Abstract
Rationale
Gamma-aminobutyric acid type A receptors (GABAARs) are the principal mediators of inhibitory transmission in the mammalian central nervous system. GABAARs can be localized at postsynaptic inhibitory specializations or at extrasynaptic sites. While synaptic GABAARs are activated transiently following the release of GABA from presynaptic vesicles, extrasynaptic GABAARs are typically activated continuously by ambient GABA concentrations and thus mediate tonic inhibition. The tonic inhibitory currents mediated by extrasynaptic GABAARs control neuronal excitability and the strength of synaptic transmission. However, the mechanisms by which neurons control the functional properties of extrasynaptic GABAARs had not yet been explored.
Objectives
We review GABAARs, how they are assembled and trafficked, the role phosphorylation has on receptor insertion and membrane stabilization. Finally, we review the modulation of GABAARs by neurosteroids and how GABAAR phosphorylation can influence the actions of neurosteroids.
Conclusions
Trafficking and stability of functional channels to the membrane surface is critical for inhibitory efficacy. Phosphorylation of residues within GABAAR subunits plays an essential role in the assembly, trafficking and cell surface stability of GABAARs. Neurosteroids are produced in the brain and are highly efficacious allosteric modulators of GABAAR mediated current. This allosteric modulation by neurosteroids is influenced by the phosphorylated state of the GABAAR which is subunit dependent, adding temporal and regional variability to the neurosteroid response. Possible links between neurosteroid actions, phosphorylation, and GABAAR trafficking remain to be explored but potential novel therapeutic targets may exist for numerous neurological and psychological disorders which are linked to fluctuations in neurosteroid levels and GABAA subunit expression.
Keywords: GABA, GABAA receptors, extrasynaptic receptors, neurosteroids, receptor trafficking, phosphorylation
Introduction
As the major source of inhibition in the adult brain GABAAR modulation has an important influence of circuit properties and in neurological conditions. GABAARs are located at synaptic sites where they produce phasic inhibition and at extrasynaptic sites where they mediate tonic inhibition of the neuron. The GABAARs located at these different sites are formed by different subunits endowing diverse properties to the GABAARs allowing them to perform distinctive tasks. Although neurosteroids enhance both phasic and tonic inhibition it appears that extrasynaptic receptors are particularly sensitive to neurosteroids. It has been known that phosphorylation of synaptic GABAARs influences trafficking. Little is known about phosphorylation and trafficking of extrasynaptic GABAARs. In addition, previous data have shown that the modulation of GABAARs by neurosteroids is also influenced by phosphorylation.
GABA: Main mediator of Neuronal Inhibition in the CNS
The neurotransmitter γ-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the mammalian central nervous system (CNS). GABA is synthesized in the brain from glutamate through the action of the enzyme L-glutamic acid decarboxylase (GAD), which catalyzes the decarboxylation of glutamic acid to form GABA (Erlander et al. 1991). In mammals, GAD exists as two isoforms: GAD65 and GAD67 (with molecular weights of 65 and 67kDa respectively). These two isoforms are encoded by different genes and have a distinct regional distribution throughout the brain (Erlander et al. 1991, Sheik et al.1999). GAD65 is membrane-bound and primarily responsible for vesicular GABA production, whereas GAD67 is located in the cytoplasm and is responsible for cytoplasmic GABA production (Erlander et al. 1991).
After release from pre-synaptic vesicles, GABA is rapidly removed from the synaptic cleft by specialized membrane-bound transporters. GABA uptake is a sodium- and chloride-dependant process mediated by a group of genetically related GABA transporters, GAT-1 to GAT-4 (Liu et al. 1993). In the mammalian brain, GABA uptake is primarily mediated by action of GAT-1 (Minelli et al. 1995). GAT-1 is localized in GABAergic axons and nerve terminals and can also be expressed in glial cells (Minelli et al. 1995). The inhibitory effects of GABA are mediated by two main classes of GABA receptors: Metabotropic G-coupled receptors (GABA type B receptors-GABABR) and GABA-gated chloride ion channels (GABA type A receptors-GABAAR).
GABAAR mediate the fast inhibitory actions of GABA
GABAARs are the main mediators of fast synaptic inhibition in the CNS. GABAARs are chloride permeable channels (Figure 1) that belong to the cys loop ligand gated ion channel super family. Members of this family include nicotinic acetylcholine receptors (nAChRs), glycine receptors, the serotonin (5-hydroxytryptamine) 5-HT3 receptor and the zinc-activated channel (ZAC) (Connolly and Wafford 2004). For this ion channel superfamily, ligand binding is followed by a change in conformation of the channel protein that allows a net inward or outward flow of ions through the membrane-spanning pore of the channel, depending on the electrochemical gradient of the ion. During early developmental stages, GABAARs are primarily depolarizing due to the high intracellular chloride concentrations compared to extracellular chloride levels. In the adult brain, the intracellular chloride concentration is lower compared to the extracellular chloride levels. Therefore in mature neurons, GABAARs are generally hyperpolarizing (Rivera et al. 2005). This chloride gradient is maintained primarily by the activity of the K+/Cl− cotransporter 2 (KCC2). In the majority of adult neurons, activation of GABAARs results in a rapid chloride ion influx that results in the hyperpolarization of the cell membrane and thus a reduction in the probability for an action potential to be generated. Therefore, GABAARs play a pivotal role in regulating cellular and network excitability in the CNS, which underlies all physiological and behavioral processes.
Fig. 1. Structure of GABAAR.
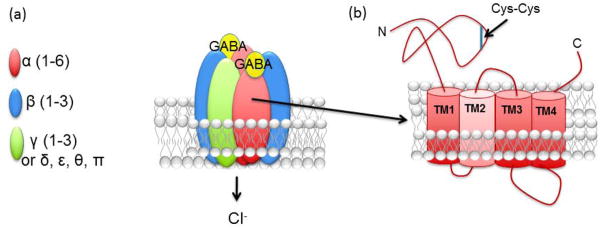
GABAAR are composed of five subunits assembled as a heteropentamer. (A) The protototypical GABAAR is composed of two α, two β, and one γ or δ. 19 GABAAR subunits have been identified to date and they are divided into seven groups on the basis of sequence similarity. (B) The common molecular GABAAR subunit structure is composed of a large N-terminal extracellular domain, four transmembrane domains, a short C-terminal extracellular domain and a long intracellular loop domain between TM3 and TM4. The intracellular domain contains critical sites for posttranslational modifications and protein-protein interactions that modulate receptor activity.
The members of cys-loop family form heteropentamers assembled from a wide range of heterologous subunits. To date, 19 GABAAR subunits have been identified. These subunits are divided into eight classes according to sequence homology; α1–6, β1–3, γ1–3, δ, ε, θ, ρ(1–3), and π (Olsen and Sieghart 2008; Sieghart and Sperk 2002).
The genomic location of the 19 genes encoding GABAAR subunits has been determined, and 14 of them are arranged in GABAAR gene clusters (Russek 1999). There are gene clusters on chromosomes 4 (α2, α4, β1 and γ1) and 5 (α1, α6, β2 and γ2) as well as on chromosomes 15 (α5, β3 and γ3) and X (α3, θ and ε). The gene for the π subunit is also located on chromosome 5 but at a site distant from the α1, α6 β2 γ2 cluster. Subunits ρ2 and ρ3 are mapped together on chromosome 6, whereas the δ and ρ3 subunits are located on their own on chromosomes 1 and 3 respectively. Each gene cluster contains gene encoding for α, β and γ/ε class (Darlison et al. 2005; Russek 1999). This gene organization has been proposed to be a result of early gene duplication events from a single ancestral αβγ GABAAR subunit cluster (Darlison et al. 2005; Russek 1999) and is believed to help coordinate gene expression (Joyce 2007).
Like all other members of the cys-loop family, all GABAAR subunits share a common structure: they have a large extended amino-terminal extracellular domain containing a cysteine loop signature, four highly conserved transmembrane domains (TMs), and a large intracellular loop domain between TM3 and TM4 (Arancibia-Carcamo and Kittler 2009; Connolly and Wafford 2004) (Figure 1). Using homology modeling based on the structure of the nicotinic acetylcholine receptor (Unwin 1995), it is believed that the lining of the water-filled channel pore is formed by the alignment of TM2 domains when subunits are arranged as a pentameric structure, and they extend through the membrane as alpha-helixes bending outwards (Corringer et al. 2000). TM1, TM3 and TM4 compose the interface with lipids and are thought to isolate TM2 lining from the hydrophobic membrane environment (Connolly and Wafford 2004).
Sites within the extracellular amino-terminal are important for oligomerization of the protein and for subunit-subunit interactions (Arancibia-Carcamo and Kittler 2009; Jacob et al. 2008). The binding site for GABA is located at the interface between α and β subunits (Connolly and Wafford 2004) (Figure 1). The accepted stoichiometry for αβγ and αβδ GABAARs is 2:2:1 (Barrera et al. 2008; Farrar et al. 1999; Patel et al. 2014), thus two β/α subunit interfaces in the pentameric structure occur which form the two GABA binding sites (Olsen and Sieghart 2009).
The intracellular domain is the most genetically divergent part of each individual GABAAR subunit. This domain is a critical site for cytoplasmic protein interactions with regulatory and signaling molecules including microtubule-binding proteins, cytoskeletal proteins, kinase-anchoring proteins and neurotransmitter transporters (Jacob et al. 2008; Luscher et al. 2011; Luscher and Keller 2004). In addition, the intracellular loop has multiple sites for post-translational modifications such as ubiquitination, palmitoylation and phosphorylation (Jacob et al. 2008; Kittler and Moss 2003). These protein interactions and post-translational modifications modulate receptor activity and trafficking.
Regulation of GABAAR oligomerization, transport, maturation and trafficking
The heterogeneity of GABAAR subtypes is restricted primarily by regulation governing the proper assembly of the receptor (Sieghart and Sperk 2002). Although a large number of GABAAR subtypes could be possible, studies suggest that only a limited number of GABAAR subunit combinations can oligomerize and reach the neuronal cell membrane (Arancibia-Carcamo and Kittler 2009).
The oligomerization of GABAARs occurs in the Endoplasmic Reticulum (ER). This process is mediated by critical assembly-domains located within the amino-terminals of GABAAR subunits and occurs within five minutes after translation (Gorrie et al. 1997; Jacob et al. 2008; Kittler et al. 2002). Receptors must be assembled and reach conformational maturity in the ER before transport to the plasma membrane. Studies in cell lines have shown that most single subunits are not capable of leaving the ER, and are targeted for proteosomal degradation (Jacob et al. 2008; Kittler et al. 2002; Moss and Smart 2001). Although some subunits (β1, β3 and γ2S) are able to reach the cell membrane, they are unable to form functional GABA-sensitive channels and are rapidly targeted for degradation (Connolly et al. 1999; Davies et al. 1997; Krishek et al. 1996; Sanna et al. 1995).
GABAARs are typically composed of two α, two β and one γ or δ subunit (Backus et al. 1993; Barrera et al. 2008; Farrar et al. 1999; Patel et al. 2014). GABAARs composed of only α and β subunits can form functional receptors in heterologous cell lines and have been suggested to exist in small numbers within neurons where they may mediate tonic inhibition (Mortensen and Smart 2006). Combinations of αγ and βγ are mostly retained in the ER and when α, β and γ are co-expressed together the formation of αβγ-GABAAR is strongly favored over receptors composed of only α and β subunits (Angelotti and Macdonald 1993).
The assembly and maturation within the ER are regulated by mechanisms involving ER-resident chaperones including Calnexin and Binding immunoglobulin protein (BiP) (Ehya et al. 2003; Jacob et al. 2008; Sarto-Jackson et al. 2006; Taylor et al. 2000). Calnexin and BiP are involved in regulating quality control of proteins within the secretory pathway (Jacob et al. 2008; Taylor et al. 2000). After oligomerization in the ER, GABAARs are then trafficked to the Golgi apparatus to be sorted into vesicles before insertion in the neuronal plasma membrane. This process is regulated by receptor-associated proteins that interact with the intracellular loop of GABAAR subunits (Jacob et al. 2008). Plic-1 (Protein that links integrin associated protein with the cytoskeleton-1) is involved in GABAAR stability in the ER and in the transport of the receptor through the secretory pathway (Figure 2). Plic-1 regulates GABAAR transport and maturation through the secretory pathway via interaction with all isoforms of α and β subunits (Bedford et al. 2001). The Plic-1 protein contains ubiquitin-like proteasome binding domains as well as ubiquitin-associated domains and is therefore able to inhibit ubiquitin-mediated proteolysis of GABAARs (Walters et al. 2002).
Fig. 2. Assembly, maturation and trafficking of GABAARs.
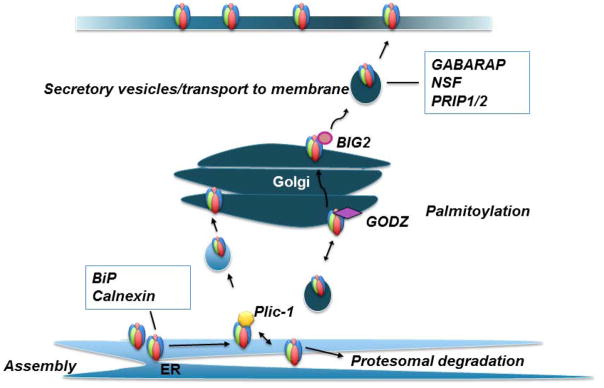
GABAARs are assembled as pentamers in the ER. This process is primarily regulated by the interaction of the ER-chaperones BiP and calnexin with residues located in the extracellular domain of GABAAR subunits. Improperly folded and unassembled GABAAR subunits are targeted for ubiquitination/proteosomal degradation. The ubiquitin-like protein Plic-1 inhibits degradation via interaction with sites located within the intracellular domain of α and β subunits. Plic-1 promotes GABAAR transport from the ER to the Golgi apparatus. In the Golgi, the palmitoytransferase GODZ palmitoylates a cysteine residue located within the intracellular loop of the γ subunit. Palmitoylation promotes GABAAR transport through the Golgi towards inhibitory synapses in the cell membrane. The translocation of GABAAR from the Golgi to the cell surface is believed to be mediated via BIG2 interaction with the intracellular domain of β subunits as well as other regulatory proteins including GABARAP and NSF.
When in the Golgi, γ2-containing GABAARs undergo palmitolation via interaction of the Golgi-specific DHHC zinc finger protein (GODZ) with cytoplasmic serine residues within the γ2 subunit. GODZ palmitolation of the γ2 subunit is critical for the subsequent accumulation of synaptic GABAARs at inhibitory synapses (Rathenberg et al. 2004).
Proper modulation of inhibition through GABAARs is dependent on receptor cycling between the cell surface and intracellular compartments (Jacob et al. 2008). To date, a number of regulatory proteins have been implicated in the cycling of GABAARs between the cell membrane and intracellular pools. These include BIG2 (Brefeldin-A inhibited GDP/GTP exchange factor 2), GABARAP (GABAAR associated protein), and components of the endocytotic machinery. BIG2 mediates the GABAARs exit from the Golgi towards the cell surface as well as GABAAR endocytic recycling via interaction with a sequence motif within the intracellular domain of β subunits (Charych et al. 2004). GABARAP is an ubiquitin-like protein that interacts with microtubules and with the intracellular loop of all γ subunits (Leil et al. 2004; Wang et al. 1999). It is involved in the translocation of GABAARs from intracellular compartments to the cell membrane (Kittler et al. 2004a; Leil et al. 2004). Other proteins involved in GABAAR trafficking are Phospholipase C-Related catalytically Inactive Proteins 1 and 2 (PRIP1/2) and N-ethylmaleimide Sensitive Factor (NSF). PRIP1/2 and NSF influence receptor trafficking indirectly through GABARAP and through a direct interaction with receptor subunit. NSF directly interacts with β subunits and can interact with GABARAP to increase receptor trafficking from the Golgi apparatus (Goto et al. 2005). In addition to binding with GABARAP, PRIP’s bind directly with β subunits and γ2 subunits, enhancing the trafficking of GABAARs to the cell surface (Mizokami et al. 2007; Terunuma et al. 2004; Uji et al. 2002).
GABAAR endocytosis and recycling
Endocytosis of GABAARs occurs primarily via mechanisms dependent on dynamin and clathrin (Figure 3). These processes are mediated by the interaction of the clathrin adaptor protein AP2 with residues located within the cytoplasmic intracellular loop of the β, γ, and δ subunits (Kittler et al. 2005; Kittler et al. 2008; Kittler et al. 2000). Work by Kittler et al. 2005, 2008 identified a ten amino acid motif within the cytoplasmic domain of all β subunits, as critical for mediating the GABAAR interaction with the μ2 subunit of the AP2 protein.
Fig. 3. GABAAR endocytosis and recycling.
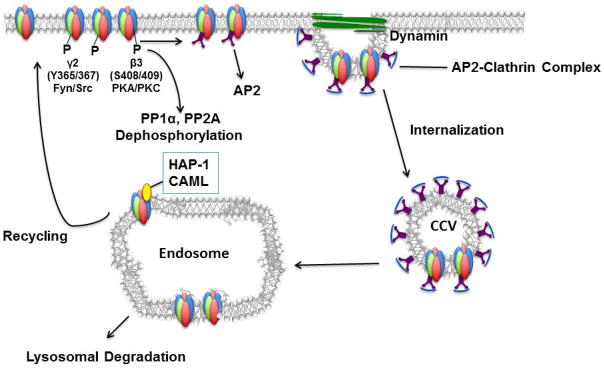
GABAAR endocytosis occurs primarily via mechanisms dependent on the formation of clathrin-coated vesicles. Clathrin- and dynamin-mediated endocytosis is regulated in a phospho-dependent manner via the interaction of specific motifs within the intracellular loop of the β and γ subunits with the μ subunit of the AP2 protein. Phosphorylation of residues within the intracellular domains of β3 and γ2 subunits (by kinases PKC/PKA or Fyn/Src, respectively) interferes with this interaction and therefore stabilizes GABAARs in the cell membrane. When endocytosed, GABAARs can be ubiquitinated and degraded via lysosomal degradation. Alternatively, the receptors can interact with regulatory lysosomal proteins such as HAP1, and CAML, which promotes the transport and recycling of receptors back to the cell surface (Arancibia-Cárcamo et al., 2009).
The interaction of the μ2 subunit with this motif within the β subunit is negatively regulated by phosphorylation of conserved serines within the AP2 binding motif (S408 in β1, S410 in β2, and S408/409 in β3). That is, AP2 can bind to GABAARs and trigger receptor internalization only when this site is dephosphorylated by protein phosphatases PP1α and PP2A (Kittler et al. 2005). The protein PRIP-1, mentioned above for its role in receptor trafficking, also binds to and inactivates PP1α, in so doing PRIP-1 can also prevent receptor internalization by preserving the phosphorylated state of the β subunit (Terunuma et al. 2004). Serines 408 and 409 in the β3 subunit can be phosphorylated by Protein Kinase A (PKA), Protein Kinase C (PKC), Calcium/Calmodulin-dependant Kinase II (CAMKII) and AKT, resulting in an increase in surface levels of β3 subunit containing GABAARs (Brandon et al. 2000; Brandon et al. 2002; McDonald et al. 1998; McDonald and Moss 1994).
The AP2 μ2 subunit also interacts with two motifs within the intracellular domain in the γ subunit. One consists of a twelve amino acid domain analogous to the AP2 binding site in the β subunit (Smith et al. 2008). The second is a high affinity γ-specific site YGYECL motif that contains a phosphorylation site at tyrosines 365/367 (Kittler et al. 2008; Smith et al. 2008). These residues can be phosphorylated by Fyn kinase and other members of the Src-family of tyrosine kinases (Jurd et al. 2010) and the phosphorylation of these sites interferes with AP2 binding and hence prevents GABAAR internalization (Boehm et al. 2004). The intracellular domain of the δ subunit also binds the AP2 μ2 subunit (Kittler et al. 2005). Recently, it has been demonstrated that the observed downregulation of extrasynaptic GABAARs in the hippocampus and dentate gyrus following ethanol administration is partly due to increased AP2 μ2 binding to the GABAAR δ subunit leading to receptor endocytosis (Gonzalez et al. 2012).
If GABAARs were to be internalized, once they are in endosomes GABAARs can become ubiquitinated and targeted for lysosomal degradation (Arancibia-Carcamo et al. 2009). Alternatively, regulatory proteins such as the Huntingtin-associated protein 1 (HAP1) (Kittler et al. 2004b; Twelvetrees et al. 2010) and the Calcium Modulating Cyclophilin Ligand (CAML) (Yuan et al. 2008) can interact with the cytoplasmic domains of the β and γ subunits respectively, and in this way facilitate vesicular transport and recycling back to the cell membrane.
We have recently shown that the cell surface expression of the α4 subunit is also modified by phosphorylation. The α4 subunit is phosphorylated by PKC within the large intracellular domain at serine 443, a covalent modification that increases cell surface stability of receptors containing α4 subunits by promoting their transport from the ER to the cell surface (Abramian et al. 2010).
Subcellular localization of GABAAR subtypes
The heterogeneity of GABAAR subunit composition is also carefully regulated by regional and temporal specificity in the patterns of expression in the CNS. This heterogeneity allows for GABAARs with different physiological and pharmacological properties as well as differential expression throughout the brain (Rudolph et al. 2001; Sieghart and Sperk 2002). GABAARs of different subunit composition have different subcellular localization (Connolly et al. 1996). GABAARs composed of the α1–3, β1–3 and γ2 are predominantly located at synaptic sites (Rudolph and Möhler 2006), whereas GABAARs composed of the α4–6, β2/3 and δ subunits are primarily localized at sites distant from synapses or extrasynaptic sites (Chandra et al. 2006; Farrant and Nusser 2005; Zheleznova et al. 2009).
The cell membrane distribution of synaptic and extrasynaptic GABAARs is dynamically regulated via the interactions with sub-synaptic scaffold molecules. During synaptic GABAAR membrane targeting, receptors are first introduced to the neuronal membrane at extrasynaptic sites, followed by subsequent lateral membrane diffusion to synaptic sites (Bogdanov et al. 2006).
Scaffold proteins located at post-synaptic localizations bind and cluster surface synaptic GABAARs at sites directly opposite GABA releasing axon terminals (Jacob et al. 2008). Gephyrin is the principal intracellular scaffolding molecule for both glycinergic and GABAergic synapses and has a critical role in regulating the clustering of synaptic GABAARs at inhibitory synapses (Fritschy et al. 2008; Levi et al. 2004; Mukherjee et al. 2011). Gephyrin interacts with proteins that regulate microfilament dynamics (such as profiling I and II) and with microtubules resulting in the formation of a hexagonal protein lattice that allows for the organization of synaptic GABAARs distribution in the cell membrane (Kirsch et al. 1995; Luscher et al. 2011; Mammoto et al. 1998). Synaptic adhesion molecules play an important role in the maturation and stabilization of inhibitory synapses (Cheng et al. 2006; Jamain et al. 2008; Ullrich et al. 1995). Tran-synaptic complexes of pre-synaptic β-neurexins and post-synaptic Neuroligin 2 (NL2) contribute to the proper alignment of pre- and post- synaptic molecules and structural maturation of inhibitory synapses (Panzanelli et al. 2011).
The cytoskeletal interactions that involve the anchoring at specific membrane localizations for those GABAAR located at extrasynaptic sites are less understood than for synaptic receptors. The cytoskeletal protein Radixin (a member of the ERM-ezrin, radixin and moesin family) has been implicated in the actin cytoskeleton anchoring of the predominantly extrasynaptic α5 subunit (Loebrich et al. 2006). However, the functional relevance of α5-containing GABAAR clustering at extrasynaptic sites is unknown. The expression of a dominant-negative radixin construct in neurons abolishes the membrane clustering of α5-containing GABAAR but has no effect on GABA- mediated currents (Loebrich et al. 2006).
Synaptic GABAARs mediate phasic inhibition
GABAARs located at synaptic and extrasynaptic sites are activated in a different manner and mediate distinct forms of inhibition. Synaptic GABAARs are activated in a transient manner after brief exposure to high concentrations of GABA released from the presynaptic membrane. This transient activation of synaptic GABAARs results in phasic inhibition (Jacob et al. 2008). Activation of synaptic GABAARs results in a rapid chloride ion influx that creates a transient or phasic, but significant reduction in the probability for an action potential to be generated due to rapid hyperpolarization of the plasma membrane.
Extrasynaptic GABAARs mediate tonic inhibition
Extrasynaptic GABAARs can be activated in a persistent, less temporally restricted manner by low ambient concentrations of GABA that either escapes from the synapse into the extracellular space or is released from non-synaptic sites such as neurogilaform cells and astrocytes (Kozlov et al. 2006; Lee et al. 2010; Olah et al. 2009). Recently it has been noted that tonic current can originate from some GABAARs that have a higher probability to transition from a closed to an open state in the absence of GABA (Wlodarczyk et al. 2013). When activated, extrasynaptic GABAARs generate an uninterrupted form of conductance that is referred to as tonic inhibition which results in a persistent reduction in the cell’s input resistance. This results in a reduction in both the size and duration of excitatory post-synaptic potentials and will in turn reduce the temporal and spatial frame for synaptic integration. Ultimately, tonic inhibition persistently reduces the likelihood for an action potential to occur.
Tonic currents mediated by extrasynaptic GABAARs have been described in various brain regions including in layer III cells of the somatosensory cortex (Salin and Prince 1996), cerebellar and dentate granule cells (Brickley et al. 1996; Nusser and Mody 2002), hippocampal interneurons and pyramidal cells (Bai et al. 2001; Semyanov et al. 2003), embryonic and developing neurons (Ge et al. 2006), neocortical layer 2/3 pyramidal cells (Drasbek et al. 2007; Drasbek and Jensen 2006), the spinal cord (Cronin et al. 2004), and in several sub cortical structures including hypothalamic (Sarkar et al. 2011; Sergeeva et al. 2005), thalamocortical neurons (Belelli et al. 2005; Cope et al. 2005) and medium spiny neurons of the striatum (Ade et al. 2008; Santhakumar et al. 2010).
The fact that GABAAR-mediated tonic inhibition occurs in many brain regions, changes during different developmental stages (Ge et al. 2006; LoTurco et al. 1995) and exhibits cell-type specific differences in magnitude suggests critical physiological roles for tonic inhibition. Indeed, tonic inhibition appears to be essential for the control of firing frequency (Rossi et al. 2003), for modulation of the firing threshold (offset), and the gain of transmission (Hamann et al. 2002; Mitchell and Silver 2003; Pavlov et al. 2009), all of which ultimately modulate network excitability (Farrant and Nusser 2005; Vida et al. 2006). During development, depolarization mediated by tonic currents has a critical role in neuronal migration, dendritic arborization, and the formation of synapses (Ge et al. 2006; Manent et al. 2005).
Extrasynaptic GABAAR properties
Extrasynaptic GABAARs exhibit different pharmacological properties from their synaptic counterparts; they are largely insensitive to benzodiazepines (Cope et al. 2005; Nusser and Mody 2002) and they are highly sensitive to THIP (4,5,6,7-tetrahydroisoxazolo [5,4-c] pyridin-3-ol/Gaboxadol) a selective GABAAR agonist (Brown et al. 2002; Wohlfarth et al. 2002). Low concentrations of the GABAAR antagonist Gabazine (SR95531) abolish inhibitory post synaptic currents while having a small or no effect on GABAAR-mediated tonic conductance (Semyanov et al. 2003; Stell and Mody 2002). Furthermore, extrasynaptic GABAARs are targets for, and principal mediators of response to, various endogenous and exogenous molecules including anticonvulsants, anesthetics and neurosteroids (Belelli et al. 2002; Bianchi and Macdonald 2003; Kretschmannova et al. 2013; Stell et al. 2003).
Extrasynaptic GABAARs are uniquely sensitive to neurosteroids
Neuroactive steroids can originate as metabolites of systemically produced progesterone or deoxycorticosterone, but can also be synthesized de novo in the brain by neurons and glia (Belelli and Herd 2003; Compagnone and Mellon 2000; Maguire and Mody 2007). The enzymes and steroid mitochondrial transporters necessary for de novo synthesis of pregnane neuorosteroids are present in many CNS regions (Mellon and Vaudry 2001). The P450cc mitochondrial cholesterol side-chain cleavage enzyme (P450cc) catalyzes the rate limiting step in de novo neurosteroid synthesis in which cholesterol is converted to pregnenolone (Mellon and Vaudry 2001). In addition, the enzymes 5α-reductase and 3α-hydroxysteroid dehydrogenase, which are required for the synthesis of 3α-hydroxy-5α-pregnane-20-one/Allopregnanolone (THPROG- from progesterone) and 3α,5α-3,21-dihydroxypregnan-20-one/Tetrahydrodeoxycorticosterone (THDOC- from deoxycorticosterone), have been shown to be expressed in the brain in a region and cell-type specific manner (Agis-Balboa et al. 2006). Unlike classical steroids, which act via their nuclear receptors to regulate gene expression, neurosteroids rapidly alter neuronal excitability via non-genomic mechanisms. Pregnane steroids containing a 3-α hydroxy ring have been shown to be potent steroselective allosteric modulators of GABAARs, having anxiolytic, anticonvulsant sedative and anesthetic effects (Majewska 1992; Paul and Purdy 1992).
Extrasynaptic GABAARs containing the δ are the most sensitive to neurosteroid modulation (Belelli et al. 2002; Bianchi and Macdonald 2003; Stell et al. 2003). Low physiological concentrations (10–100nM) of 3α,5α-THDOC greatly enhance the tonic conductance mediated by extrasynaptic GABAARs with little or no effect on the phasic conductance mediated by synaptic GABAARs, in both dentate gyrus and cerebellar granule cells (Stell et al. 2003). At the single channel level, neurosteroids increase the open duration and the frequency of GABAAR channel openings with no effect on the single channel conductance (Callachan et al. 1987; Twyman and Macdonald 1992).
Neurosteroids enhance GABAARs via a distinct binding pocket
The stereoselectivity of the potent interaction between neurosteroids and native GABAARs strongly suggested early on the possibility of a neurosteroid modulatory site on the receptor protein. Electrophysiological and radioligand binding experiments provided evidence that the modulatory site for neurosteroids on GABAARs was distinct from the binding site for benzodiazepines and other known allosteric recognition modulators (Callachan et al. 1987; Peters et al. 1988). Subsequent homology modeling studies coupled to the use of GABAAR chimeras between steroid insensitive Drosophila-RDL subunits and α subunits led to the identification of critical residues for neurosteroid modulation. These studies revealed the importance of residues with side chains that could form hydrogen bond interactions with neurosteroid molecules. The important residues are conserved among all α subunit isoforms and are glutamine (Q) located within TM1 and the residues asparagine and tyrosine located within TM4 (Hosie et al. 2006; Hosie et al. 2007). Mutating one such site in the TM1 region of the α4 subunit (Q241L) significantly abolished THDOC enhancement of the receptor (Hosie et al. 2009). Furthermore, they provided evidence that the δ subunit does not contribute to the neurosteroid modulation site and is likely to be regulating the efficacy of neurosteroid potentiation after the initial binding to the GABAAR (Hosie et al. 2009).
Tonic inhibition, Neurosteroids and Disease
Disturbances in the tonic inhibition mediated by extrasynaptic GABAARs has been observed in a wide range of psychiatric and neurological conditions including some forms of epilepsy, addiction, cognitive impairments, sleep disorders, anxiety disorders, post-partum depression and stress-related disorders (Belelli et al. 2009; Kato et al. 2007; Maguire and Mody 2008; Maguire et al. 2005; Maldonado-Aviles et al. 2009; Nie et al. 2011; Uhlhaas and Singer 2010). Mutations within the δ and other extrasynaptic GABAAR receptor subunits have been implicated in some forms of epilepsy (Macdonald et al. 2010), childhood onset of some mood disorders (Feng et al. 2010), and schizophrenia (Maldonado-Aviles et al. 2009).
Many of these disorders also involve changes in the levels of neurosteroids, that occur following physiological changes in the levels of ovarian and adrenal cortex hormones. For example, catamenial epilepsy (a form of epilepsy in which female patients show a cyclic variation in seizure intensity depending on the menstrual cycle phase) has been linked to changes in tonic inhibition during the ovarian cycle (Maguire et al. 2005). Ganaxolone (an analogue to the neurosteroid allopregnanolone) is currently being used in clinical trials for the treatment of this disorder and has been shown to be an effective anticonvulsant in an animal model (Bialer et al. 2013). Anxiety linked to premenstrual dysmorphic disorder (PMDD) has been associated with neurosteroid-mediated changes in tonic inhibition in animal models (Maguire et al. 2005; Smith et al. 1998).
Work by Maguire and Moody (2008), linked discrepancies in the number of δ-containing extrasynaptic GABAARs and the neurosteroids allopregnanolone (progesterone metabolite) with post-partum depression. Elevated allopregnanolone levels during pregnancy (as a direct result of the 100-fold increase in progesterone during this period) results in a compensatory reduction in the number of δ-containing GABAARs. In a mouse model, delay in restoring δ-containing GABAARs number to prepregnancy levels, resulted in a severe depression-like phenotype (Maguire and Mody 2008).
Of particular relevance for stress-related disorders, a recent study has shown that corticotropin-releasing hormone (CRH) neurons of the hypothalamus are modulated by the stress hormone metabolite THDOC through its actions on δ-containing GABAARs. CRH neurons are a critical component of the hypothalamic-pituitary-adrenal (HPA) axis, which mediates the physiological response to stress. Under normal physiological conditions, THDOC decreased HPA axis activity by potentiating the inhibitory effects of GABA on CRH neurons. However, during acute stress conditions THDOC activates the HPA axis. This is due to a collapse in the chloride gradient that occurs following the dephosphorylation of KCC2. THDOC actions on δ-containing GABAARs seem to constitute a positive feedback mechanism onto CRH neurons, which is required for the proper physiological response to stress (Sarkar et al. 2011).
Neurosteroids regulate changes in GABAAR subunit expression
Neurosteroids dynamically regulate changes in GABAAR subunit expression. For example, the δ and α4 subunits have been shown to undergo marked changes in expression in response to fluctuating steroid levels (Gulinello et al. 2001; Hsu et al. 2003; Maguire and Mody 2007; 2009). The levels of steroid hormones can fluctuate in a wide range of physiological states including stress, puberty, pregnancy and the menstrual cycle. Steroid-induced fluctuations in GABAAR subunit expression result in alterations in neuronal excitability and are implicated in many neurological disorders. However, the molecular mechanisms by which neurosteroids alter GABAARs subunit expression and function are not well understood.
For synaptic GABAARs, the trafficking mechanisms and protein interactions that regulate receptor cell surface localization are essential for the changes in synaptic strength mediated by GABA receptor signaling (Jacob et al. 2008). Endocytosis is a critical process for the regulation of the number of surface synaptic neurotransmitter receptors. Our laboratory has shown that phosphorylation of residues within synaptic GABAAR subunits regulates the interaction of GABAAR subunits with protein complexes that mediate endocytosis (Jacob et al. 2008; Luscher et al. 2011). Whether extrasynaptic GABAAR cell surface expression is modulated in a similar manner is not fully understood but PKC-mediated phosphorylation of the α4 subunit increases membrane surface expression of α4β3 subunit containing GABAARs in recombinant cells and in the dentate gyrus region of the hippocampus where α4/δ subunit containing GABAARs form extrasynaptic receptors (Abramian et al. 2010). Next, we explore how phosphorylation of GABAAR subunits influence the actions of neurosteroids and discuss a possible link between neurosteroids and subunit phosphorylation leading to changes in GABAAR expression.
Phosphorylation and neurosteroid action
Neurosteroids have been shown to be potent positive allosteric modulators of GABAARs and are particular efficacious on extrasynaptic GABAARs. Previous experiments have suggested that PKC activity enhances neurosteroid-mediated modulation of GABAARs, or PKC activity is required for such modulation (Fancsik et al. 2000; Harney et al. 2003; Leidenheimer and Chapell 1997). Neurosteroid modulation of IPSCs in hippocampal CA1 pyramidal neurons is dependent on both PKA and PKC, whereas in the dentate gyrus only stimulation of PKC was effective in enhancing neurosteroid effects (Harney et al. 2003). Contrary to this PKC-dependent enhancement by neurosteroids, Kia et al (2011) reported that in piriform cortex pyramidal neurons, PKC activation with the phorbal ester, PMA reduced THDOC modulation of phasic and tonic GABA-mediated currents and suggested that different isomers of PKC phosphorylated synaptic and extrasynaptic GABAARs (Kia et al. 2011). Region specific effects are likely to be observed based on region dependent expression of different subunits and kinase activity.
In addition to the well characterized positive allosteric modulation of GABAARs, neurosteroids have been demonstrated to affect subunit expression, particularly the α4 and δ subunitys. Because phosphorylation of receptor subunits greatly influences the membrane expression of GABAARs, and that we have previously found that the α4 subunit can be phosphorylated by PKC at serine 443 which results in an increase in membrane expression of α4 subunit containing GABAARs (Abramian et al. 2010), we examined if neurosteroids could influence membrane expression by changing the phosphorylated state of subunits. We demonstrated in α4 expressing recombinant cells, neurons in culture, and in brain slices that neurosteroids enhance the PKC phosphorylation of S443 within α4 subunits, and this neurosteroid-mediated effect is independent of the allosteric actions. PKC-mediated phosphorylation of α4 subunits enhances the insertion of α4 subunit containing GABAAR subtypes into the membrane, resulting in a selective and sustained elevation in the efficacy of tonic inhibition (Abramian et al. 2014). The exact mechanism behind this phospho-dependent membrane insertion of α4 subunit containing GABAARs has yet to be determined but represents a novel mechanism by which neurosteroids can alter neuronal inhibition.
Summary
Receptor expression is critical for maintaining a healthy excitation / inhibition balance. Phosphorylation of GABAAR subunits is a critical component in subunit trafficking and membrane stability of receptors. In some areas of the brain, the allosteric modulation of GABAAR-mediated currents by neurosteroids is dependent upon phosphorylation. In addition to their allosteric modulation of GABAARs, neurosteroids can exert long-term effects on neuronal excitation by dynamically regulating the expression levels of extrasynaptic GABAARs through a phosphorylation-dependent mechanism. Dysregulation of neurosteroid signaling is associated with premenstrual dysphoric disorder, panic disorder, depression, schizophrenia and bipolar disorder. Determining the mechanism by which neurosteroids modify GABAAR expression may provide novel therapeutic strategies for these conditions.
Acknowledgments
This work was supported by a grant from the Simons Foundation #206026 to S.J.M., NIH-NINDS grants, NS051195, NS056359, NS081735 (SJM), and NIH-NIMH grant, MH097446, (PAD & SJM). SJM serves as a consultant for SAGE therapeutics and AstraZeneca, relationships that are regulated by Tufts University and do not impact on this study.
Abbreviations
- AP2
clathin adaptor protein 2
- BIG2
Brefeldin-A inhibited GDP/GTP exchange factor 2
- CAML
Calcium Modulating Cyclophilin Ligand
- ER
Endoplasmic Reticulum
- GABA
γ-aminobutyric acid
- GABAARs
γ-aminobutyric acid type A receptors
- GABARAP
GABAAR associated protein
- GODZ
Golgi-specific DHHC zinc finger protein
- HAP1
Huntingtin-associated protein 1
- PKC
Protein Kinase C
- THDOC
Allotetrahydrodeoxycorticosterone
- THIP
4,5,6,7-Tetrahydroisoxazolo[5,4-c]pyridin-3-ol
References
- Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, Maguire JL, Terunuma M, Davies PA, Moss SJ. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem. 2010;285:41795–805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ade KK, Janssen MJ, Ortinski PI, Vicini S. Differential tonic GABA conductances in striatal medium spiny neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:1185–97. doi: 10.1523/JNEUROSCI.3908-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agis-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14602–7. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelotti TP, Macdonald RL. Assembly of GABAA receptor subunits: alpha 1 beta 1 and alpha 1 beta 1 gamma 2S subunits produce unique ion channels with dissimilar single-channel properties. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:1429–40. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia-Carcamo IL, Kittler JT. Regulation of GABA(A) receptor membrane trafficking and synaptic localization. Pharmacology & therapeutics. 2009;123:17–31. doi: 10.1016/j.pharmthera.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Arancibia-Carcamo IL, Yuen EY, Muir J, Lumb MJ, Michels G, Saliba RS, Smart TG, Yan Z, Kittler JT, Moss SJ. Ubiquitin-dependent lysosomal targeting of GABA(A) receptors regulates neuronal inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17552–7. doi: 10.1073/pnas.0905502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus KH, Arigoni M, Drescher U, Scheurer L, Malherbe P, Mohler H, Benson JA. Stoichiometry of a recombinant GABAA receptor deduced from mutation-induced rectification. Neuroreport. 1993;5:285–8. doi: 10.1097/00001756-199312000-00026. [DOI] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Molecular pharmacology. 2001;59:814–24. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Barrera NP, Betts J, You H, Henderson RM, Martin IL, Dunn SM, Edwardson JM. Atomic force microscopy reveals the stoichiometry and subunit arrangement of the alpha4beta3delta GABA(A) receptor. Molecular pharmacology. 2008;73:960–7. doi: 10.1124/mol.107.042481. [DOI] [PubMed] [Google Scholar]
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, Triller A, Smart TG, Moss SJ. GABA(A) receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nature neuroscience. 2001;4:908–16. doi: 10.1038/nn0901-908. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology. 2002;43:651–61. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12757–63. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABA(A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:10013–20. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:11513–20. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI) Epilepsy research. 2013;103:2–30. doi: 10.1016/j.eplepsyres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:10934–43. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Peden L, Harris RA, Blednov YA. Deletion of the fyn-kinase gene alters sensitivity to GABAergic drugs: dependence on beta2/beta3 GABAA receptor subunits. The Journal of pharmacology and experimental therapeutics. 2004;309:1154–9. doi: 10.1124/jpet.103.064444. [DOI] [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. The EMBO journal. 2006;25:4381–9. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. The Journal of biological chemistry. 2000;275:38856–62. doi: 10.1074/jbc.M004910200. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Jovanovic JN, Smart TG, Moss SJ. Receptor for activated C kinase-1 facilitates protein kinase C-dependent phosphorylation and functional modulation of GABA(A) receptors with the activation of G-protein-coupled receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:6353–61. doi: 10.1523/JNEUROSCI.22-15-06353.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. The Journal of physiology. 1996;497 ( Pt 3):753–9. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. British journal of pharmacology. 2002;136:965–74. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proceedings of the Royal Society of London Series B, Containing papers of a Biological character Royal Society. 1987;231:359–69. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15230–5. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004;90:173–89. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- Cheng VY, Bonin RP, Chiu MW, Newell JG, MacDonald JF, Orser BA. Gabapentin increases a tonic inhibitory conductance in hippocampal pyramidal neurons. Anesthesiology. 2006;105:325–33. doi: 10.1097/00000542-200608000-00015. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Frontiers in neuroendocrinology. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Uren JM, Thomas P, Gorrie GH, Gibson A, Smart TG, Moss SJ. Subcellular localization and endocytosis of homomeric gamma2 subunit splice variants of gamma-aminobutyric acid type A receptors. Molecular and cellular neurosciences. 1999;13:259–71. doi: 10.1006/mcne.1999.0746. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Wafford KA. The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function. Biochemical Society transactions. 2004;32:529–34. doi: 10.1042/BST0320529. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Wooltorton JR, Smart TG, Moss SJ. Subcellular localization of gamma-aminobutyric acid type A receptors is determined by receptor beta subunits. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9899–904. doi: 10.1073/pnas.93.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:11553–63. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annual review of pharmacology and toxicology. 2000;40:431–58. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- Cronin JN, Bradbury EJ, Lidierth M. Laminar distribution of GABAA- and glycine-receptor mediated tonic inhibition in the dorsal horn of the rat lumbar spinal cord: effects of picrotoxin and strychnine on expression of Fos-like immunoreactivity. Pain. 2004;112:156–63. doi: 10.1016/j.pain.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Darlison MG, Pahal I, Thode C. Consequences of the evolution of the GABA(A) receptor gene family. Cellular and molecular neurobiology. 2005;25:607–24. doi: 10.1007/s10571-005-4004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Kirkness EF, Hales TG. Modulation by general anaesthetics of rat GABAA receptors comprised of alpha 1 beta 3 and beta 3 subunits expressed in human embryonic kidney 293 cells. Br J Pharmacol. 1997;120:899–909. doi: 10.1038/sj.bjp.0700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasbek KR, Hoestgaard-Jensen K, Jensen K. Modulation of extrasynaptic THIP conductances by GABAA-receptor modulators in mouse neocortex. Journal of neurophysiology. 2007;97:2293–300. doi: 10.1152/jn.00651.2006. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cerebral cortex. 2006;16:1134–41. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- Ehya N, Sarto I, Wabnegger L, Sieghart W. Identification of an amino acid sequence within GABA(A) receptor beta3 subunits that is important for receptor assembly. J Neurochem. 2003;84:127–35. doi: 10.1046/j.1471-4159.2003.01509.x. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Fancsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci. 2000;20:3067–75. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Farrar SJ, Whiting PJ, Bonnert TP, McKernan RM. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. The Journal of biological chemistry. 1999;274:10100–4. doi: 10.1074/jbc.274.15.10100. [DOI] [PubMed] [Google Scholar]
- Feng Y, Kapornai K, Kiss E, Tamas Z, Mayer L, Baji I, Daroczi G, Benak I, Kothencne VO, Dombovari E, Kaczvinszk E, Besnyo M, Gadoros J, Szekely J, Kovacs M, Vetro A, Kennedy JL, Barr CL. Association of the GABRD gene and childhood-onset mood disorders. Genes, brain, and behavior. 2010;9:668–72. doi: 10.1111/j.1601-183X.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31:257–64. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Moss SJ, Olsen RW. Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of delta-containing GABAA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:17874–81. doi: 10.1523/JNEUROSCI.2535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrie GH, Vallis Y, Stephenson A, Whitfield J, Browning B, Smart TG, Moss SJ. Assembly of GABAA receptors composed of alpha1 and beta2 subunits in both cultured neurons and fibroblasts. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:6587–96. doi: 10.1523/JNEUROSCI.17-17-06587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H, Terunuma M, Kanematsu T, Misumi Y, Moss SJ, Hirata M. Direct interaction of N-ethylmaleimide-sensitive factor with GABA(A) receptor beta subunits. Molecular and cellular neurosciences. 2005;30:197–206. doi: 10.1016/j.mcn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases alpha4 GABA(A) receptor subunit levels in association with increased anxiety in the female rat. Brain research. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–33. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Harney SC, Frenguelli BG, Lambert JJ. Phosphorylation influences neurosteroid modulation of synaptic GABAA receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology. 2003;45:873–83. doi: 10.1016/s0028-3908(03)00251-x. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABA A receptors. Neuropharmacology. 2009;56:149–54. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABA(A) receptors. Pharmacology & therapeutics. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Waldeck R, Faber DS, Smith SS. Neurosteroid effects on GABAergic synaptic plasticity in hippocampus. Journal of neurophysiology. 2003;89:1929–40. doi: 10.1152/jn.00780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–43. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1710–5. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce CJ. In silico comparative genomic analysis of GABAA receptor transcriptional regulation. BMC genomics. 2007;8:203. doi: 10.1186/1471-2164-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R, Tretter V, Walker J, Brandon NJ, Moss SJ. Fyn kinase contributes to tyrosine phosphorylation of the GABA(A) receptor gamma2 subunit. Molecular and cellular neurosciences. 2010;44:129–34. doi: 10.1016/j.mcn.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Kakiuchi C, Iwamoto K. Comprehensive gene expression analysis in bipolar disorder. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2007;52:763–71. doi: 10.1177/070674370705201203. [DOI] [PubMed] [Google Scholar]
- Kia A, Ribeiro F, Nelson R, Gavrilovici C, Ferguson SS, Poulter MO. Kindling alters neurosteroid-induced modulation of phasic and tonic GABAA receptor-mediated currents: role of phosphorylation. Journal of neurochemistry. 2011;116:1043–56. doi: 10.1111/j.1471-4159.2010.07156.x. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Kuhse J, Betz H. Targeting of glycine receptor subunits to gephyrin-rich domains in transfected human embryonic kidney cells. Molecular and cellular neurosciences. 1995;6:450–61. doi: 10.1006/mcne.1995.1033. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Arancibia-Carcamo IL, Moss SJ. Association of GRIP1 with a GABA(A) receptor associated protein suggests a role for GRIP1 at inhibitory synapses. Biochemical pharmacology. 2004a;68:1649–54. doi: 10.1016/j.bcp.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, Pangalos MN, Haucke V, Yan Z, Moss SJ. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14871–6. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Kukhtina V, Vahedi-Faridi A, Gu Z, Tretter V, Smith KR, McAinsh K, Arancibia-Carcamo IL, Saenger W, Haucke V, Yan Z, Moss SJ. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit. Proc Natl Acad Sci U S A. 2008;105:3616–21. doi: 10.1073/pnas.0707920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7972–7. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Molecular neurobiology. 2002;26:251–68. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Current opinion in neurobiology. 2003;13:341–7. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proceedings of the National Academy of Sciences of the United States of America. 2004b;101:12736–41. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov AS, Angulo MC, Audinat E, Charpak S. Target cell-specific modulation of neuronal activity by astrocytes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10058–63. doi: 10.1073/pnas.0603741103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmannova K, Hines RM, Revilla-Sanchez R, Terunuma M, Tretter V, Jurd R, Kelz MB, Moss SJ, Davies PA. Enhanced tonic inhibition influences the hypnotic and amnestic actions of the intravenous anesthetics etomidate and propofol. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:7264–73. doi: 10.1523/JNEUROSCI.5475-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishek BJ, Moss SJ, Smart TG. Homomeric beta 1 gamma-aminobutyric acid A receptor-ion channels: evaluation of pharmacological and physiological properties. Molecular pharmacology. 1996;49:494–504. [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–6. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- Leidenheimer NJ, Chapell R. Effects of PKC activation and receptor desensitization on neurosteroid modulation of GABA(A) receptors. Brain Res Mol Brain Res. 1997;52:173–81. doi: 10.1016/s0169-328x(97)00255-6. [DOI] [PubMed] [Google Scholar]
- Leil TA, Chen ZW, Chang CS, Olsen RW. GABAA receptor-associated protein traffics GABAA receptors to the plasma membrane in neurons. J Neurosci. 2004;24:11429–38. doi: 10.1523/JNEUROSCI.3355-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:207–17. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Lopez-Corcuera B, Mandiyan S, Nelson H, Nelson N. Molecular characterization of four pharmacologically distinct gamma-aminobutyric acid transporters in mouse brain [corrected] J Biol Chem. 1993;268:2106–12. [PubMed] [Google Scholar]
- Loebrich S, Bahring R, Katsuno T, Tsukita S, Kneussel M. Activated radixin is essential for GABAA receptor alpha5 subunit anchoring at the actin cytoskeleton. The EMBO journal. 2006;25:987–99. doi: 10.1038/sj.emboj.7600995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–98. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacology & therapeutics. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kang JQ, Gallagher MJ. Mutations in GABAA receptor subunits associated with genetic epilepsies. The Journal of physiology. 2010;588:1861–9. doi: 10.1113/jphysiol.2010.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2155–62. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–13. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S84–90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature neuroscience. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Progress in neurobiology. 1992;38:379–95. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Maldonado-Aviles JG, Curley AA, Hashimoto T, Morrow AL, Ramsey AJ, O’Donnell P, Volk DW, Lewis DA. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. The American journal of psychiatry. 2009;166:450–9. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Sasaki T, Asakura T, Hotta I, Imamura H, Takahashi K, Matsuura Y, Shirao T, Takai Y. Interactions of drebrin and gephyrin with profilin. Biochemical and biophysical research communications. 1998;243:86–9. doi: 10.1006/bbrc.1997.8068. [DOI] [PubMed] [Google Scholar]
- Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L, Represa A. A noncanonical release of GABA and glutamate modulates neuronal migration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:4755–65. doi: 10.1523/JNEUROSCI.0553-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nature neuroscience. 1998;1:23–8. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Moss SJ. Differential phosphorylation of intracellular domains of gamma-aminobutyric acid type A receptor subunits by calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. The Journal of biological chemistry. 1994;269:18111–7. [PubMed] [Google Scholar]
- Mellon SH, Vaudry H. Biosynthesis of neurosteroids and regulation of their synthesis. International review of neurobiology. 2001;46:33–78. doi: 10.1016/s0074-7742(01)46058-2. [DOI] [PubMed] [Google Scholar]
- Minelli A, Brecha NC, Karschin C, DeBiasi S, Conti F. GAT-1, a high-affinity GABA plasma membrane transporter, is localized to neurons and astroglia in the cerebral cortex. J Neurosci. 1995;15:7734–46. doi: 10.1523/JNEUROSCI.15-11-07734.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–45. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mizokami A, Kanematsu T, Ishibashi H, Yamaguchi T, Tanida I, Takenaka K, Nakayama KI, Fukami K, Takenawa T, Kominami E, Moss SJ, Yamamoto T, Nabekura J, Hirata M. Phospholipase C-related inactive protein is involved in trafficking of gamma2 subunit-containing GABA(A) receptors to the cell surface. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:1692–701. doi: 10.1523/JNEUROSCI.3155-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic alphabeta subunit GABAA receptors on rat hippocampal pyramidal neurons. The Journal of physiology. 2006;577:841–56. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–50. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, Davies PA, Triller A, Schindelin H, Moss SJ. The residence time of GABA(A)Rs at inhibitory synapses is determined by direct binding of the receptor alpha1 subunit to gephyrin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14677–87. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic delta-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4459–64. doi: 10.1073/pnas.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. Journal of neurophysiology. 2002;87:2624–8. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–81. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacological reviews. 2008;60:243–60. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–8. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzanelli P, Gunn BG, Schlatter MC, Benke D, Tyagarajan SK, Scheiffele P, Belelli D, Lambert JJ, Rudolph U, Fritschy JM. Distinct mechanisms regulate GABAA receptor and gephyrin clustering at perisomatic and axo-axonic synapses on CA1 pyramidal cells. The Journal of physiology. 2011;589:4959–80. doi: 10.1113/jphysiol.2011.216028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Mortensen M, Smart TG. Stoichiometry of delta subunit containing GABAA receptors. British journal of pharmacology. 2014;171:985–94. doi: 10.1111/bph.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- Pavlov I, Savtchenko LP, Kullmann DM, Semyanov A, Walker MC. Outwardly rectifying tonically active GABAA receptors in pyramidal cells modulate neuronal offset, not gain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:15341–50. doi: 10.1523/JNEUROSCI.2747-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JA, Kirkness EF, Callachan H, Lambert JJ, Turner AJ. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. British journal of pharmacology. 1988;94:1257–69. doi: 10.1111/j.1476-5381.1988.tb11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathenberg J, Kittler JT, Moss SJ. Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Molecular and cellular neurosciences. 2004;26:251–7. doi: 10.1016/j.mcn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII. The Journal of physiology. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. The Journal of physiology. 2003;548:97–110. doi: 10.1113/jphysiol.2002.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Mohler H. GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–94. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Current opinion in pharmacology. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Russek SJ. Evolution of GABA(A) receptor diversity in the human genome. Gene. 1999;227:213–22. doi: 10.1016/s0378-1119(98)00594-0. [DOI] [PubMed] [Google Scholar]
- Salin PA, Prince DA. Spontaneous GABAA receptor-mediated inhibitory currents in adult rat somatosensory cortex. Journal of neurophysiology. 1996;75:1573–88. doi: 10.1152/jn.1996.75.4.1573. [DOI] [PubMed] [Google Scholar]
- Sanna E, Garau F, Harris RA. Novel properties of homomeric beta 1 gamma-aminobutyric acid type A receptors: actions of the anesthetics propofol and pentobarbital. Mol Pharmacol. 1995;47:213–7. [PubMed] [Google Scholar]
- Santhakumar V, Jones RT, Mody I. Developmental regulation and neuroprotective effects of striatal tonic GABAA currents. Neuroscience. 2010;167:644–55. doi: 10.1016/j.neuroscience.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:18198–210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarto-Jackson I, Ramerstorfer J, Ernst M, Sieghart W. Identification of amino acid residues important for assembly of GABA receptor alpha1 and gamma2 subunits. J Neurochem. 2006;96:983–95. doi: 10.1111/j.1471-4159.2005.03626.x. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nature neuroscience. 2003;6:484–90. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Sergeeva OA, Andreeva N, Garret M, Scherer A, Haas HL. Pharmacological properties of GABAA receptors in rat hypothalamic neurons expressing the epsilon-subunit. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:88–95. doi: 10.1523/JNEUROSCI.3209-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Current topics in medicinal chemistry. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Smith KR, McAinsh K, Chen G, Arancibia-Carcamo IL, Haucke V, Yan Z, Moss SJ, Kittler JT. Regulation of inhibitory synaptic transmission by a conserved atypical interaction of GABA(A) receptor beta- and gamma-subunits with the clathrin AP2 adaptor. Neuropharmacology. 2008;55:844–50. doi: 10.1016/j.neuropharm.2008.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3alpha-OH-5alpha-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor alpha4 subunit in association with increased anxiety. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:5275–84. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14439–44. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PM, Connolly CN, Kittler JT, Gorrie GH, Hosie A, Smart TG, Moss SJ. Identification of residues within GABA(A) receptor alpha subunits that mediate specific assembly with receptor beta subunits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:1297–306. doi: 10.1523/JNEUROSCI.20-04-01297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Jang IS, Ha SH, Kittler JT, Kanematsu T, Jovanovic JN, Nakayama KI, Akaike N, Ryu SH, Moss SJ, Hirata M. GABAA receptor phospho-dependent modulation is regulated by phospholipase C-related inactive protein type 1, a novel protein phosphatase 1 anchoring protein. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:7074–84. doi: 10.1523/JNEUROSCI.1323-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, Kittler JT. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. The Journal of physiology. 1992;456:215–45. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–13. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uji A, Matsuda M, Kukita T, Maeda K, Kanematsu T, Hirata M. Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2: Comparison with PRIP-1. Life sciences. 2002;72:443–53. doi: 10.1016/s0024-3205(02)02275-0. [DOI] [PubMed] [Google Scholar]
- Ullrich B, Ushkaryov YA, Sudhof TC. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. 1995;14:497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- Vida I, Bartos M, Jonas P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron. 2006;49:107–17. doi: 10.1016/j.neuron.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Walters KJ, Kleijnen MF, Goh AM, Wagner G, Howley PM. Structural studies of the interaction between ubiquitin family proteins and proteasome subunit S5a. Biochemistry. 2002;41:1767–77. doi: 10.1021/bi011892y. [DOI] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk AI, Sylantyev S, Herd MB, Kersante F, Lambert JJ, Rusakov DA, Linthorst AC, Semyanov A, Belelli D, Pavlov I, Walker MC. GABA-independent GABAA receptor openings maintain tonic currents. J Neurosci. 2013;33:3905–14. doi: 10.1523/JNEUROSCI.4193-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1541–9. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Yao J, Norris D, Tran DD, Bram RJ, Chen G, Luscher B. Calcium-modulating cyclophilin ligand regulates membrane trafficking of postsynaptic GABA(A) receptors. Molecular and cellular neurosciences. 2008;38:277–89. doi: 10.1016/j.mcn.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznova NN, Sedelnikova A, Weiss DS. Function and modulation of delta-containing GABA(A) receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S67–73. doi: 10.1016/j.psyneuen.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


