Abstract
Objective
Cardiac transplantation, along with available mechanical alternatives, are the only possible solutions for end stage cardiac disease. Unfortunately, due to the limited supply of human organs, xenotransplantation may be the ideal method to overcome this shortage. Recently we have seen significant prolongation of heterotopic cardiac xenograft survival from three months to twelve months and beyond.
Methods
Hearts from genetically engineered (GE) piglets that were alpha 1–3 galactosidase transferase knockout (GTKO) and expressed the human complement regulatory gene, CD46 (hCD46)(Group A, B, C), and the human Thrombomodulin (hTBM) gene (Group D), were heterotropically transplanted in baboons treated with anti-thymocyte globulin (ATG), cobra venom factor (CVF), anti CD20 antibody, and costimulation blockade (anti CD154 antibody (clone 5C8), in group A, or anti-CD40 antibody (clone 3A8;20mg/Kg) in group B, clone 2C10R4 (25mg/Kg) in group C, or clone 2C10R4 (50mg/Kg) in group D, along with conventional non-specific immunosuppressive agents.
Results
Group A grafts (n=8) survived for an average of 70 days with the longest survival of 236 days. Some animals in this group (n=3) developed microvascular thrombosis due to platelet activation and consumption, which resulted in spontaneous hemorrhage. The median survival time in group B (n=3) was 21 days, group C (n=6) was 80 days, and group D (n=5) was >200days. Three grafts in group D are still contracting well, with the longest ongoing graft survival surpassing the one-year mark.
Conclusion
GE pig hearts (GTKOhTg.hCD46.hTBM) with modified targeted immunosuppression (anti CD40mAb) achieved long term cardiac xenograft survival. This potentially paves the way for clinical xenotransplantation if similar survival can be reproduced in an orthotopic transplantation model.
Introduction
Patients with end stage organ failure waiting for donor organs have very limited treatment options. For those with cardiac failure mechanical assist devices provide one solution, but various complications associated with these devices have reduced their effectiveness (1). Until we learn to grow organs via tissue engineering, which is unlikely in near future, xenotransplantation seems to be a very valid approach to supplement human organ availability. Despite many setbacks over the years, two major recent developments have helped revitalized progress in the xenotransplantation field. First, is the ability to produce genetically engineered (GE) pigs (2, 3) in which certain genes that are immunogenic to humans are knocked out and human transgenes like complement regulatory proteins (hCRP) and thromboregulatory molecules (hTRP) are expressed on pig cells (4). The second achievement is the development of target specific immunosuppression that could be used in clinically in place of generalized immunosuppression (4). With these developments, cardiac xenograft survival has been prolonged to over a year (5). Due to the cost of these experiments and scarce research funding, it is not feasible to address each genetic and immunosuppressive manipulation individually. Therefore, laboratories performing experiments in the field of xenotransplantation have selectively picked specific genetic modifications in pigs, as well as, immunosuppressive drug combinations to perform xenotransplantation experiments. In this report we have summarized our results from multiple experiments to show the impact of these GE pigs and target specific immune suppression focusing on recipient B cell depletion and costimulation blockade.
Material and Methods
Animal models and genetic modifications
Specific pathogen free (SPF) baboons weighing 7–15Kg from University of Oklahoma (Norman, OK) were housed in a clean pathogen free facility. These SPF baboons are known to have lower levels of both anti non Gal IgG and IgM (6) (7). Four to eight week old genetically modified pigs that were alpha galactosidase transferase knockout and hCD46 transgenic (GTKO.hCD46), with or without TBM expression (Revivicor Inc., Blacksburg, VA), were used as heart donors. The weights of donor pigs were matched with the baboon recipient to assure adequate accommodation of the heterotopic heart.. All animals were used in compliance with guidelines provided by the National Heart, Lung and Blood Institute (NHLBI) Animal Care and Use Committee (ACUC). All transplant procedures were performed at a NHLBI core surgical facility.
Surgical procedure
Donor pig hearts were transplanted into recipient SPF baboons in a heterotopic position, as described previously (8). Briefly, the recipient baboon’s infrarenal aorta and inferior vena cava were exposed through a midline abdominal incision. Sidebiting clamps were applied; an aortotomy and venotomy made, and the end-to-side anastomosis were performed between donor and recipient aorta and donor pulmonary artery with the recipient inferior vena cava.
Immunosuppressive regimen
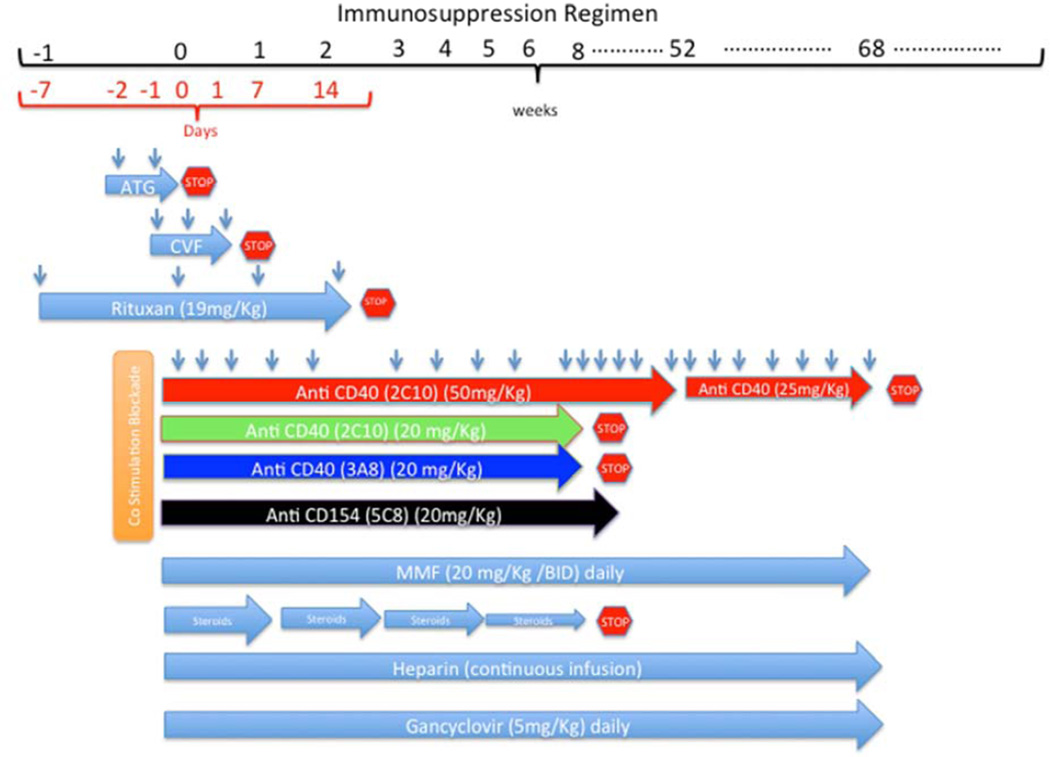
A detailed description of the immunosuppressive regimen is shown in Figure 1. In brief, it includes induction with ATG, 20 antibody (Rituxan), and costimulation blockade with either anti CD154 (5C8) or anti CD40 (3A8 or 2C10R4) monoclonal antibody (mAb). Cobra venom factor (CVF) was used to inhibit the complement activation. Mycophenolate Mofetil (MMF) and costimulation blockade antibody was also used daily and weekly as described in Figure 1 to prevent immune rejection. All recipient baboons received continuous heparin infusion to maintain the activated clotting time (ACT) level twice the baseline. Ganciclovir was administered intravenously (I/V)daily to prevent any potential viral infections. Erythropoietin (200U/Kg) I/V daily from day-7 to 7 and Cephazolin (250mg) I/V twice a day for 7 days were given.
Figure 1.
Timeline of various immunosuppressive drugs: The red stop sign indicates that the drug was stopped at this point. The duration of antibody treatment in 4 different groups is shown. Antibody in groups A and D were given until the graft was rejected or explanted.
Experimental Groups
Experimental groups are described in the table and duration of immunosuppression is illustrated in Figure 1. The main difference between the 4 groups was the type, strength, and duration of antibody used for costimulation blockade. In Group A anti CD154 (5C8) (20mg/Kg) I/V was used for the entire period of graft survival. In Group B anti CD40 (3A8) (20mg/Kg) I/V was used for a maximum of 60 days. In Group C antiCD40 (2C10) (20mg/Kg) was tapered off in 60 days. In Group D anti CD40 (2C10) (50mg/Kg) was continued for 1 year (n=2) or reduced (25mg/Kg) after 100 days (n=2).
Table.
Description of the 4 experimental groups: The difference in 4 experimental groups is described including the antibody for costimulation blockade and B cell depletion in each group.
| Group | Pig Genetics | n | Costimulation Blockade | B Cell Depletion |
|---|---|---|---|---|
| A | GTKO.hCD46Tg | 8 | Anti-CD154 (5C8) | Anti-CD20 |
| B | GTKO.hCD46Tg | 3 | Anti-CD40 (3A8) | Anti-CD20 |
| C | GTKO.hCD46Tg | 6 | Anti-CD40 (2C10) Low dose | Anti-CD20 |
| D | GTKO.hCD46Tg.hTBM | 5 | Anti-CD40 (2C10) High dose | Anti-CD20 |
Rescue therapy
If rejection was suspected by diminution of xenograft function, rescue therapy was initiated with intravenous methyl prednisolone (10–15mg/Kg) for 6 days. Heparin was also used to prevent thrombus formation and activated clotted time (ACT) was maintained twice the baseline.
Measurement of graft survival
Telemetry, manual palpation and non-invasive ultrasonography, were used to monitor the xenograft function. A telemetry device was implanted at the time of transplantation to monitor the baboon recipient’s temperature; graft left ventricular pressure (LVP), and electrocardiogram (EKG). The telemetry device data was transmitted wirelessly to a receiver attached to the animal’s cage (RMISS, Wilmington, DE). The parameters were recorded and included: peak systolic pressure (PEAK), end diastolic pressure (END), left ventricular pressure (PEAK-END) (LVP), heart rate based on LVP (LVPHR), EKG, heart rate based on EKG (EKGHR), and recipient’s body temperature (TEMP). Heart function was continuously evaluated by telemetry, and where a drop in LVP below 60 mmHg was correlated with the initiation of the rejection process, which affected the graft contractility. An LVP below 10mmHg was an indicator of complete cessation of graft contractility.
Recipients were sedated weekly for the first two months post-transplant, and biweekly thereafter, for blood collection. Palpation and ultrasound was also done on this schedule. Based on xenograft palpation, contractility of the heart was scored as ++++ (fully functional) to 0 (non functional).
Blood flow and wall motion was analyzed by echocardiography.
Hematology
White blood cell (WBC) counts, hematocrit (HCT), red blood cell count (RBC), hemoglobin (Hb), platelets, neutrophils and monocytes were analyzed. Blood chemistry was also performed weekly for the first two months and then biweekly until the graft was explanted or rejected. Activated clotting time (ACT), Prothrombin time (PT) and Troponin levels were also measured at the same intervals.
Histology
Paraffin sections from biopsies and explanted xenografts were stained with hematoxylin and eosin for light microscopy. Sections were analyzed for cardiomyocyte viability and also for the presence of hemorrhage, microvasculature thrombosis and cellular infiltrates.
Results
Graft Survival
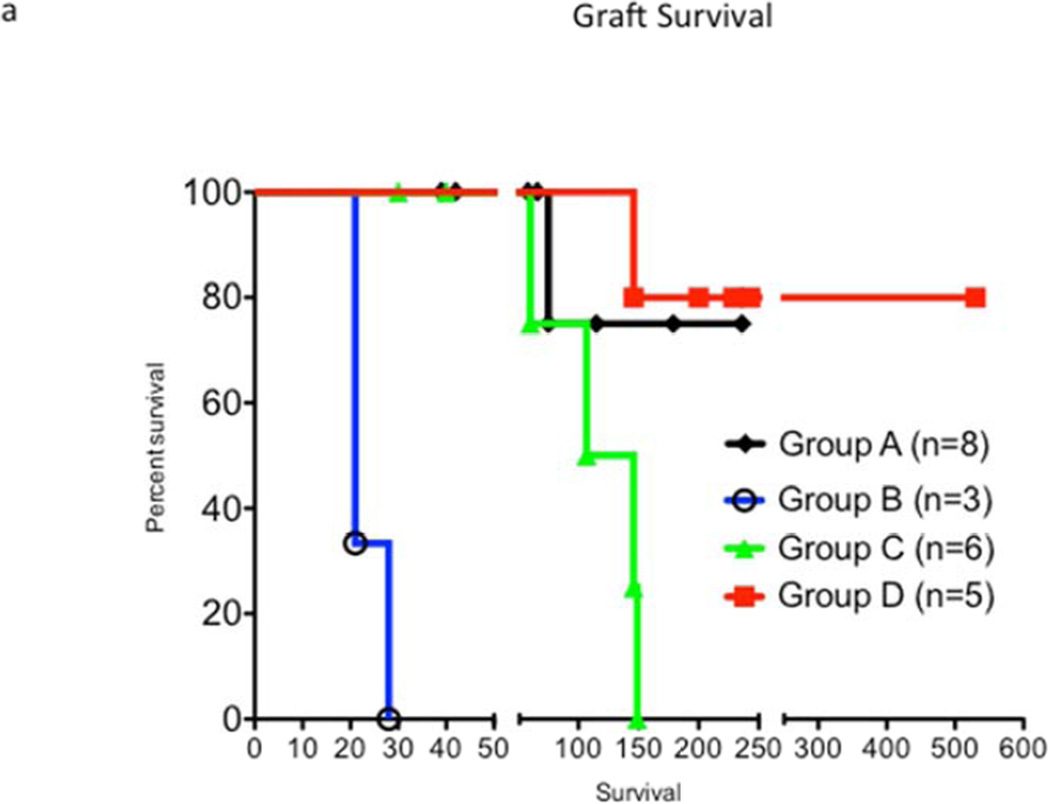
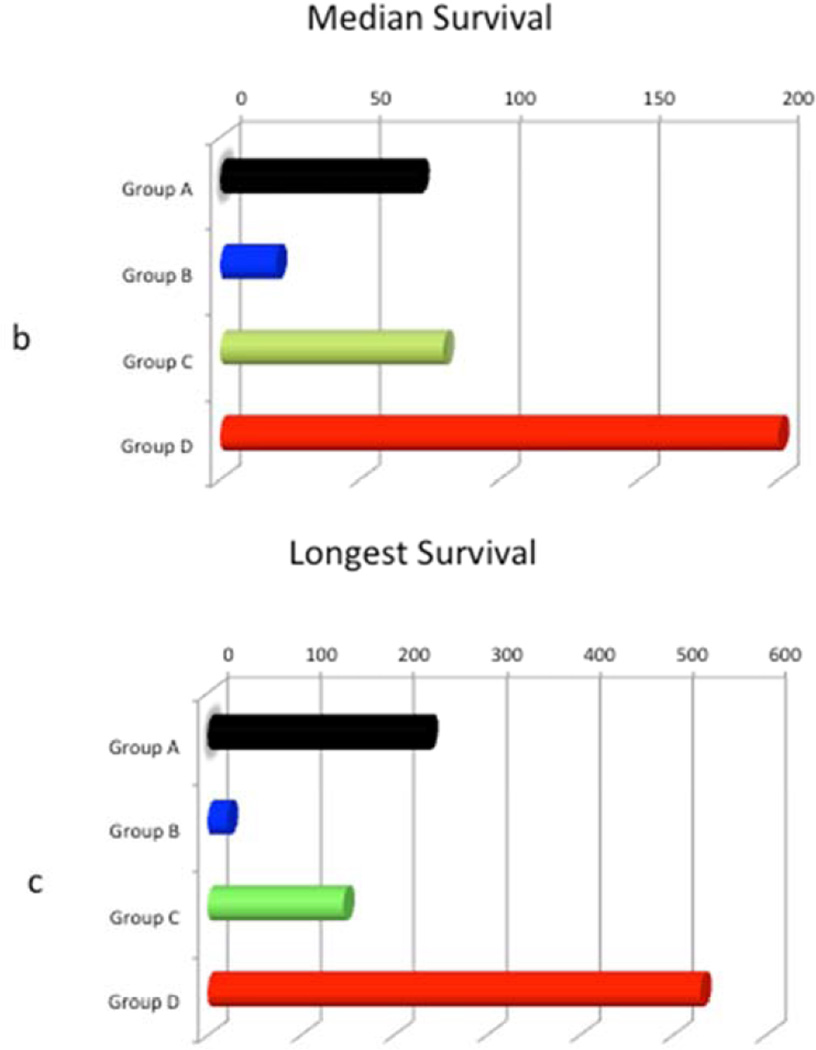
Graft survival curves for all groups are shown in Figure 2a. Median graft survivals of four groups are shown in Figure 2b, and longest survival in each group is shown in Figure 2c. Among all groups, group D, receiving the high dose of anti CD40mAb, had the longest median and individual survival. In group A, most of the grafts were still contracting at the time of recipient death or euthanasia due to various complications. All grafts in group B and C were rejected. Two out of 5 grafts in group D stopped contracting on post op days 146 and 159, but the other 3 grafts are still contracting at >200–500 days at the time of this submission.
Figure 2.
Graft survival: Figure 1a, survival days for grafts in all groups are shown. Fig 1b, Median survival time of xenografts in each group is shown. Fig 1c, longest survival time in each group is shown.
Measurement of graft contractility
Echocardiogram was only performed on group C & D (ECHO machine note available for earlier experiments) receiving 2C10R4mAb and receiving GTKO.hCD46 (group C) or GTKO.hCD46.hTBM (group D) pig heart xenografts. In all the animals excellent graft contractility was observed. The original wall thickness was maintained until the end, but in animals that stopped functioning in both groups, LV and LA walls were considerable thickened. Interestingly, there was no thrombus observed in group D receiving grafts with hTBM expression and high dose anti CD40 mAb (2C10R4) treatment.
Telemetry was used to measure LVP and EKG. The heart rate was calculated by the software based on the number of LVP peaks and QRS complexes per minute. Due to limited battery life, telemetry was useful for 200–400 days post transplantation. Three grafts in Group D were still functioning until the day of this submission and telemetry was useful in determining grafts function for 230, 250 and 400 days respectively. All the grafts in-group C rejected within 146 days and telemetry documented a loss of LV pressure and heart contractility.
Manual graft palpation scores were consistent with the findings of ultrasound and telemetry.
Depletion of T & B cells and its effect on non Gal antibody production
Initial treatment with ATG significantly reduced the number of circulating T cells but they were not totally eliminated. However, treatment with 4 weekly doses of anti CD20 effectively depleted all the circulating B cells and they stayed depleted for at least 60 days after which their number was slowly restored to pretreatment levels (6, 9). Although anti CD20 has no direct affect on plasma cells, the non Gal antibody production was maintained at a very low level by this treatment.
CBC, Chemistry and coagulation profile
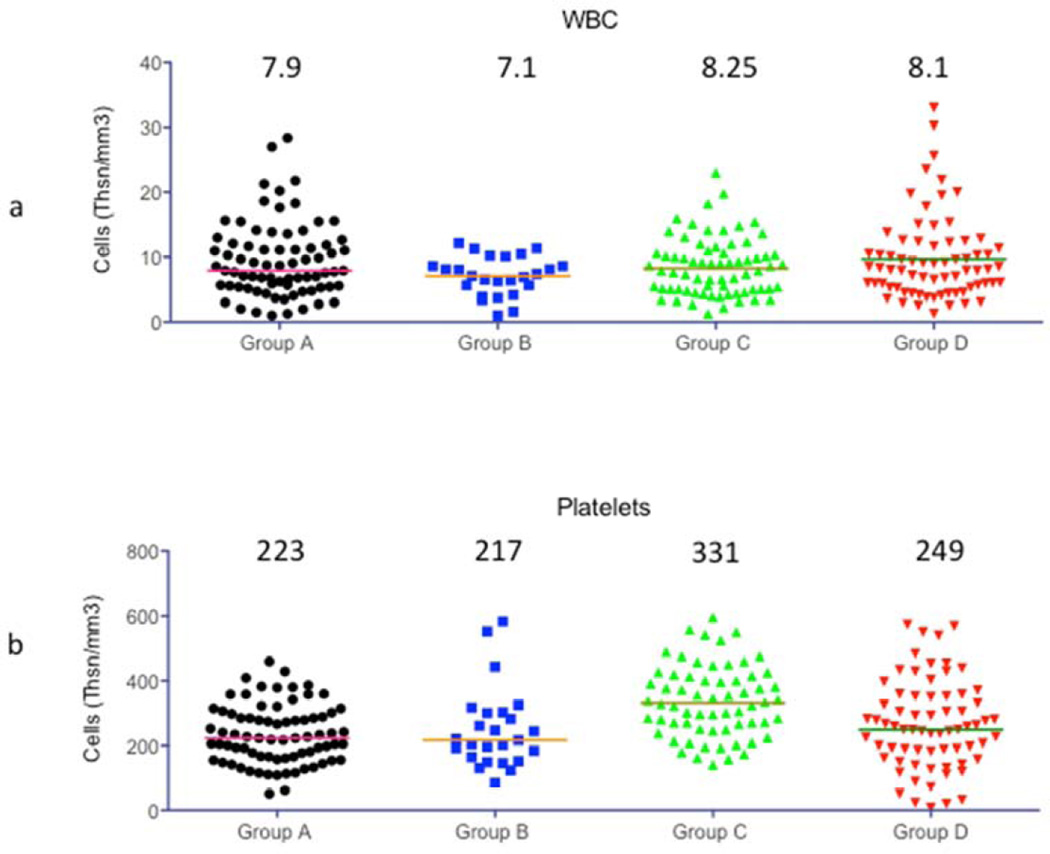
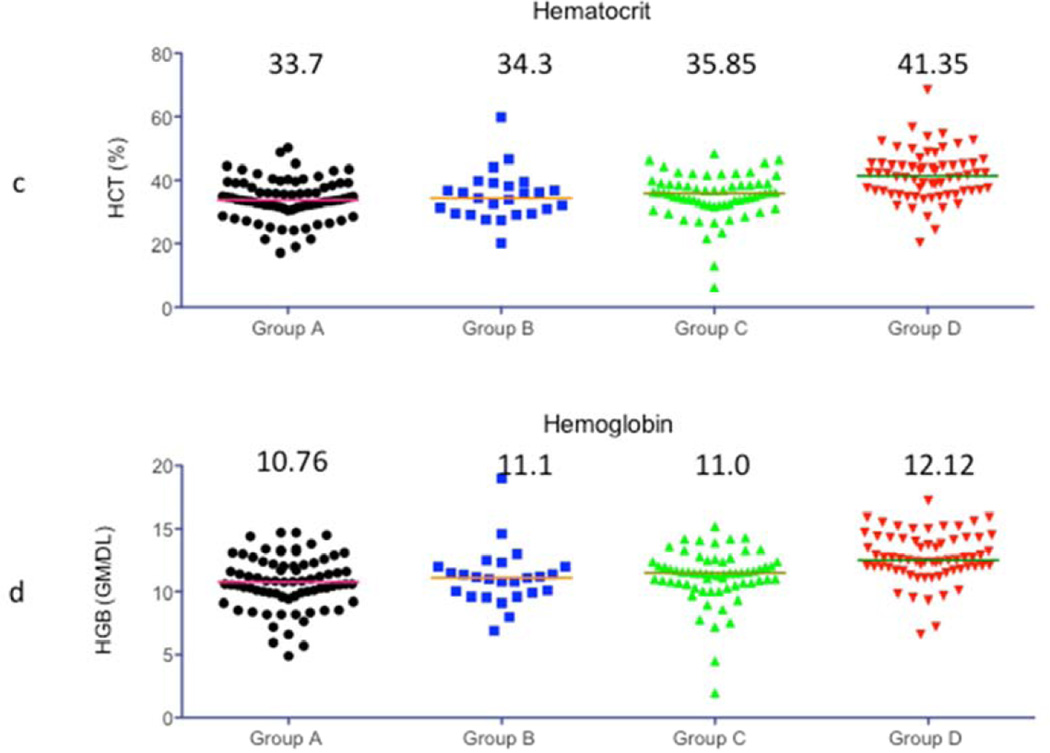
Figure 3 shows the comparison of levels of WBC count (a), Platelet count (b), hematocrit (c) and Hemoglobin (d). The treatment regimen was effective in keeping WBC counts low in all 4 groups. The median platelet numbers were maintained better with 2C10 treatment but only group C platelet counts were significantly better than the other 3 groups. The hemoglobin level and hematocrit percentage was kept close to normal in all groups and there was no significant difference between the groups. Anemia and thrombocytopenia was treated with transfusion of packed red cells (PRC) as needed.
Figure 3.
Complete blood counts: WBC and Platelet counts, hematocrit percentage and Hemoglobin levels were compared between all 4 experimental groups. Horizontal bars in each Figure indicate the median value that is also shown on the graph.
Graft Histology
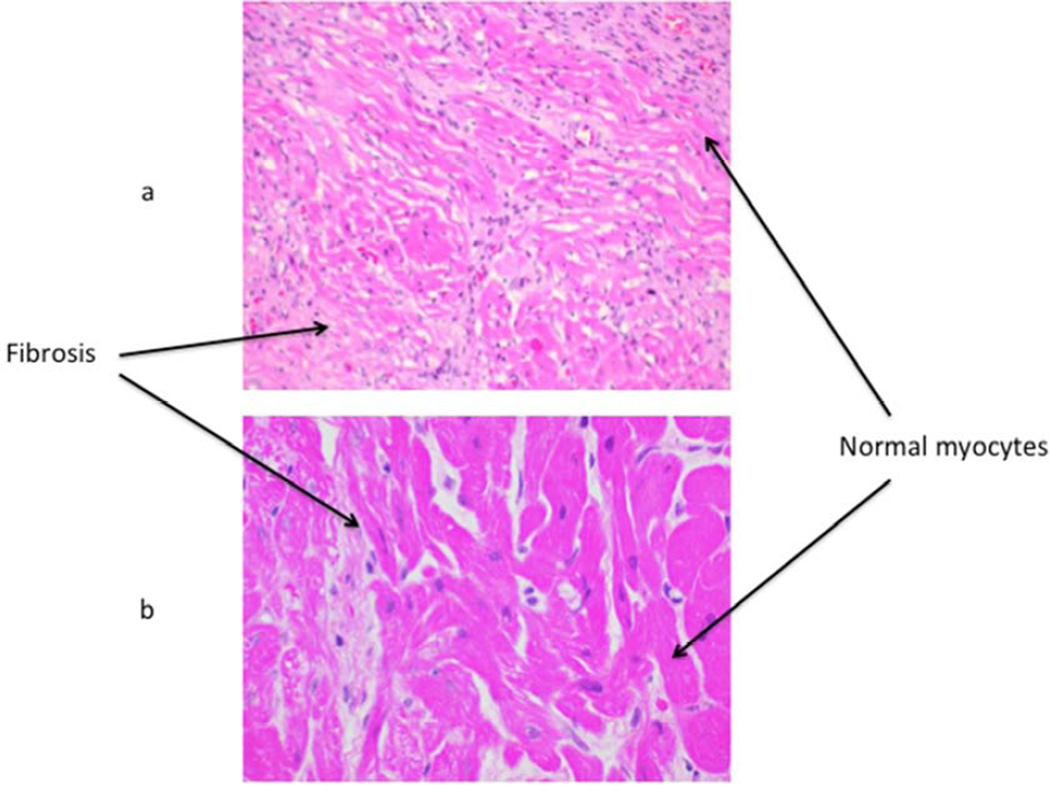
Graft histology in group A showed small areas of normal myocardium along with significant areas of hemorrhage, coagulative necrosis and fibrosis. . Some thrombotic microangiopathy (TM) was also observed in this group. No TM was seen in groups B, C or D. Rejected grafts in group B, C and D showed some myofibril loss with fibrosis, but there was only minimal hemorrhage present. Figure 4 shows one representative H & E staining of (a) left ventricle from the group A graft, showing widespread areas of fibrosis, along with some normal myocytes and (b) a biopsy specimen from a Group D heart, taken at day 182 post op, showing primarily normal myocytes along with a small area of fibrosis.
Figure 4.
Graft histology: H&E staining of a biopsy sample from one representative long term surviving graft each from group A (a) & D (b) is shown. Most of the group A graft shows fibrosis with some areas of normal myocytes, whereas mostly normal myocytes along with a small area of fibrosis is evident in group D.
Complications
Most animals in group A had to be either euthanized or died due to various complications e.g. abdominal bleeding, aspiration pneumonia, and consumptive coagulopathy. Infections were rare in all the groups and if noticed were managed by antibiotic treatment after culture and sensitivity testing was performed. Very few complications were seen in group B, C and D. In these groups most of the heart xenografts were explanted after rejection and recipient baboons were survived.
Discussion
The availability of GE pigs has transformed the field of xenotransplantation and has rejuvenated the hopes for clinical xenotransplantation. However, there are parts of this puzzle that still need to be solved (10, 11). Some of the major obstacles, after overcoming the Gal antibody barrier, include the presence of preformed and elicited non Gal antibodies against multiple antigens (12), consumptive coagulopathy (CC) caused by rapid depletion of platelets (13), and TM due to an induced hypercoagulable state (14).
Our laboratory, in collaboration with other groups, has made several inroads in this field. Conventional immunosuppression has been previously tried in this model and these agents reasonably prolonged the graft survival (15, 16). We have tried to improve upon the conventional immunosuppression with the use of target specific immunosuppressive agents, which, along with the use of newly available GE pigs, have significantly improved the graft survival of pig cardiac xenografts in baboons.
The very first step toward improvement in graft survival was observed with B cell depletion via anti CD20 mAb. The possible mechanisms of depletion include direct lysis via NK cells / macrophages, antibody mediated cell cytotoxicity, and complement dependent cytotoxicity (17). Only 4 doses of anti CD20 mAb on days - 7,0, 7 and 14 were able deplete the B cell population for two months. The research group at the Mayo Clinic, in Rochester, MN, has shown that one dose can deplete circulating B cells effectively but 4 doses are required to deplete B cells from the lymphoid organs (personal communication with Gerry Byrne). Although this anti B cell treatment was not effective for plasma cell depletion, as plasma cells do not express CD20, the levels of both anti non Gal IgM and IgG remained at a very low levels due to this treatment, and signs of antibody mediated rejection were not seen in any animals receiving anti CD20 mAb treatment. It should be noted; we have reported that the cardiac xenografts do show some minor deposition of anti non Gal antibodies (6). There was a moderate increase in non-Gal antibody levels after 60 days of survival but no obvious signs of rejection were observed. Perhaps the xenograft is “accommodated” by this time or a state of “immune tolerance” is induced. Currently studies are being performed to evaluate these mechanisms. There may be a benefit of continuous suppression of B cells by anti CD20 antibody beyond 60 days. The anti CD40 antibody used in these experiments also suppress the B cell response and may be sufficient to keep the B cell response under control after 60 days.
The costimulation blockade pathway also was critical to graft survival, as suppression of this pathway modulates the response of both B and T cells. In the initial experiments, we targeted the CD40-CD40Ligand pathway by using anti CD154 mAb, which yielded the previous longest cardiac xenograft survival of 236 days. Unfortunately, use of this antibody in some cases, due to expression of CD154 on platelets, and binding of anti CD154 to the platelets resulting in their activation and aggregation, was accompanied by fatal coagulopathies leading to euthanasia or death of recipient baboons before graft rejection (6, 18). This is a known complication of this antibody, and as a result anti CD154 mAb is not recommended for clinical use (19, 20). However, the experiments with this antibody demonstrated the beneficial effect of costimulation blockade in suppressing the xenogeneic immune response and extending xenograft survival. Fortunately, the use of anti CD40 mAb allowed blockade of the same CD40-CD40 ligand costimulation pathway without encountering the complications of thrombosis. Two available anti CD40 mAbs were used for this purpose, one generated in mouse (clone 3A8), and another recombinant mouse-rhesus chimeric antibody (clone 2C10R4). Neither clones had the thrombogenic characteristics seen with the anti CD154 (5C8) clone. The antibody from the 3A8 clone was not able to extend graft survival beyond 28 days (9). However, the graft survival was significantly prolonged with the 2C10R4 clone, irrespective of the pig genetics used. A higher dose of this antibody was required in these experiments to produce consistent long term graft survival, perhaps due to lower affinity of this antibody for baboon cells. We also observed a quicker recovery time from surgery in groups utilizing anti CD40 mAb. No complications were observed in group C or group D. A humanized form of antiCD40 antibody is under production and will be available for clinical use very soon (personal communication with Keith Reimann)
Additional donor pig genetic modifications employed, besides alpha Gal knockout, were transgenic expression of human CD46 and TBM. Our results indicate that TBM expression along with anti CD40 treatment was crucial in long term graft survival. TBM expression successfully avoided the TM and other coagulation issues. It is difficult to dissect the preferential role of either of the two modifications used here, and further experiments are required for this clarification.
Infections were rarely seen in our animals despite the long-term infusion of maintenance drugs via jugular catheter. More complications were related to technical problems with the tether system, than the recipient baboon themselves. No perioperative graft dysfunction was observed. Some pig viruses like Cytomegalovirus virus (CMV) has shown to affect graft survival in pig allograft models but we have not seen any active CMV disease in any of our baboons except one baboon where CMV inclusion bodies were found in the testes on necropsy. The source of these inclusion bodies was not determined and no evidence of active CMV disease was found in this baboon. Some other complications experienced over the length of the project included abdominal bleeding. GI bleeding, aspiration pneumonia, seizures, thrombocytopenia and anemia (21).
Finally additional technical improvements contributed favorably to aiding graft survival. Video and telemetric monitoring allowed early identification and management of complications expeditiously (22). For example, infections were detected early by monitoring recipient temperature via telemetry. Video monitoring allowed us continuous surveillance of the baboons and triggered prompt response to emergencies like, infusion pump malfunction, tether / jacket problems and seizures. These methods retrospectively enabled us to evaluate the onset of any complication.
In summary, significant improvement in graft survival from179 days (23), to 8 months (8), and as reported here, to over one year, by using newly available GE pigs, and an immunosuppression regimen based on a NHP-mouse chimeric anti CD40mAb (2C10R4) along with the histology showing preservation of heart architecture in long-term grafts is very encouraging and indicates that these xenograft hearts can be tested in the orthotopic position for their ability to sustain life (word count 2552).
Acknowledgements
Authors are thankful to the following people and programs for their support: Billeta Lewis,BS for animal care, Tannia Clak, DVM for help in performing ECHO, Michael Eckhaus, DVM for pathology, Caleb Seavey for developing analyzing software, Suyapa Ball, and Carol Phelps,PhD (Revivicor, INC.) for production of GE pigs, Eckhard Wolf and Nikolai Klymiuk (Ludwig-Maximillians University, Munich) for collaboration on GE pig cells, Keith Reimann, PhD for providing the anti CD40 mAb, Animal Surgery Resource, NHLBI for their help in surgical procedure, Flow Cytometry Core, NHLBI, Division of Veterinary Resource, NIH for animal care and Patricia Jackson for her administrative help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These results were presented at the AATS 94th Annual Meeting in Toronto, Canada
Authorship and Scientific responsibility
MMM: Designed the study, performed experiments, collected and interpreted data, wrote and submitted manuscript
AKS: Performed experiments, collected and analyzed data
PCC: Performed experiments and collected data
RFH: Helped in surgical procedures and animal management
MLT: Managed animal care, collected data
DA: Provided materials for the experiment and analyzed data
KAH: Helped design study, assisted in experiments, analyzed data and revised manuscript.
Disclosure:
David Ayares is the Executive Vice President and Chief Scientific Officer of Revivicor, Inc. All other authors have nothing to disclose.
References
- 1.Allen SJ, Sidebotham D. Postoperative care and complications after ventricular assist device implantation. Best Pract Res Clin Anaesthesiol. 2012 Jun;26(2):231–246. doi: 10.1016/j.bpa.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003 Jan 17;299(5605):411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekser B, Cooper DK. Overcoming the barriers to xenotransplantation: prospects for the future. Expert Rev Clin Immunol. 2010 Mar;6(2):219–230. doi: 10.1586/eci.09.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satyananda V, Hara H, Ezzelarab MB, Phelps C, Ayares D, Cooper DK. New concepts of immune modulation in xenotransplantation. Transplantation. 2013 Dec 15;96(11):937–945. doi: 10.1097/TP.0b013e31829bbcb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, 3rd, Lewis BG, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014 Feb;14(2):488–489. doi: 10.1111/ajt.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohiuddin MM, Corcoran PC, Singh AK, Azimzadeh A, Hoyt RF, Jr, Thomas ML, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Mar;12(3):763–771. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H, Iwase H, Wolf RF, Ekser B, Ezzelarab M, Hara H, et al. Are there advantages in the use of specific pathogen-free baboons in pig organ xenotransplantation models? Xenotransplantation. 2014 Feb 18; doi: 10.1111/xen.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu G, Pfeiffer S, Schroder C, Zhang T, Nguyen BN, Lea W, et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation. 2005;12(3):197–208. doi: 10.1111/j.1399-3089.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 9.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, 3rd, Lewis BG, et al. Role of anti-CD40 antibody-mediated costimulation blockade on non-Gal antibody production and heterotopic cardiac xenograft survival in a GTKO.hCD46Tg pig-to-baboon model. Xenotransplantation. 2013 Oct 29; doi: 10.1111/xen.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vadori M, Cozzi E. Immunological Challenges and Therapies in Xenotransplantation. Cold Spring Harb Perspect Med. 2014 Mar 10; doi: 10.1101/cshperspect.a015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol Rev. 2014 Mar;258(1):241–258. doi: 10.1111/imr.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tazelaar HD, Byrne GW, McGregor CG. Comparison of Gal and non-Gal-mediated cardiac xenograft rejection. Transplantation. 2011 May 15;91(9):968–975. doi: 10.1097/TP.0b013e318212c7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CC, Ezzelarab M, Shapiro R, Ekser B, Long C, Hara H, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010 Jul;10(7):1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Connell PJ. Thrombotic microangiopathy: the next big hurdle for xenotransplantation. J Am Soc Nephrol. 2005;16(9):2529–2530. doi: 10.1681/ASN.2005070735. [DOI] [PubMed] [Google Scholar]
- 15.McGregor CG, Davies WR, Oi K, Teotia SS, Schirmer JM, Risdahl JM, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005;130(3):844–851. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Ekser B, Kumar G, Veroux M, Cooper DK. Therapeutic issues in the treatment of vascularized xenotransplants using gal-knockout donors in nonhuman primates. Curr Opin Organ Transplant. 2011 Apr;16(2):222–230. doi: 10.1097/MOT.0b013e3283446c3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bluml S, McKeever K, Ettinger R, Smolen J, Herbst R. B-cell targeted therapeutics in clinical development. Arthritis Res Ther. 2013;15(Suppl 1):S4. doi: 10.1186/ar3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Pillars article: long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. 1996. J Immunol 2011 Mar 1;186(5):2693-7. [DOI] [PubMed] [Google Scholar]
- 19.Buhler L, Alwayn IP, Appel JZ, III, Robson SC, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism. Transplantation. 2001;71(3):491. doi: 10.1097/00007890-200102150-00028. [DOI] [PubMed] [Google Scholar]
- 20.Koyama I, Kawai T, Andrews D, Boskovic S, Nadazdin O, Wee SL, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77(3):460–462. doi: 10.1097/01.TP.0000110291.29370.C0. [DOI] [PubMed] [Google Scholar]
- 21.Corcoran PC, Horvath KA, Singh AK, Hoyt RF, Jr, Thomas ML, III, Eckhaus MA, et al. Surgical and nonsurgical complications of a pig to baboon heterotopic heart transplantation model. Transplant Proc. 2010;42(6):2149–2151. doi: 10.1016/j.transproceed.2010.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horvath KA, Corcoran PC, Singh AK, Hoyt RF, Carrier C, Thomas ML, III, et al. Left ventricular pressure measurement by telemetry is an effective means to evaluate transplanted heart function in experimental heterotopic cardiac xenotransplantation. TransplantProc. 2010;42(6):2152–2155. doi: 10.1016/j.transproceed.2010.05.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. NatMed. 2005;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]