Abstract
Background
Skin trauma may play a role in the development of morphea lesions. The association between trauma and the distribution of cutaneous lesions has never been examined.
Objective
Determine whether patients enrolled in the Morphea in Adults and Children (MAC) cohort exhibit skin lesions distributed in areas of prior (isotopic) or ongoing (isomorphic) trauma.
Methods
Cross-sectional analysis of the MAC cohort.
Results
Of 329 patients in the MAC cohort, 52 (16%) had trauma associated lesions at the onset of disease. Patients with lesions in an isotopic distribution had greater clinical severity as measured by a clinical outcome measure (mean modified Rodnan Skin Score of 13.8 vs. 5.3, P=0.004, 95% CI=3.08-13.92) and impact on life quality (mean Dermatology Life Quality Index 8.4 vs. 4.1, P=0.009, 95% CI 1.18-7.50) than those with an isomorphic distribution. Most frequent associated trauma were chronic friction (isomorphic) and surgery/isotopic.
Limitations
Recall bias for patient reported events.
Conclusion
Sixteen percent of patients in the MAC cohort developed initial morphea lesions at sites of skin trauma. If these findings can be confirmed in additional series, they suggest that elective procedures and excessive skin trauma or friction might be avoided in these patients.
Keywords: Morphea, localized scleroderma, Morphea in Adults and Children cohort, skin trauma, DLQI, mRSS
Introduction
Morphea (localized scleroderma) is a heterogeneous sclerosing disorder of the skin and subcutaneous tissue of uncertain pathogenesis. Several case reports and series have reported precipitating events including friction from clothing, injection, herpes zoster infection, and radiation therapy prior to the onset of morphea.1-4 However, the association of morphea with skin trauma has not been systematically investigated.
Prior reports in morpheaform chronic graft-versus-host disease (GVHD), a disorder clinically similar to morphea, indicate that these lesions may be distributed in areas of skin trauma and have used the terms isotopic and isomorphic to describe the distribution of these lesions.5, 6 The isotopic response is defined as the development of a second, unrelated disease in the same area as previous healed disease or injury (Figure 1).6 This is in contrast to the isomorphic response of Koebner, which describes skin lesions appearing in areas of repeated trauma (Figure 2).5 Given that there is an association between skin trauma and the distribution of skin lesions in morpheaform GVHD, it is reasonable to expect this may also be true in morphea. Determining whether there is an association between morphea and skin trauma has important implications for both pathogenesis and clinical care.
Figure 1.
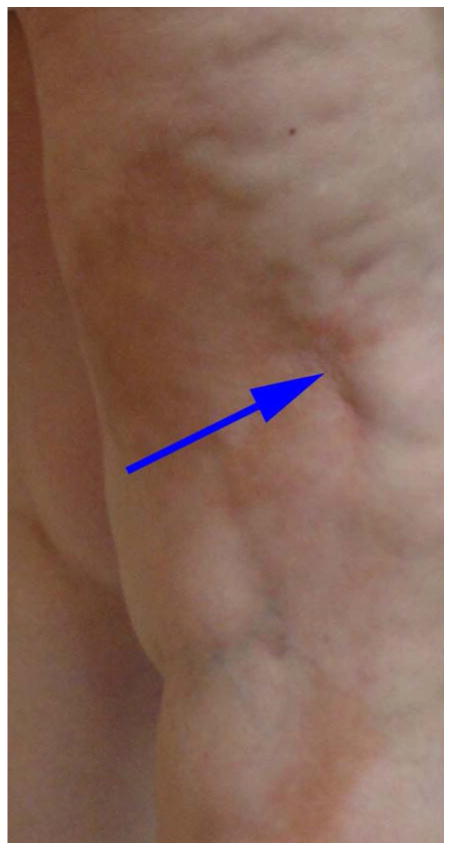
Isotopic morphea on the right leg. The solid arrow points to a surgical scar where the lesion began.
Figure 2.

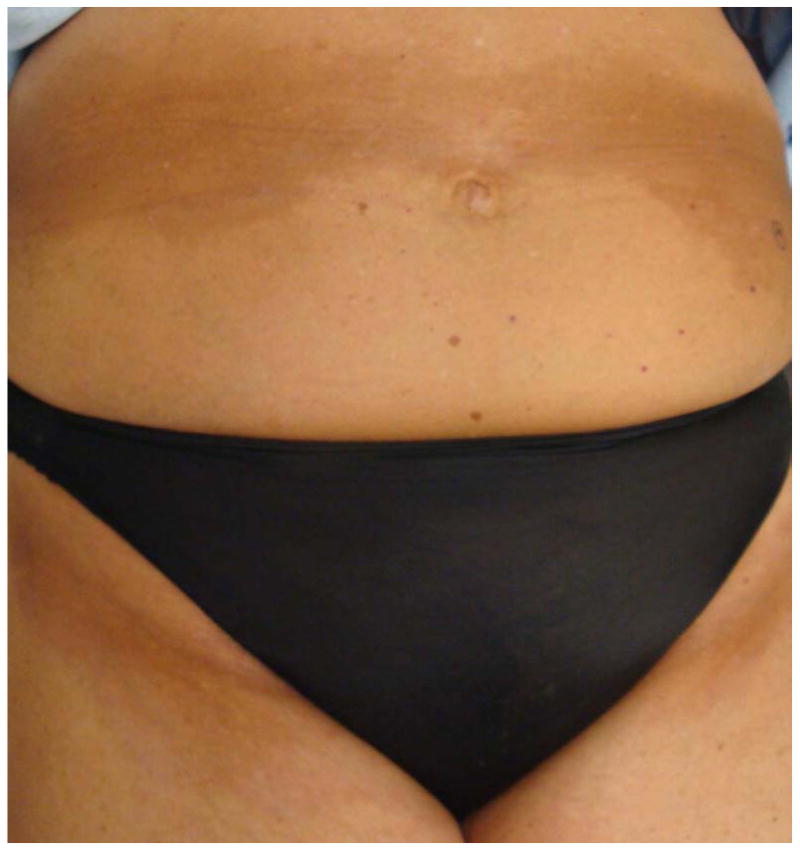
a: Isomorphic morphea in the brassiere line of a Caucasian female undergoing phototherapy
b: Isomorphic morphea in the waist band and inguinal areas in the same patient.
The Morphea in Adults and Children (MAC) cohort was designed to examine demographic, clinical, and autoimmune features of adults and children with morphea. Following patients prospectively, we aimed to define the effect of these features on clinical outcomes. The objective of this study was to determine the frequency of the presence of lesions distributed in sites of skin trauma (in an isotopic or isomorphic distribution) in patients enrolled in the MAC cohort. We also examined patient demographics, clinical characteristics, and impact on quality of life in patients with trauma-induced morphea lesions.
Methods
Patients
The institutional review board-approved Morphea in Adults and Children (MAC) cohort contained 329 adults (18 years or older at enrollment) and children (≤17 years old at enrollment) as of March 2012. Patients were prospectively recruited from within the University of Texas Southwestern Medical Center system and from private practitioners providing patients of varied disease severity, subtypes, and socioeconomic backgrounds. Criteria for inclusion in this study included: eligibility for enrollment in the MAC cohort, age four years or older at time of enrollment, the presence of sufficient information for analysis, an inciting traumatic event at the initial site of morphea, and the presence of trauma induced lesions. The latter two variables were obtained from the predetermined Case Report Form (CRF) which specifically queried patients for any skin trauma occurring in the site of their lesions prior to disease onset. If patients answered “yes” further inquiries were made including questions regarding the type of traumatic event and the location and timing of the event in relation to the morphea onset and location of the initial lesion. In addition, the site was examined by one author (HJ) to ascertain residua of trauma (surgical scar, radiation markings, scar from trauma, etc.) Medical records were obtained for verification of date and type of procedure. In cases of radiation therapy related to morphea lesions, biopsies were performed to exclude cutaneous metastasis and radiation induced dermatitis. Isotopic patients were defined as those who had trauma occur at the site of the initial lesion within 6 months of onset of morphea with confirmation by medical records and/or physical examination. Isomorphic patients were those with lesions distributed exclusively in areas of friction in the brassiere, waistband, and inguinal crease. These classifications were based on definitions in the literature5, 6 and recent studies of sclerotic-type GVHD.7, 8
Variables of Interest
Data was collected using a CRF that included demographic, clinical, medical history, Dermatology Life Quality Index (DLQI), and physician-based determinants of disease severity --modified Rodnan Skin Score (mRSS) and Localized Scleroderma Cutaneous Assessment Tool (LoSCAT).9, 10 The mRSS was included because the Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) was not available at the inception of the cohort and is the only clinical score available for all MAC cohort patients. Medical records were obtained and reviewed for confirmation of patient reported findings including any reported skin trauma. All patients were examined by one examiner with significant expertise in morphea (HJ), who assigned the subtype and scores. Clinical classification was based on the preliminary proposed criteria established by Laxer and Zulian into linear, plaque, and generalized subtypes.11 The linear subtype was defined as a linear induration affecting the dermis and may include the subcutaneous tissue and underlying muscle. Generalized morphea is indurated plaques that have become confluent on at least two anatomic sites whereas plaque morphea is an indurated plaque that has not become confluent.
Quality of Life
Disease-related quality of life over the preceding 7 days was measured with the 10-item validated DLQI (range 0-30, with 30 reflecting greatest effect). Scores were divided into categories: 0-1 = no effect, 2-5 = small effect, 6-10 = moderate effect, 11-20 = very large effect, and 21-30 = extremely large effect from their skin disease.12 Itching and pain were assessed on a visual analog scale (VAS)13 of 1-10, with 10 indicating greatest severity.
Statistical Analysis
In this cross-sectional study, the association with all outcomes of interest was compared between the trauma induced groups (isomorphic and isotopic) as well as the overall MAC cohort. Fisher's exact test was used for comparisons of categorical variables and an unpaired t-test was used for comparisons of continuous variables between groups with P values < 0.05 considered significant. Means along with confidence intervals were calculated for continuous variables. GraphPad (GraphPad Software Inc, La Jolla, CA) was used for all analyses.
Results
Of the 329 patients in the MAC cohort, a total of 52 (16%) met the inclusion criteria -- 21 (6%) isotopic and 31 (9%) isomorphic. Patients (n=277) were excluded from the study due to the following: age <4 years (n=9), insufficient data on variables of interest (n=29), indeterminate subtype (n=8), and lack of inciting event or isomorphic distribution of lesions (n=231). The demographics of the patients included in the study are shown in Table I. The mean age of onset for the isotopic group was 44.4 versus a mean age of 52.4 in the isomorphic group (P=0.1). Both groups were female predominant (86% of the isotopic group and 100% of the isomorphic were female).
Table I. Demographics of patients.
| Trauma induced morphea | P-value | MAC overall | |||
|---|---|---|---|---|---|
|
| |||||
| Isotopic n(%) | Isomorphic n(%) | n(%) | P-value* | ||
| Total number of patients | 21 | 31 | 329 | ||
| Mean age of onset (SEM) | 44.4 (4.4) | 52.4 (2.5) | 0.097 | 30.4 (1.2) | <0.001 |
| 0-17, n (%) | 3 (14) | 1 (3) | 92 (28) | ||
| 18 and over, n (%) | 18 (86) | 30 (97) | 0.291 | 237 (72) | 0.001 |
| Sex, n (%) | |||||
| Male | 3 (14) | 0 (0) | 56 (17) | ||
| Female | 18 (86) | 31 (100) | 0.06 | 273 (83) | 0.039 |
| Race, n (%) | |||||
| White | 19 (90) | 25 (81) | 234 (71) | ||
| Black | 0 (0) | 1 (3) | 0.542 | 14 (4) | 0.125 |
| Other | 2 (10) | 5 (16) | 81 (25) | ||
P-value comparing Trauma induced morphea (Isotopic and Isomorphic combined) to MAC cohort
In comparing the trauma-induced morphea group (both isotopic and isomorphic) with the overall MAC cohort, the trauma-induced group had a mean age of 49.13 (9 -78) when compared with the overall cohort 30.4 (0.2 – 78) (p<0.0001). Of the trauma-induced morphea patients 87% presented with the generalized subtype in contrast to the MAC cohort in which patients presented with the following subtypes: 52% linear, 33% generalized, and 14% plaque. Relative to the MAC cohort overall, children were underrepresented in the trauma induced group (4/52% vs. 92/329%). The trauma-induced group also had a higher mean mRSS of 6.7 (5.1 - 8.3) compared to 6.0 (5.4 - 6.6) in the overall MAC cohort, but this difference was not statistically significant (P=0.47). Both groups had similar DLQI scores with 5.98 (4.39 - 7.57) for the trauma-induced group and 6.06 (5.30-6.82) for the overall MAC cohort (P=0.93).
The morphea subtypes, clinical features, and autoimmune associations are shown in Table II. In both groups, the majority of patients had generalized morphea -- 71% isotopic and 97% isomorphic however the isomorphic group had a statistically higher proportion of generalized and a lower proportion of linear morphea than the isotopic group (P=0.02). Patients with isotopic morphea had increased disease severity when compared to the isomorphic group with a mRSS of 9.8 versus 5.3, respectively (P=0.01). Additionally patients with isotopic morphea had a decreased QoL as shown in the DLQI with a score of 9.1 versus 4.1 for the isomorphic group (P=0.002). Despite differences in QOL, both trauma-induced groups reported similar symptoms of itch and pain with isotopic patients reporting a mean of 4.7 (3.0 – 6.4) and 3.7 (2.1 – 5.2) on the VAS respectively compared to 4.3 (3.0 – 5.7) and 2.8 (1.8 – 3.8), (P=0.74 and P=0.30) for the isomorphic patients. Both trauma induced groups had an equal proportion of positive ANA patients (P=1).
Table II. Secondary characteristics: morphea subtype, clinical features, and autoimmune associations.
| Trauma induced morphea | P value | ||
|---|---|---|---|
|
| |||
| Isotopic | Isomorphic | ||
| Mean MRSS (SEM), n | 9.8 (2.4), 13 | 5.3 (0.5), 30 | 0.01 |
| LoSCAT, n | 8 | 17 | |
| Mean LoSSI (SEM) | 45.9 (18.3) | 24.6 (4.8) | 0.14 |
| Mean LoSDI (SEM) | 24.4 (9.4) | 20.2 (3.4) | 0.61 |
| Mean DLQI (SEM), n | 9.1 (1.7), 21 | 4.1 (0.6), 31 | 0.002 |
| Itching, mean (SEM) | 4.7 (0.8), 21 | 4.3 (0.7), 31 | 0.74 |
| Pain, mean (SEM) | 3.7 (0.7), 21 | 2.8 (0.5), 31 | 0.30 |
| Mean # of sites (SEM) | 7.7 (1.7) | 9.3 (0.9) | 0.38 |
| ANA, n (%) | 1 | ||
| Positive ANA | 3 (25) | 5 (23) | |
| Negative ANA | 9 (75) | 17 (77) | |
| Concomitant autoimmune, n (%) | 7 (33) | 12 (39) | 0.77 |
| Morphea subtype, n (%) | 0.02 | ||
| Linear | 4 (19) | 0 (0) | |
| Plaque | 2 (10) | 1 (3) | |
| Generalized | 15 (71) | 30 (97) | |
The putative inciting events in isotopic patients are provided in Table III. Previous surgeries were the most common antecedent events prior to the development of lesions. Surgical procedures performed on patients in this cohort included mastectomy, lumpectomy, breast augmentation, gastric bypass, rhinoplasty, coronary artery bypass surgery, and arthroplasty. Penetrating trauma, injections, and radiation were less frequent. Of note, the majority of these lesions occurred in the chest or in the case of women, breasts (Table IV).
Table III. Putative inciting events in patients with isotopic morphea.
| Inciting event | Patients, n (%) |
|---|---|
| Surgery | 9 (43%) |
| Penetrating trauma | 4 (19) |
| Injection | 3 (14) |
| Herpes Zoster | 2 (10) |
| Radiation therapy | 1 (5) |
| Diagnostic x-ray | 1 (5) |
| Extreme exercise | 1 (5) |
Table IV. Distribution of Morphea Lesions.
| Trauma induced morphea | ||
|---|---|---|
|
| ||
| Isotopic | Isomorphic | |
| Abdomen (%) | 3 (14) | 26 (57) |
| Arms (%) | 2 (10) | 0 (0) |
| Chest (%) | 10 (48) | 20 (43) |
| Face (%) | 4 (19) | 0 (0) |
| Lower Back (%) | 1 (5) | 0 (0) |
| Legs (%) | 1 (5) | 0 (0) |
Discussion
The objective of this cross-sectional study was to determine the frequency of skin trauma-associated lesions in the MAC cohort and the associated demographic and clinical features in these patients. We found that of 329 patients, 52 (16%) had evidence for skin trauma or friction in the clinical distribution of skin lesions at the onset of disease. This was present most frequently in adults with the subtype of generalized morphea (Table II) implicating the possibility of an isotopic/isomorphic response in the pathogenesis of these lesions.
Although the prevalence of trauma-induced lesions was not previously assessed in a prospective cohort of morphea patients, our results are consistent with prior case reports and case series suggesting friction from clothing, injection, herpes zoster infection, and radiation therapy in the development of morphea lesions.1-4 Similar associations are reported in other sclerosing skin conditions including systemic sclerosis and morpheaform cGVHD. Sclerotic type cGVHD has been shown to occur in an isotopic fashion with ionizing radiation, repeated needle sticks, and varicella-zoster virus infection as well as in an isomorphic fashion at areas of skin friction (waistband and brassiere line).7, 8 As seen in Table IV, greater than 40% of patients with trauma induced lesions had them on the breast implying a potential preferential distribution, however its significance is difficult to ascertain due to the strong female predominance of affected patients who would be more likely to have procedures in those areas. Also of interest, children, in which the subtype is predominantly linear, were underrepresented in the trauma induced morphea group relative to the overall MAC cohort. This implies trauma may be a less important factor in pathogenesis in children, although further study is needed. In addition, the isotopic patients had a higher MRSS and a lower mean number of body sites affected implying increased induration of these lesions, however this was not supported by the LoSDI scores. Further research is needed to determine the difference in induration of lesions.
The results of the present study suggest the possibility of an isotopic or isomorphic response in morphea. The underlying mechanism has yet to be defined, but studies in the pathogenesis of fibrosis have shown that tissue damage from recurrent or sustained trauma upregulates damage-associated endogenous toll-like receptor ligands. In turn, this activates fibroblast innate immune signaling which enhances fibrinogenic responses and establishes a self-amplifying cycle of fibrogenesis.14 Additionally, patients with scleroderma have reduced or defective intrinsic protective signals such as peroxisome proliferator-activated receptor – gamma (PPAR-gamma) which acts as a brake on fibroblast activation.14 These findings support current hypotheses for the role of aberrant wound healing and stress in the development of fibrosis, as well as provide insight into the pathogenesis of morphea in this patient cohort.
Conclusions from the present study are limited by a small sample size particularly when dividing trauma into isomorphic and isotopic subgroups. In addition, despite concerted efforts to document trauma and the development of skin lesions, we had to rely on patient report for the timing of the onset of morphea related to trauma.
The present study found a clear association between trauma and morphea at the site of antecedent injury in a relatively sizable proportion of morphea patients, suggesting the possibility of an isotopic or isomorphic response. If these findings can be confirmed in additional series, they suggest that elective procedures and excessive skin trauma or friction might be avoided in these patients.
Acknowledgments
Funding Sources: Research for this manuscript was supported in part by NIH Grant No. K23AR056303-4. This work was conducted with support from UT-STAR, NIH/NCRR/NCATS Grant Number UL1RR024982. The content is solely the responsibility of the authors and does not necessarily represent the official views of UT-STAR, The University of Texas Southwestern Medical Center at Dallas and its affiliated academic and health care centers, the National Center for Research Resources, or the National Institutes of Health.
Abbreviations and Acronyms
- cGVHD
Chronic Graft-Versus-Host Disease
- CRF
Clinical Report Form
- DLQI
Dermatology Life Quality Index
- GVHD
Graft-Versus-Host Disease
- HJ
Heidi Jacobe, MD, MSCS
- LoSCAT
Localized Scleroderma Cutaneous Assessment Tool
- LoSSI
Localized Scleroderma Skin Severity Index
- LoSDI
Localized Scleroderma Skin Damage Index
- MAC
Morphea in Adults and Children cohort
- mRSS
Modified Rodnan Skin Scores
- VAS
Visual Analog Scale
Footnotes
Conflict of Interest Disclosure: The authors have no conflict of interest to declare.
Prior presentation/publication: This study has not been presented or published previously.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniel Grabell, Email: daniel.grabell@utsouthwestern.edu.
Clifford Hsieh, Email: chsieh27@uchicago.edu.
Rachel Andrew, Email: randrew112@gmail.com.
Kathryn Martires, Email: kathryn.martires@kp.org.
Andrew Kim, Email: andy.kim@gmail.com.
Rebecca Vasquez, Email: rebecca.vasquez@phhs.org.
Heidi Jacobe, Email: heidi.jacobe@utsouthwestern.edu.
References
- 1.Ueda T, Niiyama S, Amoh Y, Katsuoka K. Linear scleroderma after contusion and injection of mepivacaine hydrochloride. Dermatol Online J. 2010;16(5):11. [PubMed] [Google Scholar]
- 2.Ehara M, Oono T, Yamasaki O, Matsuura H, Iwatsuki K. Generalized morphea-like lesions arising in mechanically-compressed areas by underclothes. Eur J Dermatol. 2006;16(3):307–9. [PubMed] [Google Scholar]
- 3.Forschner A, Metzler G, Rassner G, Fierlbeck G. Morphea with features of lichen sclerosus et atrophicus at the site of a herpes zoster scar: another case of an isotopic response. Int J Dermatol. 2005;44(6):524–5. doi: 10.1111/j.1365-4632.2005.01625.x. [DOI] [PubMed] [Google Scholar]
- 4.Verbov J. Post-irradiation morphoea. Br J Dermatol. 1989;121(6):819–20. doi: 10.1111/j.1365-2133.1989.tb08227.x. [DOI] [PubMed] [Google Scholar]
- 5.Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol. 1990;29(6):401–10. doi: 10.1111/j.1365-4362.1990.tb03821.x. [DOI] [PubMed] [Google Scholar]
- 6.Wolf R, Brenner S, Ruocco V, Filioli FG. Isotopic response. Int J Dermatol. 1995;34(5):341–8. doi: 10.1111/j.1365-4362.1995.tb03616.x. [DOI] [PubMed] [Google Scholar]
- 7.Patel AR, Pavletic SZ, Turner ML, Cowen EW. The isomorphic response in morphealike chronic graft-vs-host disease. Arch Dermatol. 2008;144(9):1229–31. doi: 10.1001/archderm.144.9.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martires KJ, Baird K, Citrin DE, Hakim FT, Pavletic SZ, Cowen EW. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury: potential insight into the mechanism of isomorphic and isotopic responses. Arch Dermatol. 2011;147(9):1081–6. doi: 10.1001/archdermatol.2011.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22(7):1281–5. [PubMed] [Google Scholar]
- 10.Arkachaisri T, Pino S. Localized scleroderma severity index and global assessments: a pilot study of outcome instruments. J Rheumatol. 2008;35(4):650–7. [PubMed] [Google Scholar]
- 11.Laxer RM, Zulian F. Localized scleroderma. Curr Opin Rheumatol. 2006;18(6):606–13. doi: 10.1097/01.bor.0000245727.40630.c3. [DOI] [PubMed] [Google Scholar]
- 12.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 13.Vasquez R, Jacobe H. Something new under the sun: Phototherapy for sclerosing skin conditions. Expert Rev Dermatol. 2011;6(6):595–612. [Google Scholar]
- 14.Bhattacharyya S, Wei J, Varga J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat Rev Rheumatol. 2012;8(1):42–54. doi: 10.1038/nrrheum.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]


