Abstract
Rationale
Hypothalamic-pituitary-adrenal (HPA) axis hormones have neuroactive metabolites with receptor activity similar to ethanol.
Objectives
The present study related HPA hormones in naïve monkeys to ethanol self-administration.
Methods
Morning plasma adrenocorticotropic hormone (ACTH), cortisol, deoxycorticosterone (DOC), aldosterone and dehydroepiandrosterone-sulfate (DHEA-S) were measured longitudinally in male rhesus macaques (Macaca mulatta) induced to drink ethanol followed by access to ethanol (4% w/v, in water) and water 22 hours/day for 12 months.
Results
During ethanol access, DOC increased among non-heavy (average intake over 12 months ≤ 3.0 g/kg/day, n = 23) but not heavy drinkers (> 3.0 g/kg/day, n = 9), aldosterone was greater among heavy drinkers after 6 months. The ratio of DOC/aldosterone decreased only among heavy drinkers after 6 or12 months of ethanol self-administration. ACTH only correlated significantly with DHEA-S, the ratio of cortisol/DHEA-S and DOC after the onset of ethanol access, the former two just in heavy drinkers. Baseline hormones did not predict subsequent ethanol intake over 12 months, but baseline DOC correlated with average blood-ethanol concentrations (BEC), among all monkeys and heavy drinkers as a group. During ethanol access, aldosterone and DOC correlated and tended to correlate, respectively, with 12-month average ethanol intake.
Conclusions
Ethanol self-administration lowered ACTH and selectively altered its adrenocortical regulation. Mineralocorticoids may compensate for adrenocortical adaptation among heavy drinkers and balance fluid homeostasis. As DOC was uniquely predictive of future BEC) and not water intake, to the exclusion of aldosterone, GABAergic neuroactive metabolites of DOC may be risk factors for binge drinking to intoxication.
Keywords: ethanol, self-administration, rhesus monkeys, hypothalamic-pituitary-adrenal axis, ACTH, cortisol, deoxycorticosterone, dehydroepiandrosterone-sulfate, aldosterone
Ethanol is considered a stressor because it can activate the hypothalamic-pituitary-adrenal (HPA) axis. Corticotropin-releasing hormone (CRH) from the median eminence of the hypothalamus is necessary for an increase in adrenocorticotropic hormone (ACTH; rats, Rivier et al. 1984), and ACTH is necessary for ethanol to increase adrenal-derived hormones in plasma (Boyd et al. 2010). In turn, there are adrenal-derived, neuroactive metabolites of steroidal hormones controlled by ACTH that are released in response to stressful events (Boyd et al., 2010). Although there are limited data in non-human primates, there are clear conditions under which ethanol can activate the HPA axis. In ethanol-naïve monkeys, the combination of removal from the home cage, restraint for blood sampling and non-contingent ethanol administration (1.0–3.0 g/kg, i.v.) increased plasma ACTH from 125 pg/ml to 225 pg/ml and higher doses of ethanol (2.0–2.2 g/kg; i.v.) increased cortisol from 25 µg/dl to 50 µg/dl (Schwandt et al. 2011). In contrast, in monkeys under ethanol self-administration procedures with indwelling catheters for ethanol infusion and blood sampling, an average intake of 2.4 g/kg (i.v.) slightly lowered levels of ACTH and cortisol compared to saline self-administration (Broadbear et al. 2005). Likewise, in monkeys that have chronically self-administered ethanol, and have habituated to awake blood sampling, ethanol self-administration resulted in decreased cortisol compared to pre-drinking baseline levels (Cuzon-Carlson et al. 2011; Helms et al. 2012a). Acute ethanol ingestion in humans (0.75 g/kg) did not increase ACTH or cortisol in most (6/8) young adult, nonalcoholic men (Waltman et al. 1993). Further, compared to controls, human alcoholics had no difference in plasma cortisol but increased ACTH (Wand and Dobs 1991). However, compared to social drinkers, 28-day abstinent alcoholics had greater basal salivary cortisol (Sinha et al. 2009). Finally, there was similar serum cortisol levels in alcoholics and controls during a baseline phase, but cortisol increased when subjects were given ethanol every four hours for four consecutive days (Mendelson and Stein 1966). Thus, it appears that acute ethanol can result in an activation of the HPA axis, whereas chronic ethanol self-administration in non-human primates slightly suppresses basal activity of the HPA axis, and findings from humans are inconclusive.
Activation of the HPA axis by ethanol has mixed effects on circulating neuroactive steroids in primates. After acute ethanol administration, the precursor to all steroid hormones, pregnenolone, was decreased in macaque monkeys (Porcu et al. 2006) and increased in humans who were social drinkers (Pierucci-Lagha et al. 2006). Likewise, following acute ethanol in cynomolgus monkeys or humans, the neuroactive steroid precursors progesterone and DOC, as well as the neuroactive metabolite 3α,5α-pregnanolone and the mineralocorticoid aldosterone are either not increased or are decreased (Porcu et al., 2006; 2010; Pierucci-Lagha et al. 2006; Nieminen et al. 1981). Lastly, ethanol challenge did not increase the neurosteroid DHEA in human males (Pierucci-Lagha et al. 2006). Collectively the findings from an ethanol challenge in non-dependent primates suggest that the response of adrenal hormones either adapts to below baseline (cortisol) or is unchanged (neuroactive steroids). However, there are few data sets available to draw this conclusion.
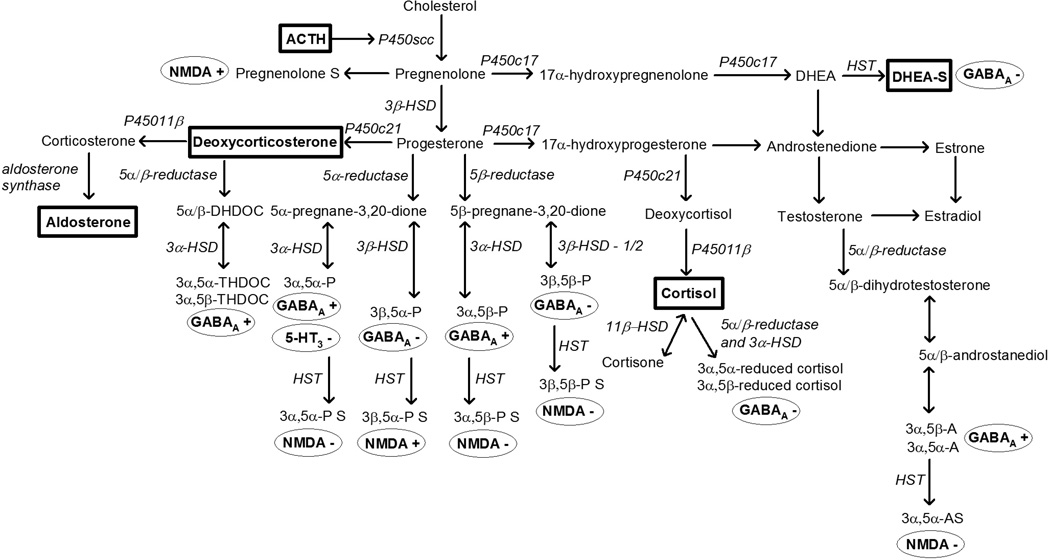
Some neuroactive steroids have similar neuronal receptor mechanisms as ethanol (Figure 1). As demonstrated using drug discrimination, metabolites of progesterone (3α,5α- and 3α,5β-pregnanolone; Grant et al. 1996, 1997, 2008a), deoxycorticosterone (DOC) (3α,5α-tetrahydrodeoxycorticosterone, THDOC; Ator et al. 1993) and testosterone (3α,5α-androsterone; Grant et al. 2008a) that positively modulate γ-aminobutyric acid (GABA)A receptors produce discriminative stimulus effects that substitute for ethanol. Other hormones such as dehydroepiandrosterone-sulfate (DHEA-S) and cortisol metabolites (3α,5α- and 3α,5β-reduced cortisol) negatively modulate GABAA receptors and do not substitute for ethanol (Helms et al., 2012c). The influence of physiological levels of neuroactive steroids on the subjective effects of ethanol was suggested by a study showing endogenous variation of progesterone during the menstrual cycle in monkeys alters sensitivity to ethanol in drug discrimination procedures (Grant et al. 1997). These studies suggest marked consequences of variation in endogenous hormones on the behavioral pharmacology of ethanol that is mediated by GABAA receptor mechanisms.
Figure 1.
Hormones measured in the present study (in rectangles) in the steroidogenic pathway selected to measure hypothalamic-pituitary-adrenal (HPA) axis activity (adrenocorticotropic hormone, ACTH; cortisol), mineralocorticoids (deoxycorticosterone, aldosterone) or androgens (dehydroepiandrosterone-sulfate, DHEA-S). Metabolites of hormones can be neuroactive as positive (+) or negative (−) modulators at γ-aminobutyric acid (GABA)A, N-methyl-D-aspartate (NMDA) or 5-HT receptors which are known to be involved in the behavioral pharmacological effects of ethanol.
The overlapping receptor mechanisms of ethanol and neuroactive steroids suggest a prominent interaction that may affect ethanol self-administration. Endogenous GABAergic neurotransmission, which is modulated by neuroactive steroids, inhibits the HPA axis and alters responses to ethanol. For example, antagonism of GABAA receptors in the paraventricular nucleus of the hypothalamus of rats increased corticosterone, and time- and dose-dependently decreased ethanol self-administration (Li et al. 2011). Interestingly, repeated corticosterone administration in rats resulted in rightward shifts in the dose-response curve indicating decreased efficacy and potency of ethanol to produce discriminative stimulus effects (Besheer et al. 2012). Further, neuroactive steroids that have similar receptor mechanisms as ethanol (e.g., GABAA receptor positive modulation) are self-administered in monkeys (Rowlett et al. 1999) and if used as a pretreatment, can increase ethanol self-administration (Sinnott et al. 2002). Thus, shared receptor mechanisms between stress-responsive adrenal hormones and ethanol (Biggio et al. 1990), imply a common homeostasis of these processes.
The present study extends our understanding of ethanol-neuroactive steroid interactions from drug discrimination to self-administration by documenting individual differences in baseline HPA axis activity and then studying subsequent ethanol drinking. Using the same procedure as the present study, we previously reported that oral ethanol self-administration during a schedule-induced polydipsia procedure (Grant et al., 2008) flattens ACTH and blunts cortisol diurnal rhythms (Helms et al. 2013). Subsequent ethanol self-administration (22 hours/day) resulted in a greater proportion of heavy drinkers that were of subordinate or intermediate compared to dominant social rank (Helms et al. 2012a), which could be related to differences between social ranks in hormonal response to ACTH (Czoty et al. 2009). Here we hypothesized that baseline HPA axis activity would correlate with the dose of self-administered ethanol, and the direction of correlation would depend on whether the neuroactive metabolites are positive or negative modulators of GABAA receptors (Figure 1). Hormones metabolized to GABAA negative modulators should attenuate the effects of ethanol, and were expected to positively correlate with ethanol self-administration. Hormones metabolized to GABAA positive modulators should potentiate the effects of ethanol, and were expected to negatively correlate with ethanol self-administration. In addition, repeated ethanol use appears to alter the threshold for stress responses and activation of the HPA axis in some studies (e.g., Berman et al. 1990; Dai et al. 2007; Porcu et al. 2008). However, there are no longitudinal studies of chronic oral ethanol self-administration and alterations of HPA axis hormones that are neuroactive steroid precursors. Therefore, this study characterized HPA axis adaptation with ethanol dose-dependent effects across long-term daily drinking, and the dose relationship of ethanol intake to HPA axis activity.
Methods
Animals
Experimentally-naïve male rhesus monkeys (Macaca mulatta, n = 32, 4–11 years, 6–8 kg) were subjects in this experiment. Throughout the experiment, animals were individually housed in quadrant cages (0.8 × 0.8 × 0.9 m) with constant temperature (20–22°C), humidity (65%) and light cycle (11 hours lights on, 13 hours lights off). Animals had visual, auditory and olfactory contact with other conspecifics and interacted extensively with the laboratory staff and the operant panel. Body weights were measured weekly. All procedures were conducted in accordance with the NIH and the Guide for the Care and Use of Laboratory Animals and approved by the Oregon National Primate Research Center IACUC. Data analysis on ethanol self-administration and MRI structural brain imaging from these animals have been published (Helms et al. 2014; Kroenke et al. 2014).
Apparatus
Each monkey had an operant panel on one wall of the home cage that provided access to food and fluid. The panels were controlled by a computerized system (Dell Computer Corporation, Round Rock, TX, USA, with interface and programming environment from National Instruments Corporation, Austin, TX, USA). As previously described (Vivian et al. 2001; Grant et al. 2008b), each panel had two fluid spouts, each beneath a set of three horizontally parallel lights (red, white and green), a centrally located recessed dowel with an associated stimulus light and an infrared finger-poke (OTBVR811, Banner Engineering, Minneapolis, MN). Each drinking spout was connected via tubing to a 1-L fluid reservoir placed on a digital scale (AV4101C, Ohaus Corporation, Pine Brook, NJ, USA). Electronic valves controlled fluid availability. Both the valves and scales were connected to a computer interface.
Ethanol Access
Schedule-induced polydipsia (Grant et al. 2008b, Vivian et al. 2001) was used to induce animals to self-administer 4% (w/v, in water) ethanol. Overall, one banana-flavored pellet was delivered every 300 seconds until a volume of water equal to 1.5g/kg 4% w/v ethanol solution was consumed, after which pellets were available on a fixed-ratio 1 (FR-1) schedule following a 2-hour period during which only water was available. The animals were then introduced to 4% w/v ethanol and required to consume increasing doses in 30-day increments beginning with 0.5 g/kg/day, 1.0 g/kg/day and finally 1.5 g/kg/day. Following induction, ethanol and water were available concurrently and animals were able to self-administer fluids for 22 hours/day. During concurrent access pellets were delivered on a FR-1 schedule in three equal meals with 2-hour intervals in between.
Sample Collection
After acclimating to the laboratory and staff, training for awake venipuncture was performed twice daily and advanced for each animal individually as they performed each step with minimal distress. As described by Porcu et al. (2006), training was conducted using positive reinforcement (fresh fruit) as the animal sat at the front of the cage and presented its leg through an opening (10 × 10 cm). Once comfortable, a dental pick was used to simulate a needle stick at the femoral vein. Finally, blood was collected (3 ml, 22-g × 2.54-cm Vacutainer needle and hematology tube; Becton Dickinson, Franklin Lakes, NJ) once per week for assay of cortisol, adrenocorticotropic hormone (ACTH), deoxycorticosterone (DOC), aldosterone and dehydroepiandrosterone sulfate (DHEA-S). Due to circadian variation of some of the hormones assayed, all samples were obtained when the lights came on in the morning, which was consistent within subjects, but varied slightly between groups of monkeys (6:00–8:00 am). Blood samples (3 ml) were chilled on ice then centrifuged (3000 rpm, 15 min, 4°C), aliquoted and stored at −80°C. Samples were selected throughout the timeline for assay, 2–4 samples/monkey during baseline (116–118 days, 7–42 days in between samples) and 7–10 samples/monkey during 22 hours/day access to ethanol (358–404 days, 34–69 days in between samples). Approximately every fifth day, samples for blood-ethanol concentration (BEC) were collected 7 hours after session onset during 22 hours/day access to ethanol using gas chromatography (see Grant et al. 2008 for details). This timing of sample collection coincided with the lights turning off in the housing room (off for 13 hours) and is associated with a high correlation (r ≥ 0.80) between 7-hour ethanol intake and BEC (Grant et al. 2014). A BEC was used to indicate intoxication according to the NIAAA definition of 80 mg/dl as being legally intoxicated.
Hormone assays
The Oregon National Primate Research Center Endocrine Technology Services Laboratory conducted all assays. A Roche Cobas e411 automatic clinical platform was used to assay ACTH (sensitivity, 1–2000 pg/ml; inter-assay variation, 0.8%), cortisol (sensitivity, 0.036–63.4 µg/dl; inter-assay variation, 1.1%) and dehydroepiandrosterone-sulfate (DHEA-S; sensitivity, 0.001–10 µg/ml; inter-assay variation, 4.4%). Commercial assays were used for aldosterone (enzyme-linked immunosorbent assay with sensitivity of 0–1600 pg/ml and inter-assay variation of 7.8%), and for deoxycorticosterone (DOC; extraction radioimmunoassay with sensitivity of 0–1000 ng/ml and inter-assay variation of 4.5%).
Statistical analysis
Hormone values, except DOC, were log transformed prior to analysis to accommodate the assumption of a normal distribution. Pearson’s correlations were used to evaluate the relationship between hormones by experimental phase. Discriminant analysis was applied with hormones as independent variables and drinking status [defined by Grant et al. 2008b as > 3.0 g/kg/day (n = 9) and < 3.0 g/kg/day (n = 23), respectively, over months of 22 hours/day ethanol access] as the dependent variable. Discriminant analysis undertakes the same task as multiple linear regression by predicting an outcome when the dependent variable is categorical and the independent variable is continuous. The aim was to find an underlying structure that characterizes drinking phenotype based on a weighted linear sum of hormone concentrations for each monkey. A discriminant score was calculated for each monkey based on standardized canonical discriminant function coefficients of each hormone. Hormones with large coefficients stand out as those that strongly predict drinking phenotype. Centroid values, defined by the group means of predicted values, were used to distinguish heavy and non-heavy drinkers. Hormones identified using discriminant analysis were compared across experimental phase using linear mixed models with monkey as the subject variable, drinking status (heavy, non-heavy) as the between-subjects effect and experimental phase (baseline, first 6 months of ethanol access, second 6 months of ethanol access) as the within-subjects effect. Schwarz’s Bayesian Information Criteria was used to determine the best-fit covariance structure. Age of heavy and non-heavy drinkers were compared using an independent-groups t-test with Satterthwaite approximated t-value to accommodate unequal variance, and age was included as a covariate in the mixed model. The ratio of DOC/ALD was calculated and served as a dependent variable based on the results of the discriminant analysis showing the greatest distinction between these two hormones during chronic ethanol self-administration. The ratio of cortisol/DHEA-S served as dependent variables as an index of allostatic load, and based on relationships to neuropsychiatric conditions (Maninger et al. 2009) and laboratory models of anxiety (fear-potentiated startle; Grillon et al. 2006). Main effects and interactions were evaluated using Bonferroni-corrected t-tests. Analyses were conducted using SAS 9.2 (Cary, NC), α < 0.05.
Results
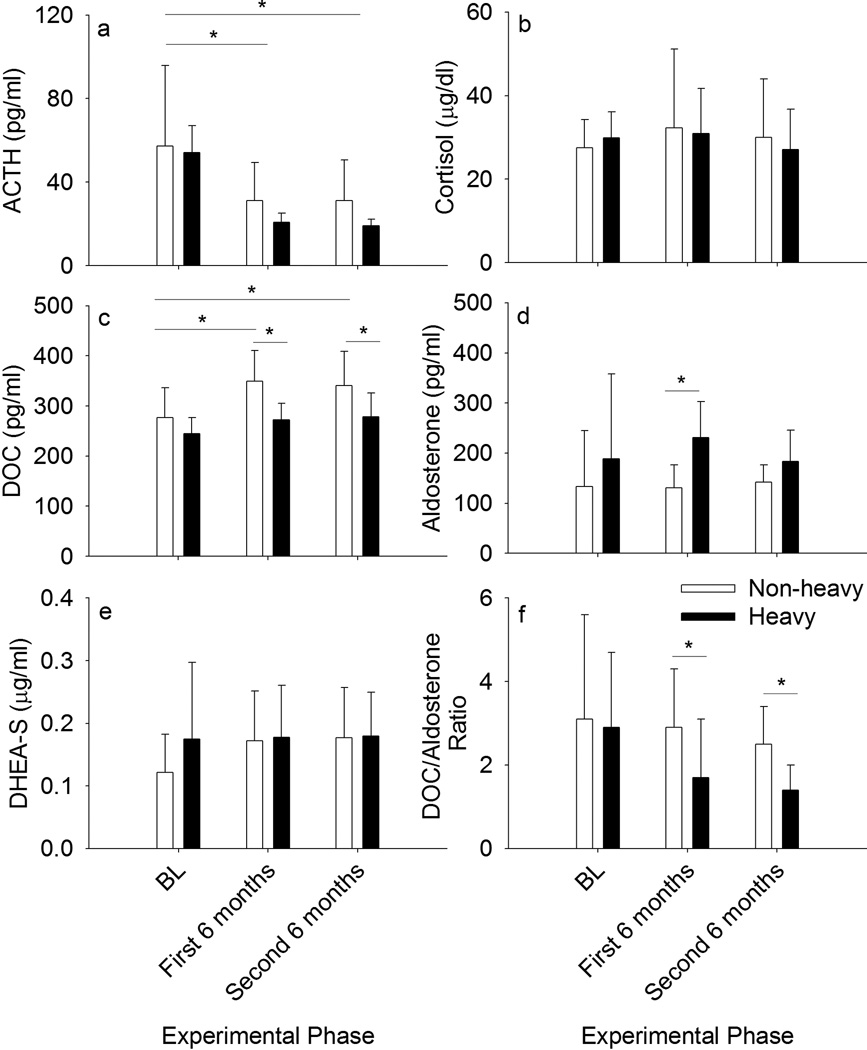
At baseline, ACTH was greater compared to after 6 or 12 months of ethanol self-administration, F(2, 60) = 141, p < 0.0001 [t(59) = −14.7, p = 0.0004 and t(59) = −15.6, p = 0.0004, respectively; Figure 2a]. Heavy and non-heavy drinkers did not differ. Cortisol and DHEA-S on average did not differ between the experimental phases or between the groups (Figures 2b and 2e, respectively). On the other hand, DOC increased during ethanol self-administration, F(2, 60) = 18.3, p < 0.0001, only in non-heavy drinkers as indicated by an interaction between group and experimental phase. F(2, 60) = 3.9, p = 0.03 [BL versus first 6 months: t(59) = −8.9, p = 0.0006; BL versus second 6 months: t(59) = −8.1, p = 0.0006; Figure 2c]. Age at the onset of ethanol access significantly differed between heavy (mean ± SD, 5.2 ± 0.7 years) and non-heavy drinkers (6.6 ± 1.8 years), t(30) = 3.2, p = 0.003, but the above results were significant after accounting for age. Aldosterone was significantly greater among heavy compared to non-heavy drinkers during the first 6 months of ethanol access, F(2, 60) = 6.5, p = 0.003, also after accounting for age differences [t(59) = −4.1, p = 0.0003; Figure 2d].
Figure 2.
Hormone concentrations (a–e) and the ratio of mineralocorticoids deoxycorticosterone (DOC) and aldosterone (f) at baseline (BL), during the first and during the second 6 months of access to ethanol among rhesus macaques that became non-heavy (mean daily dose of self-administered ethanol ≤ 3.0 g/kg/day over 12 months) and heavy (> 3.0 g/kg/day; Grant et al. 2008b) drinking. The data are mean ± SD.
Discriminant analysis revealed centroid values based on coefficients for each hormone indicating that the greatest difference between heavy and non-heavy drinkers was in DOC and aldosterone concentrations. In other words, heavy drinkers tended to be on the positive side of the dimension (aldosterone concentrations), while non-heavy drinkers tended toward the more negative side (DOC). Based on this result, the ratio of DOC/aldosterone was calculated and compared across heavy and non-heavy drinkers. The ratio of DOC/aldosterone varied across experimental phase and with drinking status, F(2, 60) = 7.7, p = 0.001 (Figure 2f). In the first 6 months, ethanol intake and BEC were (mean ± SEM) 2.2 ± 0.01 g/kg/day and 53.9 ± 0.3 mg/dl, respectively, including all monkeys, and 2.4 ± 0.01 g/kg/day and 71.8 ± 0.4 mg/dl, respectively, in the second 6 months. Heavy and non-heavy drinkers had a similar DOC/aldosterone ratio at baseline, but after accounting for age differences, this ratio was significantly lower among heavy drinkers both during the first [t(59) = −6.0, p = 0.0003] and second [t(59) = −3.1, p = 0.008] 6 months of ethanol access. On an individual basis, the increase in aldosterone during ethanol self-administration was greater than the increase in DOC, particularly among heavy drinkers, accounting for the lower DOC/aldosterone ratio during these experimental phases.
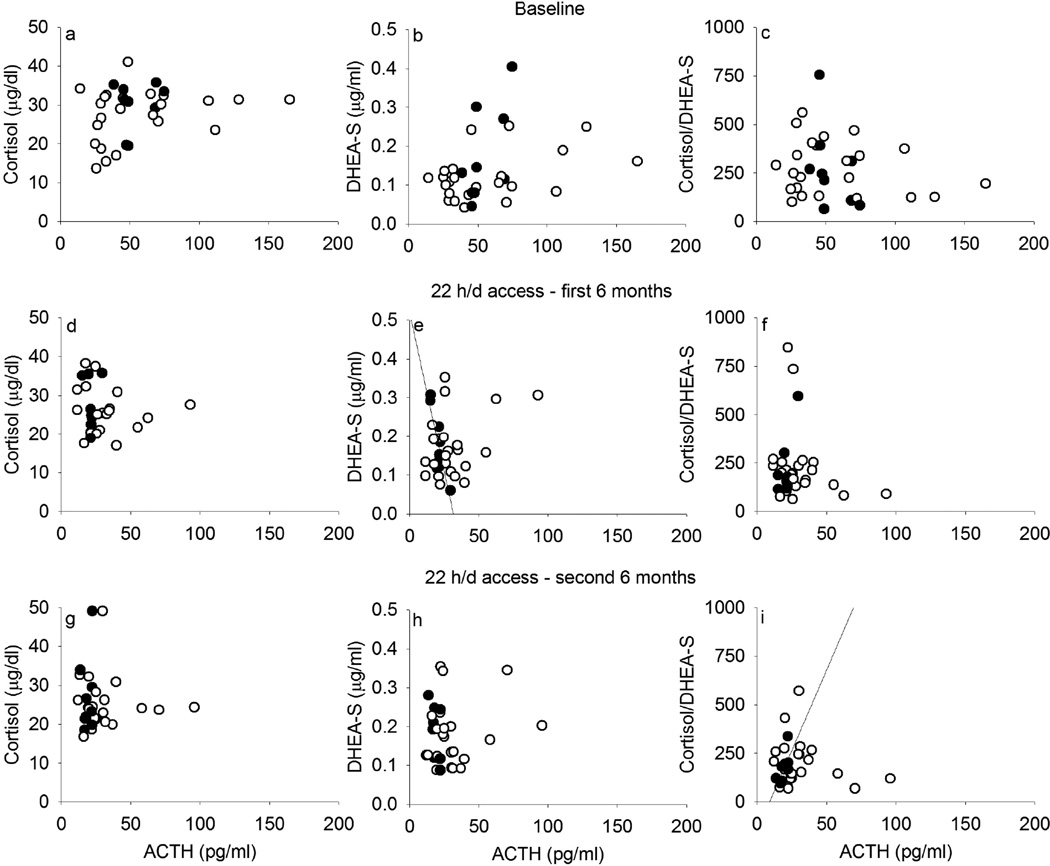
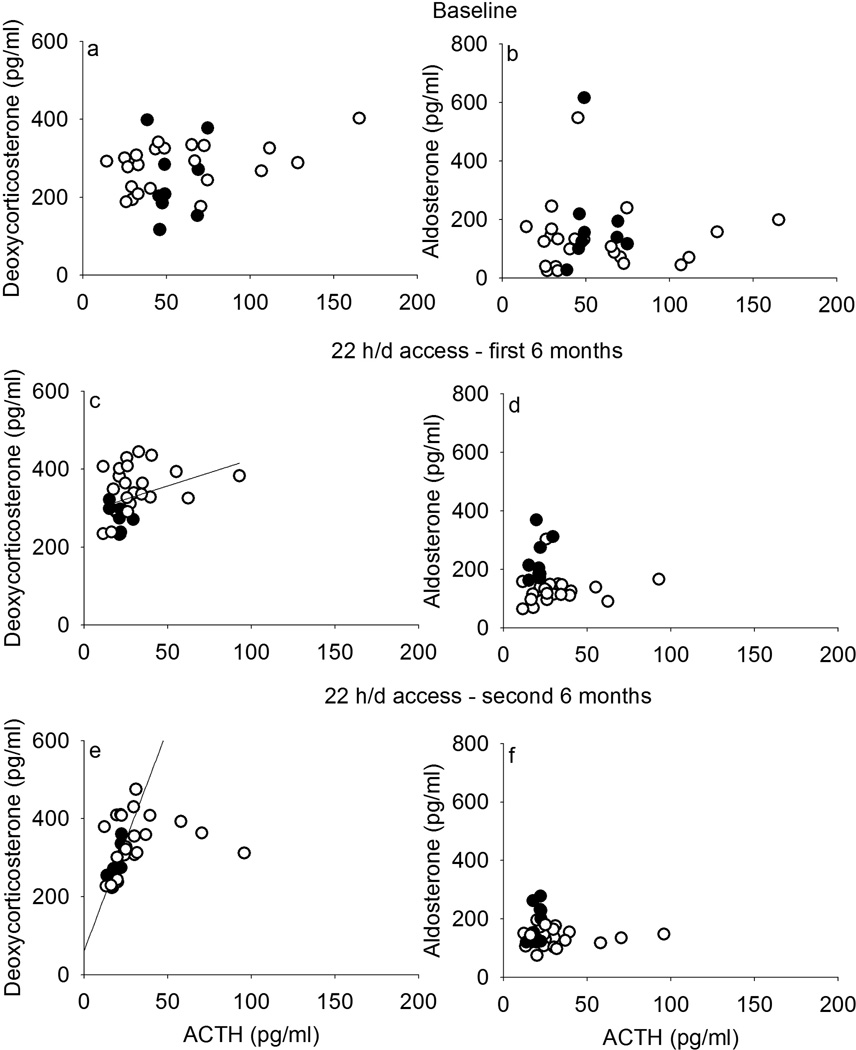
At baseline, there was a trend for ACTH to correlate with cortisol (r = 0.29, p = 0.11; Figure 3a) and with DHEA-S (r = 0.32, p = 0.07; Figure 3b), which did not differ between monkeys that later became heavy or non-heavy drinkers. The ratio of cortisol/DHEA-S did not correlate with ACTH before ethanol access (r = −0.20, p = 0.27; Figure 3c), which was similar for eventual heavy and non-heavy drinkers (non-heavy: r = −0.17, p = 0.45; heavy: r = −0.46, p = 0.21). After the onset of ethanol access, ACTH regulation of cortisol (Figure 3d, 3g) and DHEA-S (Figure 3e, 3h) was also absent, except for heavy drinkers, for whom in the first 6 months of ethanol access, ACTH correlated with DHEA-S (r = −0.87, p = 0.002) and in the second 6 months, cortisol/DHEA-S ratio (r = 0.73, p = 0.03). In contrast, correlations between ACTH and the mineralocorticoids DOC (Figure 4a, 4c, 4e) or aldosterone (Figure 4b, 4d, 4f), were not observed at baseline but there were weak correlations between ACTH and DOC including all monkeys during ethanol self-administration (first 6 months: r = 0.37, p = 0.04; second 6 months: r = 0.43, p = 0.02), and a positive correlation between ACTH and DOC only among heavy drinkers during the second 6 months of self-administration (r = 0.73, p = 0.03). Also, a lack of correlation between DOC and aldosterone was observed at every phase of the experiment for heavy drinkers, though for non-heavy drinkers, DOC and aldosterone correlated during the first (r = 0.57, p = 0.005) and second (r = 0.46, p = 0.03) 6 months of access to ethanol (data not shown).
Figure 3.
Adrenocorticotropic hormone (ACTH) correlations with cortisol produced in the zona fasciculata of the adrenal gland (left) and with dehydroepiandrosterone-sulfate (DHEA-S) produced in the zona reticularis of the adrenal gland, and their ratio (right), at baseline (a–c), during the first 6 months (d–f) and the second 6 months (g–i) of 22 hours/day access to ethanol in rhesus monkeys that became non-heavy (open circles, ≤ 3.0 g/kg/day) and heavy (closed, > 3.0 g/kg/day) drinkers on average over 12 months of access.
Figure 4.
Adrenocorticotropic hormone (ACTH) did not correlate with deoxycorticosterone (DOC, left) or aldosterone (right), mineralocorticoids produced in the zona glomerulosa (both) and zona fasciculata (DOC) of the adrenal gland, at baseline (a–b), during the first 6 months (c–d) or during the second 6 months (e–f) of 22 hours/day access to ethanol in rhesus monkeys that became non-heavy (open circles, ≤ 3.0 g/kg/day) and heavy (closed, > 3.0 g/kg/day) drinkers on average over 12 months of access.
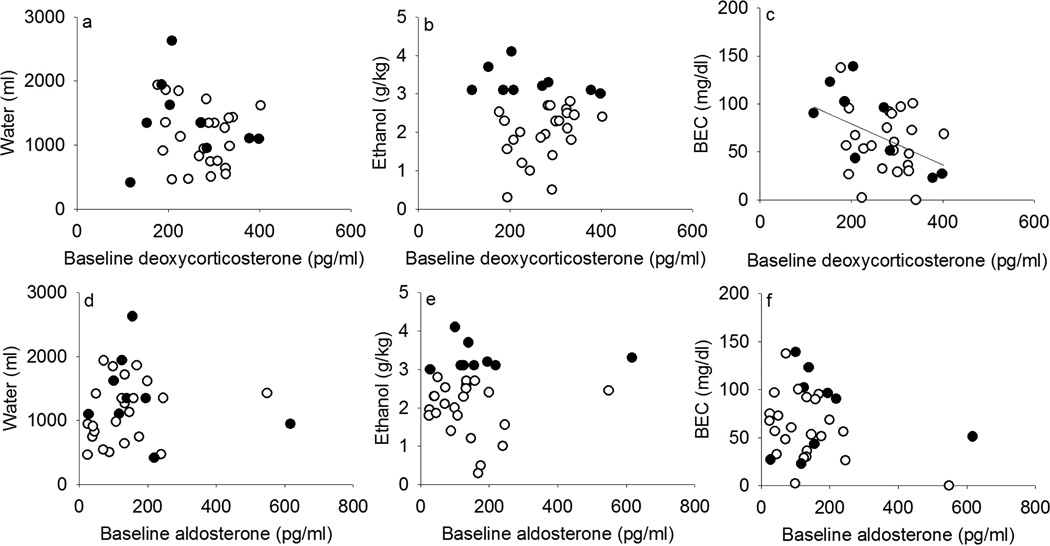
Mean DOC concentration at baseline was negatively correlated with future BEC (r = −0.42, p = 0.02; Figure 5c), which was 64.9 ± 6.4 mg/dl across all subjects (mean ± SEM; min–max, 0–139 mg/dl). This correlation was upheld when all monkeys were included, and when future non-heavy drinkers were excluded (r = −0.74, p = 0.02), even though only 9 monkeys were heavy drinkers. In contrast, DOC did not correlate with future daily ethanol intake (Figure 4b; mean ± SEM: 2.3 ± 0.2; min-max, 0.3–4.1 g/kg/day) or water intake (Figure 5a; 1204 ± 93; 416–2628 ml/day). Baseline aldosterone did not correlate with water (Figure 5d), ethanol intake (Figure 5e) or BEC (Figure 5f). Baseline concentrations of the other hormones measured did not correlate with subsequent water intake or ethanol intake over 12 months, or with BEC (data not shown), regardless of whether all monkeys were included or whether future heavy and non-heavy drinkers were analyzed separately. In addition, the average daily ethanol (r = −0.58, p = 0.0005) and water (r = −0.47, p = 0.006) intake across 12 months of access including both heavy and non-heavy drinkers correlated significantly with the DOC/aldosterone ratio, with a trend for BEC (r = −0.33, p = 0.07).
Figure 5.
Baseline deoxycorticosterone and aldosterone in relation to mean daily water self-administration (a, d), ethanol self-administration (b, e) and blood-ethanol concentration measured every fifth day (BEC, c, f) during 12 months of access to ethanol and water among rhesus macaques that became non-heavy (open circles, ≤ 3.0 g/kg/day) and heavy (closed, > 3.0 g/kg/day) drinkers on average over 12 months of access. Regression lines indicate significant correlations.
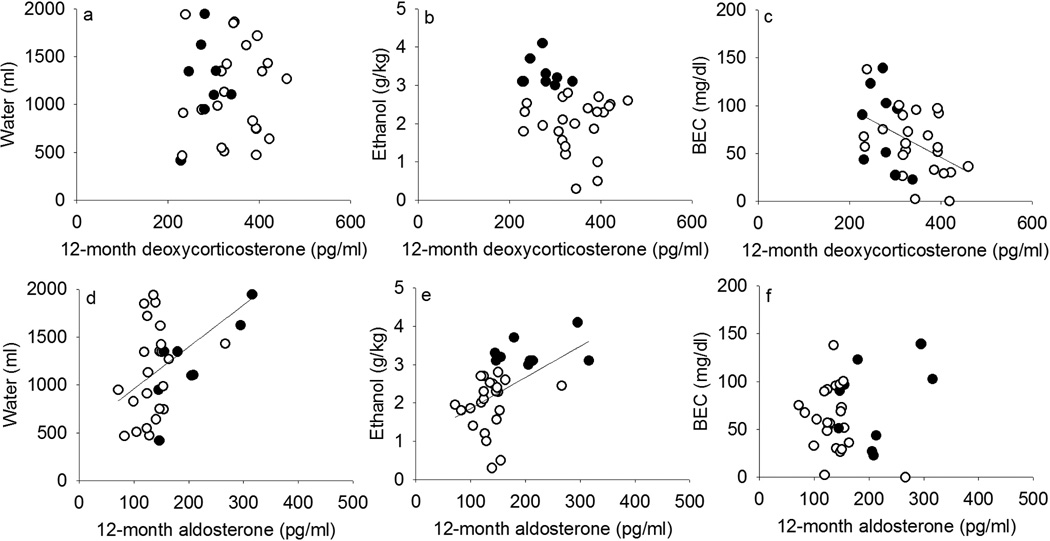
Figure 6 also shows correlations between hormone concentration averaged over 12 months of ethanol access and ethanol intake. Over this time frame, ethanol intake showed a trend (r = −0.33, p = 0.07) and BEC was significantly correlated (r = −0.45, p = 0.009) with DOC. When analyzed separately, this correlation was significant in non-heavy (r = −0.44, p = 0.04) but not heavy (r = −0.39, p = 0.31) drinkers, though in the same direction for these 9 monkeys. Aldosterone over 12 months was moderately correlated with ethanol intake (r = 0.49, p = 0.004) but not BEC (r = 0.07), only when including both heavy and non-heavy drinkers. These data are consistent with the claim that aldosterone reflected fluid homeostasis instead of intoxication as suggested by BEC, as aldosterone correlated with water intake (r = 0.46, p = 0.008) regardless of drinking status.
Figure 6.
Average deoxycorticosterone and aldosterone during 12 months of access to ethanol and water in relation to mean daily water self-administration (a, d), ethanol self-administration (b, e) and blood-ethanol concentration measured every fifth day (BEC, c, f) across the same time among rhesus macaques that became non-heavy (open circles, ≤ 3.0 g/kg/day) and heavy (closed, > 3.0 g/kg/day) drinkers on average over 12 months of access. Regression lines indicate significant correlations.
Discussion
As the balance of neuroactive steroids that regulate GABAergic neurotransmission may be an important variable mediating the effects of ethanol, the present study related circulating neuroactive steroid precursors in rhesus macaques as a predictor of chronic ethanol drinking. These results distinguish the contributions of the mineralocorticoids aldosterone and DOC on a basal hormonal milieu that may be a risk factor for ethanol self-administration. This longitudinal study was not designed to evaluate the contribution of day-to-day hormonal variation on ethanol intake, as a maximum of 10 plasma samples throughout the 12 months of ethanol access were assayed. Instead, this study shows that baseline hormonal milieu is related to the average level of intoxication monkeys attain as indicated by BEC.
Mechanistically, the GABAergic neuroactive metabolite of DOC, THDOC, is expected to contribute to homeostasis of GABAergic neurotransmission in the brain, as plasma DOC strongly correlated with brain DOC in mice (Porcu et al. 2011). Past studies showed that THDOC positively modulates GABAA receptor currents in parvocellular neurons of the paraventricular nucleus of the hypothalamus (Womack et al. 2006). Fluctuation in neurosteroid concentration is associated with altered neural expression of GABAA receptor subtypes (Concas et al. 1998) and GABA release (for a review, see Helms et al. 2012c). In mice, basal DOC was positively correlated with ethanol-induced loss of righting reflex, ethanol-induced ataxia and ethanol-induced corticosterone, and measures of anxiety-like behavior (Porcu et al. 2011). These positive correlations were interpreted to indicate that DOC was metabolized to corticosterone instead of an anxiolytic neuroactive metabolite (Bitran et al. 1991). Overall, circulating DOC appears to contribute to individual set-points for ethanol sensitivity, and in the present study, extended to BEC after ethanol self-administration in rhesus macaques.
In previous studies, abstinent alcoholics (also nicotine dependent) and controls were shown to have similar baseline plasma DOC (Porcu et al. 2008), but it is not possible to know whether differences could have existed prior to the onset of ethanol use or abstinence. Besides function of its metabolite THDOC, DOC itself has activity at mineralocorticoid and glucocorticoid receptors (Vinson 2011). Mineralocorticoid receptors are decreased in the brains of patients with major depression in post-mortem analysis (Klok et al. 2011) and preclinical studies indicate that chronic (11 days) of mineralocorticoid receptor antagonism decreased anxiety-like behavior and corticosterone response to stress (Hlavacova et al. 2010). Chronic corticosterone treatment in rats resulted in neuroadapative changes associated with transiently increased ethanol self-administration (Besheer et al. 2013). Even though the effects of chronic DOC treatment on ethanol self-administration and related behaviors has not been studied, based on the present findings, DOC could be hypothesized to protect against drinking to intoxication independently of the average daily intake. Heavy (> 3.0 g/kg/day) and non-heavy (≤ 3.0 g/kg/day) drinkers had a similar range of BEC taken at 7 hours into the drinking session indicating that the drinking patterns on the session where BEC was analyzed was similar across groups. However, the individual average BECs were also highly variable suggesting variable patterns of alcohol intake within-individuals as well as between individuals. Baseline DOC correlated inversely with future BEC averaged over 12 months of access to ethanol, indicating a relationship between DOC and a future pattern of drinking leading to intoxication.
The data in the present study extend and confirm this relationship between circulating DOC and binge drinking, as suggested by BEC, although additional studies are needed to confirm using independent behavioral measures of intoxication. The present findings are in line with our hypothesis that hormones metabolized to GABAA positive modulators (DOC) should negatively correlate with ethanol self-administration. Originally, we reported that glucocorticoid regulation of DOC by dexamethasone was negatively correlated with subsequent daily ethanol intake averaged over 12 months (Porcu et al. 2006). That is, lower suppression of DOC by glucocorticoid feedback to the HPA axis was a risk factor for greater ethanol self-administration. In the current study, baseline DOC (morning samples prior to dexamethasone, before the onset of ethanol access), in the absence of stress- or pharmacological-induced negative feedback of the HPA axis, did not correlate with 12-month intake, in contrast to a negative correlation with BEC. The lack of correspondence between hormone concentration with ethanol intake, and hormone concentration with BEC, is not surprising as there were few hormone samples compared to daily ethanol intake averaged from 12 months of access and BEC averaged from samples taken every fifth day over the same time. On sample days, BEC is highly correlated with the self-administered dose of ethanol in monkeys (Grant et al. 2008b). A dissociation between average dose of ethanol consumed and average BEC over all 12 months of access is expected because patterns of ethanol intake contribute to BEC in addition to the dose consumed. On the other hand, baseline DOC was also not correlated with water drinking, excluding an interpretation based on the role of DOC in fluid homeostasis. The correlation between baseline DOC and 12-month BEC suggests that an individual’s waking DOC and/or its metabolites contributes to a risk of ethanol drinking to intoxication rather than overall ethanol intake.
Aldosterone levels appear to be related to its role in fluid homeostasis, as baseline aldosterone was correlated with both water and ethanol intake. The results of a discriminant analysis showing that concentrations of these mineralocorticoids differed between heavy and non-heavy drinkers lead us to examine the balance of DOC and aldosterone, being that DOC is a precursor of aldosterone. Prior to ethanol exposure, this ratio did not differ between monkeys that became heavy or non-heavy drinkers. During daily ethanol drinking, however, heavy drinkers had lower DOC/aldosterone ratios after 6 and after 12 months of self-administration. Significant correlations in the present study extended this finding to show that aldosterone is a linear function of the quantity of water or ethanol consumed over many months, accounting for correlations with fluid intake and the DOC/aldosterone ratio. Indeed, these were the only significant or trending correlations between the hormones measured and average daily ethanol intake in this study. These data indicate that total fluid consumption and fluid homeostasis is related to the altered balance between DOC and aldosterone.
The data suggested changes in basal HPA axis activity and hormonal milieu with chronic ethanol intake based on altered correlations between hormones. The relationship between circulating ACTH and steroidogenesis differs between the layers of the primate adrenal cortex. Secretion of DOC is mediated by ACTH, angiotensin II and potassium ions, being synthesized in the zona fasciculata and zona glomerulosa of the adrenal gland, the latter also the site of aldosterone synthesis (Brown et al. 1972). In the present study, circulating levels of the mineralocorticoids DOC and aldosterone were correlated only among heavy drinkers consuming ethanol, and with plasma ACTH during the second 6 months of ethanol self-administration. Correlations that were significant only for heavy drinkers are particularly strong because only 9 monkeys were included in this group, and suggest an interaction between patterns of ethanol self-administration and intoxication and endocrine activity. In the absence of a stressor or challenge, but in an awake and active animal, mineralocorticoid production by the zona glomerulosa may be primarily regulated by vasopressin (Grazzini et al. 1998). The present data suggest steroidogenic effects of ethanol at different regions of the adrenal gland. Low circulating levels of ACTH did not appear to stimulate adrenocortical activity at the level of the zona fasciculata as suggested by the absence of correlation with circulating cortisol, which was similar between heavy and non-heavy drinkers. Under HPA axis challenge, ACTH increases circulating cortisol (e.g., Wand and Dobs 1991; Czoty et al. 2009), so the absence of a correlation between ACTH and cortisol in this study suggests that the monkeys were calm at baseline sampling. Administration of ACTH increases DHEA (e.g., Radant et al. 2009) and its long-lasting sulfated form, DHEA-S, after a delay (Griffing et al. 1985). Individuals vary in this capacity with age (Baulieu 1996) and possibly with ethanol consumption. Additional studies are needed to confirm, in the present study, the effect of chronic heavy ethanol self-administration on ACTH and DHEA-S, and to determine the effect of chronic ethanol on adrenocortical stimulation of DHEA-S.
The basal hormone concentrations reported in the present study reflect individual differences in endogenous HPA axis in a basal state, instead of reactivity to a challenge. Except for DOC, the hormones measured in this study indicated that individual differences in endogenous HPA axis activity was similar between monkeys that became heavy and non-heavy drinkers, and could be related to temperament or some other enduring characteristic of the monkeys. Indeed, HPA axis reactivity correlates with temperament in normal healthy humans (Tyrka et al. 2008). An association between drinking to intoxication and basal individual differences in neuroactive steroids may be established early in life. In an experimental model of stress using social isolation at weaning, rats showed decreased concentrations of neuroactive steroids including the DOC metabolite, THDOC, in plasma and cerebral cortex compared to group-housed controls (Serra et al. 2000). Increased adrenocortical response to stress in rats after maternal deprivation during post-natal days 2–10 is decreased by treatment with THDOC (Patchev et al. 1997), suggesting that early life stress affects sensitivity to the neuroendocrine and behavioral effects of GABAergic ligands including neuroactive steroids, and possibly ethanol. However, the effects of early life stress on responding for ethanol and ethanol consumption in rodents are variable across protocols (McCool and Chappell 2009; Pisu et al. 2009; Butler et al. 2014), and these are not optimized to determine the effects of stress and HPA axis activity on clinically relevant, heavy alcohol drinking. Additional studies that capture wider natural or treatment-induced variation in HPA axis activity are needed to experimentally test the role of these baseline traits and future ethanol intake in monkeys.
Greater differences in basal HPA axis activity emerged between heavy and non-heavy drinkers after the onset of ethanol access in the present study. With repeated ethanol use and dependence, the threshold for stress responses and activation of the HPA axis appears to adapt (e.g., Berman et al. 1990; Inder et al. 1995; Dai et al. 2007; Porcu et al. 2008). In the present study, ACTH was weakly related to adrenocortical activity at the level of the zona reticularis as indicated by a correlation with DHEA-S. However, this correlation was only present during ethanol access for heavy drinkers. During ethanol access, ACTH concentrations clustered around lower values and the variation in DHEA-S remained consistent across experimental phases, aligning with ACTH in heavy drinkers after 6 months of self-administration. Altered ACTH regulation of adrenocortical activity at the level of the zona fasciculata, namely cortisol, has been observed in abstinent alcoholics by administering cosyntropin, a synthetic derivative of ACTH (Adinoff et al. 2005). An earlier study from these same investigators found no difference in basal DHEA-S between alcoholics abstinent for 1–3 weeks, alcoholics abstinent for 3 weeks to 6 months and controls, although the DHEA after ovine CRH administration appeared to be greater among abstinent alcoholics (Adinoff et al. 1996). The ratio of cortisol/DHEA-S is a clinically significant marker of self-rated depression among abstinent alcoholics (Heinz et al. 1999). The hormonal correlates of this ratio distinguish heavy and non-heavy drinkers only after the onset of ethanol self-administration, as ACTH concentrations correlated with DHEA-S and the cortisol/DHEA-S ratio among heavy drinkers. In normal healthy humans, there is a positive correlation between plasma ACTH and cortisol, ACTH and DHEA and cortisol and DHEA, but not among patients developing inflammatory joint disease (Kanik et al. 2000). We previously showed that chronic ethanol self-administration alters plasma concentrations of many cytokines and immune-regulating proteins, and their correlations with ACTH and cortisol in monkeys (Helms et al. 2012b). Correlations of ACTH with cortisoland DHEA-S, and with DOC, during ethanol self-administration in the current study may be due to pathogenesis involving inflammatory processes. Overall, the data suggest that the ratios of ACTH, cortisol and DHEA-S could be very informative for documenting ethanol-induced alterations in HPA axis homeostasis.
Acknowledgements
This research was supported by NIH grants OD011092, AA109431, AA010760, AA013510.
Footnotes
Some of the data in this manuscript were presented at the meeting of the Research Society on Alcoholism, Orlando, FL, June 22–27, 2013, and at the meeting of the American Society for Pharmacology and Experimental Therapeutics, Boston, MA, April 20–24, 2013.
References
- Adinoff B, Kiser JMC, Martin PR, Linnoila M. Response of dehydroepiandrosterone to corticotropin-releasing hormone stimulation in alcohol-dependent subjects. Biol Psychiatry. 1996;40:1305–1307. doi: 10.1016/s0006-3223(96)00381-2. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: adrenocortical and pituitary glucocorticoid responsiveness. Alcohol Clin Exp Res. 2005;29:517–527. doi: 10.1097/01.ALC.0000158940.05529.0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu E-E. Dehydroepiandrosterone (DHEA): a fountain of youth? J Clin Endocrinol Metab. 1996;81:3147–3151. doi: 10.1210/jcem.81.9.8784058. [DOI] [PubMed] [Google Scholar]
- Berman JD, Cook DM, Buchman M, Keith LD. Diminished adrenocorticotropin response to insulin-induced hypoglycemia in nondepressed, actively drinking male alcoholics. J Clin Endocrinol Metab. 1990;71:712–717. doi: 10.1210/jcem-71-3-712. [DOI] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Grondin JJM, Cannady R, Hodge CW. The effects of repeated corticosterone exposure on the interoceptive effects of alcohol in rats. Psychopharmacology. 2012;220:809–822. doi: 10.1007/s00213-011-2533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Lindsay TG, Cannady R. Transient increase in alcohol self-administration following a period of chronic exposure to corticosterone. Neuropharmacology. 2013;72:139–147. doi: 10.1016/j.neuropharm.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJM, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens in required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2010;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJM, Salling MC, Spanos M, Stevenson RA, Hodge CW. Interoceptive effects of alcohol require mGluR5 receptor activity in the nucleus accumbens. J Neurosci. 2009;29:9582–9591. doi: 10.1523/JNEUROSCI.2366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggio G, Concas A, Corda MG, Girogi O, Sanna E, Serra M. GABAergic and dopaminergic transmission in the rat cerebral cortex: effect of stress, anxiolytic and anxiogenic drugs. Pharmacol Ther. 1990;48:121–142. doi: 10.1016/0163-7258(90)90077-f. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Butler TR, Ariwodola OJ, Weiner JL. The impact of social isolation on HPA axis function, anxiety-like behaviors, and ethanol drinking. Front Integ Neurosci. 2014;7:1–11. doi: 10.3389/fnint.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein SR, Chrousos GP. Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: neural and immune inputs. J Clin Endocrinol Metab. 1999;84:1729–1736. doi: 10.1210/jcem.84.5.5631. [DOI] [PubMed] [Google Scholar]
- Boyd KN, Kumar S, O’Buckley TK, Porcu P, Morrow AL. Ethanol induction of steroidogenesis in rat adrenal and brain is dependent upon pituitary ACTH release and de novo adrenal StAR synthesis. J Neurochem. 2010;112:784–796. doi: 10.1111/j.1471-4159.2009.06509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Woods JH. Self-administration of methohexital, midazolam and ethanol: effects on the pituitary-adrenal axis in rhesus monkeys. Psychopharmacology. 2005;178:83–91. doi: 10.1007/s00213-004-1986-4. [DOI] [PubMed] [Google Scholar]
- Brown RD, Strott CA, Liddle GW. Site of stimulation of aldosterone biosynthesis by angiotensin and potassium. J Clin Investigation. 1972;51:1413–1418. doi: 10.1172/JCI106937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Nat Acad Sci. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Nader MA. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis) J Neuroendocrinol. 2009;21:68–76. doi: 10.1111/j.1365-2826.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Santella S, Gianoulakis C. Response of the HPA-axis to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Psychoneuroendocrinology. 2007;32:293–305. doi: 10.1016/j.psyneuen.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Bowen CA, Mirkis S, Purdy RH. Ethanol-like discriminative stimulus effects of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in female Macaca fascicularis monkeys. Psychopharmacology. 1996;124:340–346. doi: 10.1007/BF02247439. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Shively CA, Purdy RH. Discriminative stimulus effects of ethanol and 3α-hydroxy-5α-pregnan-20-one in relation to menstrual cycle phase in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology. 1997;130:59–68. doi: 10.1007/s002130050211. [DOI] [PubMed] [Google Scholar]
- Grant KA, Ferguson B, Helms CM, McClintick M. Drinking to dependence risk factors in nonhuman primates. In: Changhai C, Noronha A, Harris A, Crabbe J, editors. Neurobiology of Alcohol Dependence. Oxford: Elsevier; 2014. pp. 407–424. [Google Scholar]
- Grant KA, Helms CM, Rogers LSM, Purdy RH. Neuroactive steroid stereospecificity of ethanol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2008a;326:354–361. doi: 10.1124/jpet.108.137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LSM, Gonzales SW. Drinking typography established by sche-duled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008b;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazzini E, Boccara G, Joubert D, Trueba M, Durroux T, Guillon G, Gallo-Payet N, Chouinard L, Payet MD, Serradeil Le Gal C. Vasopressin regulates adrenal functions by acting through different vasopressin receptor subtypes. Adv Exp Med Biol. 1998;449:325–334. doi: 10.1007/978-1-4615-4871-3_41. [DOI] [PubMed] [Google Scholar]
- Griffing GT, Allen J, Pratt H, Melby JC. Discordance of plasma DHEA-S, DHEA, and cortisol responses with various ACTH regimens. Metab. 1985;34:631–636. doi: 10.1016/0026-0495(85)90090-3. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Baas JMP, Lawley M, Ellis V, Charney DS. Cortisol and DHEA-S are associated with startle potentiation during aversive conditioning in humans. Psychopharmacology. 2006;186:434–441. doi: 10.1007/s00213-005-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Weingartner H, George D, Hommer D, Wolkowitz OM, Linnoila M. Severity of depression in abstinent alcoholics is associated with monoamine metabolites and dehydroepiandrosterone-sulfate concentrations. Psychiatry Res. 1999;89:97–106. doi: 10.1016/s0165-1781(99)00099-2. [DOI] [PubMed] [Google Scholar]
- Helms CM, McClintick M, Grant KA. Social rank, chronic ethanol self-administration and hypothalamic-pituitary-adrenal axis response in monkeys. Psychopharmacology. 2012a;224:133–143. doi: 10.1007/s00213-012-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Messaoudi I, Freeman WM, Vrana KE, Grant KA. A longitudinal analysis of circulating stress-related proteins and chronic ethanol self-administration in cynomolgus macaques. Alcohol Clin Exp Res. 2012b;36:995–1003. doi: 10.1111/j.1530-0277.2011.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Shaw J, Rau A, Stull C, Gonzales SW, Grant KA. The effects of age at the onset of drinking to intoxication and chronic ethanol self-administration in male rhesus macaques. Psychopharmacology. 2014;231:1853–1861. doi: 10.1007/s00213-013-3417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Rossi DJ, Grant KA. Neurosteroid influences on sensitivity to ethanol. Front Endocrinol. 2012c;3:1–19. doi: 10.3389/fendo.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Shaw J, Rau A, Stull C, Gonzales SW, Grant KA. The effects of age at the onset of drinking to intoxication and chronic ethanol self-administration in male rhesus macaques. Psychopharmacology. 2014 doi: 10.1007/s00213-013-3417-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlavacova N, Bakos J, Jezova D. Eplerenone, a selective mineralocorticoid receptor blocker, exerts anxiolytic effects accompanied by changes in stress hormone release. J Psychopharmacology. 2010;24:779–786. doi: 10.1177/0269881109106955. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Cox AA, Bratt AM, Camarini R, Iller K, Kelley SP, Mehmert KK, Nannini MA, Klok MD, Alt SR, Irurzun Lafitte AJM, Turner JD, Lakke EAFJ, Huitinga I, Muller CP, Zitman FG, de Kloet ER, DeRijk RH. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. J Psychiatric Res. 2011;45:871–878. doi: 10.1016/j.jpsychires.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Inder WJ, Joyce PR, Ellis MJ, Evans MJ, Livesey JH, Donald RA. The effects of alcoholism on the hypothalamic-pituitary-adrenal axis: interaction with endogenous opioid peptides. Clin Endocrinology. 1995;43:283–290. doi: 10.1111/j.1365-2265.1995.tb02033.x. [DOI] [PubMed] [Google Scholar]
- Kanik KS, Chrousos GP, Schumacher HR, Crane ML, Yarboro CH, Wilder RL. Adrenocorticotropin, glucocorticoid, and androgen secretion in patients with new onset synovitis/rheumatoid arthritis: relations with indices of inflammation. J Clin Endocrinol Metab. 2000;85:1461–1466. doi: 10.1210/jcem.85.4.6534. [DOI] [PubMed] [Google Scholar]
- Kroenke CD, Rohlfing T, Park B, Sullivan EV, Pfefferbaum A, Grant KA. Monkeys that voluntarily and chronically drink alcohol damage their brains: a longitudinal MRI study. Neuropsychopharmacology. 2014;39:823–830. doi: 10.1038/npp.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye J-H. Blockade of GABAA receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addiction Biol. 2011;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Experimentally induced intoxication in alcoholics: a comparison between programmed and spontaneous drinking. J Pharmacol Exp Ther. 1970;173:101–116. [PubMed] [Google Scholar]
- Mendelson JH, Stein S. Serum cortisol levels in alcoholic and nonalcoholic subjects during experimentally induced ethanol intoxication. Psychosomatic Med. 1966;28:616–626. doi: 10.1097/00006842-196601000-00001. [DOI] [PubMed] [Google Scholar]
- Nieminen MM, Fyhrquist F, Linkola J, Tikkanen I, Tontti K. Renin-aldosterone axis in ethanol intoxication. Pharmacol Biochem Behav. 1981;15:879–882. doi: 10.1016/0091-3057(81)90047-2. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Montkowski A, Rouskova D, Koranyi L, Holsboer F, Almeida OFX. Neonatal treatment of rats with the neuroactive steroid tetrahydrodeoxycorticosterone (THDOC) abolishes the behavioral and neuroendocrine consequences of adverse early life events. J Clin Investigation. 1997;99:962–966. doi: 10.1172/JCI119261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, Marx CE, Shampine AL, Marx CE, Shampine LJ, Kranzler JR. Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of ethanol. Psychopharmacology. 2006;186:451–461. doi: 10.1007/s00213-005-0231-0. [DOI] [PubMed] [Google Scholar]
- Pisu MG, Mostallino MC, Dore R, Maciocco E, Secci PP, Serra M. Effects of voluntary ethanol consumption on emotional state and stress responsiveness in socially isolated rats. Eur Neuropsychopharmacology. 2011;21:414–425. doi: 10.1016/j.euroneuro.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, Grant KA, Green HL, Rogers LSM, Morrow AL. Hypothalamic-pituitary-adrenal axis and ethanol modulation of deoxycorticosterone levels in cynomolgus monkeys. Psychopharmacology. 2006;186:293–301. doi: 10.1007/s00213-005-0132-2. [DOI] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL. Simultaneous quantification of GABAergic 3α,5α/3α,5β neuroactive steroids in human and rat serum. Steroids. 2009;74:463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, Morrow AL. Differential effects of ethanol on serum GABAergic 3α,5α/3α,5β neuroactive steroids in mice, rats, cynomolgus monkeys and humans. Alcohol Clin Exp Res. 2010;34:432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Morrow AL, Adinoff B. Differential hypothalamic-pituitary-adrenal activation of the neuroactive steroids pregnenolone sulfate and deoxycorticosterone in healthy controls and alcohol-dependent subjects. Psychoneuroendocrinology. 2008;33:214–226. doi: 10.1016/j.psyneuen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Song SC, Harenza JL, Lu L, Wang X, Williams RW, Miles MF, Morrow A. Genetic analysis of the neurosteroid deoxycorticosterone and its relation to alcohol phenotypes: identification of QTLs and downstream gene regulation. PLoS One. 2011;6:e18405. doi: 10.1371/journal.pone.0018405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radant AD, Dobie DJ, Peskind ER, Murburg MM, Petrie EC, Kanter ED, Raskind MA, Wilkinson CW. Adrenocortical responsiveness to infusions of physiological doses of ACTH is not altered in posttraumatic stress disorder. Front Behav Neurosci. 2009;3:1–8. doi: 10.3389/neuro.08.040.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- Rowlett JK, Winger G, Carter RB, Wood PL, Woods JH, Woolverton WL. Reinforcing and discriminative stimulus effects of the neuroactive steroids pregnanolone and Co 8-7071 in rhesus monkeys. Psychopharmacology. 1999;145:205–212. doi: 10.1007/s002130051050. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology. 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Higley JD, Suomi SJ, Heilig M, Barr CS. OPRM1 gene variation influences hypothalamic-pituitary-adrenal axis function in response to a variety of stressors in rhesus macaques. Psychoneuroendocrinology. 2011;36:1303–1311. doi: 10.1016/j.psyneuen.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Wier LM, Price LH, Rikhye K, Ross NS, Anderson GM, Wilkinson CW, Carpenter LL. Cortisol and ACTH responses to the DEX/CRH test: influence of temperament. Horm Behav. 2008;53:518–525. doi: 10.1016/j.yhbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson GP. The mislabeling of deoxycorticosterone: making sense of corticosteroid structure and function. J Endocrinol. 2011;211:3–16. doi: 10.1530/JOE-11-0178. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Waters CA, Szeliga KT, Jordan K, Grant KA. Characterization of the discriminative stimulus effects of N-methyl-D-aspartate ligands under different ethanol training conditions in the cynomolgus monkey (Macaca fascicularis) Psychopharmacology. 2002;162:273–281. doi: 10.1007/s00213-002-1086-2. [DOI] [PubMed] [Google Scholar]
- Waltman C, Blevins LS, Jr, Boyd G, Wand GS. The effects of mild ethanol intoxication on the hypothalamic-pituitary-adrenal axis in nonalcoholic men. J Clin Endocrinol Metab. 1993;77:518–522. doi: 10.1210/jcem.77.2.8393888. [DOI] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic-pituitary-adrenal axis in actively drinking alcoholics. J Clin Endocrinology Metab. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Womack MD, Pyner S, Barrett-Jolley R. Inhibition by alpha-tetrahydrodeoxycorticosterone (THDOC) of pre-sympathetic parvocellular neurones in the paraventricular nucleus of rat hypothalamus. Br J Pharmacol. 2006;149:600–607. doi: 10.1038/sj.bjp.0706911. [DOI] [PMC free article] [PubMed] [Google Scholar]