Abstract
BACKGROUND
To investigate perceived barriers to mammography among underserved women, we asked participants in the Siteman Cancer Center Mammography Outreach Registry – developed in 2006 to evaluate mobile mammography's effectiveness among the underserved – why they believed women did not get mammograms.
METHODS
The responses of approximately 9000 registrants were analyzed using multivariable logistic regression. We report adjusted odds ratios (OR) and 95% confidence intervals (CI) significant at two-tailed p<0.05.
RESULTS
Fears of cost (40%), mammogram-related pain (13%), and bad news (13%) were the most commonly reported barriers. Having insurance was associated with not perceiving cost as a barrier (OR 0.44, 95%CI 0.40–0.49) but with perceiving fear of both mammogram-related pain (OR 1.39, 95%CI 1.21–1.60) and receiving bad news (OR 1.38, 95%CI 1.19–1.60) as barriers.
CONCLUSION
Despite free services, underserved women continue to report experiential and psychological obstacles to mammography, suggesting the need for more targeted education and outreach in this population.
Keywords: breast cancer, health beliefs, health disparities, mobile mammography, screening
INTRODUCTION
Mirroring national trends, breast cancer is the most commonly diagnosed non-cutaneous malignancy and the second most common cause of cancer-related mortality among women in Missouri.1 Since 2000, there has been a significant decline in breast-cancer-specific mortality throughout the state,1 but this overall improvement masks statewide disparities. Compared with whites and in contrast to the nation as a whole, African-American women in Missouri have higher incidence rates of breast cancer than whites (142.5 versus 122.1 breast-cancer diagnoses for every 100,000 women in 2005–2009), and the age-adjusted breast-cancer mortality rate among African-American women is also higher (24.4 versus 23.1 breast-cancer-specific deaths for every 100,000 women in 2005–2009).2 In addition, disproportionately high rates of late-stage (locally advanced or metastatic) breast cancer have been reported among women in North St. Louis, an urban, low-income, and predominantly African-American community,3–5 while in the rural, southeastern Bootheel region of Missouri, women have lower mammography utilization rates and higher breast-cancer mortality rates than women in almost any other part of the state.6
As part of an effort to redress these disparities in the breast-cancer continuum of care, several outreach efforts to medically underserved communities have been initiated by the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, the only National Cancer Institute (NCI)-designated Comprehensive Cancer Center in the state of Missouri. Over the past 10 years, Siteman's Mammography Van has significantly expanded its geographic catchment area throughout the St. Louis metropolitan area and in the Missouri Bootheel region.7 Furthermore, women found to have breast abnormalities through van mammograms are now referred directly to Siteman for follow-up, regardless of insurance coverage, rather than being referred back to their primary care physicians.7 Finally, Siteman has worked with two organizations in order to fund mammograms for low-income and under/uninsured women: Show Me Healthy Women (SMHW), a program providing free breast- and cervical-cancer screening for Missouri residents over the age of 35,8 and the St. Louis Affiliate of Susan G. Komen for the Cure, which has funded the Breast Health Care for At-Risk Communities (BHCAC) program at Siteman for 15 years.9
To assess and enhance the effectiveness of Siteman's mammography services among the medically underserved, Siteman's Mammography Outreach Registry was established in 2006. Any woman who received a free or reduced-cost mammogram at Siteman's Joanne Knight Breast Health Center (BHC) or on the mammography van was included in the registry. Because many of the Outreach Registry participants reside in zip codes where screening participation has historically been low, we wanted to explore registrants' health beliefs regarding mammography, with the hope that their beliefs might shed light on factors associated with screening nonparticipation in their communities. In formulating our questions, we utilized the Health Belief Model for assessing health behavior.
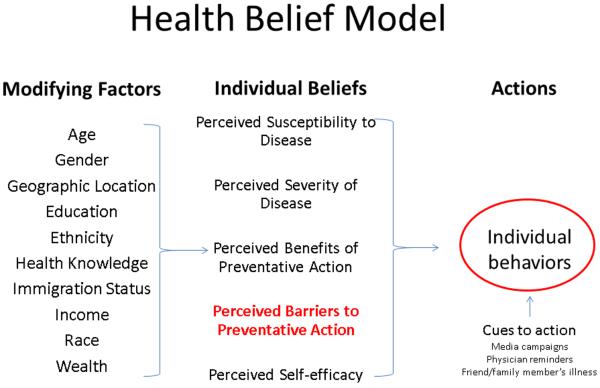
The Health Belief Model (HBM, Figure 1), first developed by social psychologists in the United States (US) Public Health Service in the 1950s, is a theory of health behavior widely used not only to examine why people do or do not take action to prevent, screen for, or treat disease but also to guide the development of interventions aimed at improving healthcare participation.10 Over the past 30 years, the HBM has been advanced by Victoria Champion, Celette Skinner, and others as a means through which to improve rates of breast-cancer screening amongst the underserved.11 Indeed, in large part because of community-based interventions informed by the HBM, racial disparities in mammography utilization in the US have essentially been eliminated.12 However, as already discussed, geographic pockets of disparity remain both in the state of Missouri and throughout the nation.
Figure 1. Health Belief Model Components and Linkages.
The major constructs of the Health Behavior Model are perceived susceptibility, severity, benefits, barriers, and self-efficacy (middle column). Modifying factors (left column) affect these perceptions, as do cues to action (right column). The combination of beliefs and cues to action leads to behavior. Perceived barriers (red text) have been demonstrated to be the single most powerful predictor of health behavior.11,39,40
Previous studies have demonstrated that within the HBM (Figure 1), perceived barriers represent the set of health beliefs most likely to predict health behavior.11 Thus, in order to efficiently evaluate the efficacy of our mobile mammography program and assess the potential for increased screening participation in the communities served by the mammography van, we asked registrants to share with us their impressions of why women in general might be more or less likely to undergo a screening mammogram: that is, what psychological, logistical, and/or experiential factors did they believe women were likely to perceive as barriers to getting screened? Here, we report the results of our prospective cohort study, which represents the largest review ever conducted of a mobile mammography population.
METHODS
Population studied
Beginning in 2006, women who received screening mammograms funded by SMHW or Komen's BHCAC programs were registered in the Siteman Mammography Outreach Registry during their first screening visit at either the Siteman Mammography Van or at the BHC. Participation in the registry was not required for receipt of services, but the vast majority of women – approximately 99% – who presented for mammograms agreed to participate.
Data collection
At registration, each participant completed a 6-item questionnaire about their experiences with mammography in general and as part of the Siteman Mammography Outreach Registry in particular. The questionnaire was written at a sixth-grade reading level and was designed by the nurse manager of the BHC and a public-health trained breast radiologist. In accordance with Siteman's institutional policy guaranteeing interpreter services to all who need them, the questionnaire was interpreted on an ad hoc basis for all women with limited English proficiency. The first item of the questionnaire – “What is the reason women don't get a mammogram?” – was included for the purpose of assessing perceived barriers to mammography, and respondents were encouraged to select one response from a set of 11 standardized responses (Table 1) to this query.
Table 1.
Questionnaire Item 1: “What is the reason women don't get a mammogram?”
| Standardized Responses |
|---|
| Fear of cost |
| Being too busy |
| Fear of mammogram-related pain |
| Lack of transportation |
| Not being able to get time off work |
| Fear of getting bad news |
| Not knowing they needed a mammogram |
| Lack of childcare |
| People they knew were getting mammograms every yeara |
| Not having health insurance |
| Other/unspecified barriers |
Only question response that is NOT a perceived barrier but rather a positive perception associated with mammography utilization.
Registrants also agreed to provide access to their medical record information and to share demographic information. Women who had been previously screened through a Siteman provider, whose mammograms were provided during employee-targeted van visits to corporate sites, whose mammograms were covered entirely by private insurance, or who were under the age of 18 years were excluded. The Washington University Human Research Protection Office (i.e., institutional review board) approved this study. Verbal consent was obtained by a trained member of the BHC or mammography van staff prior to the questionnaire's being administered.
Statistical Analysis
Bivariate associations between demographic characteristics and registrants' perceived barriers to mammography – i.e., responses to the first item of the questionnaire – were analyzed using chi-square (χ2) tests for which two-tailed p<0.05 was considered significant. Demographic characteristics found to have statistically significant associations in bivariate tests were included as independent variables in multivariable logistic regression analyses modeling each of the most commonly reported barriers to mammography as a yes/no binary outcome; non-significant predictors were removed via stepwise elimination to reach the final regression models. The total number of respondents for each regression model reflects the number of registrants for whom complete demographic data and questionnaire responses were available. We report adjusted odds ratios (OR) and 95% confidence intervals (CI) significant at two-tailed p<0.05. Statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, North Carolina).
In addition, participants' responses were geocoded to assess the regional distribution of perceived obstacles to mammography participation. Zip-code-specific distributions of the most commonly reported barriers were calculated and translated into color-coded maps created through ArcGIS 10 (ESRI, Inc., Redlands, California).
RESULTS
Between April 2006 and May 2011, a total of 9082 women (Table 2) entered the Siteman Mammography Outreach Registry. Mean age of registrants was 52.04 years (standard deviation 8.65). The majority of registrants were black (54%), uninsured (74%), screened on the mammography van (83%), resided in the greater St. Louis region (85%), and reported a good or excellent experience as part of the outreach program (92%).
Table 2.
Siteman Mammography Outreach Registry Participants' Characteristics, April 2006–May 2011
| Demographic Variable (n=9082) | n (%) |
|---|---|
| Age, years (n=8873) | |
| 21–39 | 104 (1.2) |
| 40–45 | 2189 (24.7) |
| 46–55 | 3774 (42.5) |
| 56–65 | 2218 (25.0) |
| >65 | 588 (6.6) |
| Race/Ethnicity (n=8870) | |
| Non-Hispanic Black | 4816 (54.3) |
| Non-Hispanic White | 2966 (33.4) |
| Hispanic | 363 (4.1) |
| Other | 725 (8.2) |
| Annual Income (n=806) | |
| <$10,000 | 365 (45.3) |
| $10–20,000 | 336 (41.7) |
| >$20,000 | 105 (13.0) |
| Education [years of education completed] (n=853) | |
| < High School [0–11] | 246 (28.8) |
| Completed High School [12] | 376 (44.1) |
| Some Post-Secondary Education [13–16] | 231 (27.1) |
| Marital status (n= 8364) | |
| Married | 2310 (27.6) |
| Unmarried | 6054 (72.4) |
| Insurance coverage (n= 8860) | |
| Insured | 2278 (25.7) |
| Uninsured | 6582 (74.3) |
| Site of Residence (n=8908) | |
| St. Louis City | 3980 (44.7) |
| St. Louis County | 3597 (40.4) |
| Bootheel and Other MO | 1331 (14.9) |
| Site of Screening (n=8873) | |
| Mammography Van | 7334 (82.7) |
| Breast Health Center | 1539 (17.3) |
The three most commonly reported barriers to mammography were fear of cost (n=3537, 40%), fear of mammogram-associated pain (n=1152, 13%), and fear of getting bad news (n=1178, 13%). These barriers were further examined in multivariable logistic regression models (n=8739).
As shown in Table 3, registrants who were employed (OR 1.11) or who lived in the Missouri Bootheel (OR 2.32) were more likely to perceive fear of cost as a barrier to mammography, while those who had health insurance (OR 0.44) or who were (as compared with non-Hispanic [NH] whites) NH black (OR 0.58) or Hispanic (OR 0.34) were less likely to report fear of cost as a potential barrier.
Table 3.
Fear of Cost as a Barrier to Mammography (n=8739)a
| Demographic Variable | Odds Ratio (OR) | 95% CIb | p-value |
|---|---|---|---|
| Employed vs. not employed | 1.11 | 1.01–1.22 | 0.032 |
| Site of Residence | |||
| North STL vs. Not North STLc | 0.89 | 0.79–1.00 | 0.059 |
| Bootheel vs. Not Bootheeld | 2.31 | 1.86–2.88 | <0.001 |
| Insured vs. uninsured | 0.44 | 0.40–0.49 | <0.001 |
| Race/Ethnicity e | |||
| NH Black vs. NH White | 0.58 | 0.52–0.64 | <0.001 |
| Hispanic vs. NH White | 0.34 | 0.26–0.43 | <0.001 |
| Other vs. NH White | 0.65 | 0.55–0.77 | <0.001 |
Wald χ2df 11= 513.00, p<0.0001
Multivariable logistic regression model included the following variables (variable values in parentheses with reference group underlined): employment (employed, unemployed), residence in high-mortality North St. Louis zip code (resident, non-resident), residence in Bootheel zip code (resident, non-resident), insurance coverage (insured, uninsured), and race/ethnicity (non-Hispanic Black, non-Hispanic White, Hispanic, Other).
CI = confidence interval
North STL = one of 8 North St. Louis zip codes with disproportionately high rates of both late-stage breast cancer diagnoses and breast-cancer-specific mortality3–5
Bootheel = southernmost, rural area of Missouri
NH = Non-Hispanic
As shown in Table 4, registrants who were screened on the van (OR 1.63), had health insurance (OR 1.39), or were NH Black (OR 1.32) were more likely to report fear of mammogram-related pain as a potential barrier to getting a mammogram. However, registrants who reported Other race/ethnicity were less likely to perceive fear of mammogram-related pain as a barrier (OR 0.57).
Table 4.
Fear of Mammogram-related Pain as a Barrier to Mammography (n=8739)a
| Demographic Variable | Odds Ratio (OR) | 95% CIb | p-value |
|---|---|---|---|
| Site of Screening | |||
| Van vs. BHCc | 1.63 | 1.33–2.01 | <0.001 |
| Insured vs. uninsured | 1.39 | 1.21–1.60 | <0.001 |
| Race/Ethnicity d | |||
| NH Black vs. NH White | 1.32 | 1.15–1.52 | <0.001 |
| Hispanic vs. NH White | 1.05 | 0.73–1.49 | 0.811 |
| Other vs. NH White | 0.57 | 0.42–0.78 | <0.001 |
Wald χ2df 5= 103.63, p<0.0001
Multivariable logistic regression model included the following variables (variable values in parentheses with reference group underlined): site of mammogram provision (on van, at Breast Health Center), insurance coverage (insured, uninsured), and race/ethnicity (non-Hispanic Black, non-Hispanic White, Hispanic, Other).
CI = confidence interval
BHC = Joanne Knight Breast Health Center at Siteman Cancer Center
NH = Non-Hispanic
As shown in Table 5, having insurance (OR 1.38), being NH Black (OR 2.46), and being Hispanic (OR 2.98) predicted perceiving fear of receiving bad news as a barrier, while older women (OR 0.99) and women who were screened on the van (OR 0.77) were less likely to report this concern.
Table 5.
Fear of Receiving Bad News as a Barrier to Mammography (n=8739)a
| Demographic Variable | Odds Ratio (OR) | 95% CIb | p-value |
|---|---|---|---|
| Age | 0.99 | 0.98–0.99 | <0.001 |
| Site of Screening | |||
| Van vs. BHCc | 0.77 | 0.65–0.91 | 0.003 |
| Insured vs. uninsured | 1.38 | 1.19–1.60 | <0.001 |
| Race/Ethnicity d | |||
| NH Black vs. NH White | 2.46 | 2.10–2.87 | <0.001 |
| Hispanic vs. NH White | 2.98 | 2.22–4.00 | <0.001 |
| Other vs. NH White | 0.89 | 0.65–1.23 | 0.486 |
Wald χ2df 11= 205.73, p<0.0001
Multivariable logistic regression model included the following variables (variable values in parentheses with reference group underlined): age (continuous variable), site of mammogram provision (on van, at Breast Health Center), insurance coverage (insured, uninsured), and race/ethnicity (non-Hispanic Black, non-Hispanic White, Hispanic, Other).
CI = confidence interval
BHC = Joanne Knight Breast Health Center at Siteman Cancer Center
NH = Non-Hispanic
Insurance status was the only demographic characteristic significantly associated with all three of the most commonly reported barriers, but its directionality of association was not consistent: having insurance was associated with a lower likelihood of perceiving cost as a potential barrier to mammography, but it was associated with a greater likelihood of reporting fears of mammogram-related pain and receiving bad news as barriers.
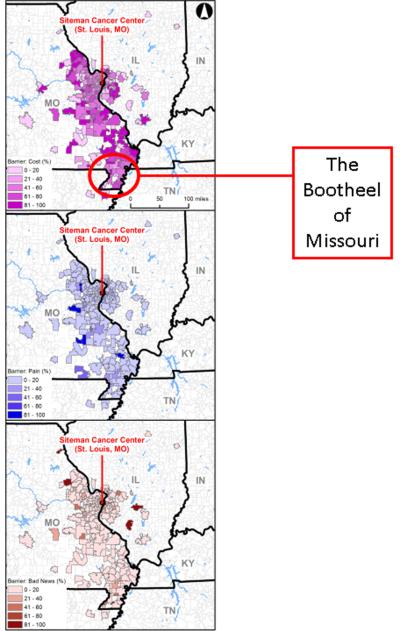
The proportions of patients reporting fears of cost, pain during mammography, and receiving bad news as barriers to mammography were also calculated based on registrants' residential zip codes. A total of 8916 registrants resided in 282 zip codes at the time of registration (mean and median of 31.61 and 4 registrants, respectively, per zip code; range of 1 to 542 registrants per zip code). The mean proportions of women per zip code reporting fears of cost, mammogram-related pain, and receiving bad news as barriers were 49%, 9%, and 10%, respectively (Figure 2). As noted above, residence in the Missouri Bootheel region was the only geographic factor found to be associated with a perceived barrier – fear of cost – in regression analysis.
Figure 2.
Geographic distribution of fear of cost (top), fear of mammogram-related pain (middle), and fear of receiving bad news (bottom) as perceived barriers to mammography
DISCUSSION
In this study, a variety of demographic variables were associated with perceived barriers to mammography as reported by registry participants. Most of the registrants were NH black and/or uninsured, and both race and socioeconomic status (for which insurance coverage can serve as a proxy measure) have historically been associated with disparities in breast screening utilization.13–21 Yet, while registrants share demographic characteristics with population subgroups at risk for screening avoidance, study participants obviously differed from screening nonparticipants by virtue of having received a screening mammogram. It is notoriously difficult to engage screening non-participants in research studies precisely because they are unlikely to have routine, let alone research-specific, contact with the medical establishment.19,22 However, there is evidence that screeners and non-screeners from the same communities are more likely to differ with regards to the intensity rather than the absence or presence of particular emotions or beliefs known to impact health behavior. For example, a recent qualitative study comparing the characteristics of colorectal screening participants and nonparticipants – all of whom had health insurance – demonstrated that while fear of cost was the most common barrier reported by non-screeners, it was also the second-most commonly reported barrier among screeners.23 In addition, it would be a mistake to think that our registrants, having been screened for the first time, do not remain at high risk for future nonparticipation. Lopez et al.'s examination of screeners and non-screeners in Mississippi revealed that the two groups reported similar barriers to mammography, indicating that the divide between those who get mammograms and those who do not s is less fixed and finite than one might initially think.24 The members of our registry are largely women who belong to demographic groups that are traditionally underrepresented in screening populations. We feel that they represent an important non-screener proxy group heretofore uncaptured at this scale (>9000 women) or in this region (few screening participation studies have been conducted in the lower Midwestern and Southern US). To learn from their responses, we need to explore what characteristics might make our study participants different from nonscreeners that are otherwise similar to them. But we also need to determine whether or not the perceived barriers study participants reported were representative of the beliefs held by nonscreeners within their communities. Specifically, to interpret our study participants' perceptions of possible barriers to mammography and to contextualize their responses in light of known risk factors for mammography nonparticipation, we must consider not only how fears and perceived barriers to screening translate into health-related behaviors but also the extent to which the pervasiveness and degree of these barriers might differ between screening participants and screening nonparticipants.
Fear – sometimes described interchangeably with anxiety and worry – is the most commonly studied emotional and psychological regulator of screening behavior.13 Fear of cost is a commonly reported barrier to health-related behaviors in general, and to screening in particular, and it was the most commonly reported perceived barrier in our cohort. Consistent with our participants' responses, fear of cost has been shown to persist even when women are provided with free or low-cost mammograms, as was the case in our sample, or if they have insurance coverage, particularly if women are African-American, low-income, older, and/or from an inner-city area.21,25,26 In addition, fear of cost must be further separated into fear of the cost of the screening procedure itself and fear of the costs associated with abnormal screenings and a potential cancer diagnosis, including wages lost while treatment is being conducted. Employed registrants may have been more likely than unemployed registrants to report fear of cost as a perceived barrier because they were all too aware of the wages they might lose not only while getting a mammogram but also while receiving breast cancer treatment, as was found to be the case for early-stage breast cancer patients in a recent study.27
In our study, registrants from the Missouri Bootheel were also more likely to report fear of cost as a possible deterrent to mammography. For these women, who live in a rural area marked by high levels of poverty and a paucity of healthcare facilities offering screening,28 the financial costs associated with physically accessing both a screening provider and a site for breast-cancer care might prove particularly prohibitive. Regardless of which components of breast-cancer detection and management contribute to reported fear of cost, it appears to be a perceived barrier among both screening participants and nonparticipants. But for the women in our registry, fear of cost was not sufficient to deter them from obtaining a mammogram, suggesting that free mobile mammography is a successful strategy to address the cost barrier among underserved populations. Lack of knowledge of such services might explain why many women in the registry had not been screened previously. Furthermore, cost may continue to be perceived as a barrier among registry participants because if free screening were not available, these women might not feel able to afford future mammograms.
The extent to which fear of mammography-related pain promotes or deters mammography utilization may depend on the relationship between this type of fear, other fears and perceived barriers, and emotional coping mechanisms in a given individual.13,29,30 For example, when coupled with a strong or catastrophic fear of receiving bad news after screening, fear of mammography-related pain has been demonstrated in various studies to be greater, and the emotional distress associated with both anticipating and receiving a mammogram is heightened.31 In previous studies, African-American and Hispanic women have been found to be more likely than white women to report fear of procedural pain as a concern that might keep them from getting mammograms.19 In our sample, NH black race was predictive of seeing mammogram-related pain as a potential barrier to mammography participation, but Hispanic ethnicity was not (Table 4). This is likely due to the registry's small Hispanic sample, which reflects the size of Missouri's Hispanic population. According to the 2010 census, less than 4% of Missourians reported Hispanic ethnicity as compared to 17% of the US population.32 It is unclear to what extent certain unmeasured demographic characteristics (e.g., country of origin, degree of acculturation, legal immigration status) within our sample's Hispanic participants are comparable to those characteristics reported in other studies exploring mammography barriers among Latinas,16,33–35 but such differences might help explain why Hispanic ethnicity was not associated with procedure-related pain in our analysis.
It is important to note that insured women in our study were significantly more likely to report fear of pain as a possible barrier, though there is no evidence in the literature on screening of a connection between having health insurance and fearing procedural pain. This finding further validates the concern that free screening programs and health insurance coverage do not necessarily alleviate anxiety about the logistical and personal challenges associated with obtaining a screening mammogram.
Fear of “receiving bad news”, i.e., of abnormalities being found during cancer screening, has been found in some studies to be associated with decreased screening utilization, particularly among black and Hispanic patients,15–17,20 but fear of finding cancer per se has not been universally found to be a deterrent to cancer-screening participation.36 Indeed, fear of being diagnosed with cancer has been shown in some studies to be a motivating factor in promoting regular screening utilization.36,37 Fear of getting cancer – which reflects both perceptions of personal susceptibility and disease severity according to the HBM (Figure 1) – may be an important distinguishing factor between women who do not plan to get a mammogram, women who plan on getting mammograms, and women who actually receive mammograms.18 Thus, the fact that fear of receiving bad news was one of the three most commonly reported perceived barriers in our cohort may not be a particularly surprising finding given that all of our study participants actually underwent screening mammography. Indeed, it may very well be that for these women, fear proved to be a protective emotion, prompting them to get screened. However, study participants might have reported fear of receiving bad news out of recognition that the same fear that ultimately motivated them to get screened might very well be a deterrent to screening among their friends and family and might even at one time have been a personal deterrent for the registrants themselves.
Thus, a potentially powerful strategy for improving screening utilization in the communities from which registrants come is to incentivize women who sign up to get a mammogram to return in future years and also to refer a friend, in much the same way retailers encourage customers to refer their favorite vendors to their friends and receive some kind of reward for doing so. The incentivization would ideally be trivial and/or in the form of an experience rather than remuneration, but would be enough to encourage members of the Outreach Registry to be especially proactive in recruiting their family members and friends. Getting one's mammogram would be depicted as something friends and family did together to help themselves and each other.
With regards to targeted education, steps are already underway to specifically address the fear of getting bad news that might deter women not only from getting screening mammograms but also from getting diagnostic imaging to follow up abnormal screening results. In the St. Louis region, efforts have been made to develop a media campaign that would publicize the curability of breast cancer, the importance of getting annual screening mammograms and of pursuing follow-up of abnormal mammograms, and the necessity of undergoing treatment once breast cancer has been diagnosed. This effort and similar “No Fear” campaigns should be developed in collaboration with cancer centers, community health organizations, survivor support groups, and primary care providers and would feature both screening participants who have never had cancer as well as breast-cancer survivors from demographic groups with historically low mammography participation. Testimonials from women who look like them would no doubt help demystify the process of getting a mammogram for women in the medically underserved communities represented by our registrants.
Our study is novel for a number of reasons. First of all, most studies looking at screening focus on demographic properties that correlate with participation. While we report these demographic traits as well, by choosing to look at perceived barriers, we can investigate mindsets, which – unlike race or education level – are somewhat mutable, though the ways in which these perspectives, values, beliefs, and preferences are modified must be informed by the demographic characteristics that help seat a person in a particular culture. Second, our study is geographically based in the Illinois/Missouri Bi-state region, and there is a relative paucity of literature on screening participation from Midwestern and Southern regions of the United States. Furthermore, our inclusion of geographic information system (GIS) analyses helped illustrate where registrants' perceived barriers were most prominent in our service region, allowing for geographically targeted interventions. Third, we believe our study represents the largest review ever conducted of a mobile mammography population and uses a much larger sample size than previous studies examining barriers to mammography as perceived by women from medically underserved communities. Fourth, ours is a prospectively collected database through which we will be able to observe longitudinally the impact of education and outreach interventions, of changing demographics in the region, and of implementation of the Affordable Care Act on mammography participation in an increasingly diverse region with areas of both urban and rural medical need. Historically, studies examining racial differences in mammography screening have reported complex, sometimes contradictory findings,24 and our analysis was no exception. But we hope that the complexity of our findings is embraced as a tool with which to shape solutions regarding disparities in breast cancer rather than as an indication that these problems cannot be solved.
With the evolution of breast imaging and, as a result, an enhanced ability to diagnose breast cancer at an early stage, we surgeons are increasingly able to cure breast-cancer patients through the operations we perform. Accordingly, we believe that promotion of mammography is a public health goal that should be actively promoted by all surgeons performing breast surgery. Furthermore, lessons learned from our experience with mammography among the medically underserved could be applicable to other types of screening (e.g., colonoscopy for colorectal cancer) that are systematically underutilized by particular groups in particular regions.
Limitations
Our study had some limitations. First, the outreach registry was not created for the purpose of conducting research but rather as part of a quality improvement initiative. Thus, although the process for collecting both objective data and subjective information about registrants' perceptions and experiences was informed by the HBM, it was not based on validated research questionnaires; indeed, the public-health trained physician who helped design our registry's questionnaire was unable to find a validated questionnaire on mobile mammography at the time the registry was first established in 2006. Nonetheless, the fact that the results of our statistical analyses of patients' responses were largely concordant with findings from the screening literature reassured us that our sample was not especially biased with regards to the perceptions we hoped to study and that the questions and response prompts from the questionnaire were sufficiently clear to most patients.
Second, no statistical sampling strategy was developed for recruiting patients to the registry, nor was a target recruitment goal set based on a priori power calculations. Women whose mammograms were funded by two initiatives developed to provide mammograms to low-income women were included in our study, and as most of these women were screened on the mammography van, our registry largely consists of mobile-mammography patients from all parts of the Missouri/Illinois bi-state region. As far as we know, our study is the largest review of mobile mammography patients ever conducted. We feel that our large sample of approximately 9000 participants should somewhat mitigate concerns about selection bias within our cohort.
CONCLUSION
In summary, women in the Siteman Mammography Outreach Registry reported perceived barriers to mammography that were similar to concerns that have been expressed by screening nonparticipants in other studies, though fear of receiving bad news – specifically of being diagnosed with cancer – might be a concern that not only is more common but also, somewhat paradoxically, serves as a motivating sentiment among women who receive screening mammograms. Passage of the Affordable Care Act will make important strides toward addressing cost as a barrier to mammography,38 but the responses of our registrants indicate that neither availability of free screening nor having health insurance necessarily mitigate women's perceived inability to obtain mammograms.
Structural and demographic factors such as age, income, marital status, and ethnicity cannot be directly or easily modified. Hence, although the study of these variables can help identify those at risk for poor screening participation, such research offers little direction in terms of viable interventions.29 It seems increasingly clear that if we are to improve screening rates, we must develop interventions that target variables that are both amenable to change and for which there is room for improvement. Screening-averse perceptions and psychological barriers – unlike race/ethnicity and other immutable demographic variables associated with screening avoidance – have the potential to be modified through public-health interventions13 including educational materials and outreach programs developed to specifically connect with and influence people who have potentially deterrent health beliefs.18 Having identified fears of cost, mammogram-related pain, and receiving bad news as significant concerns among women in the registry, we hope not only to improve the services we provide to the medically underserved women already using our mobile mammography program but also to encourage peer-to-peer recruitment and to enhance messaging and outreach in targeted ways that will help expand mammography utilization among medically underserved women throughout the region.
ACKNOWLEDGEMENTS
The work of Dr. Fayanju was supported by the National Institutes of Health (NIH) Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant 5T32CA009621-22. The work of Drs. Goodman and Drake was supported by the Siteman Cancer Center and NIH via National Cancer Institute (NCI) grant U54CA153460. The work of Dr. Oka was supported by the NCI Centers for Transdisciplinary Research on Energetics and Cancer (TREC) (U54CA155496). The authors would like to thank the St. Louis Affiliate of Susan G. Komen for the Cure, the Show Me Healthy Women initiative, and the Program for the Elimination of Cancer Disparities (PECaD) of the Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine for their support. We would also like to thank Dione Farria, MD, MPH, for co-writing the registry questionnaire; Priyanka Garg, MPH, and Xumei Si, MS, MPH, for their assistance with our data analysis; Donna Jeffe, PhD, and Mary Politi, PhD, for assisting with earlier drafts of the manuscript; and Graham Colditz, MD, DrPH, for his overall support for this initiative. Portions of this study's findings were presented at the 2012 Breast Cancer Symposium of the American Society of Clinical Oncology (ASCO), September 13–15, 2012, San Francisco, CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. [Accessed 5 October 2012];The Burden of Cancer in Missouri: A comprehensive analysis and plan, 2010–2015. 2012 http://health.mo.gov/living/healthcondiseases/chronic/chronicdisease/cancerburdenreport.pdf.
- 2.U.S. Cancer Statistics Working Group [Accessed 23 July 2013];United States Cancer Statistics: 1999–2009 Incidence and Mortality Web-based Report. 2013 www.cdc.gov/uscs.
- 3.Schootman M, Jeffe DB, Gillanders WE, Yan Y, Jenkins B, Aft R. Geographic Clustering of Adequate Diagnostic Follow-Up after Abnormal Screening Results for Breast Cancer among Low-income Women in Missouri. Ann Epidemiol. 2007;17:704–712. doi: 10.1016/j.annepidem.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Lian M, Jeffe DB, Schootman M. Racial and Geographic Differences in Mammography Screening in St. Louis City: A Multilevel Study. Journal of Urban Health. 2008;85:677–692. doi: 10.1007/s11524-008-9301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quesada LC. [Accessed 16 October 2012];Public Health: Understanding Our Needs - The City of Saint Louis Department of Health. 2007 http://stlouis-mo.gov/government/departments/health/documents/upload//UON_VOL_3_20072.pdf.
- 6.Gehlert SJ, Beers CE, Colditz GA. Coordinated care model to reduce rural disparities in breast cancer mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(10 Suppl):B65. [Google Scholar]
- 7.Fayanju OM, Jeffe DB, Elmore L, Ksiazek DN, Margenthaler JA. Patient and Process Factors Associated with Late-Stage Breast Cancer Diagnosis in Safety-Net Patients: A Pilot Prospective Study. Annals of Surgical Oncology. 2013;20:723–732. doi: 10.1245/s10434-012-2558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [Accessed 14 October 2012];Show Me Healthy Women. 2012 http://health.mo.gov/living/healthcondiseases/chronic/showmehealthywomen/index.php.
- 9. [Accessed 14 October 2012];St. Louis Affiliate of Susan G. Komen for the Cure: Grant Recipients 2012–13. 2012 http://www.komenstlouis.org/site/PageServer?pagename=grants_recipients.
- 10.Champion VL. Instrument development for health belief model constructs. Advances in Nursing Science. 1984;6(3):73–85. doi: 10.1097/00012272-198404000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Champion VL, Skinner CS. The Health Belief Model. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education: Theory, Research, and Practice. 4th ed John Wiley & Sons; San Francisco: 2008. [Google Scholar]
- 12. [Accessed 30 November 2012];Disparities in Breast Cancer Screening. 2012 http://ww5.komen.org/BreastCancer/RacialEthnicIssuesinScreening.html.
- 13.Consedine NS, Magai C, Krivoshekova YS, Ryzewicz L, Neugut AI. Fear, Anxiety, Worry, and Breast Cancer Screening Behavior: A Critical Review. Cancer Epidemiol Biomarkers Prev. 2004;13:501–510. [PubMed] [Google Scholar]
- 14.von Wagner C, Good A, Whitaker KL, Wardle J. Psychosocial Determinants of Socioeconomic Inequalities in Cancer Screening Participation: A Conceptual Framework. Epidemiol Rev. 2011;33:135–147. doi: 10.1093/epirev/mxq018. [DOI] [PubMed] [Google Scholar]
- 15.Ochoa-Frongia L, Thompson HS, Lewis-Kelly Y, Deans-McFarlane T, Jandork L. Breast and Cervical Cancer Screening and Health Beliefs Among African American Women Attending Educational Programs. Health Promot Pract. 2012;13:447–453. doi: 10.1177/1524839910385900. [DOI] [PubMed] [Google Scholar]
- 16.Austin L, Ahmad F, McNally M, Steward D. Breast and cervical cancer screening in Hispanic women: a literature review using the Health Belief Model. Women's Health Issues. 2002;12:122–128. doi: 10.1016/s1049-3867(02)00132-9. [DOI] [PubMed] [Google Scholar]
- 17.Bloom JR, Hayes WA, Saunders F, Flatt S. Cancer awareness and secondary prevention practices in Black Americans: Implications for intervention. Fam Community Health. 1987;10:19–30. [Google Scholar]
- 18.Russell KM, Monahan P, Wagle A, Champion V. Differences in Health and Cultural Beliefs by Stage of Mammography Screening Adoption in African American Women. Cancer. 2007;109(2 Suppl):386–395. doi: 10.1002/cncr.22359. [DOI] [PubMed] [Google Scholar]
- 19.Schueler KM, Chu PW, Smith-Bindman R. Factors Associated with Mammography Utilization: A Systematic Quantitative Review of the Literature. Journal of Women's Health. 2008;17:1477–1498. doi: 10.1089/jwh.2007.0603. [DOI] [PubMed] [Google Scholar]
- 20.Vernon SW, Laville EA, Jackson GL. Participation in breast screening programs: a review. Social Science and Medicine. 1990;30:1107–1118. doi: 10.1016/0277-9536(90)90297-6. [DOI] [PubMed] [Google Scholar]
- 21.Young RF, Severson RK. Breast cancer screening barriers and mammography completion in older minority women. Breast Cancer Research and Treatment. 2005;89:111–118. doi: 10.1007/s10549-004-1476-8. [DOI] [PubMed] [Google Scholar]
- 22.Meissner HI, Breen N, Taubman ML, Vernon SW, Graubard BI. Which women aren't getting mammograms and why. Cancer Causes Control. 2007;18:61–70. doi: 10.1007/s10552-006-0078-7. [DOI] [PubMed] [Google Scholar]
- 23.Medina GG, McQueen A, Greisinger AJ, Bartholomew LK, Vernon SW. Gastroenterology Research and Practice. 2012. What Would Make Getting Colorectal Cancer Screening Easier? Perspectives from Screeners and Nonscreeners. doi 10.1155/2012/895807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez EDS, Khoury AJ, Dailey AB, Hall AG, Chisholm LR. Screening Mammography : A Cross-Sectional Study to Compare Characteristics of Women Aged 40 and Older From the Deep South Who Are Current, Overdue, and Never Screeners. Women's Health Issues. 2009;19:434–445. doi: 10.1016/j.whi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Kiefe CI, McKay SV, Halevy A, Brody BA. Is Cost a Barrier to Screening Mammography for Low-Income Women Receiving Medicare Benefits?: A Randomized Trial. Archives of Internal Medicine. 1994;154:1217–1224. [PubMed] [Google Scholar]
- 26.Urban N, Anderson GL, Peacock S. Mammography Screening: How Important Is Cost as a Barrier to Use? American Journal of Public Health. 1994;84:50–55. doi: 10.2105/ajph.84.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauzier S, Maunsell E, Drolet M, et al. Wage Losses in the Year After Breast Cancer: Extent and Determinants Among Canadian Women. Journal of the National Cancer Institute. 2008;100:321–332. doi: 10.1093/jnci/djn028. [DOI] [PubMed] [Google Scholar]
- 28.Rural Policy Research Institute Demographic and Economic Profile; Missouri: 2006. [Accessed 6 November 2012]. http://www.rupri.org/Forms/Missouri.pdf. [Google Scholar]
- 29.Aro AR, de Koning HJ, Absetz P, Schreck M. Two distinct groups of non-attenders in an organized mammography screening program. Breast Cancer Res Treat. 2001;70:145–153. doi: 10.1023/a:1012939228916. [DOI] [PubMed] [Google Scholar]
- 30.Keefe FJ, Hauck ER, Egert J, Rimer B, Kornguth P. Mammography pain and discomfort: a cognitive-behavioral perspective. Pain. 1994;56:247–260. doi: 10.1016/0304-3959(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 31.Shelby RA, Scipio CD, Somers TJ, Soo MS, Weinfurt KP, Keefe FJ. Prospective Study of Factors Predicting Adherence to Surveillance Mammography in Women Treated for Breast Cancer. J Clin Oncol. 2012;30:813–819. doi: 10.1200/JCO.2010.34.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.United States Census Bureau State & County QuickFacts; Missouri: 2012. [Accessed 6 November 2012]. http://quickfacts.census.gov/qfd/states/29000.html. [Google Scholar]
- 33.Kagay CR, Quale C, Smith-Bindman R. Screening mammography in the American elderly. Am J Prev Med. 2006;31:142–149. doi: 10.1016/j.amepre.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Mandelblatt JS, Yabroff KR. Breast and cervical cancer screening for older women: recommendations and challenges for the 21st century. J Am Med Womens Assoc. 2000;55:210–215. [PubMed] [Google Scholar]
- 35.Smith-Bindman R, Miglioretti DL, Lurie N, et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144:541–553. doi: 10.7326/0003-4819-144-8-200604180-00004. [DOI] [PubMed] [Google Scholar]
- 36.Consedine NS, Magai C, Neugut AI. The contribution of emotional characteristics to breast cancer screening among women from six ethnic groups. Preventive Medicine. 2004;38:64–77. doi: 10.1016/j.ypmed.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Edwards NI, Jones DA. Uptake of breast cancer screening in older women. Age and Ageing. 2000;29:131–135. doi: 10.1093/ageing/29.2.131. [DOI] [PubMed] [Google Scholar]
- 38. [Accessed 2 February 2013];Women and the Affordable Care Act. 2013 http://www.healthcare.gov/law/information-for-you/women.html.
- 39.Becker MH, Haefner DP, Kasl SV, Kirscht JP, Maiman LA, Rosenstock IM. Selected psychosocial models and correlates of individual health-related behaviors. Medical care. 1977 May;15(5 SUPPL):27–46. doi: 10.1097/00005650-197705001-00005. [DOI] [PubMed] [Google Scholar]
- 40.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health education quarterly. 1988 Summer;15(2):175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]