-Hz test, pentylenetetrazol test, maximal electroshock test
Introduction
Certain endogenous steroid hormone metabolites are positive allosteric modulators of GABAA receptors at low concentrations and directly gate these receptors at higher concentrations (Lambert et al., 2009). As is the case for other agents that enhance inhibitory GABAergic function, such neurosteroids protect against seizures in diverse animal models, and there is emerging evidence that agents of this type are effective in the treatment of seizures and epilepsy in humans (Reddy and Rogawski, 2012; Bialer et al., 2013). The anticonvulsant potency of such steroids is closely correlated with their activity at GABAA receptors (Morrow et al., 1990; Kokate et al., 1994). Although the pregnanes allopregnanolone [(3α,5α)-3-hydroxypregnan-20-one)] and tetrahydrodeoxycorticosterone [(3α,5α)-3,21-dihydroxypregnan-20-one)] were the first endogenous GABAA receptor modulatory neurosteroids to be identified (Paul and Purdy, 1992), it is now recognized that a number of structurally related endogenous steroids have similar actions on GABAA receptors. In particular, the 17-ketosteroid androsterone (5α,3α-A; Fig. 1) potentiatates GABAA receptor responses albeit more weakly than allopregnanolone (Turner et al., 1989; Hawkinson et al., 1994; Anderson et al., 2000), and we demonstrated that it, as well as its 5β-epimer etiocholanolone (5β,3α-A), exhibit anticonvulsant activity in animal seizure models (Kaminski et al., 2005).
Fig. 1.
Structures of the natural 17-keto androgen class neurosteroids androsterone and etiocholanolone and their unnatural enantiomers.
Most neurosteroids show enantioselectivity in which the natural form is more potent than the corresponding unnatural enantiomer. Notably, the enantiomers of allopregnanolone and its 5β-epimer pregnanolone are much less effective at potentiating GABAA receptor responses than the corresponding natural steroids (Wittmer et al., 1996; Covey et al., 2000; Covey, 2009). Recently, Katona et al. (2008) made the unexpected discovery that the enantiomers of androsterone and etiocholanolone (ent-5α,3α-A and ent-5β,3α-A, respectively) are more potent than the natural steroids as (i) inhibitors of [35S]TBPS binding (a functional measure GABAA receptor activity), (ii) enhancers of rat α1β2γ2L GABAA receptor currents in Xenopus laevis oocytes, and (iii) anesthetics causing loss of righting reflex in tadpoles. As of yet, the enhanced potency of the enantiomers has not been verified in mammals. Therefore, in the present study we examined the activity of the enantiomers in comparison with their natural counterparts in mice in several seizure models. The metabolism of neurosteroids is complex and we reasoned that the enantiomers might be less susceptible to bioinactivation or elimination than their natural counterparts. Accordingly, it was of interest to assess the time course of action of the enantiomers.
Materials and methods
Animals
Male NIH Swiss mice (22–30 g) were housed four per cage. Animals were kept in a vivarium under controlled laboratory conditions (temperature, 22–26 °C; humidity, 40–50%) with an artificial 12-h light/dark cycle and free access to food and water. Animals were allowed to acclimate to the vivarium for ≥5 days. The experiments were performed during the light phase of the light/dark cycle after a ≥30-min period of acclimation to the experimental room. Animals were maintained in facilities fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all studies were performed under protocols approved by the University of California, Davis, Institutional Animal Care and Use Committee in strict compliance with the Guide for the Care and Use of Laboratory Animals of the National Research Council (National Academy Press, Washington, DC; http://www.nap.edu/readingroom/books/labrats/). Mice that survived testing were euthanized with CO2.
Test substances and drug administration
Solutions of 5α,3α-A, 5β,3α-A (Sigma-Aldrich, St. Louis, MO, U.S.A.) and ent-5α,3α-A and ent-5β,3α-A (synthesized by K.K. in the laboratory of D.F.C.; see Katona et al., 2008) were made fresh daily in 40% hydroxypropyl-β-cyclodextrin (Trappsol®; Cyclodextrin Technologies Development, High Springs, FL, U.S.A.) in sterile 0.9% saline. Further dilutions were made by using sterile saline. The convulsant agent pentylenetetrazol (PTZ; Sigma-Aldrich) was dissolved in saline immediately before use. All drug solutions were administered in a volume equaling 0.01 ml/g body weight. In the PTZ, 6-Hz and MES seizure tests, the steroids or vehicle were administered intraperitoneally 15 min before PTZ or electrical stimulation. The pretreatment interval was based on the time of maximal effect in the time-course experiment in the 6-Hz model (Fig. 3). Vehicle alone did not affect seizures in any of the models.
6-Hz electroshock seizure test
Testing was carried out as described previously (Kaminski et al., 2004). In brief, 3-s corneal stimulation (200-μs duration, 32-mA monopolar rectangular pulses at 6 Hz) was delivered by a constant-current device (ECT Unit 5780; Ugo Basile, Comerio, Italy). Ocular anesthetic (0.5% tetracaine) was applied to the corneas 15 min before stimulation. Immediately before stimulation, the corneal electrodes were wetted with saline to provide good electrical contact. The seizures were often preceded by a period of intense locomotor agitation (wild running and jumping). The animals then exhibited a “stunned” posture associated with rearing (bipedal standing), forelimb automatic movements and clonus, twitching of the vibrissae, and Straub-tail. The duration of the seizure activity ranged from 60 to 120 s in untreated animals. Animals resumed their normal exploratory behavior after the seizure. The experimental end point was protection against the seizure: an animal was considered to be protected if it resumed its normal exploratory behavior within 10 s of stimulation.
PTZ seizure test
Testing was carried out as previously described (Kokate et al., 1994). In brief, mice were injected subcutaneously with PTZ (80 mg/kg) and were observed for a 30-min period. Mice failing to show clonic seizures lasting >5 s were scored as protected.
Maximal electroshock (MES) seizure test
Animals were subjected to a 0.2-s, 60-Hz electrical stimulus through corneal electrodes (as described earlier). The electroshock unit was adjusted to deliver a constant current of 50 mA. Animals failing to show tonic hindlimb extension were scored as protected (Kokate et al., 1994).
Motor toxicity test
Steroids were evaluated for motor toxicity by using a modification of the horizontal screen test as described previously (Kokate et al., 1994). Mice were placed on a horizontally oriented grid (consisting of parallel 1.5-mm diameter rods situated 1 cm apart) and the grid was inverted. Animals that fell from the grid within 10 s were scored as impaired.
Data analysis
To construct dose–response curves, steroids were tested at several doses spanning the dose producing 50% protection (ED50) or motor impairment (TD50). ED50 and TD50 values and their corresponding 95% confidence limits were determined by log-probit analysis using the Litchfield and Wilcoxon method (PHARM/PCS Version 4.2; Micro- Computer Specialists, Philadelphia, PA, U.S.A.). The protective index (PI), a measure of relative toxicity, was calculated as the ratio TD50/ED50. Half-time (t½) was estimated by non-linear single-exponential fits to the falling phase of the time-course data. The eudismic ratio was taken as the ratio of the ED50 or TD50 values of the less active enantiomer (distomer) to that of the more potent enantiomer (eutomer).
Results
6-Hz test
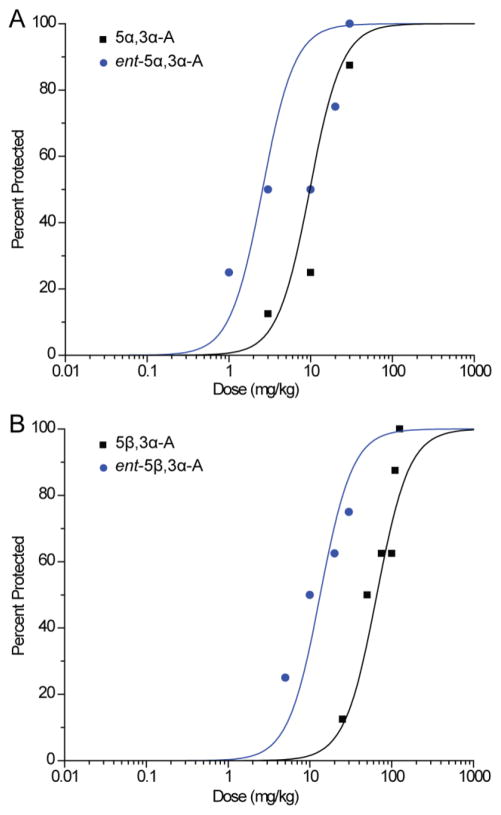
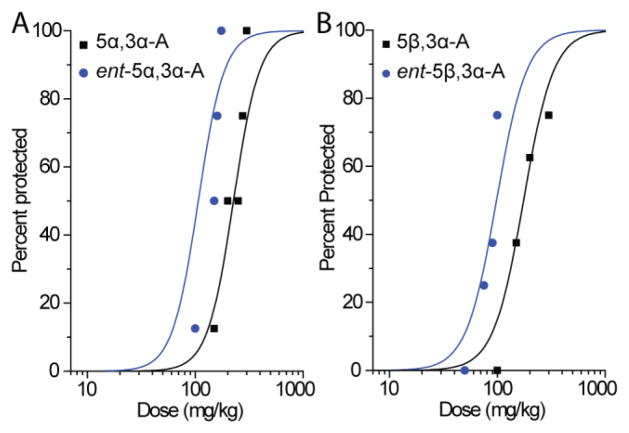
In confirmation of our previous study (Kaminski et al., 2005), we found that pretreatment with 5α,3α-A conferred protection in the 6-Hz test in a dose-dependent fashion (Fig. 2A). Ent-5α,3α-A also conferred protection in a dose-dependent fashion, but was somewhat more potent (Fig. 2A and Table 1). Similarly, 5β,3α-A was effective in the 6-Hz test, but modestly less potent than its epimer 5α,3α-A (Fig. 2B). The enantiomer ent-5β,3α-A was more potent than its natural counterpart.
Fig. 2.
A, Dose–response relationships for protective activity of androsterone (5α,3α-A) and entandrosterone (ent-5α,3α-A) in the 6-Hz seizure test. Steroids were administered 15 min before electrical stimulation. Data points indicate percentage of animals protected. Each point represents eight mice. B, Dose–response relationship for protective activity of etiocholanolone (5β,3α-A) and ent-etiocholanolone (ent-5β,3α-A) in the 6-Hz test. Each point represents eight mice.
Table 1.
ED50 values of androsterone (5α,3α-A), ent-androsterone (ent-5α,3α-A), etiocholanolone (5β,3α-A) and ent-etiocholanolone (ent-5β,3α-A) for protection in the 6-Hz electrical stimulation, pentylenetetrazol (PTZ) and maximal electroshock (MES) seizure tests in mice
| Steroid | ED50 (95% confidence intervals), mg/kga [PIb] |
||
|---|---|---|---|
| 6-Hz | PTZ | MES | |
| 5α,3α-A | 12.10 (6.04–24.30) [15.13] |
51.80 (32.57–82.46) [3.53] |
222.90 (180.20–275.80) [0.82] |
| ent-5α,3α-A | 4.95 (1.40–17.60) [14.18] |
22.80 (18.40–28.10) [3.08] |
140.10 (115.13–170.43) [0.50] |
| 5β,3α-A | 57.57 (39.60–83.70) [2.61] |
109.07 (100.31–118.60) [1.37] |
177.07 (118.34–264.94) [0.85] |
| ent-5β,3α-A | 11.78 (5.98–23.20) [3.07] |
20.38 (13.70–30.32) [1.78] |
89.87 (79.05–102.16) [0.40] |
ED50 values (with 95% confidence intervals in parentheses) represent the dose in mg/kg that is estimated to protect 50% of animals.
PI (protective index) is the ratio between TD50 (the dose estimated to produce motor impairment in 50% of animals in the horizontal screen test) value and the ED50 value. The TD50 values (95% confidence intervals) for 5α,3α-A and ent-5α,3α-A were 183.1 (136.5–245.8) mg/kg and 70.2 (42.6– 115.6) mg/kg, respectively. The TD50 values for 5β,3α-A and ent-5β,3α-A were 150.0 (84.1–267.4) mg/kg and 36.2 (24.1–54.4) mg/kg, respectively.
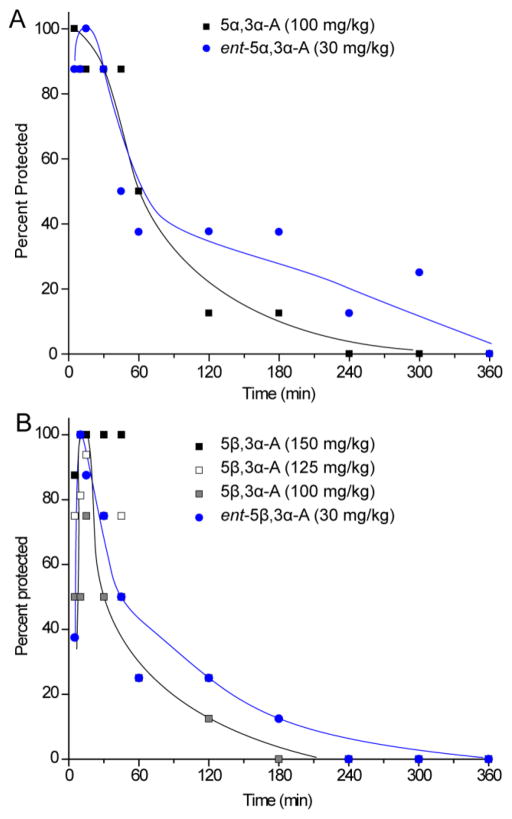
We used the 6-Hz test to assess the time course of action of the steroids. As shown in Fig. 3A, 5α,3α-A (100 mg/kg) conferred seizure protection in all animals at 5 min, the earliest time point tested, and substantial protection up to 45 min; protection dropped over the next 135 min and was no longer evident at 240 min. A lower dose of ent-5α,3α-A (30 mg/kg) was used to achieve a similar degree of peak seizure protection. At this dose, nearly complete protection was achieved at time points up to 30 min. Protection then dropped at successive time points but protection was still evident at 300 min. Thus, the tail of the response to the enantiomer was more prolonged than that of its natural counterpart even though a substantially lower dose was used. The fraction of animals protected at time points ≥2 h by ent-5α,3α-A was significantly greater than those protected by 5α,3α-A (p=0.048 by Fisher’s exact test). The t½ values for 5α,3α-A and ent-5α,3α-A were 67.1 ± 10.0 min and 80.6 ± 13.9 min, respectively.
Fig. 3.
A, Time course for protection by 5α,3α-A and ent-5α,3α-A at doses of 100 and 30 mg/kg respectively in the 6-Hz (32 mA, 3 s) seizure test. The interval between the steroid injection and the electrical stimulus is plotted on the abscissa and the percentage of animals protected against seizures is plotted on the ordinate. B, Time course for protection by 5β,3α-A at a doses of 150, 125, 100 mg/kg and ent-5β,3α-A at a dose of 30 mg/kg in the 6-Hz test. Each point represents at least eight mice. Curves are arbitrary fits to the data; the curve representing 5β,3α-A is to the 100 mg/kg group.
Fig. 3B show time course results with various doses of the epimer 5β,3α-A. The higher doses (150 and 125 mg/kg) produced substantial protection up to 45 min: the highest dose conferred full protection from 10 to 45 min, whereas the 125 mg/kg was modestly less effective, peaking at 94 percent at 15 min. Seizure protection with the lowest dose (100 mg/kg) also peaked at 15 min but the maximum protection achieved was only 75 percent. A lower dose of ent-5β,3α-A (30 mg) chosen to just achieve full seizure protection, exhibited 100 percent protection at 10 min. Seizure protection then fell progressively but was still evident at 180 min. Therefore, ent-5β,3α-A even at the substantially lower dose had a slightly more prolonged duration of action although the difference did not reach statistical significance. The t½ values for 5α,3α-A (125 mg/kg) and ent-5α,3α-A were 55.0 ± 11.7 min and 72.4 ± 46.2 min, respectively.
PTZ seizure test
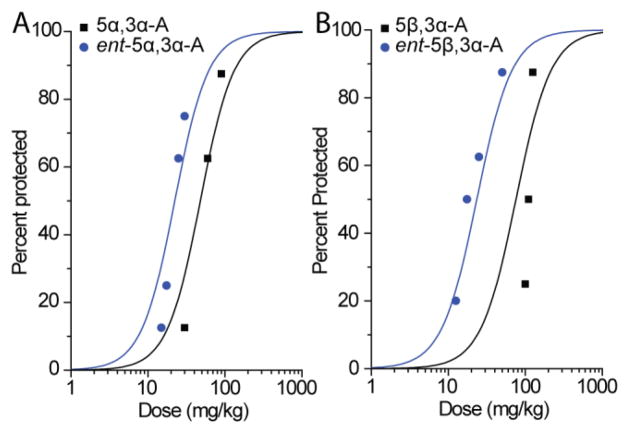
As in our earlier study (Kaminski et al., 2005), 5β,3α-A and 5β,3α-A conferred protection in the PTZ seizure test in a dose-dependent fashion (Fig. 4). The enantiomers also conferred dosedependent protection but were considerably more potent (Fig. 4; Table 1).
Fig. 4.
A, Dose–response relationship for protective activity of 5α,3α-A and ent-5α,3α-A in the pentylenetetrazol (PTZ) test. Steroids were administered 15 min before injection of PTZ. Data points indicate percentage of animals protected against clonic seizures. Each point represents eight mice. B, Dose–response relationship for protective activity of 5β,3α-A and ent-5β,3α-A in the PTZ test. Each point represents eight to ten mice.
MES test
GABAA receptor active neurosteroids generally have low potency in the MES test (Kokate et al., 1994). The 17-ketosteroids are no different in this regard (Kaminski et al., 2005). As was the case in the other seizure models, the enantiomers were more potent than their natural counterparts in the MES test (Fig. 5; Table 1).
Fig. 5.
A, Dose–response relationships for protection by 5α,3α-A and ent-5α,3α-A in the maximal electroshock (MES; 50 mA, 0.2 s, 60 Hz) test. Steroids were administered 15 min before electrical stimulation. Data points indicate percentage of animals protected against seizures. Each point represents eight mice. B, Dose–response relationships for protection by 5β,3α-A and ent-5β,3α-A in the MES test. Each point represents eight mice.
Motor toxicity
All of the steroids caused motor impairment in the horizontal screen test (Table 1). As in the seizure tests, the enantiomers were more potent than their corresponding natural analogs for inducing positive effects in the horizontal screen test. Doses causing motor impairment were greater than those effective in the 6-Hz and PTZ test but lower than those effective the MES test. We calculated the “protective index” (PI = TD50/ED50) as a measure of the separation between the dose protecting against seizures and the toxic dose. For each natural steroid and its enantiomer, the PI values in the various models were similar.
Discussion
The 17-ketosteroids androsterone (5α,3α-A) and its 5β-epimer etiocholanolone (5β,3α-A), the major excreted metabolites of testosterone, are weak positive modulators of GABAA receptors. In contrast to other A-ring reduced steroids with GABAA receptor modulatory activity, Katona et al. (2008) reported that the enantiomers ent-5α,3α-A and ent-5β,3α-A were more potent than their natural counterparts as functional modulators of GABAA receptors and also as anesthetics in Xenopus laevis tadpoles. A recent study indicated that GABAA receptor modulation by the ent-forms occurs at the same interaction site in the transmembrane domain of the α-subunit where the natural steroids act (Hosie et al., 2009; Krishnan et al., 2012). Interestingly, the ent-steroids appear to bind co-planar in a “flipped” orientation relative to their natural counterparts, with the hydroxy groups at position 3 in both molecules (which are critical for activity at GABAA receptors) situated so that they can interact with the same site on the protein receptor.
We now confirm the atypical potency relationship between the enantiomers of the androsterone epimers and their natural counterparts. We used the 6-Hz test and the PTZ seizure test, which are sensitive to GABAA receptor positive modulatory neurosteroids (Kokate et al., 1994; Kaminski et al., 2004). In addition, we used the MES test, which is a widely used and well-accepted model to identify antiseizure agents (Krall et al., 1978). In the three mouse seizure models and in a test of motor impairment, which reflects sedation or muscle relaxation, the unnatural enantiomers were more potent than their natural counterparts. The eudismic ratio, a measure of the degree of enantioselectivity in the specific test (Lehmann et al., 1976), ranged from 1.6 to 5.3 (Table 2). As previously reported for the androgen derived A-ring reduced steroids (Kaminski et al., 2005) and also for other structurally related steroids (Kokate et al., 1994) with GABAA receptor activity, the α-orientation of the 5-H is generally preferred to the β-orientation, but only modestly so. This relationship is less clear for the enantiomers. While ent-5α,3α-A was possibly slightly more potent than ent-5β,3α-A in the tadpole assay and in the 6-Hz test, this was not the case for the other tests. A relationship of note that is similar in the present study in mice to that of the previous work in vitro and in tadpoles is that the enantioselectivity of the 5β-enantiomers exceeds that of the 5α-enantiomers. Thus, in all four tests, the eudismic ratio of the 5β-enantiomers is greater than that of the 5α-enantiomers (Table 2), suggesting that ent-5β,3α-A has higher complementarity to its receptor target than does ent-5α,3α-A.
Table 2.
Eudismic ratios for enantiomer pairs in the seizure tests and the test of motor toxicity
| Enantiomer Pairs | Eudismic Ratioa
|
|||
|---|---|---|---|---|
| 6-Hzb | PTZb | MESb | Horizontal Screenc | |
| 5α,3α-A– ent-5α,3α-A | 2.4 | 2.3 | 1.6 | 2.6 |
| 5β,3α-A– ent-5β,3α-A | 4.9 | 5.3 | 2.0 | 4.1 |
Ratio of the ED50 or TD50 values from Table 1 of distomer (natural steroids) to that of eutomer (entsteroids).
Seizure tests.
Test of motor toxicity reflecting sedation or muscle relaxation.
The correspondence in the structure-activity relationships (SAR) observed in the present study and the SAR in the prior study of functional effects on GABAA receptors in vitro (Katona et al., 2008) is consistent with the conclusion that the steroids exert their protective activity in the seizure and motor toxicity tests through effects on GABAA receptors. Prior SAR studies have similarly found that the relative potency ranking of GABAA receptor modulating neurosteroids in the PTZ and 6-Hz tests is closely related to their potencies as GABAA receptor modulators assessed by patch clamp recording in vitro (Kokate et al., 1994; Kaminski et al., 2004; 2005). The results of the present study bolster the conclusion that the anticonvulsant and sedative actions of GABAA receptor modulatory neurosteroids and their analogs occurs through effects on GABAA receptors.
While the natural 17-ketosteroids are generally weaker than other GABAA receptor modulatory neurosteroids in in vivo seizure tests, the unnatural enantiomers had potencies comparable to the most potent known neurosteroids, including allopregnanolone and its synthetic 3β-methyl analog ganaxolone, which is in clinical development for the treatment of epilepsy (Kaminski et al., 2005; Bialer et al., 2013). In the 6-Hz test, ent-5α,3α-A was slightly more potent than ganaxolone (ED50, 6.3 mg/kg) and ~3-fold more potent than allopregnanolone (ED50, 14.2 mg/kg; Kaminski et al., 2004), whereas in the PTZ test, ent-5α,3α was modestly weaker than either ganaxolone or allopregnanolone (Reddy and Rogawski, 2000; Kokate et al., 1994). In general, GABAA receptor modulating neurosteroids have been found to exhibit similar potencies in the 6-Hz and PTZ tests (Kaminski et al., 2004). The reason for the relatively weaker activity in the PTZ test compared with the 6-Hz test in the present study is not apparent.
A particularly interesting outcome of the present study is the observation that the ent-forms were more potent in the MES test than their natural counterparts despite the fact that all steroids tested in this model were relatively weakly active. In the past, we have obtained results suggesting that the weak activity of certain GABAA receptor modulating neurosteroids in the MES test could be due to off-target pharmacological effects distinct from their actions on GABAA receptors (Kokate et al., 1999; Reddy and Rogawski, 2002). Agents with protective activity in the MES test often modulate voltage-gated sodium channels raising the possibility that they or another MES-relevant target could play a role. However, the corresponding enantioselectivity of the 17-ketosteroids in the MES test and for functional effects on GABAA receptors strongly suggests that for these steroids GABAA receptors are the relevant molecular target conferring activity in the MES test. This conclusion is likely generalizable to other GABAA modulatory neurosteroids, supporting the view that protective activity in the MES test, albeit weak, is truly due to effects on GABAA receptors.
An important characteristic of the ent-forms is that equieffective doses had more prolonged durations of action than their natural counterparts. All of the steroids exhibited peak responses from 5 to 15 min after administration. However, the durations of action differed. Thus, ent-5α,3α-A at a dose (30 mg/kg) that produced full protection in the 6 Hz test exhibited some degree of protection for about ~5 h (t½, 80.6 min) whereas 5α,3α-A at a dose (100 mg/kg) that exhibited similar peak protective activity conferred protection for only ~3 h (t½, 67.1 min) (see also Kaminski et al., 2005). Similarly, ent-5β,3α-A had protective activity that persisted about ~3 h (t½, 72.4 min) whereas for 5β,3α-A protection persisted ~2 h (t½, 55.0 min for 125 mg/kg group). The natural forms of the 17-ketosteroids exhibit a duration of action comparable to other structurally similar neurosteroids such as allopregnanolone and tetrahydrodeoxycorticosterone (Kokate et al., 1994; Reddy and Rogawski, 2002; Kaminski et al., 2005). We speculate that the more prolonged duration of action of the unnatural enantiomers reflects their reduced susceptibility to metabolism by steroid metabolic enzymes. Alternatively, the prolonged duration of action could be due to differences in distribution or elimination or to conversion of the longer acting steroids into active metabolites.
All steroids evaluated in the present study produced motor impairment at doses that were greater than those conferring seizure protection in the 6-Hz and PTZ tests but at lower doses than were effective in the MES test. The PI values reported in Table 1 provide a measure of the separation between the doses conferring therapeutic efficacy and adverse effects. The PI values of the 17-ketosteroids and their enantiomers are comparable to those obtained with other steroids acting on the GABAA receptor such as the pregnane steroids allopregnanolone and tetrahydrodeoxycorticosterone and their 5β-epimers (Kokate et al., 1994). In our prior study, the 5α-pregnane steroids had modestly greater PI values than their 5β-epimers. A similar situation applies for the 17-ketosteroids. It is noteworthy that despite the enhanced potency of the ent-steroids, they do not provide an improvement in PI. The PI values for both natural steroids are similar to those of the corresponding enantiomer.
In conclusion, the present study confirms the greater potency of the unnatural enantiomers of 17-keto androgen class neurosteroids in several in vivo mouse models. We have found that in addition to greater potency, the eutomers have more prolonged durations of action likely as a result of greater metabolic stability. Unnatural enantiomers of such steroids could have therapeutic utility and may provide advantages over the corresponding natural isomers due to enhanced potency and improved pharmacokinetic characteristics.
Acknowledgments
Supported in part by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number NS079202 (M.A.R.) and the National Institute of General Medical Sciences of the National Institutes of Health under award number GM47969 (D.F.C.). The experiments were conducted in compliance with all applicable laws and regulations.
Abbreviations
- 5α,3α-A
androsterone
- 5β,3α-A
etiocholanolone
- ent-5α,3α-A
ent-androsterone
- ent-5β,3α-A
ent-etiocholanolone
- PTZ
pentylenetetrazol
- MES
maximal electroshock
- PI
protective index
- SAR
structure-activity relationship
Footnotes
The authors declare no conflicts of interest.
References
- Anderson A, Boyd AC, Clark JK, Fielding L, Gemmell DK, Hamilton NM, Maidment MS, May V, McGuire R, McPhail P, Sansbury FH, Sundaram H, Taylor R. Conformationally constrained anesthetic steroids that modulate GABAA receptors. J Med Chem. 2000;43:4118–4125. doi: 10.1021/jm000977e. [DOI] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI) Epilepsy Res. 2013;103:2–30. doi: 10.1016/j.eplepsyres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Covey DF. ent-Steroids: novel tools for studies of signaling pathways. Steroids. 2009;74:577–585. doi: 10.1016/j.steroids.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DF, Nathan D, Kalkbrenner M, Nilsson KR, Hu Y, Zorumski CF, Evers AS. Enantioselectivity of pregnanolone-induced γ-aminobutyric acidA receptor modulation and anesthesia. J Pharmacol Exp Ther. 2000;293:1009–1016. [PubMed] [Google Scholar]
- Hawkinson JE, Kimbrough CL, Belelli D, Lambert JJ, Purdy RH, Lan NC. Correlation of neuroactive steroid modulation of [35S]t-butylbicyclophosphorothionate and [3H]flunitrazepam binding and γ-aminobutyric acidA receptor function. Mol Pharmacol. 1994;46:977–985. [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology. 2009;6:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABAA receptors protect against partial seizures induced by 6 Hz electrical stimulation in mice. Epilepsia. 2004;45:1–4. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Marini H, Kim WJ, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–827. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona BW, Krishnan K, Cai ZY, Manion BD, Benz A, Taylor A, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 12. Potent enhancement of GABA15 mediated chloride currents at GABAA receptors by ent-androgens. Eur J Med Chem. 2008;43:107–113. doi: 10.1016/j.ejmech.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Banks MK, Magee T, Yamaguchi S, Rogawski MA. Finasteride, a 5α-reductase inhibitor, blocks the anticonvulsant activity of progesterone in mice. J Pharmacol Exp Ther. 1999;288:679–684. [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Krall RL, Penry JK, White BG, Kupferberg HJ, Swinyard EA. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia. 1978;19:409–428. doi: 10.1111/j.1528-1157.1978.tb04507.x. [DOI] [PubMed] [Google Scholar]
- Krishnan K, Manion BD, Taylor A, Bracamontes J, Steinbach JH, Reichert DE, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 17. Inverted binding orientations of androsterone enantiomers at the steroid potentiation site on γ-aminobutyric acid type A receptors. J Med Chem. 2012;55:1334–1345. doi: 10.1021/jm2014925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JJ, Cooper MA, Simmons RD, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABAA receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S48–58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Lehmann FPA, Rodrigues de Miranda JF, Ariëns EJ. Stereoselectivity and affinity in molecular pharmacology. Prog Drug Res. 1976;20:101–142. doi: 10.1007/978-3-0348-7094-8_4. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Pace JR, Purdy RH, Paul SM. Characterization of steroid interactions with γ-aminobutyric acid receptor-gated chloride ion channels: evidence for multiple steroid recognition sites. Mol Pharmacol. 1990;37:263–270. [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000;294:909–915. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAA receptor function and seizure susceptibility. J Neurosci. 2002;22:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroids – Endogenous regulators of seizure susceptibility and role in the treatment of epilepsy (Chapter 77) In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s basic mechanisms of the epilepsies. 4. Oxford University Press; 2012. pp. 984–1002. [Google Scholar]
- Turner DM, Ransom RW, Yang JS, Olsen RW. Steroid anesthetics and naturally occurring analogs modulate the γ-aminobutyric acid receptor complex at a site distinct from barbiturates. J Pharmacol Exp Ther. 1989;248:960–966. [PubMed] [Google Scholar]
- Wittmer LL, Hu Y, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Enantioselectivity of steroid-induced γ-aminobutyric acidA receptor modulation and anesthesia. Mol Pharmacol. 1996;50:1581–1586. [PubMed] [Google Scholar]