Abstract
Sea turtles are a charismatic and ancient ocean species and can serve as key indicators for ocean ecosystems, including coral reefs and sea grass beds as well as coastal beaches. Genotoxicity studies in the species are absent, limiting our understanding of the impact of environmental toxicants on sea turtles. Hexavalent chromium (Cr(VI)) is a ubiquitous environmental problem worldwide, and recent studies show it is a global marine pollutant of concern. Thus, we evaluated the cytotoxicity and genotoxicity of soluble and particulate Cr(VI) in hawksbill sea turtle cells. Particulate Cr(VI) was both cytotoxic and genotoxic to sea turtle cells. Concentrations of 0.1, 0.5, 1, and 5 μg/cm2 lead chromate induced 108, 79, 54, and 7 percent relative survival, respectively. Additionally, concentrations of 0, 0.1, 0.5, 1, and 5 μg/cm2 lead chromate induced damage in 4, 10, 15, 26, and 36 percent of cells and caused 4, 11, 17, 30, and 56 chromosome aberrations in 100 metaphases, respectively. For soluble Cr, concentrations of 0.25, 0.5, 1, 2.5, and 5 μM sodium chromate induced 84, 69, 46, 25, and 3 percent relative survival, respectively. Sodium chromate induced 3, 9, 9, 14, 21, and 29 percent of metaphases with damage, and caused 3, 10, 10, 16, 26, and 39 damaged chromosomes in 100 metaphases at concentrations of 0, 0.25, 0.5, 1, 2.5, and 5 μM sodium chromate, respectively. These data suggest that Cr(VI) may be a concern for hawksbill sea turtles and sea turtles in general.
Keywords: Chromate, chromium, hexavalent chromium, genotoxicity, hawksbill sea turtle
1. INTRODUCTION
Sea turtles are a charismatic species that inhabit both coastal and pelagic ecosystems. They have long lives, up to 80 years, and have the potential to bioaccumulate pollutants from food, sediment, and water, as well as air. The hawksbill sea turtle (Eretmochelys imbricata) is considered critically endangered and at risk of extinction (IUCN, 2013). Hunting and loss of egglaying habitat have been key factors in their decline (Meylan and Donnelley 1999). Efforts have been undertaken to reduce these factors by banning the trade of materials from hawksbills and protecting some egg laying sites. However, illegal trade and continued coastal development continues to impair their recovery.
Superimposed on these two factors is the concern that ocean pollution may put the struggling hawksbill population at further risk. It is increasingly clear that ocean pollution has reached even the remotest regions and, if they are sufficiently exposed, pollution could impair the ability of the hawksbill and other similarly endangered sea turtles and sea life to survive, reproduce and thrive. For example, we recently identified chromium (Cr) as a global marine pollutant using sperm whales (Physeter macrocephalus) as an indicator species. Human and rodent studies indicate that Cr can damage DNA and induce reproductive and developmental toxicity (Al-Hamood et al., 1998; Bataineh et al., 1997; Chowdhury et al., 1995; Mancuso, 1997; Witmer et al., 1989, 1991). Such outcomes could lead to disease in an individual and a reduction in reproductive success for a population, both of which could seriously impair a critically endangered species like the hawksbill.
Metal pollutants have been found in tissue from several sea turtle species (Storelli et al., 1998, 2008; Anan et al., 2002; Franzelliti et al., 2004; Maffucci et al., 2005; Gardner et al., 2006; Frias-Expericuet et al., 2006; Andreani et al., 2008; Garcia-Fernandez et al., 2009; Jerez et al., 2010). Only two of these studies measured Cr. One study reported low Cr levels (0.039 μg/ml) in the plasma of captive hawksbills (Suzuki et al., 2012). The other reported average Cr levels from 12 stranded loggerhead turtles in the Mediterranean of 1.05, 1.57, and 1.43 mg/kg dry weight in liver, kidney and muscle, respectively, with the highest levels found in lung tissue (2.29 mg/kg) (Storelli et al., 1998).
Only two studies considered the impact of metal exposure in sea turtle model systems. Both were focused on green turtles. One study correlated increased carapace metal levels with adverse health markers in green turtles from San Diego Bay (Kormoroske et al., 2011). The other study considered the cytotoxic effects of four metals, including Cr, in green sea turtle cell lines and found that cadmium and Cr were the most cytotoxic (Tan et al., 2010). It appears none have considered impacts in hawksbill turtles.
Presumably, one reason for this lack of effects data is the intent to avoid losing any individual turtles to scientific studies and thereby preserve as many as possible. While such reasoning makes sense, it remains possible to conduct controlled toxicology studies and gain valuable species-specific insights into the potential toxicological response and impact by using cells cultured from sea turtle tissues in a manner that does not harm the animal or is collected from a recently deceased animal. Accordingly, to begin developing a better understanding of ocean pollution impacts on the hawksbill in particular and sea turtles in general, we investigated the cytotoxicity and genotoxicity of Cr in skin cells developed from a hawksbill sea turtle. Because the marine environment favors the hexavalent form of Cr (Geisler and Schmidt, 1992; Pettine and Millero, 1990), and because in humans the particulate Cr(VI) forms are more potent than soluble ones (IARC, 1990; Holmes et al., 2008; Wise, S et al., 2008), we focused our study on particulate and soluble Cr(VI) compounds.
2. METHODS
2.1 Chemicals and Reagents
RPMI was purchased from Mediatech (Manassas, VA). Penicillin/streptomycin, Gurr’s buffer, and trypsin/EDTA were purchased from Invitrogen Corporation (Grand Island, NY). Crystal violet, acetic acid, and methanol were purchased from J.T. Baker (Phillipsburg, NJ) and Fetal Bovine Serum (FBS) was purchased from Gibco Life Technologies (Grand Island, NY). Tissue culture dishes, flasks, and plasticware were purchased from BD (Franklin Lakes, NJ). Lead chromate, sodium chromate, potassium chloride (KCl) and demecolchicine were purchased from Sigma/Aldrich. Giemsa stain was purchased from Biomedical Specialties Inc. (Santa Monica, CA).
2.2. Cells and Cell Culture
Hawksbill sea turtle fibroblast cells were established from a skin biopsy of a healthy juvenile hawksbill sea turtle (Fukuda et al., 2012). Cells were grown in RPMI with 10% FBS and maintained in 5% CO2 at 26°C. Cell cultures were maintained and all experiments performed as subconfluent monolayers. They were fed at least twice a week and subcultured at least once a week. Cells were tested routinely for mycoplasma contamination. All experiments were conducted on logarithmically growing cells.
2.3. Preparation of Chemicals
Sodium chromate (CAS #7775-11-3, ACS reagent minimum 98% purity), a soluble hexavalent Cr compound was administered as a solution in water as previously described (Wise J et al., 2002). Lead chromate (CAS# 7758-97-6, ACS reagent minimum 98% purity), a particulate Cr(VI) compound was administered as a suspension in water as previously described (Wise J et al., 2002). Lead chromate does not fully dissolve in tissue culture while sodium chromate does (Holmes et al., 2005; Wise S. et al., 2005). Thus, direct comparisons of the two chemicals using a common unit of measure is difficult. If dissolution had been complete the concentrations for lead chromate (0.1, 0.5, 1, and 5 μg/cm2) would be 0.42, 2.1, 4.2 and 21 μg/ml, and the concentrations for sodium chromate (0.25, 0.5, 1, 2.5, and 5 μM) would be 0.04, 0.08, 0.16, 0.4, and 0.8 μg/ml.
2.4. Cytotoxicity
Cytotoxicity was established using a clonogenic assay based on our published methods (Wise J et al., 2008). Briefly, cells were seeded in each well of a 6-well tissue-culture dish and treated for 24 h with either lead chromate or sodium chromate. After treatment, cells were resuspended in fresh medium and reseeded at 1000 cells in four 100 mm dishes per treatment group. Once colonies formed (about 14 days), dishes were fixed and stained with crystal violet and colonies counted. Each experiment was repeated at least three times.
2.5. Clastogenicity
Clastogenicity was determined using a chromosomal aberration assay based on our published methods (Wise J et al., 2008). Briefly, log phase cells were seeded into 100 mm dishes and treated for 24 h with either lead chromate or sodium chromate. Demecolcine (0.1 g/ml) was added 1 h before the end of treatment to arrest the cells in metaphase. Cells were then collected by trypsinization, spun down and resuspended in a 0.075M KCl hypotonic solution for 20 min followed by fixation with 3:1 methanol:acetic acid. The fixative was changed twice then cells were dropped onto clean wet slides and stained with 5% Giemsa stain in Gurr’s buffer. One hundred metaphases per treatment were analyzed in each experiment and chromosome aberrations were scored by standard criteria (Wise J et al., 2008). All experiments were repeated three times.
2.6. Statistics
Dose-response curves were estimated using regression analysis. Ninety-five percent Wald confidence intervals were calculated, as were Wald chi square tests of statistical significance. Differences between pairs of dose levels were assessed using t-tests.
3. RESULTS
3.1. Cytotoxicity
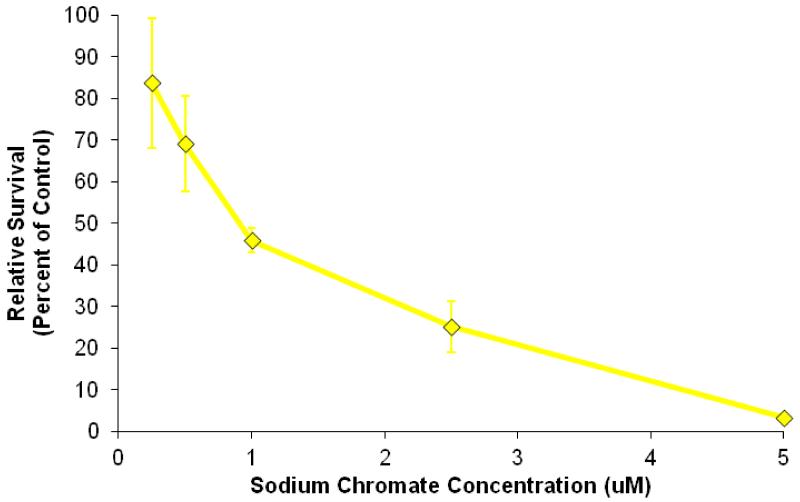
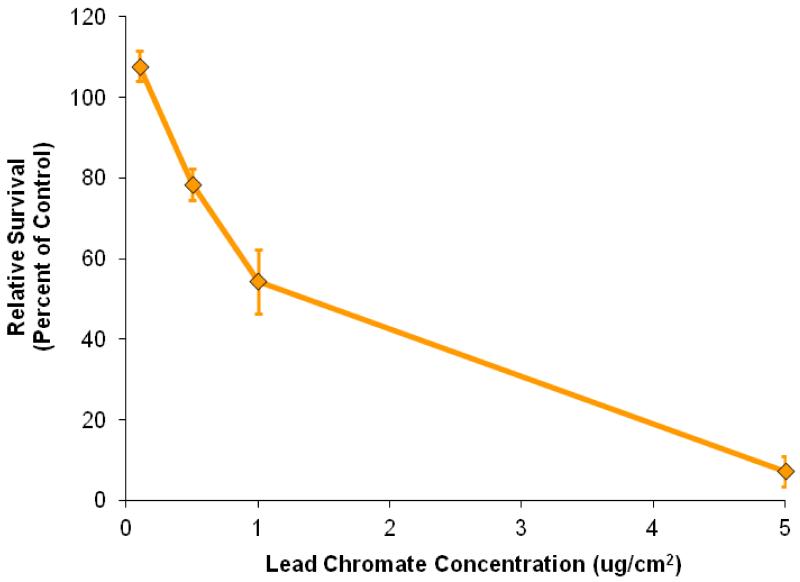
Sodium chromate induced a concentration-dependent decrease in cell survival (Figure 1). Specifically, 0.25, 0.5, 1, 2.5, and 5 μM sodium chromate induced 84, 69, 46, 25, and 3 percent relative survival, respectively, in hawksbill sea turtle cells after a 24 h treatment. Lead chromate also induced a concentration-dependent decrease in cell survival (Figure 2). Specifically, concentrations of 0.1, 0.5, 1, and 5 μg/cm2 induced 108, 79, 54, and 7 percent relative survival, respectively, in hawksbill sea turtle cells after 24 h exposure. The estimated LC50s for sodium chromate and lead chromate were 1.2 μM (95% confidence interval: 0.9 to 1.5) and 1.1 μg/cm2 (95% confidence interval: 0.9 to 1.3), respectively.
Figure 1. Sodium Chromate Is Cytotoxic to Hawksbill Sea Turtle Cells.
This figure shows that soluble sodium chromate is cytotoxic to hawksbill sea turtle cells. Data represent 3 experiments ± the standard error of the mean. All concentrations were significantly different from control, except for 0.25 and 0.5 μM (p < 0.0001 for 1, 2.5 and 5 μM). 0.25 μM was significantly different from 2.5, and 5 μM (p < 0.02 and p < 0.01, respectively). 0.5 μM was significantly different from 2.5, and 5 μM (p < 0.02 and p < 0.01, respectively). 1 μM was significantly different from 2.5, and 5 μM (p < 0.03 and p < 0.0003, respectively). 2.5 μM was also significantly different from 5 μM (p < 0.03).
Figure 2. Lead Chromate Is Cytotoxic to Hawksbill Sea Turtle Cells.
This figure shows that particulate lead chromate is cytotoxic to hawksbill sea turtle cells. Data represent 3 experiments ± the standard error of the mean. All concentrations except for 0.10 μg/cm2 were significantly different from control (p< 0.008). All concentrations were significantly different from each other (p< 0.04 for 0.5 compared to 1 μg/cm2; all others - p < 0.005).
3.2. Clastogenicity
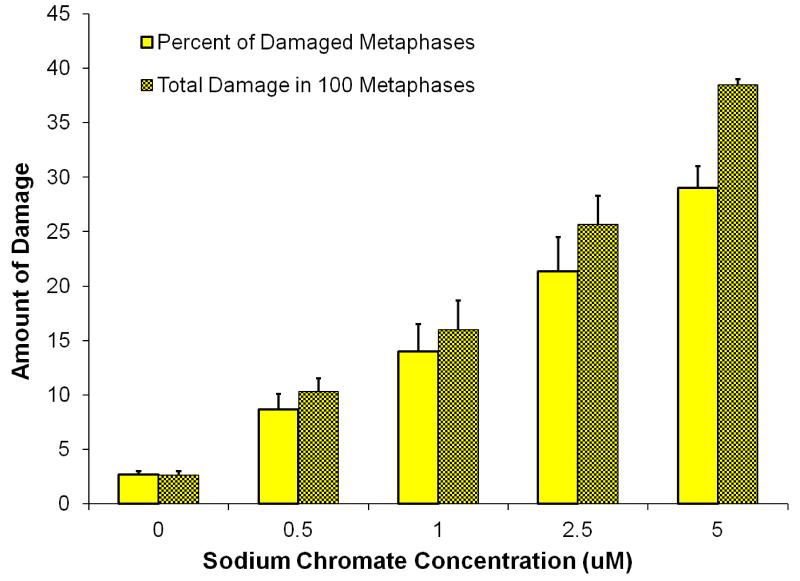
Clastogenicity was used as a measure of genotoxicity and expressed as percent of damaged metaphases and as the total damage observed in 100 metaphases. Sodium chromate induced a concentration-dependent increase in clastogenicity (Figure 3, Table 1). Sodium chromate induced 3, 9, 9, 14, 21, and 29 percent of metaphases with damage at concentrations of 0, 0.25, 0.5, 1, 2.5, and 5 μM sodium chromate, respectively. At the same concentrations we found 3, 10, 10, 16, 26, and 39 damaged chromosomes in 100 metaphases, respectively.
Figure 3. Sodium Chromate Is Genotoxic to Hawksbill Sea Turtle Cells.
This figure shows that soluble sodium chromate is genotoxic to hawksbill sea turtle cells. Data represent 3 experiments ± the standard error of the mean. For the percent of metaphases with damage, all concentrations were significantly different from control (p < 0.001). For the percent of metaphases with damage, 0.5 μM was significantly different from 2.5 μM (p < 0.04) and 5 μM (p < 0.01); and 1 was significantly different from 5 μM (p < 0.01). For the total damage in 100 metaphases, all concentrations were significantly different from control (p< 0.009 for 0.25 μM; p<0.0001 for all other comparisons). For the total damage in 100 metaphases, 0.5 μM was significantly different from 2.5 μM (p < 0.01) and 5 μM (p < 0.0005); 1 was significantly different from 2.5 μM (p < 0.05) and 5 μM (p < 0.01); and 2.5 was significantly different than 5 μM (p < 0.03).
Table 1. Spectrum of Chromosome Aberrations.
| Sodium Chromate Concentration1 |
Chromatid Lesion |
Isochromatid Lesion |
Chromatid Exchange |
Ring | Double Minute |
Acentric Fragment |
Dicentric |
|---|---|---|---|---|---|---|---|
| 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 7 | 4 | 0 | 0 | 0 | 0 | 0 |
| 2.5 | 20 | 5 | 0 | 0 | 0 | 0 | 1 |
| 5 | 33 | 6 | 0 | 0 | 0 | 0 | 0 |
| Lead Chromate Concentration1 |
Chromatid Lesion |
Isochromatid Lesion |
Chromatid Exchange |
Ring | Double Minute |
Acentric Fragment |
Dicentric |
|---|---|---|---|---|---|---|---|
| 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 |
| 0.1 | 8 | 1 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 19 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 17 | 5 | 0 | 0 | 0 | 0 | 0 |
| 5 | 39 | 10 | 0 | 0 | 0 | 0 | 0 |
Data represent one experiment for each chemica
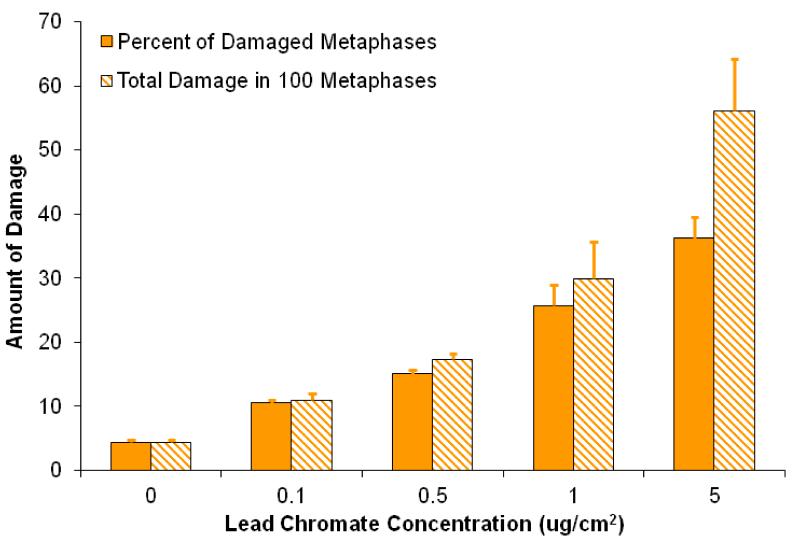
Lead chromate induced a concentration-dependent increase in clastogenicity (Figure 4, Table 1). At concentrations of 0, 0.1, 0.5, 1, and 5 μg/cm2 lead chromate, we found 4, 10, 15, 26, and 36 percent of metaphases with damage, respectively, and 4, 11, 17, 30, and 56 damaged chromosomes in 100 metaphases, respectively.
Figure 4. Lead Chromate Is Genotoxic to Hawksbill Sea Turtle Cells.
This figure shows that particulate lead chromate is genotoxic to hawksbill sea turtle cells. Data represent 3 experiments ± the standard error of the mean. 0.1. μg/cm2 is the mean of 2 experiments. For the percent of metaphases with damage, all concentrations were significantly different from control (p< 0.02). For the percent of metaphases with damage, all concentrations were significantly different from each other (p < 0.03), except for 0.5 compared to 1 μg/cm2 (p < 0.08); and 1 compared to 5 μg/cm2 (p < 0.07). For the total damage in 100 metaphases, for all concentrations were significantly different from each other (p < 0.03), except for 0.5 compared to 01 and 1 μg/cm2 (p < 0.078 and p < 0.1, respectively); and 1 compared to 5 μg/cm2 (p < 0.06).
The spectrum of chromosome aberrations for both compounds consisted of mostly chromatid lesions (Table 1). There were more total aberrations than percent of metaphases with damage at treatments of 2.5 and 5 μM sodium chromate and in 1 and 5 μg/cm2 lead chromate, indicating that at these concentrations cells began to show multiple aberrations per cell.
4. DISCUSSION
Our data are the first to report genotoxicity in any sea turtle species. Cr(VI) is a well-known genotoxicant in humans and terrestrial animals with the respiratory and reproductive systems as primary targets (Witmer et al., 1989, 1991; Mancuso, 1997). More recently it has also been shown to be a concern for other marine species such as north Atlantic right whales, sperm whales and stellar sea lions (Li Chen et al., 2009, 2012; Wise S et al., 2009; Wise J et al., 2011). We found that Cr(VI) induced significant levels of chromosomal aberrations. The induction of chromosome aberrations is a standard accepted short-term test for cancer, and thus, this outcome indicates that Cr(VI) is a carcinogen in sea turtles, as it is in humans and rodents.
Such an outcome also raises concern about the impact of Cr on hawksbill sea turtle reproduction and development. If genotoxicity were to occur during key reproduction or embryogenesis stages it could cause offspring loss or impact hatchling development (El-Makawy et al., 2006; Keshava and Ong, 1999; Nayak et al., 1989). These outcomes would reduce the ability of an affected individual to survive or reproduce, resulting in detrimental effects to the population which is small in number and endangered.
Our study is also the first to consider the cytotoxic effects of a chemical in hawksbill sea turtle cells and the first to consider particulate Cr(VI) in any sea turtle species. Our data are consistent with the only other study that considered metal cytotoxicity (including Cr(VI)) in sea turtles (Tan et al., 2010). Specifically, that study focused on fibroblasts from brain, eye, heart, tumor, lung, liver, spleen, testes and bladder tissues from a green sea turtle suffering from severe fibropapilloma as well as pooled embryo fibroblasts. They found Cr(VI) was the most cytotoxic of the four metals considered including cadmium, Cr, zinc, and copper. Cytotoxicity was the only endpoint they considered.
While our outcomes are consistent with their study i.e. Cr(VI) was cytotoxic, the potency of Cr(VI) differed. More specifically, in their most sensitive cell line, green sea turtle brain fibroblasts, they reported an LC50 of 22 μM for a soluble Cr(VI) compound. In their most resistant cell line, green sea turtle liver fibroblasts, they reported an LC50 above 100 μM. In contrast, we found an LC50 of 1.2 μM (95% CI: 0.9 to 1.5) for soluble Cr(VI), which is two orders of magnitude lower. There are several possible explanations for this observed difference in potency. First, the difference could reflect a species effect with green sea turtles more resistant to Cr(VI) than hawksbill sea turtles. Second, it may reflect a difference in cell type as our study considered skin cells, which may be more sensitive to Cr(VI) than the cells in their study. Third, it may reflect a difference in the type of cytotoxicity assay. The green sea turtle study measured cytotoxicity using the MTT and Coomassie blue assays, which are less sensitive than the colony-forming assay used our present study. Finally, it may reflect the health of the animals from which the cells were isolated. Our cell line was isolated from a healthy animal with a stable, normal chromosome count. By contrast, the cell lines used by Tan et al., were isolated from a turtle with severe fibropapilloma and that had abnormal chromosome counts in various tissues (Lu et al., 1999). It may be that the disease or the abnormal chromosome complement made the animal more resistant to Cr(VI). We note that results from our study are consistent with cytotoxicity levels for Cr(VI) in other vertebrates cell lines (DeFlora 1990; Li Chen et al., 2009; Wise S et al., 2009; Wise, J et al., 2011) and likely are a more accurate representation of the cytotoxic effects of Cr in healthy sea turtles.
We do note that the particulate Cr(VI) compound we considered was lead chromate. We chose this compound because it is commonly used as a representative particulate Cr(VI) compound due to its known carcinogenicity and frequent use in products. But, it does raise a question about the possible role of lead in the toxicity of the compound. Our previous studies in human cells demonstrated that lead does not contribute to the cytotoxic and genotoxic effects of lead chromate because it is poorly absorbed by cells (Wise, S. et al., 2004; 2005). We did check sea turtle cells and found very low lead uptake after lead chromate exposure (data not shown). Moreover, studies measuring lead (Pb) levels in sea turtle tissues have reported overall low to non-detectable levels of Pb suggesting it may not be a concern for sea turtles (Storelli et al 2005; Gardner et al 2006; Garcia-Fernandez et al 2009; Paez-Osuna et al 2010). Thus, we do not believe lead exposure was a factor in the cytotoxicity or genotoxicity of lead chromate in the sea turtle cells used in this study.
Overall, these data are consistent with observations that Cr(VI) is cytotoxic and genotoxic to primary human and marine mammal skin cells. However, compared to previous studies, hawksbill cells were more sensitive to soluble Cr(VI) than sperm whale cells, but more resistant than human cells (Li Chen et al., 2012; Wise, J et al., 2002, 2011). For example, in hawksbill, 0.5, 1, and 5 μg/cm2 particulate Cr(VI) induced a total of 17, 30, and 56 aberrations in 100 metaphases, respectively. By contrast, in sperm whale cells, these concentrations induced a total of 5, 11 and 16 aberrations in 100 metaphases, respectively. But, in human cells 0.5 and 1 μg/cm2 induced a total of 32 and 43 aberrations in 100 metaphases, respectively. At 5 μg/cm2 no metaphases were observed. Similarly, concentrations of 1, 2.5 and 5 μM sodium chromate induced a total of 16, 26 and 39 aberrations in 100 metaphases, respectively, in hawksbill sea turtle skin cells. In sperm whale skin cells, these concentrations induced a 1.5-5-fold lower amount of aberrations and in human skin cells they induced 1.8-2.4 fold more aberrations, depending on dose. The explanation for these differences are uncertain, but may indicate that hawksbill cells absorb more Cr than whale cells but less Cr than human cells, or that turtles may have a more proficient DNA repair system than humans, but not as proficient as whales.
We chose concentrations for Cr(VI) based on a standard toxicological approach for studies in cultured cells. The concentrations were chosen based on their relative toxicity in the system i.e. spanning from very low to moderate toxicity in the sea turtle cells to ensure we were not considering supratoxic concentrations. As described above, we also took previous reports in humans and other wildlife into consideration and our concentrations were chosen to match those. With that approach, we have established the first report of genotoxicity in a sea turtle model system. The next challenge is determining the relevance of this dose range to the turtles’ environment.
It is difficult to ascertain the exposure of hawksbill sea turtles to Cr(VI) or even total Cr as there are insufficient data to accurately determine it. Cr(VI) levels have not been determined in the sea turtle environment. One approach is to consider dietary exposure based on total Cr levels. Hawksbill turtles consume about 1.5 kg/day of prey and primarily feeding on marine sponges as they age and (NMFS, 2014). Total Cr levels were measured in 6 species of marine sponges in the Mediterranean Sea and found to range from 0.6 to 9.1 ug total Cr/g sponge tissue dry weight (Perez et al., 2004). Converting to wet weight by multiplying by a factor of 0.25 (Wise, J et al., 2011), and considering the daily amount of prey consumption, one can calculate that hawksbill sea turtles could be exposed to 225 to 3,412 ug total Cr in a 24 h period from their diet [For example 0.6 ug total Cr/g sponge tissue dry weight × 0.25 moisture × 1000 g/kg × 1.5 kg sponge tissue/d = 225 ug total Cr/day].
In our study, we exposed cells to 27-273 ug of total Cr from lead chromate and 0.52 to 10 ug of total Cr from sodium chromate for a 24 h period. Therefore, based on this calculation and exposure scenario, our treatment concentrations range from 21 percent higher than the amount of Cr ingested in a day for our highest concentration of lead chromate, down to 4.1 to 6,500-fold lower than the amount of Cr ingested in a day for all of our other concentrations of lead chromate and all of our concentrations of sodium chromate. Of course, the effective amount of Cr released from lead chromate would be much lower. This scenarios is strictly hypothetical for context and actual exposure will vary based on environmental conditions and prey selection as well as contributions from marine air. However, it does show that although the precise amount of Cr exposure is uncertain for the hawksbill sea turtle, our concentrations in this study reflect a plausible exposure range.
It is clear from data in marine mammals that Cr, has become a significant concern in the marine environment and are at such high tissue levels significant exposure to Cr(VI) is anticipated to have occurred (Wise, J et al., 2008, 2009). In sea turtles, however, Cr is are much less studied. Several studies have recognized metals as a threat to sea turtles; however, Cr levels in sea turtles have only been reported in two studies. One study found low levels of Cr in blood plasma of hawksbill sea turtles (Suzuki et al., 2012). This outcome is not surprising as in humans and other terrestrial animals blood plasma levels are low (ATSDR 2012).
The second study (Storelli et al., 1998) showed that Cr was most highly accumulated in the lungs. This outcome is consistent with observations in human workers that Cr accumulates in the lungs after inhalation exposure to particulate Cr (Holmes et al., 2008). Liver, kidney and muscle were also measured in this study and were low relative to lung. These data suggest that for sea turtles respiration is likely a primary source for Cr exposure. When considering potential sources of Cr exposure in sea turtles, respiratory exposure is rarely addressed. However, sea turtles are air breathing animals and they dive for extended periods of time, which is likely to increase the retention time of airborne pollutants in their lungs while they hold their breath. One report suggests that coastal marine animals might be more exposed to atmospheric pollution than humans due to the concentration of particles in the air-water interface (Rawson et al., 1991). More research is needed to determine the exposure levels of Cr for sea turtles.
In summary, our data demonstrate that Cr(VI) is cytotoxic and genotoxic to Hawksbill sea turtle cells and indicate it is a health concern for exposed individuals and the population overall. Further work is needed to determine the extent and how hawksbill sea turtles are exposed.
Highlights.
Particulate Cr(VI) is cytotoxic and clastogenic to hawksbill sea turtle cells.
Soluble Cr(VI) is cytotoxic and clastogenic to hawksbill sea turtle cells.
Cr(VI) may be a risk factor for hawksbill sea turtle health.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Alexandra Smith for technical assistance and Shouping Huang and Christy Gianios, Jr. for administrative and information technology support, respectively. We would also like to thank The Vieques Conservation and Historical Trust, TICATOVE, and the Vieques office of the United Stated Fish and Wildlife Service for their support with this work. Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number R01ES016893 (JPW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional funding was provided by the Maine Space Grant Consortium (JPW), the Ocean Foundation (JPW), the Henry Foundation (JPW), the Curtis and Edith Munson Foundation (JPW), and the Maine Center for Toxicology and Environmental Health (JPW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agency for Toxic Substances and Disease Research . Top 20 hazardous substances: ATSDR/EPA priority list for 1997. U.S. Department of Health and Human Services Public Health Service/U.S. Environmental Protection Agency; 2012. [Google Scholar]
- Al-Hamood MH, Elbetieha A, Bataineh H. Sexual maturation and fertility of male and female mice exposed prenatally and postnatally to trivalent and hexavalent chromium compounds. Reprod. Fertil. Dev. 1998;10:179–183. doi: 10.1071/r97001. [DOI] [PubMed] [Google Scholar]
- Anan Y, Kunito T, Sakai H, Tanabe S. Subcellular distribution of trace elements in the liver of sea turtles. Marine. Poll. Bull. 2002;45:224–229. doi: 10.1016/s0025-326x(02)00106-6. [DOI] [PubMed] [Google Scholar]
- Andreani G, Santoro M, Cottignoli S, Fabbri M, Carpene E, Isani G. Metal distribution and metallothionein in loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles. Sci. Total Environ. 2008;390:287–294. doi: 10.1016/j.scitotenv.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Bataineh H, Al-Hamood MH, Elbetieha A, Bani Hani I. Effect of long-term ingestion of chromium compounds on aggression, sex behavior and fertility in adult male rat. Drug Chem. Toxicol. 1997;20:133–149. doi: 10.3109/01480549709003875. [DOI] [PubMed] [Google Scholar]
- Chowdhury AR, Mitra C. Spermatogenic and steroidogenic impairment after chromium treatment in rats. Indian J. Exp. Biol. 1995;33:480–484. [PubMed] [Google Scholar]
- DeFlora S, Bagnasco M, Serra D, Zanacchi P. Genotoxicity of chromium compounds. A review. Mutat. Res. 1990;238:99–172. doi: 10.1016/0165-1110(90)90007-x. [DOI] [PubMed] [Google Scholar]
- El-Makawy A, Radwan HA, Ghaly IS, El-Raouf AA. Genotoxical, teratological and biochemical effects of anthelmintic drug oxfendazole Maximum Residue Limit (MRL) in male and female mice. Reprod., Nutr., Dev. 2006;46:139–156. doi: 10.1051/rnd:2006007. [DOI] [PubMed] [Google Scholar]
- Franzellitti S, Locatelli C, Gerosa G, Vallini C, Fabbri E. Heavy metal in tissues of loggerhead turtles (Carrtta caretta) from the northwestern Adriatic Sea. Comp. Biochem. Physio. Part C. 2004;138:187–194. doi: 10.1016/j.cca.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Frias-Expericuet MG, Osuna-Lopez JI, Ruiz-Telles A, Quitero-Alvarez JM, Lopez-Lopez G, Izaguirre-Fierro G, Voltolina D. Heavy metals in the tissues of the sea turtle Lepidochelys olivacea from a nesting site of the northwest coast of Mexico. Bull. Environ. Contamin. Toxicol. 2006;77:179–185. doi: 10.1007/s00128-006-1048-1. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Kurita J, Saito T, Yuasa K, Kurita M, Donai K, Nitto H, Soichi M, Nishimori K, Uchida T, Isogai E, Onuma M, Sone H, Oseko N, Inoue-Murayama M. Efficient establishment of primary fibroblast cultures from the hawksbill sea turtle (Eretmochelys imbracata) J. In Vitro Cell. Dev. Biol. Anim. 2012;48(10):660–665. doi: 10.1007/s11626-012-9565-1. [DOI] [PubMed] [Google Scholar]
- Gardner SC, Fitzgerald SL, Vargas BA, Rodriguez LM. Heavy metal accumulation in four species of sea turtles from the Baja California peninsula, Mexico. BioMetals. 2006;19:91–99. doi: 10.1007/s10534-005-8660-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez AJ, Gomez-Ramirez P, Matinez-Lopez E, Hernandez-Garcia A, Maria-Mojica P, Romero D, Jimenez P, Castillo JJ, Bellido JJ. Heavy metals in tissues from loggerhead turtles (Caretta caretta) from the southwestern Mediterranean (Spain) Ecotox. Envion. Safety. 2009;72:557–563. doi: 10.1016/j.ecoenv.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Geisler CD, Schmidt D. An overview of chromium in the marine environment. Dt. Hydrogr. Z. 1991;44:185–196. [Google Scholar]
- Holmes AL, Wise SS, Xie H, Gordon N, Thompson WD, Wise JP., Sr. Lead ions do not cause human lung cells to escape chromate-induced cytotoxicity. Toxicol. Appl. Pharmol. 2005;203:167–176. doi: 10.1016/j.taap.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Wise SS, Wise JP., Sr. Carcinogenicity of hexavalent chromium. Ind. J. Med. Res. 2008;128:353–372. [PubMed] [Google Scholar]
- IARC . Monographs on the Evaluation of Carcinogenic Risks to Humans: Chromium, Nickel and Welding. Vol. 49. International Agency for Research on Cancer; Lyons, France: 1990. [PMC free article] [PubMed] [Google Scholar]
- IUCN IUCN Red List of Threatened Species. Version 2013.2. 2013 < www.iucnredlist.org>. Downloaded on 08 April 2014.
- Jerez S, Motas M, Canovas RA, Talavera J, Almela RM, del Rio AB. Accumulation and tissue distribution of heavy metals and essential elements in loggerhead turtles (Caretta caretta) from Spanish Mediterranean coastline of Murcia. Chemosphere. 2010;78:256–264. doi: 10.1016/j.chemosphere.2009.10.062. [DOI] [PubMed] [Google Scholar]
- Keshava N, Ong T. Occupational exposure to genotoxic agents. Mutat. Res., Rev. Mutat. Res. 1999;437:175–194. doi: 10.1016/s1383-5742(99)00083-6. [DOI] [PubMed] [Google Scholar]
- Komoroske LM, Lewison RL, Seminoff JA, Deheyn DD, Dutton PH. Pollutants and the health of green sea turtles resident to an urbanized estuary in San Diego, CA. Chemosphere. 2011;84:544–552. doi: 10.1016/j.chemosphere.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Li Chen T, Wise SS, Kraus S, Shaffiey F, Grau M, Thompson WD, Zheng T, Zhang Y, Romano T, O’Hara T, Wise JP., Sr. Particulate hexavalent chromium is cytotoxic and genotoxic to the North Atlantic right whale (Eubalaena glacialis) lung and skin fibroblasts. Environ. Mol. Mutagen. 2009;50:387–393. doi: 10.1002/em.20471. [DOI] [PubMed] [Google Scholar]
- Li Chen T, LaCerte C, Wise SS, Holmes A, Martino J, Wise JP, Jr., Thompson WD, Wise JP., Sr. Comparative cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human and sperm whale (Physeter macrocephalus) skin cells. Comp. Biochem. Physio. Part C: Toxicol Pharmacol. 2012;155:143–150. doi: 10.1016/j.cbpc.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Nerurkar VR, Aguirre AA, Work TM, Balazs GH, Yanagihara R. Establishment and characterization of 13 cell lines from a green turtle (Chelonia mydas) with fibropapillomas. Vitro Anim. Cell Dev. Biol. 1999;35(7):389–393. doi: 10.1007/s11626-999-0113-6. [DOI] [PubMed] [Google Scholar]
- Maffucci F, Caurant F, Bustamante P, Bentivegna F. Trace element (Cd, Cu, Hg, Se, Zn) accumulation and tissue distribution in loggerhead turtles (Caretta caretta) from the western Mediterranean sea (southern Italy) Chemosphere. 2005;58:535–542. doi: 10.1016/j.chemosphere.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Mancuso TF. Chromium as an industrial carcinogen: Part II. Chromium in human tissues. Am. J. Ind. Med. 1997;2:140–147. doi: 10.1002/(sici)1097-0274(19970204)31:2<140::aid-ajim2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Meylan A, Donnelly M. Status justification for listing the hawksbill turtle (Eretmochelys imbricata) as critically endangered on the 1996 IUCN Red List of Threatened Animals. Chelonian Conserv. Biol. 1999;3(2):200–224. [Google Scholar]
- Nayak BN, Ray M, Persaud TV, Nigli M. Relationship of embrytoxicity to genotoxicity of lead nitrate in mice. Exp. Pathol. 1989;36(2):65–73. doi: 10.1016/s0232-1513(89)80116-1. [DOI] [PubMed] [Google Scholar]
- National Marine Fisheries Service [accessed May 29, 2014];Office of Protected Resources website. http://www.nmfs.noaa.gov/pr/species/turtles/hawksbill.htm.
- Paez-Osuna F, Calderon-Campuzano MF, Soto-Jimenez MF, Ruelas-Inzunza JR. Lead in blood and eggs of sea turtle, Lepidochelus olivacea, from the Eastern Pacific: concentration, isotopic composition and maternal transfer. Marine Poll. Bull. 2010;60:433–439. doi: 10.1016/j.marpolbul.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Pettine M, Millero FJ. Chromium speciation in seawater: The probable role of hydrogen peroxide. Limnol. Oceanogr. 1990;35:730–736. [Google Scholar]
- Perez T, Vacelet J, Rebouillon P. In Situ Comparative Study of Several Mediterranean Sponges as Potential Biomonitors of Heavy Metals. Boll. Mus. Ist. Biol. Univ. Genova. 2004;68:517–525. [Google Scholar]
- Storelli MM, Ceci E, Marcotrigiano GO. Distribution of heavy metal residues in some tissues of the Caretta carretta (Linnaeus) specimen beached along the Adriatic Sea (Italy) Bull. Environ. Contam. Toxicol. 1998;60:546–552. doi: 10.1007/s001289900660. [DOI] [PubMed] [Google Scholar]
- Storelli MM, Storelli A, D’Addabbo R, Marano C, Bruno R, Marcotrigiano GO. Trace elements in loggerhead turtles (Caretta caretta) from the eastern Mediterranean Sea: overview and evaluation. Environ. Poll. 2005;135:163–170. doi: 10.1016/j.envpol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Storelli MM, Barone G, Storelli GO, Marcotrigiano GO. Total and subcellular distribution of trace elements (Cd, Cu and Zn) in the liver and kidney of green turtles (Chelonia mydas) from the Mediterranean Sea. Chemosphere. 2008;70:908–913. doi: 10.1016/j.chemosphere.2007.06.069. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Noda J, Yanagisawa M, Kawazu I, Sera K, Fukui D, Asakawa M, Yokota H. Relationships between carapace sizes and plasma major and trace element status in captive hawksbill sea turtles (Eretmochelys imbricata) J. Vet. Med. Sci. 2012;74:167–1680. doi: 10.1292/jvms.12-0099. [DOI] [PubMed] [Google Scholar]
- Tan F, Wang M, Wang W, Alonso Aguirre A, Lu Y. Validation of an in vitro cytotoxicity test for four heavy metals using cell lines derived from a green sea turtle (Chelonia mydas) Cell Bio. Toxicol. 2010;26:255–263. doi: 10.1007/s10565-009-9130-1. [DOI] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Ketterer ME, Hartsock W, Fomchencko E, Katsifas SP, Thompson WD, Wise JP., Sr. Chromium is the proximate clastogenic species for lead chromate-induced clastogenicity in human bronchial cells. Mutat Res. 2004;560:79–89. doi: 10.1016/j.mrgentox.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Moreland JA, Xie H, Sandwick SJ, Stackpole MM, Fomchenko E, Teufack S, May AJ, Jr., Katsifas SP, Wise JP., Sr. Human lung cell growth is not stimulated by lead ions after lead chromate-induced genotoxicity. Mol. Cell. Biochem. 2005;279:75–84. doi: 10.1007/s11010-005-8217-0. [DOI] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Wise JP., Sr. Hexavalent chromium-induced DNA damage and repair mechanisms. Rev. Environ. Health. 2008;23:39–57. doi: 10.1515/reveh.2008.23.1.39. [DOI] [PubMed] [Google Scholar]
- Wise SS, Shaffiey F, LaCerte C, Goertz CEC, Dunn JL, Gulland FMD, Abouiessa A, Zheng T, Wise JP., Sr. Particulate and soluble hexavalent chromium are cytotoxic and genotoxic to Stellar sea lion lung cells. Aquatic Tox. 2009;91:329–335. doi: 10.1016/j.aquatox.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr., Wise SS, Little JE. The cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human lung cells. Mutat. Res. 2002;517:221–229. doi: 10.1016/s1383-5718(02)00071-2. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr., Wise SS, Kraus S, Shaffiey F, Grau M, Chen T, Li Perkins C, Thompson WD, Zheng T, Zhang Y, Romano T, O’Hara T. Hexavalent chromium is cytotoxic and genotoxic to the North Atlantic right whale (Eubalaena glacialis) lung and testes fibroblasts. Mutat. Res. 2008;650:30–38. doi: 10.1016/j.mrgentox.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr., Payne R, Wise SS, LaCerte C, Wise J, Gianios C, Jr., Thompson WD, Perkins C, Zheng T, Zhu C, Benedict L, Kerr I. A global assessment of chromium pollution using sperm whales (Physeter macrocephalus) as an indicator species. Chemosphere. 2009;75:1461–1467. doi: 10.1016/j.chemosphere.2009.02.044. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr., Thompson WD, Wise SS, LaCerte C, Wise J, Gianios C, Jr., Perkins C, Zheng T, Zhu C, Benedict L, Mason MD, Payne R, Kerr I. A Global Assessment of Gold, Titanium, Strontium and Barium Pollution Using Sperm Whales (Physeter macrocephalus) as an Indicator Species. J. Ecosys. Ecograph. 2011;1:101. doi: 10.1016/j.chemosphere.2009.02.044. doi:10.4172/2157-7625.1000101. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr., Wise SS, LaCerte C, Wise JP, Jr., Abouissa A. The genotoxicity of particulate and soluble chromate in sperm whale (Physeter macrocephalus) skin fibroblasts. Environ. Mol. Mutat. 2011;52:43–49. doi: 10.1002/em.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer CM, Harris R, Shupack SI. Oral bioavailability of chromium from a specific site. Environ. Health Perspect. 1991;92:105–110. doi: 10.1289/ehp.92-1519374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer CM, Harris R, Shupack SI. Mutagenicity and disposition of chromium. Sci. Total Environ. 1989;86:131–148. doi: 10.1016/0048-9697(89)90200-3. [DOI] [PubMed] [Google Scholar]