Abstract
Objectives:
The effect of direct restorative materials on caries lesion formation was investigated with an 8-week in situ study with split-mouth design, testing the hypothesis that no difference in mineral loss next to a restoration would be found between different composite-based-materials and amalgam.
Methods:
Six groups (n=18) of restored dentin samples were prepared using amalgam, a microhybrid, a nanohybrid and a silorane composite. The composites were adhesively bonded with systems with or without an antibacterial monomer (Clearfil-SE-Protect, Clearfil-SE-bond, respectively), except for the silorane group (Silorane- System-Adhesive). Non-restored dentin samples were used as control (primary caries). Samples were inserted into slots, in lower prosthesis especially made for the experiment. Subjects were instructed to dip the lower prosthesis in a sucrose solution 4 times per day. At baseline and 8 weeks, samples were radiographed extra-orally and the integrated mineral loss was calculated. Data were statistically analyzed using multiple linear regression with a multilevel model (p=0.05).
Results:
Nine subjects were selected, and only outer lesions were observed. The hypothesis was partially rejected, as the microhybrid composite bonded with the antibacterial system and the nanohybrid composite presented statistically significant lower mineral loss compared to amalgam. Also, no significant differences were seen for these groups compared to control.
Conclusion:
Within the limits of this study, the restorative material may influence outer lesion progression. Amalgam was not found to be related to lower secondary caries progression in dentin compared to composite-based materials after 8 weeks in situ.
Keywords: Dentin-Bonding Agents, Dental caries, Dental materials, Microradiography, Tooth demineralization
1. Introduction
Secondary or recurrent caries is defined as a caries lesion developing adjacent to a dental restoration1 and is along with fracture the predominant reason for failure of posterior restorations.2,3 It has been proposed that secondary caries lesions develop as outer lesions on the tooth surface next to the restoration margins and as wall lesions, within the tooth/restoration interface4,5 While wall lesion formation would occur when interfacial gaps are present,6,7 outer lesions would develop similarly to primary caries on the tooth surface.8
Secondary caries has been often related to the restorative material used. In clinical studies, failure for secondary caries has been less frequently found for amalgam than composite restorations.9,10 Some factors could contribute to this finding, such as the surface deterioration of resin composites leading to an increase in surface roughness11 and decrease in surface hardness,12 the elution of unpolymerized monomers from composites and dentin-bonding agents stimulating the growth of cariogenic microorganisms,13 and the polymerization shrinkage, leading to microgap formation14,15 and microleakage.14
Developments in biomaterials science frequently aim to counteract those shortcomings. Of the strategies in use, some have shown beneficial properties, at least in vitro. The use of smaller inorganic fillers in nanocomposites were found to promote lower surface roughness,16 while silorane-based composites showed lower polymerization shrinkage17 and lower quantity of adhering streptococci compared to methacrylate-based restorative materials.18 Another proposed strategy is to add antibacterial components into the adhesive system and composites to reduce the bacterial growth over the surfaces,19 to inhibit the metabolic activity of cariogenic microorganisms20 and to disinfect cavities from residual bacteria.21
In the present study, secondary lesion formation next to different restorative materials was investigated in situ. The null hypothesis tested was that no effect in lesion development next to a restoration would be found between different composite-based materials and amalgam.
2. Materials and methods
The study was submitted to an Ethical Committee Board and approved (CMO code NL 33526091-11).
2.1. Study Design
This was a mono-center, randomized (regarding teeth distribution and sample holders among patients), single blinded (statistician) in situ study, with split-mouth design regarding materials. Independent variables were the restorative materials with varying bonding modalities and unrestored dentin (control), whereas the outcome variable was integrated mineral loss.
2.2. Sample Size
The present study was exploratory, and therefore having a proper sample size calculation was not possible. However, the number of patients was at some level estimated based on the study of Thomas et al. (2007).8 In that study, average lesion progression in dentin samples restored with composite was 83.9 μm (SD 23 μm). We worked under the concept that differences on lesion progression lower than 30% (25.17 μm) would not be meaningful. Then, since a split mouth design would be used, the equation applied was n=f(α,β)*δ2/(μ1-μ2)2,22 from which a sample size of 9 patients was obtained for 5% significance level with 90% power.
2.3. Volunteers
For inclusion, the following criteria were applied: subjects between the ages of 18 and 75 years wearing full prosthesis; good general health; salivary flow ≥ 0.2 ml/min (unstimulated) and ≥ 0.7 ml/min (stimulated). Exclusion criteria were medication that affects immunological system or salivary glands, systemic diseases influencing oral and salivary function and subjects categorized as ASA > 2 (according to the physical status classification system adopted by American Society of Anesthesiologists). The recruiting of volunteers was completed within 2 weeks at Arnhem Dental (Arnhem, NL) and all subjects gave written informed consent. A copy of the lower prosthesis – the trial prosthesis, was made for each volunteer.
2.4. Samples
Dentine samples (A- sized 3.0 · 2.0 · 1.0 mm) and half that size dentine samples (B-1.5 · 2.0 · 1.0 mm) were prepared from extracted sound human molars (Fig. 1a). The enamel portion was removed by grinding in a vertical plate under water cooling and the exposed dentin was prepared using 600-grid papers (Siawat Abrasives, Bern, Switzerland), also under water cooling. Approximately 4 dentin samples were obtained from each tooth at the middle third (2 from the mesial site and 2 from the distal site) using a water cooled diamond saw at low speed. The whole sized dentine samples (A) were left unrestored to provide a primary caries development control group. The half-sized sections (B) were randomly distributed and built up with different restorative materials/techniques, with the orientation of the dentin tubuli positioned perpendicular to the outer surface, assessed under magnification lenses. This resulted in whole-sized (3.2 · 2.0 · 1.0 mm) samples of dentine/material (Fig. 1a). Materials selected were: amalgam (Tytin), two methacrylate-based composites – one microhybrid (Clearfil AP-X) and one nanohybrid composite (Filtek Supreme), and a silorane-based composite (Filtek Silorane). In total, 6 groups of dentin/material were prepared, which are described in table 1. The silorane group was bonded with its own adhesive system, whereas the microhybrid composite, used in three groups, was adhesively bonded to dentin with systems with or without the antibacterial monomer MDPB – methacryloyloxydodecylpyridinium bromide (Clearfil SE Protect, Clearfil SE bond, respectively). One microhybrid group (bonded with Clearfil SE Protect) received 6 layers of Clearfil SE Protect over the composite surface, which was made in an attempt to simulate an antibacterial composite. The nanohybrid composite was bonded with Clearfil SE bond. The amalgam group was not bonded, and the mechanical retention was accomplished by having two small apertures on the left and right sides of the acrylic matrix, forming two amalgam pins. All procedures were performed according to the manufacturers' instructions.
Fig. 1.
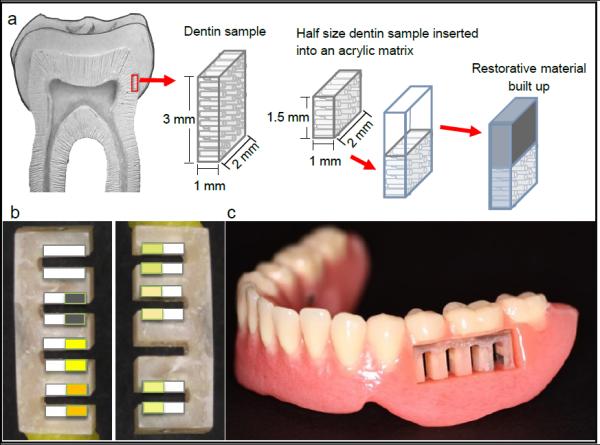
a. Schematic representation of the preparation of the samples; b. acrylic sample holders with restored and unrestored samples from the same group positioned facing each other across a simulated interdental space; c. sample holders inserted into the (pre)molar space on either side of the trial prosthesis.
Table 1.
Restorative materials used; description of groups and protocol according to manufactures' instructions.
| Restorative Material |
Adhesive System | Group | Protocol |
|---|---|---|---|
| Clearfil AP-X (Kuraray Medical Inc., Okayama, Japan) + |
Clearfil SE Bond (Kuraray) |
Microhybrid +SE |
a) Primer for 20 s, dry with mild air flow, bond, air flow gently and light cure (LED) for 10 s. b) Composite in one increment within an acrylic matrix with the dentin, light cure for 30 s under a polyester matrix. |
|
| |||
| Clearfil SE Protect (Kuraray) |
Microhybrid +P |
||
|
| |||
| Clearfil SE Protect – with 6 layers |
Microhybrid +P+6 |
a + b + After the normal procedure of priming, bonding and composite placement*, 6 additional layers of primer and bond were applied over the composite surface. |
|
|
| |||
| Filtek Supreme (3M ESPE, St. Paul, MN, USA) + |
Clearfil SE Bond | Nanohybrid +SE |
a + b |
|
| |||
| Filtek Silorane (3M ESPE) + |
Silorane System Adhesive (3M ESPE) |
Silorane | Primer for 15 s with agitation of the brush, dry with mild air flow, light cure for 10 s, apply the bond, gently air flow and light cure for 10 s + b |
|
| |||
| Tytin amalgam (Kerr Corporation, Orange, CA, USA) |
No bond | Amalgam | Condensed into an acrylic matrix with the dentin. |
Note.
the composite built up was inserted leaving approximately 0.3 mm space on top for the adhesive system layers.
Eighteen samples were made for each group, 2 per subject, and embedded into acrylic sample holders. The 2 samples from the same group were positioned facing each other with the purpose of simulating the interdental space (Fig. 1b). In the first sample holder pair (right and left) the groups were randomly assigned to the slots according to a computer-generated randomization list (Random Allocation Software v.1.0.0, M. Saghaei, Isfahan, IR). To generate minimum unevenness between groups with regard to slot location while maintaining randomness, in subsequent place holders the assignment of groups to locations was rotated. One of the sample holder spaces was left without samples being a polymethylmethacrylate group for future analysis (biofilm profiles). To ensure correct orientation of the sample surfaces for T-WIM pictures, the holder was fixed into a device where a high speed handpiece was coupled and surfaces were finished with cylindrical diamond burs (fine and extra fine) under water cooling. They were sterilized before use by ethylene oxide gas at WIMAC (Kliniekdiensten B.V., Rotterdam, The Netherlands) according to ISO 9001:2000 and EN 13485:2003. The sample holder pairs were randomly allocated (Random Allocation Software v.1.0.0) and inserted into a space in the (pre)molar area on either side of the trial prosthesis (Fig. 1c). Patients and groups were coded by one researcher using a 4- digit alpha-numeric system.
2.5. In situ Protocol
All instructions were given orally and in writing. The volunteers were given a "trial kit", which contained the instructions, a diary, sugar and a measuring bottle for the sucrose solution, a prosthesis container, fluoride toothpaste (1400 ppm) and toothbrush. They were instructed to wear the trial prosthesis for 8 weeks, 24 h a day. They should keep their normal diet and additionally immerse the trial prosthesis in a freshly prepared 20% sucrose solution (using tap water), 4 times per day for 5 minutes between meals, in order to ensure standardized baseline of cariogenic challenge. Instruction was given to clean the device once a day with fluoride toothpaste, by brushing the denture and covering the samples with the toothpaste slurry for 2 minutes. They were instructed not to clean the sample holders, but were allowed to rinse the prosthesis with running water as often as they wished. A diary was provided, in which subjects recorded the time of the sucrose immersions and cleaning of the device. Subjects were not blinded regarding materials since amalgam samples present a different color, easily perceived. However, subjects were unaware of the study aims as well as each one had all sample groups and it would not be possible to interfere with the outcome of a particular group. They attended the appointments for the study (at 28th and 56th day after commencement) at Radboud university medical center (Nijmegen, NL) where the data were collected. At the last appointment the original prosthesis was returned to the volunteers.
2.6. Transversal Wavelength Independent Microradiography (T-WIM)
T-WIM radiographs were made at baseline (T0), after 4 weeks (T4), and after 8 weeks (T8) using the method of Thomas et al., 2006.23 The follow-up of lesion progression was performed within the each sample, since it is a non-destructive method. For the interim analysis (T4), the sample holders were detached from the trial prosthesis, microradiographed and placed back into the prosthesis. These measurements were performed to evaluate the need to increase sucrose exposure. The settings for the microradiography were 60 kV, 30 mA at an exposure time of 8 seconds. A stepwedge with the same absorption coefficient as tooth material (94% Al / 6% Zn alloy) was used to calculate the integrated mineral loss (ML). After exposure, the films (FUJI, Fine grain Positive film 71337, Fuji Photo Film Co., Tokyo, Japan) were developed (10 min), fixed (7 min), rinsed and dried. A digital image of each sample was recorded with a light microscope Leica M50 (Leica Microsystems, Germany) with a magnification of 10x and a digital SLR camera (Canon EOS 50D, Japan). Microradiographs were visually assessed for the presence of wall lesions and surface lesions. A lesion with a progressing front parallel to the outer surface of the tooth sample was considered an outer surface lesion. A wall lesion was defined as a lesion progressing perpendicularly to the tooth-restoration interface. Three digital scans perpendicular to the outer dentine surface and three digital scans to the tooth restoration interface were made. From these scans the mineral profiles were calculated, and lesion depth (μm) and mineral loss (vol%.μm) determined using a custom made software program (T-WIM calculation program, version 5.25, J.de Vries, Groningen/NL). Results of the three scans for the sites in each sample and also for the two opposing samples were averaged for each subject, to yield a mineral profile for a specific material/ group. This procedure was carried out by a researcher who was blinded regarding subject, material group, and sample position in the sample holder and who also performed the statistical analysis. Baseline mineral profiles (T0) were subtracted from the mineral profiles after 8 weeks wearing the prosthesis (T8) to calculate the specific mineral loss values and lesion depth after 8 weeks.
2.7. Statistical Analysis
Mineral loss values were analyzed using multiple linear regression. To correct for the clustering of surfaces in one subject a multilevel model was used, with the subject as random effect. All materials were modeled as dichotomous variables with amalgam as reference category. Comparisons within materials that both are not the reference – Amalgam, cannot be read from the regression results directly. To facilitate for these comparisons, t-tests can be performed using the estimated effect and its standard error, which can be seen directly in the regression model. To allow for a possible slot location effect (position in the sample holder more towards the mesial or the distal), the central site position in the sample holder was used as reference, with a dichotomous distal and mesial variable in the model. To quantify the subject effect, the intraclass correlation coefficient (ICC) was calculated.
3. Results
A total of 9 subjects were included in the study, 4 female and 5 male, with an age ranging between 45 and 71 years. After 4 weeks all subjects were seen, and no serious adverse events were reported. One subject reported slight pressure sores, which were treated by correcting the denture. After 8 weeks no adverse events were reported.
Visual evaluation of the microradiographs showed no signs of wall lesion development, and only outer lesions were analyzed. Figure 2a-c presents an example of mineral profiles at T0, T4 and T8, illustrating lesion progression. Figure 2d shows the average mineral loss for the groups at T8. When comparing secondary to primary lesion progression (control group dentin), Amalgam, Microhybrid+SE, and Silorane showed statistically significant higher mineral loss.
Fig. 2.
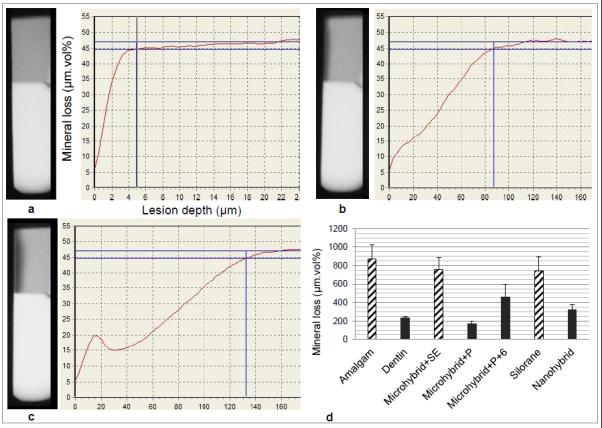
a-c: Examples of T-WIM radiographs of one sample (top half is dentine, bottom is composite) and resulting mineral profiles showing lesion progression baseline (a), 4 weeks (b) and 8 weeks (c); d: Average mineral loss values (and standard errors) for each group, with bar patterns indicating the statistical grouping according to the regression model and t tests (Table 2).
The results of the multiple linear regression analysis can be found in Table 2, with amalgam as the reference material. Except for Silorane and Microhybrid+SE, all the other groups presented statistically significant lower mineral loss increments when compared to amalgam. Within groups that were significantly different from amalgam, no statistically significant differences were seen between groups with the lowest (Microhybrid+P) and highest (Microhybrid+P+6) mineral loss values (p=0.075). The intraclass correlation coefficient (ICC=0.302) showed 30% of the variability to be related to subject factors, i.e. factors not included in the regression model.
Table 2.
The estimate effect of material in mineral loss increment is presented with multiple linear regression, considering patient and sample position in the model.
| Material | Effect | p | 95% CI of effect | |
|---|---|---|---|---|
| (SE) | Lower | Upper | ||
| Intercept* | 693 (173) | 355 | 1032 | |
| Dentin | −676 (126) | <0.001 | −923 | −429 |
| Microhybrid+P | −771 (124) | <0.001 | −1014 | −529 |
| Microhybrid+P+6 | −444 (136) | 0.001 | −710 | −178 |
| Microhybrid+SE | −170 (124) | 0.171 | −413 | 73 |
| Silorane | −115 (124) | 0.354 | −358 | 128 |
| Nanohybrid+SE | −487 (126) | <0.001 | −735 | −240 |
| Mesial site | 597 (83) | <0.001 | 434 | 759 |
| Distal site | 148 (84) | 0.079 | −17 | 312 |
Amalgam is used as reference material for analysis with the specimen positioned in a central site in the sample holder.
The analysis refers to 356 observations in 9 patients.
4. Discussion
This study investigated the effects of different restorative materials in caries formation in dentin adjacent to restorations. The use of in situ models provides standardized conditions, simultaneously maintaining the individual variability of the oral cavity complexity. This particular model was described previously8 and few modifications were performed. The samples were positioned in a parallel plane within each other, instead of the "V" configuration, because it showed to interfere with the shape of outer lesion formation8, probably due to differences in the biofilm stagnation. During the 8- week period, the subjects were asked to immerse the trial prosthesis into a sucrose solution, ensuring a standard baseline cariogenic challenge. The 4-week microradiographs were performed in order to evaluate the frequency of sucrose exposure (4 × / day), which was found to be sufficient to detect mineral loss under a daily fluoride exposure. The use of fluoride-containing dentifrice was included because of its widespread use and to model more closely the in vivo situation. Typical sub-surface lesion formation was observed (Figure 2c). Thus, the present in situ model was considered suitable for testing the possible effects of restorative materials under cariogenic conditions.
Considering the assumption that the presence of a material per se would predispose to secondary caries in the adjacent tissue, two different situations were found in the present study. Lesion progression next to Microhybrid+P, Microhybrid+P+6 and Nanohybrid+SE groups was similar to primary caries lesions in dentin (control). However, next to Amalgam, Silorane and Microhybrid+SE groups, secondary lesion progression was significantly higher than primary lesions (Figure 2d). To the authors’ knowledge there are no previous studies that have investigated the effect of these materials on mineral loss in adjacent dentin, making direct comparisons impossible.
The null hypothesis under investigation was rejected for some materials. Significantly lower mineral loss was observed next to composite using the adhesive system with antibacterial component (Microhybrid+P/Microhybrid+P+6), which may be attributed to the antibacterial properties of the adhesive system. In the Microhybrid+P group the adhesive system was restricted to the tooth/restoration interface, whereas the Microhybrid+P+6 group had additional primer/adhesive layers applied over the composite surface in an attempt to simulate an antibacterial composite. Although mineral loss values were not significantly different between these groups, the higher mineral loss values found for Microhybrid+P+6 were unexpected. A possible explanation for this result could be related to loss of the adhesive coating during the experiment, leading to surface defects which may have promoted biofilm stagnation areas. Therefore, the effect of antibacterial composites on secondary caries lesions progression remains to be investigated. Also, the effect of nanohybrid composite on adjacent mineral loss was significantly different from that of amalgam. The small filler particles of this material could be related to these findings, since surface characteristics can affect biofilm formation.24 A recent in situ study25 assessed the surface of different materials including amalgam and the nanocomposite included in the present investigation, showing higher roughness values and a less regular surface for amalgam.25 Since surface roughness evaluations were not performed in the present study, mineral loss findings cannot be directly related to these data. Future investigation should explore the relationship between surface profiles and adjacent mineral loss.
This study did not support previous clinical reports showing that amalgam restorations are less susceptible to secondary caries than composite restorations.9,26 The fact that the clinical diagnosis of secondary caries next to composites can be easily mistaken for marginal defects or discoloration may systematically compromise the clinical judgment.1,27 Similar to the present findings, Sousa et al. (2009)28 investigated the effect of dental materials in secondary caries development using a 14 days in situ model, and found comparable mineral loss next to amalgam and a microhybrid composite. It may be speculated that the very high rate of caries development in in situ studies does not allow for factors related to ageing of materials. Although a much longer study period of 8 weeks in situ was used, this may still be too short to sufficiently incorporate ageing effects. An alternative explanation may lie in the observation that no wall lesions occurred in the present study. The difference between the clinical caries susceptibility of amalgam and composite restorations may be due to differences in gap formation and wall lesion development, which were not included in this model.
Apart from the effect of materials on caries progression, 30% of the effect in caries progression was subject-related. This was to some extent expected, as cariogenic challenge was only standardized to a limited extent and individual habits and diet of the subjects were included in the model. The between-subject variation was previously also reported regarding lesion progression in situ, where no sucrose exposures were added to the model at all.8 Although the current study focused on material as the factor under investigation, caries development and progression is related to behavioral aspects,29 which were present in this study. The split-mouth set- up of the study, however, allowed for comparisons between materials, independent of the underlying rate of caries progression, and significant material effects could be shown even in this small sample population.
Secondary caries in the clinical setting may ultimately depend on individual habits and different patterns of oral pathogens prevalence within the biofilm, whereas the material may play a smaller role as suggested in the present study. Nonetheless, a different scenario may be found for secondary caries in the shape of wall lesions, since interfacial gaps and loading can create distinct niches,30 which were not present in this study. In this sense, future research should also focus on secondary caries in interfacial gaps.
5. Conclusion
In conclusion, within the limits of this study, a restorative material may influence outer lesion progression. Under cariogenic challenge, amalgam was not found to be related to lower secondary caries progression in dentin compared to composite-based materials after 8-weeks in situ.
Clinical Significance.
Although patient factors play a major role in caries progression, the restorative material may affect outer secondary lesion progression.
ACKNOWLEDGMENTS
This study was funded by the National Institutes for Health (NIH), grant number 1R01DE021383-01, under call RFA-DE-10-005 Increasing the service life of dental resin composites. XXX XXXXX XXXXXX XXXXXXXX XXXXXXXXXXX XXXXXXXXXXXXXX XXXXX XXX XXXXXXX XXXXXXXX XXXXXXXX XXXXXX XXXX XXX XXXXXXXXXXXX XXX XXX XXXXXXXXXXX XX XXXXXX XXXXX XXXXXXXXX XXXXXXXX, XXXXXX. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Authors acknowledge the contribution of T. Jetten for making the trial prostheses and J. Ruben for his contribution on T-WIM procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Françoise H. van de Sande, Radboud university medical center, Philips van Leydenlaan 25, 6525 EX Nijmegen, The Netherlands and Graduate Program in Dentistry, School of Dentistry, Federal University of Pelotas, Gonçalves Chaves 457, 96015-560, Pelotas, RS, Brazil.
Niek J.M. Opdam, Radboud university medical center Philips van Leydenlaan 25, 6525 EX Nijmegen, The Netherlands.
Gert Jan Truin, Radboud university medical center Philips van Leydenlaan 25, 6525 EX Nijmegen, The Netherlands.
Ewald M. Bronkhorst, Radboud university medical center Philips van Leydenlaan 25, 6525 EX Nijmegen, The Netherlands.
Johannes J. de Soet, Department of Preventive Dentistry, Academic Centre for Dentistry Amsterdam (ACTA) Gustav Mahler Laan 3004, 1081 LA, Amsterdam, The Netherlands.
Maximiliano S. Cenci, Graduate Program in Dentistry, School of Dentistry, Federal University of Pelotas Gonçalves Chaves 457, 96015-560, Pelotas, RS, Brazil.
Marie-Charlotte Huysmans, Radboud university medical center Philips van Leydenlaan 25, 6525 EX Nijmegen, The Netherlands.
REFERENCES
- 1.Mjor IA, Toffenetti F. Secondary caries: a literature review with case reports. Quintessence Int. 2000;31(3):165–79. [PubMed] [Google Scholar]
- 2.Manhart J, Chen H, Hamm G, Hickel R. Buonocore Memorial Lecture. Review of the clinical survival of direct and indirect restorations in posterior teeth of the permanent dentition. Oper Dent. 2004;29(5):481–508. [PubMed] [Google Scholar]
- 3.Demarco FF, Correa MB, Cenci MS, Moraes RR, Opdam NJ. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater. 2012;28(1):87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Hals E, Nernaes A. Histopathology of in vitro caries developing around silver amalgam fillings. Caries Res. 1971;5(1):58–77. doi: 10.1159/000259733. [DOI] [PubMed] [Google Scholar]
- 5.Hals E, Andreassen BH, Bie T. Histopathology of natural caries around silver amalgam fillings. Caries Res. 1974;8(4):343–58. doi: 10.1159/000260123. [DOI] [PubMed] [Google Scholar]
- 6.Cenci MS, Pereira-Cenci T, Cury JA, Ten Cate JM. Relationship between gap size and dentine secondary caries formation assessed in a microcosm biofilm model. Caries Res. 2009;43(2):97–102. doi: 10.1159/000209341. [DOI] [PubMed] [Google Scholar]
- 7.Diercke K, Lussi A, Kersten T, Seemann R. Isolated development of inner (wall) caries like lesions in a bacterial-based in vitro model. Clin Oral Investig. 2009;13(4):439–44. doi: 10.1007/s00784-009-0250-z. [DOI] [PubMed] [Google Scholar]
- 8.Thomas RZ, Ruben JL, ten Bosch JJ, Fidler V, Huysmans MC. Approximal secondary caries lesion progression, a 20-week in situ study. Caries Res. 2007;41(5):399–405. doi: 10.1159/000104799. [DOI] [PubMed] [Google Scholar]
- 9.Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitao J, et al. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc. 2007;138(6):775–83. doi: 10.14219/jada.archive.2007.0265. [DOI] [PubMed] [Google Scholar]
- 10.Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: findings From the New England Children's Amalgam Trial. J Am Dent Assoc. 2007;138(6):763–72. doi: 10.14219/jada.archive.2007.0264. [DOI] [PubMed] [Google Scholar]
- 11.Heintze SD, Forjanic M, Ohmiti K, Rousson V. Surface deterioration of dental materials after simulated toothbrushing in relation to brushing time and load. Dent Mater. 2010;26(4):306–19. doi: 10.1016/j.dental.2009.11.152. [DOI] [PubMed] [Google Scholar]
- 12.Barbosa RP, Pereira-Cenci T, Silva WM, Coelho-de-Souza FH, Demarco FF, Cenci MS. Effect of cariogenic biofilm challenge on the surface hardness of direct restorative materials in situ. J Dent. 2012;40(5):359–63. doi: 10.1016/j.jdent.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Khalichi P, Singh J, Cvitkovitch DG, Santerre JP. The influence of triethylene glycol derived from dental composite resins on the regulation of Streptococcus mutans gene expression. Biomaterials. 2009;30(4):452–9. doi: 10.1016/j.biomaterials.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 14.Sun J, Eidelman N, Lin-Gibson S. 3D mapping of polymerization shrinkage using X-ray micro-computed tomography to predict microleakage. Dent Mater. 2009;25(3):314–20. doi: 10.1016/j.dental.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peutzfeldt A, Asmussen E. Determinants of in vitro gap formation of resin composites. J Dent. 2004;32(2):109–15. doi: 10.1016/j.jdent.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 16.de Moraes RR, Goncalves Lde S, Lancellotti AC, Consani S, Correr-Sobrinho L, Sinhoreti MA. Nanohybrid resin composites: nanofiller loaded materials or traditional microhybrid resins? Oper Dent. 2009;34(5):551–7. doi: 10.2341/08-043-L. [DOI] [PubMed] [Google Scholar]
- 17.Lien W, Vandewalle KS. Physical properties of a new silorane-based restorative system. Dent Mater. 2010;26(4):337–44. doi: 10.1016/j.dental.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Buergers R, Schneider-Brachert W, Hahnel S, Rosentritt M, Handel G. Streptococcal adhesion to novel low-shrink silorane-based restorative. Dent Mater. 2009;25(2):269–75. doi: 10.1016/j.dental.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent Mater J. 2009;28(1):11–9. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 20.Izutani N, Imazato S, Nakajo K, Takahashi N, Takahashi Y, Ebisu S, et al. Effects of the antibacterial monomer 12-methacryloyloxydodecylpyridinium bromide (MDPB) on bacterial viability and metabolism. Eur J Oral Sci. 2011;119(2):175–81. doi: 10.1111/j.1600-0722.2011.00817.x. [DOI] [PubMed] [Google Scholar]
- 21.Imazato S, Kuramoto A, Takahashi Y, Ebisu S, Peters MC. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent Mater. 2006;22(6):527–32. doi: 10.1016/j.dental.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Pandis N. Sample calculation for split-mouth designs. Am J Orthod Dentofacial Orthop. 2012;141(6):818–9. doi: 10.1016/j.ajodo.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Thomas RZ, Ruben JL, de Vries J, ten Bosch JJ, Huysmans MC. Transversal wavelength-independent microradiography, a method for monitoring caries lesions over time, validated with transversal microradiography. Caries Res. 2006;40(4):281–91. doi: 10.1159/000093186. [DOI] [PubMed] [Google Scholar]
- 24.Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8(9):881–90. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padovani G, Fucio S, Ambrosano G, Sinhoreti M, Puppin-Rontani R. In Situ Surface Biodegradation of Restorative Materials. Oper Dent. 2014 doi: 10.2341/13-089-C. [DOI] [PubMed] [Google Scholar]
- 26.Opdam NJ, Bronkhorst EM, Loomans BA, Huysmans MC. 12-year survival of composite vs. amalgam restorations. J Dent Res. 2010;89(10):1063–7. doi: 10.1177/0022034510376071. [DOI] [PubMed] [Google Scholar]
- 27.Mjor IA. Clinical diagnosis of recurrent caries. J Am Dent Assoc. 2005;136(10):1426–33. doi: 10.14219/jada.archive.2005.0057. [DOI] [PubMed] [Google Scholar]
- 28.Sousa RP, Zanin IC, Lima JP, Vasconcelos SM, Melo MA, Beltrao HC, et al. In situ effects of restorative materials on dental biofilm and enamel demineralisation. J Dent. 2009;37(1):44–51. doi: 10.1016/j.jdent.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369(9555):51–9. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 30.Kuper NK, Opdam NJ, Bronkhorst EM, Ruben JL, Huysmans MC. Hydrodynamic flow through loading and in vitro secondary caries development. J Dent Res. 2013;92(4):383–7. doi: 10.1177/0022034513481040. [DOI] [PubMed] [Google Scholar]


