Abstract
Rationale
Reproductive mood disorders, including premenstrual dysphoria (PMD) and postpartum depression (PPD), are characterized by affective dysregulation that occurs during specific reproductive states. The occurrence of illness onset during changes in reproductive endocrine function has generated interest in the role of gonadal steroids in the pathophysiology of reproductive mood disorders, yet the mechanisms by which the changing hormone milieu triggers depression in susceptible women remain poorly understood.
Objectives
This review focuses on one of the neurosteroid metabolites of progesterone – allopregnanolone (ALLO) – that acutely regulates neuronal function and may mediate affective dysregulation that occurs concomitant with changes in reproductive endocrine function. We describe the role of the ‘neuroactive’ steroids estradiol and progesterone in reproductive endocrine-related mood disorders to highlight the potential mechanisms by which ALLO might contribute to their pathophysiology. Finally, using existing data, we test the hypothesis that changes in ALLO levels may trigger affective dysregulation in susceptible women.
Results
Although there is no reliable evidence that basal ALLO levels distinguish those with PMD or PPD from those without, existing animal models suggest potential mechanisms by which specific reproductive states may unmask susceptibility to affective dysregulation. Consistent with these models, initially euthymic women with PMD and those with a history of PPD show a negative association between depressive symptoms and circulating ALLO levels following progesterone administration.
Conclusions
Existing animal models and our own preliminary data suggest that ALLO may play an important role in the pathophysiology of reproductive mood disorders by triggering affective dysregulation in susceptible women.
Keywords: reproductive mood disorders, premenstrual dysphoria, postpartum depression, neurosteroids, gonadal steroids, estradiol, progesterone, allopregnanolone, animal models
Introduction
Reproductive mood disorders are characterized by affective dysregulation and functional impairment that occur during specific reproductive states. Dysregulated affect in reproductive mood disorders includes increased negative affect (i.e., irritability, anger, sadness, and anxiety), decreased positive affect (i.e., anhedonia), and affective lability (Pearlstein et al. 2005; Tuohy and McVey 2008), while functional impairment is defined by clinically significant distress or disability in social, occupational, or other important activities (American Psychiatric Association and DSM-5 Task Force 2013). One such disorder, premenstrual dysphoric disorder (PMDD), affects 2-5% of women and is characterized by a recurrent, predictable pattern of distressing emotional and somatic symptoms that begin during the mid- to late-luteal phase of the menstrual cycle, when estradiol and progesterone levels are relatively high, and remit after the onset of menses, when estradiol and progesterone levels are relatively low and stable (Epperson et al. 2012). Prior to DSM-5 recognition of PMDD, many researchers studied “premenstrual dysphoria” (PMD). In our research, diagnosis of PMD required prospective daily assessment of mood symptoms over the course of three consecutive menstrual cycles. PMD was defined by a 30% increase in mean negative mood during the week before menses compared with the week after menses, a more stringent criterion than that of DSM-5. For the purpose of this review, we will use the term PMD to refer to both PMDD and PMD. A second disorder, postpartum depression (PPD), affects between 8% and 19% of women following delivery, frequently begins during pregnancy, when estradiol and progesterone levels increase dramatically, and is exacerbated during the postpartum period, when hormone levels rapidly decline (Gavin et al. 2005). The occurrence of illness onset during these specific reproductive states understandably has generated interest in the role of gonadal steroids in the pathophysiology of reproductive mood disorders. In this paper, we will focus on one of the neurosteroid metabolites of progesterone – allopregnanolone (ALLO) – that acutely regulates neuronal function and that theoretically could mediate affective dysregulation that occurs concomitant with changes in reproductive endocrine function during the menstrual cycle and pregnancy. We will discuss gonadal steroid regulation of mood as a model useful for understanding the role of neurosteroids, and ALLO in particular, in reproductive mood disorders. We will also describe and integrate the results of neuroimaging studies that provide evidence of the effects of neurosteroid regulation on those brain circuits implicated in mood disorders. Finally, we will present new data demonstrating the role of ALLO in triggering affective dysregulation in women with PMD and PPD. This review does not include the third reproductive mood disorder, perimenopausal depression, because less research has been conducted on the role of ALLO in this disorder and our new data address only PMD and PPD.
What Are Neurosteroids?
Neurosteroids are metabolites of cholesterol-derived steroid hormones that are synthesized in the brain and nervous system and modulate the major inhibitory and excitatory central nervous system (CNS) neurotransmitter systems: γ-aminobutyric acid (GABA) and glutamate, respectively. Neurosteroids are among the most potent and effective modulators of GABAA receptors. Although neurosteroids have distinct, characteristic effects, which may be either excitatory or inhibitory (for a full review see Carver and Reddy 2013), they are widely recognized for their role in augmenting GABAergic inhibition (Belelli and Lambert 2005). The powerful anxiolysis observed to accompany this potentiation of GABAA receptors has led to the speculation that neurosteroid dysregulation plays a central role in the etiology of affective disorders, including the reproductive mood disorders PMD and PPD, which will be the focus of this review.
Neurosteroids act quickly and locally to affect brain function and behavior, which is distinct from the classical view of steroid actions as more diffuse and sub-acute. Neurosteroids are distinguished from “neuroactive steroids,” which are also capable of modifying neural activities, independent of their origin. Increasingly, the distinction between neurosteroids and neuroactive steroids is blurring, as several neuroactive steroids, like estradiol and progesterone, are synthesized in brain and are capable of acute neuromodulatory effects through, among other means, binding membrane steroid receptors and ion channels. For purposes of this review, we will describe the role of the gonadal ‘neuroactive’ steroids estradiol and progesterone in reproductive endocrine-related mood disorders to highlight the potential mechanisms by which one neurosteroid — ALLO — might contribute to the pathophysiology of these disorders. For a summary of the current literature on GABAA receptor plasticity as a function of neurosteroid change, the interested reader is directed to a review by MacKenzie and Maguire (in press).
Gonadal Steroid Regulation of Affect
Gonadal steroids not only regulate the menstrual cycle, pregnancy, and maternal behavior, but as well they play a major role in basic emotion processing, arousal, cognition, and motivation. Estradiol, for example, regulates virtually every neurotransmitter system implicated in depression (McEwen 2002, Rubinow et al, l998) and influences many of the same neuroregulatory processes as classic antidepressants and electroconvulsive therapy. In the forebrain and hippocampus, ovariectomy decreases and estradiol increases brain-derived neurotrophic factor (BDNF) levels (Sohrabji et al. 1994b), which are decreased by depression and stress and increased by antidepressants (Shimizu et al. 2003). Estradiol also increases cAMP response element-binding (CREB) protein activity (Zhou et al. 1996) and the neurotrophin receptor protein trkA (Sohrabji et al. 1994a), and it decreases GSK-3 beta activity (Cardona-Gomez et al. 2004) in the rat brain similar to antidepressant medications. Progesterone also regulates neurotransmitter synthesis, release, and transport (Finocchi and Ferrari 2011). For example, progesterone up-regulates BDNF expression in the hippocampus and cerebral cortex (Pluchino et al. 2013). Rodent models have strongly implicated changes in estradiol and progesterone in the pathogenesis of anxiety and depression-like behavior (for comprehensive reviews of extant animal models and biochemical mechanisms, see Walf and Frye 2006; Frye 2009; Foy et al. 2010). The relevance of gonadal steroids to affective regulation is further suggested by modulatory effects on stress and the HPA axis, neuroplasticity, cellular energetics, immune activation, and cortical activity (Rubinow and Girdler 2011), all processes that have been implicated as dysfunctional in depression. Of particular note are the manifold effects of gonadal steroids on brain function as revealed by brain imaging studies. These studies, employing positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) in asymptomatic women, have demonstrated that physiologic levels of gonadal steroids modulate the neurocircuitry involved in normal and pathological affective states. In a study of healthy women, regional cerebral blood flow (rCBF) was attenuated in the dorsolateral prefrontal cortex, inferior parietal lobule, and posterior inferior temporal cortex during GnRH agonist-induced hypogonadism, whereas the characteristic pattern of cortical activation reemerged during both estradiol and progesterone addback (Berman et al. 1997). Studies of neural activity during the menstrual cycle have compared activation across menstrual phases within subjects. Goldstein et al. (2005) found increased amygdala activity during the late follicular phase (higher estradiol levels) compared to the early follicular phase (lower estradiol levels), and Protopopescu et al. (2005) demonstrated increased activity in the medial orbitofrontal cortex (a region that exerts inhibitory control over amygdalar function) during the luteal phase (higher estradiol levels) compared with the follicular phase (relatively lower estradiol levels). The opposite was true for the lateral orbitofrontal cortex, suggesting that sensory and evaluative neural functions are suppressed in the days prior to menstruation (Protopopescu et al. 2005). Ovarian hormones also modulate neural reward function in humans, with increased follicular phase activation (compared with the luteal phase) of the superior orbitofrontal cortex and amygdala during reward anticipation and of the midbrain, striatum, and left ventrolateral prefrontal cortex during reward delivery (Dreher et al. 2007).
Brain Activation in PMD and PPD
Brain imaging is one of the most powerful tools for exploring the neurobiology of affective dysregulation, and this strategy (e.g., electroencephalography (EEG), functional magnetic resonance imaging (fMRI), or positron emission tomography (PET)) has been employed to detect abnormalities that distinguish women with PMD from healthy, regularly menstruating control women. Using EEG, Baehr et al. (2004) demonstrated that women with PMD had greater cerebral asymmetry during the luteal phase compared with the follicular phase, a pattern that was absent in control women. This result is consistent with prior EEG research linking left frontal hypoactivity, behavioral approach deficits, and major depressive disorder (Davidson 1998; Tomarken et al. 2004) and highlights the temporal relevance of mood symptoms in PMD, which are confined to the luteal phase and distinguish PMD from major depressive disorder. In two independent fMRI studies, women with PMD showed increased amygdala responses to negative stimuli when compared with healthy control women (Protopopescu et al. 2008; Gingnell et al. 2012). Other studies have demonstrated that PMD is characterized by decreased responsiveness to behavioral inhibition in the medial orbitofrontal cortex during the premenstrual phase (Protopopescu et al. 2008), increased right cerebellar blood flow during the luteal phase compared with the follicular phase (Rapkin et al. 2011), and decreased activity of the left insula during the follicular phase (both compared with controls and with the luteal phase) (Bannbers et al. 2012). To directly assess whether gonadal steroids influence brain function in PMD, Baller et al. (2013) examined brain responsivity to a working memory task using fMRI and PET under conditions of experimentally induced hypogonadism, estradiol addback, and progesterone addback in women with and without PMD. Across all hormone conditions, women with PMD showed greater activation of the dorsolateral prefrontal cortex, medial prefrontal gyrus, and cerebellum. Among the women with PMD, dorsolateral prefrontal cortical activation was negatively associated with global assessment of functioning, earlier age of onset, and greater pre-intervention menses-related changes in negative affect (Baller et al. 2013). Taken together, these findings suggest that PMD is characterized by a combination of stable, trait-like functional abnormalities in the dorsolateral prefrontal cortex and amygdala, areas responsible for cognitive, emotional, and social functions, as well as menstrual phase-specific abnormalities that may result from changing gonadal steroid levels.
PPD is characterized by abnormal activation of the same brain regions implicated in non-puerperal major depression: the amygdala, insula, striatum, orbitofrontal cortex, and dorsomedial prefrontal cortex (Silverman et al. 2007; Moses-Kolko et al. 2010; Moses-Kolko et al. 2011). Further, there is evidence of reduced connectivity between the amygdala and prefrontal regions in women with PPD, which implicates dysregulation of the limbic system in the neural pathophysiology of PPD (Moses-Kolko et al. 2010). Despite evidence of functional neural abnormalities in women with PPD, none of the existing studies has examined the role of estradiol and progesterone in the neural dysregulation that characterizes PPD.
Steroid Triggering and Sensitivity: Role of Gonadal Steroids in PMD and PPD
As a rule, basal hormone studies of PMD and PPD have been singularly unrevealing, with no consistently observed differences between patients and controls. Indeed, there is virtually no convincing evidence for a hormone abnormality in these disorders. Gonadal steroids can, nonetheless, be demonstrated to trigger PMD and PPD, albeit in the context of an antecedent susceptibility. In a study employing GnRH agonist-induced ovarian suppression and hormone addback, Schmidt et al. (1998) demonstrated that elimination of the menstrual cycle eliminated PMD symptoms, while hormone (estradiol and progesterone) addback precipitated symptom return. The same hormone manipulation was without effect on mood in women lacking a history of PMD. Thus, ovarian steroids triggered PMD symptoms, but only in a group of women that were otherwise susceptible to the mood destabilizing effects of estradiol and progesterone. In a similar study, Bloch et al. (2000) created a scaled-down model of the puerperium in which euthymic women with or without a history of PPD were blindly administered high dose estradiol and progesterone during ovarian suppression and then abruptly withdrawn. Again, women with a history of PPD became depressed during hormone withdrawal, but women lacking a history of PPD experienced no perturbation of mood despite identical hormonal conditions. Both of these studies suggest that normal levels or changes in gonadal steroid hormones can precipitate symptoms in a vulnerable subpopulation of women. Finally, in a recently completed study employing ovarian suppression and continuous hormone addback for three months, Schmidt et al. (unpublished data) demonstrated that it was the change in hormone and not the hormone level itself that was the critical signal in precipitating affective symptoms in women with a history of PMD. It is this finding that is particularly intriguing regarding a role for neurosteroids in reproductive mood disorders.
The Role of ALLO in Affective Regulation
There are several reasons to speculate that ALLO plays a role in affective regulation and dysregulation. First, the GABA receptor, which mediates anxiolysis as described above, is positively modulated by the 5α- and β-reduced metabolites of progesterone (ALLO and pregnanolone, respectively) (Majewska et al. 1986). Second, progesterone withdrawal in rats produces anxiety and insensitivity to benzodiazepines due to withdrawal of ALLO, with consequent induction of GABAA α4-subunit levels and inhibition of GABA currents (Smith et al. 1998a; Smith et al. 1998b). Third, decreased ALLO levels are seen in major depressive disorder, with an increase seen in both plasma and CSF following successful antidepressant treatment (Uzunova et al. 1998; Romeo et al. 1998; Ströhle et al. 1999; Schüle et al. 2005; Eser et al. 2006; Schüle et al. 2007). Fourth, ALLO displays anxiolytic effects in several animal anxiety models (Bitran et al. 1991; Wieland et al. 1991; Bitran et al. 1993). Fifth, antidepressants may promote the reductive activity of one of the synthetic enzymes (3-α-hydroxysteroid oxidoreductase), thus favoring the formation of ALLO (Uzunov et al. 1996; Griffin and Mellon 1999).
ALLO also modulates the biological processes dysregulated in major depressive disorder. In animals, ALLO increases in response to stress, reduces pain sensitivity, and is thought to restore physiologic homeostasis following stress (Frye and Duncan 1994; Morrow et al. 1995). ALLO administration reduces circulating levels of corticotropin releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and corticosterone, thereby suppressing the hypothalamic-pituitary-adrenal (HPA) axis (Patchev et al. 1994; Patchev et al. 1996; Barbaccia et al. 1997; Kehoe et al. 2000). ALLO administered during stress prevents the onset of depression-like behavior in rodents and preserves neurogenesis in the hippocampus (Evans et al. 2012). ALLO also exerts neuroprotective effects by reducing the expression of pro-apoptotic proteins and apoptotic DNA fragmentation (Djebaili et al. 2005; Sayeed et al. 2009), thereby reducing the cell death and gliosis associated with depression (Glantz et al. 2010; Shelton et al. 2011). Neuroprotection is mediated by immune regulation in depression (Licinio and Wong 1999), and ALLO reduces the expression of the pro-inflammatory cytokine TNF-α (He et al. 2004), which is elevated in individuals with major depression (Dowlati et al. 2010). Thus, ALLO may exert antidepressant effects by reducing the physiological impact of stress, promoting neuroprotection, and protecting against the pro-inflammatory immune activation and cytokine hypersecretion associated with major depressive disorder.
ALLO regulates the neural circuits implicated in depression. ALLO is present in brain regions dysregulated in depression, including the amygdala, hippocampus, ventral striatum, and prefrontal cortex, with the highest concentrations found in the substantia nigra, basal hypothalamus, and amygdala (Bixo et al. 1997). Despite the distribution of ALLO throughout the brain, the amygdala appears to play a central role in its anxiolytic effects. ALLO infusions to the central nucleus of the amygdala produced anxiolysis in two animal models of anxiety (Akwa et al. 1999), suggesting a potential role for ALLO in modulating mood and anxiety symptoms in humans.
ALLO may be of particular relevance to reproductive mood disorders not only because it is derived from progesterone but also because gonadal steroids modulate the enzymes responsible for neurosteroid production. For example, 5α-reductase gene expression is transcriptionally regulated in the female mouse brain by progesterone, which promotes ALLO production (Matsui et al. 2002), and short-term treatment with estradiol results in reduced expression of neurosteroid enzyme genes, including CYP12A1, in the hippocampus of ovariectomized non-human primates (Sorwell et al. 2012). Although the regulation of steroidogenesis has not been explored in the human CNS, estradiol acting via estrogen receptor alpha downregulates the transcription of CYP17A1 and decreases local steroidogenesis in ovarian follicles (Taniguchi et al. 2007). Estradiol may similarly downregulate CYP17A1 transcription in the human CNS, thereby modulating neurosteroidogenesis.
ALLO Serves as an Affective Switch in PMD and PPD
The majority of research on neurosteroids in reproductive mood disorders has focused on ALLO (for comprehensive reviews of the role of neurosteroids and neuroactive steroids in affective disorders see Dubrovsky 2006 and Schüle et al. 2011). Researchers have examined abnormalities in circulating ALLO in PMD with varying results. Although several investigators observed decreased serum ALLO levels in women with PMD compared to controls on menstrual cycle day 26 (Rapkin et al. 1997), during the luteal phase only (Monteleone et al. 2000), or during the follicular phase only (Bičíková et al. 2007), PMD patients in the last two studies had lower progesterone levels, which may explain the observed decreased ALLO levels. This explanation is supported by the observation of Girdler et al. (2001) that women with PMD had both higher progesterone and ALLO levels during the luteal phase compared with controls. Successful treatment with antidepressant medication was associated with reduced ALLO levels in a study by Freeman et al. (2002), but it is unclear whether baseline differences in ALLO or progesterone may account for this finding. Other studies showed neither diagnosis-related differences in ALLO or pregnanolone (Schmidt et al. 1994; Wang et al. 1996) nor any difference in ALLO levels in women with PMD before and after successful treatment with citalopram (Sundström and Bäckström 1998). The use of different assays, antibodies, and methods of extraction across studies may account for some the variability in findings. Nonetheless, there is no reliable evidence that peripheral ALLO levels distinguish those with PMD from those without.
In the absence of consistent basal neurosteroid abnormalities in PMD, researchers have examined the ALLO response to various challenges, including the administration of neurosteroids, benzodiazepines, and mental stress. One reliable neurophysiologic measure of GABAA receptor sensitivity is saccadic eye velocity (SEV), which characterizes the speed of eye movements that bring the retinal image being viewed onto the fovea. SEV is correlated with self-reported sedation and suppressed by benzodiazepines (Sundström et al. 1997). Women with PMD show differences from controls in pregnanolone-modulated SEV and sedation in the luteal phase (Sundström et al. 1998), although these differences may be attributable to SEV response to vehicle in those with PMD and blunted sedation in controls during the follicular phase. Patients with severe PMD show blunted SEV and sedation responses to the GABAA receptor modulators pregnanolone and midazolam compared with women with less severe PMD (Sundström et al. 1997; Sundström et al. 1998), which may indicate an altered profile of GABAA receptor subunits. Midazolam insensitivity, in particular, could suggest increased expression of the α4 and/or δsubunits (Sundström Poromaa et al. 2003). However, neurosteroid levels were not presented for women with more and less severe PMD, making it difficult to draw conclusions about whether the effects may have been attributable to variation in baseline hormone levels given the risk of increased sampling variability with small samples (n=6). Although women with PMD also show a blunted ALLO response to stress (Girdler et al. 2001), as mentioned above, the lack of an expected increase in ALLO following stress may be attributed to elevated progesterone and ALLO levels at baseline in the women with PMD. Similarly, women with PMD demonstrate altered metabolism of progesterone to ALLO (Klatzkin et al. 2006), although women with PMD had an elevated ALLO/progesterone ratio at baseline, and the administration of progesterone appeared to correct the ratio of progesterone to ALLO such that women with PMD and controls showed the same pattern of results following progesterone administration (Klatzkin et al. 2006). The concept of altered steroidogenesis conferring vulnerability to reproductive-related illness is supported by research in polycystic ovarian syndrome, which is characterized by enhanced peripheral 5α-reductase activity and results in hyperandrogenism (Fassnacht et al. 2003) and attendant behavioral symptoms, including depression and anxiety (Deeks et al. 2010). Steroid synthesis may be similarly altered in PMD such that mood symptoms result from decreased enzymatic conversion of progesterone to ALLO by 5α- reductase and 3α-hydroxysteroid dehydrogenase (3α-HSD). One potential mechanism that could account for differential enzymatic metabolism of progesterone to ALLO is suggested by Bortolato et al. (2011). They demonstrated that early life stress reduces the expression of 5α-reductase isoforms in the rat nucleus accumbens and medial prefrontal cortex, thereby reducing the synthesis of ALLO from progesterone.
Results are similar for PPD, although fewer studies have been conducted. Epperson et al. (2006) showed that cortical GABA and ALLO are reduced in postpartum women, regardless of the presence of PPD, compared with healthy women in the follicular phase. Despite similar levels of circulating progesterone and ALLO, women with PPD show reduced resting state functional connectivity between the anterior cingulate cortex, amygdala, hippocampus, and dorsolateral prefrontal cortex in the context of the postnatal decline in ALLO (Deligiannidis et al. 2013). Altogether, there is no consistent evidence of abnormalities in basal circulating ALLO levels or in ALLO responsiveness to pharmacological or psychological interventions in PMD or PPD.
A third approach to examining ALLO abnormalities in reproductive mood disorders has been to examine the timing of symptom onset or exacerbation relative to changes in ALLO levels. Circulating ALLO levels fluctuate during the menstrual cycle, pregnancy, and the postpartum period because ALLO is a progesterone metabolite. As such, plasma ALLO concentrations are highest during the mid-luteal phase, decline to low levels in the late luteal phase and follicular phase, and are correlated with progesterone levels in healthy women (Schmidt et al. 1994; Genazzani et al. 1998). The same is true during pregnancy: in healthy women, ALLO levels increase during pregnancy, decrease in the postpartum period, and are correlated with progesterone levels (Gilbert Evans et al. 2005). Because of the potent anxiolytic effects of ALLO, one would expect decreased mood symptoms during periods of elevated progesterone and ALLO levels (i.e., the mid-luteal phase of the menstrual cycle and during pregnancy). However, PMD symptoms emerge during the mid-luteal phase (during elevated ALLO concentrations) and remit after the onset of menses (during decreased ALLO concentrations) (Pearlstein et al. 2005). Progesterone administration is either ineffective or exacerbates affective symptoms in women with PMD (Sampson 1979; Freeman et al. 1990; Freeman et al. 1995; Schmidt et al. 1998; Tiemstra and Patel 1998). In addition, drugs that prevent ovulation, and thus prevent the subsequent increase in progesterone and ALLO levels, prevent PMD symptoms (Schmidt et al. 1998). Similarly, PPD most commonly begins during pregnancy (when ALLO levels are relatively high) (Gotlib et al. 1989), and anxiety and depressive symptoms during pregnancy are the strongest predictors of PPD (O’Hara and Swain 1996). Progesterone administration increases affective symptoms in the postnatal period and precipitates the onset of depression in women with a history of PPD (Bloch et al. 2000; Lawrie et al. 2000). Although the timing of symptom onset and response to progesterone administration in both PMD and PPD seemingly conflict with the literature on the anxiolytic effects of ALLO, two recent models of ALLO function explain how increasing circulating progesterone levels, both during the menstrual cycle and during pregnancy, may trigger affective dysregulation in susceptible women.
First, Smith et al. (2006) demonstrated that under certain hormonal and behavioral conditions ALLO exerts anxiogenic effects. ALLO withdrawal followed by ALLO administration results in anxiogenesis in response to an aversive stimulus. This effect is mediated by an increase in GABAA receptor α4 expression, which is associated with insensitivity to the benzodiazepine agonist lorazepam (Smith et al. 2006). Thus, ALLO withdrawal modified the GABAA receptor such that the reintroduction of ALLO, in combination with an aversive stimulus, triggers hippocampal excitability rather than inhibition, resulting in an amplified stress response. One possible cellular mechanism for the abnormal response to ALLO administration after ALLO withdrawal is presented by Shen et al. (2007), in which a particular configuration of subunits (α4β2δ) of GABAA receptors, a configuration induced by changes in ALLO levels, reverses the effects of ALLO from enhancing to inhibiting GABA-gated current.. Thus, ALLO released by stress during the luteal phase (and following the rise in ALLO post ovulation) may paradoxically increase negative mood symptoms. These findings may help explain the timing of affective symptom onset during the luteal phase of the menstrual cycle and during pregnancy, the negative mood effects of progesterone administration in both PMD and PPD, and the salutary effects of ovulation suppression in women PMD.
Second, Maguire and Mody (2008) demonstrated a GABAA receptor subunit knock-out that is behaviorally silent until an animal is exposed to pregnancy and the postpartum state, following which the dam displays depression-like behavior and cannibalizes its young. Thus, in this model reproductive events unmask the genetic susceptibility to affective dysregulation. Alterations in the GABAA receptor δ-subunit occur as ovarian hormone levels fluctuate during the menstrual cycle, pregnancy, and the postpartum period (Maguire et al. 2005; Maguire and Mody 2008; Maguire et al. 2009). During pregnancy, the expression of the GABAA receptor δ-subunit is downregulated as ALLO levels increase, and at parturition, the expression of the GABAA receptor δ-subunit is recovered in response to rapidly declining neurosteroid levels (Maguire et al. 2009). The failure to regulate these receptors during pregnancy and the postpartum, consequent to the knock-out of the GABAA receptor δ-subunit, appears to provoke behavioral abnormalities consistent with PPD. Thus, GABAA receptor δ-subunit deficient mice exhibit normal behaviors prior to pregnancy, but they show insensitivity to ALLO during pregnancy, depression-like and anxiety-like behavior, and abnormal maternal behavior (Maguire and Mody 2008). These models suggest that changes in neurosteroid concentrations are capable of provoking affective dysregulation, particularly in those with a genetically determined susceptibility.
Consistent with these models, women with PMD show abnormalities in cortical inhibition and GABA levels across the menstrual cycle. Cerebral cortical inhibition increases during the luteal phase in healthy control women, a presumed effect of increased ALLO levels and a finding that is absent in women with PMD (Smith et al. 2002; Smith et al. 2003). Women with PMD show reduced GABA levels in the occipital cortex during the follicular phase and increasing levels from the follicular to the luteal phase, whereas healthy controls show decreasing levels from the follicular to the luteal phase (Epperson et al. 2002). Healthy control women also show a significant negative association between ALLO and cortical GABA levels, whereas women with PMD do not (Epperson et al. 2002). These findings are consistent with an abnormal response to ALLO in women with PMD.
Reanalysis of existing data from our prior studies also supports the role of ALLO in reproductive-related affective dysregulation. We examined ALLO levels and depressive symptoms in a previous study of ovarian suppression with depot leuprolide acetate (a GnRH agonist) and subsequent progesterone addback in women with PMD (Schmidt et al. 1998). During baseline screening women were identified as having PMD, which was defined as an increase of at least 30% in negative mood (i.e., depression, anxiety, irritability) in the 7 days before menses compared with the 7 days after the end of menses in at least 2 of 3 cycles. Following eight or 12 weeks of hypogonadism maintained by leuprolide, progesterone (200 mg bid by vaginal suppository) was added in double-blind fashion for four weeks as part of a larger study in which estradiol, progesterone, or placebo was administered along with leuprolide. Blood samples were drawn and mood ratings were obtained after the second and fourth week of progesterone addback. Circulating progesterone levels decreased between the second and fourth week of progesterone addback in both women with PMD and controls. Repeated measures ANOVA revealed a significant time by diagnosis interaction (F1,21=4.22, p<.05), reflecting a greater decrease in ALLO over time in women with PMD compared with controls (t21=5.57, p<.01 on post hoc testing). Further, the change in ALLO was negatively correlated with clinician-rated total premenstrual symptoms at a trend level in women with PMD (r=-.58, p<.1; Figure 1a) but not in controls, which suggests that declining levels of ALLO worsened symptoms in those with PMD.
Fig. 1.
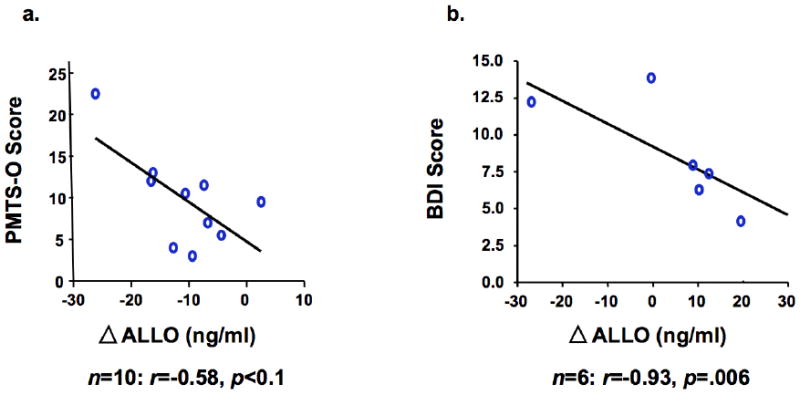
Correlations between psychiatric symptoms and the change in circulating ALLO concentrations between the second and fourth week of progesterone addback in women with (a) current PMD and (b) a history of PPD. Note: PMD = premenstrual dysphoria. PPD = postpartum depression. PMTS-O = Premenstrual Tension Scale – Other Rating. BDI = Beck Depression Inventory. ALLO = allopregnanolone.
We found remarkably similar results when reexamining existing data from an analogous study of ovarian suppression with depot leuprolide (a GnRH agonist) and subsequent progesterone and estradiol addback in women with a history of PPD (Bloch et al. 2000). In that study, euthymic, parous women with either a history of PPD (and without non-puerperal major depressive disorder) or no psychiatric history were enrolled. Following eight weeks of hypogonadism maintained by leuprolide, progesterone (400–900 mg/day) and estradiol (4–10 mg/day) were added in a double-blind fashion for four weeks. Blood samples were drawn and mood ratings were obtained following the second and fourth week of hormone addback. Depressive symptoms, as measured with the Beck Depression Inventory, were negatively correlated with the change in ALLO following progesterone addback in women with a history of PPD (r=-.93, p=.006; Figure 1b), an effect that was absent in control women. Taken together, these preliminary results support the hypothesis that changes in ALLO levels may dysregulate affective state, thereby serving as an “affective switch,” in susceptible women. Indeed, a separate, recently completed study revealed the ability of stabilization of ALLO to prevent the appearance of PMD symptoms (Martinez et al., unpublished). The animal models discussed above suggest two potential mechanisms by which ALLO withdrawal may trigger mood symptoms in susceptible women. First, ALLO withdrawal is sufficient to change inhibitory neural signals to excitatory signals in the context of stress (Smith et al. 2006). Second, genetic susceptibility to affective dysregulation may be unmasked during periods of reproductive hormone change (Maguire and Mody 2008). Although few genomic studies of reproductive mood disorders have been conducted, preliminary research has implicated genes containing multiple estrogen binding sites (Mahon et al. 2009) and estradiol-enhanced DNA methylation patterns related to hippocampal synaptic plasticity in PPD (Guintivano et al. 2013). In addition, polymorphic variants of the estrogen receptor alpha gene are associated with PMD (Huo et al. 2007). Further research will be necessary to determine whether ALLO withdrawal precipitates the onset of depressive symptoms or is simply a correlate of affective dysregulation. Moreover, given the differences in basal ALLO levels associated with PMD symptoms across studies, potentially as a result of different assay techniques, results should be confirmed in other laboratories.
Conclusion
ALLO may play an important role in the pathophysiology of reproductive mood disorders by triggering affective dysregulation in susceptible women. Although the source of this susceptibility remains unclear, animal models suggest that ALLO may trigger mood symptoms as a function of GABAA receptor α4 and/or δ subunit alterations that are heritable or consequent to ALLO withdrawal combined with stress (Maguire et al. 2005; Smith et al. 2006; Shen et al. 2007; Maguire and Mody 2008; Maguire et al. 2009). In humans, preliminary evidence is accumulating in support of a genetic vulnerability to affective dysregulation in the context of specific reproductive states. Understanding the source of individual susceptibility is critical to both preventing the onset of illness and developing novel, individualized treatments for reproductive-related affective dysregulation. The capacity of changes in neurosteroids to function as behavioral switches suggests a potentially important role of these hormone metabolites in reproductive endocrine-related mood disorders.
References
- Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/S0166-43289900101-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Association; Arlington, Va: 2013. [Google Scholar]
- Baehr E, Rosenfeld P, Miller L, Baehr R. Premenstrual dysphoric disorder and changes in frontal alpha asymmetry. Int J Psychophysiol Off J Int Organ Psychophysiol. 2004;52:159–167. doi: 10.1016/j.ijpsycho.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Baller EB, Wei S-M, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170:305–314. doi: 10.1176/appi.ajp.2012.12030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannbers E, Gingnell M, Engman J, et al. The effect of premenstrual dysphoric disorder and menstrual cycle phase on brain activity during response inhibition. J Affect Disord. 2012;142:347–350. doi: 10.1016/j.jad.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, et al. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br J Pharmacol. 1997;120:1582–1588. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, et al. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94:8836–41. doi: 10.1073/pnas.94.16.8836. Clinical Trial Controlled Clinical Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bičíková M, Dibbelt L, Hiill M, et al. Allopregnanolone in Women with Premenstrual Syndrome. Horm Metab Res. 2007;30:227–229. doi: 10.1055/s-2007-978871. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-J. [DOI] [PubMed] [Google Scholar]
- Bitran D, Purdy RH, Kellog CK. Anxiolytic effect of progesterone is associated with increases in cortical alloprenanolone and GABAA receptor function. Pharmacol Biochem Behav. 1993;45:423–428. doi: 10.1016/0091-30579390260-Z. [DOI] [PubMed] [Google Scholar]
- Bixo M, Andersson A, Winblad B, et al. Progesterone, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997;764:173–178. doi: 10.1016/S0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, et al. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Devoto P, Roncada P, et al. Isolation rearing-induced reduction of brain 5α-reductase expression: Relevance to dopaminergic impairments. Neuropharmacology. 2011;60:1301–1308. doi: 10.1016/j.neuropharm.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez P, Perez M, Avila J, et al. Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Mol Cell Neurosci. 2004;25:363–373. doi: 10.1016/j.mcn.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABAA receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology (Berl) 2013;230:151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology. 1998;35:607–614. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- Deeks AA, Gibson-Helm ME, Teede HJ. Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertil Steril. 2010;93:2421–2423. doi: 10.1016/j.fertnstert.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Deligiannidis KM, Sikoglu EM, Shaffer SA, et al. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: A preliminary study. J Psychiatr Res. 2013;47:816–828. doi: 10.1016/j.jpsychires.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, et al. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, et al. A Meta-Analysis of Cytokines in Major Depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, et al. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104:2465–70. doi: 10.1073/pnas.0605569104. Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky B. Neurosteroids, neuroactive steroids, and symptoms of affective disorders. Pharmacol Biochem Behav. 2006;84:644–655. doi: 10.1016/j.pbb.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Epperson C, Haga K, Mason G, et al. Cortical γ-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: A proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Gueorguieva R, Czarkowski KA, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl) 2006;186:425–433. doi: 10.1007/s00213-006-0313-7. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual Dysphoric Disorder: Evidence for a New Category for DSM-5. Am J Psychiatry. 2012;169:465–475. doi: 10.1176/appi.ajp.2012.11081302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser D, Schüle C, Baghai TC, et al. Neuroactive Steroids in Depression and Anxiety Disorders: Clinical Studies. Neuroendocrinology. 2006;84:244–254. doi: 10.1159/000097879. [DOI] [PubMed] [Google Scholar]
- Evans J, Sun Y, McGregor A, Connor B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology. 2012;63:1315–1326. doi: 10.1016/j.neuropharm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Fassnacht M, Schlenz N, Schneider SB, et al. Beyond Adrenal and Ovarian Androgen Generation: Increased Peripheral 5α-Reductase Activity in Women with Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2003;88:2760–2766. doi: 10.1210/jc.2002-021875. [DOI] [PubMed] [Google Scholar]
- Finocchi C, Ferrari M. Female reproductive steroids and neuronal excitability. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2011;32(Suppl 1):S31–35. doi: 10.1007/s10072-011-0532-5. [DOI] [PubMed] [Google Scholar]
- Foy MR, Baudry M, Akopian GK, Thompson RF. Regulation of hippocampal synaptic plasticity by estrogen and progesterone. Vitam Horm. 2010;82:219–239. doi: 10.1016/S0083-67291082012-6. [DOI] [PubMed] [Google Scholar]
- Freeman E, Rickels K, Sondheimer S, Polansky M. Ineffectiveness of progesterone suppository treatment for premenstrual syndrome. JAMA. 1990;264:349–353. doi: 10.1001/jama.1990.03450030073035. [DOI] [PubMed] [Google Scholar]
- Freeman E, Rickels K, Sondheimer S, Polansky M. A double-blind trial of oral progesterone, alprazolam, and placebo in treatment of severe premenstrual syndrome. JAMA. 1995;274:51–57. doi: 10.1001/jama.1995.03530010065036. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Frye CA, Rickels K, et al. Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J Clin Psychopharmacol. 2002;22:516–520. doi: 10.1097/00004714-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Frye CA. Neurosteroids’ effects and mechanisms for social, cognitive, emotional, and physical functions. Psychoneuroendocrinology. 2009;34(Supplement 1):S143–S161. doi: 10.1016/j.psyneuen.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duncan JE. Progesterone metabolites, effective at the GABAA receptor complex, attenuate pain sensitivity in rats. Brain Res. 1994;643:194–203. doi: 10.1016/0006-89939490025-6. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–83. doi: 10.1097/01.AOG.0000183597.31630.db. Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, P.H.S. Review. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, et al. Circulating Levels of Allopregnanolone in Humans: Gender, Age, and Endocrine Influences. J Clin Endocrinol Metab. 1998;83:2099–2103. doi: 10.1210/jc.83.6.2099. [DOI] [PubMed] [Google Scholar]
- Gilbert Evans SE, Ross LE, Sellers EM, et al. 3alpha-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2005;21:268–279. doi: 10.1080/09513590500361747. [DOI] [PubMed] [Google Scholar]
- Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62:400–406. doi: 10.1016/j.yhbeh.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–97. doi: 10.1016/s0006-3223(00)01044-1. Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Overstreet DH, et al. Pro-apoptotic Par-4 and dopamine D2 receptor in temporal cortex in schizophrenia, bipolar disorder and major depression. Schizophr Res. 2010;118:292–299. doi: 10.1016/j.schres.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci Off J Soc Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Whiffen VE, Mount JH, et al. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57:269–274. doi: 10.1037/0022-006X.57.2.269. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci. 1999;96:13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guintivano J, Arad M, Gould TD, et al. Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Evans C-O, Hoffman SW, et al. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004;189:404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Huo L, Straub RE, Roca C, et al. Risk for Premenstrual Dysphoric Disorder Is Associated with Genetic Variation in ESR1, the Estrogen Receptor Alpha Gene. Biol Psychiatry. 2007;62:925–933. doi: 10.1016/j.biopsych.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe P, Mallinson K, McCormick CM, Frye CA. Central allopregnanolone is increased in rat pups in response to repeated, short episodes of neonatal isolation. Dev Brain Res. 2000;124:133–136. doi: 10.1016/S0165-38060000106-1. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Leslie Morrow A, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31:1208–1219. doi: 10.1016/j.psyneuen.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Lawrie TA, Herxheimer A, Dalton K. Oestrogens and progestogens for preventing and treating postnatal depression. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD001690. CD001690. [DOI] [PubMed] [Google Scholar]
- Licinio J, Wong ML. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatry. 1999;4:317–327. doi: 10.1038/sj.mp.4000586. [DOI] [PubMed] [Google Scholar]
- MacKenzie G, Maguire J. The role of ovarian hormone-derived neurosteroids on the regulation of GABAA receptors in affective disorders. Psychopharmacology (Berl) :1–10. doi: 10.1007/s00213-013-3423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Ferando I, Simonsen C, Mody I. Excitability Changes Related to GABAA Receptor Plasticity during Pregnancy. J Neurosci. 2009;29:9592–9601. doi: 10.1523/JNEUROSCI.2162-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABAAR Plasticity during Pregnancy: Relevance to Postpartum Depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mahon P, Payne J, MacKinnon D, et al. Genome-wide linkage and follow-up association study of postpartum mood symptoms. Am J Psychiatry. 2009;166:1229–1237. doi: 10.1176/appi.ajp.2009.09030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, et al. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Matsui D, Sakari M, Sato T, et al. Transcriptional regulation of the mouse steroid 5α-reductase type II gene by progesterone in brain. Nucleic Acids Res. 2002;30:1387–1393. doi: 10.1093/nar/30.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Basic neurobiology of ovarian steroids: clinical implications. Dialogues Clin Neurosci. 2002;4:163–176. [Google Scholar]
- Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol Eur Fed Endocr Soc. 2000;142:269–273. doi: 10.1530/eje.0.1420269. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Devaud LL, Purdy RH, Paul SM. Neuroactive Steroid Modulators of the Stress Response. Ann N Y Acad Sci. 1995;771:257–272. doi: 10.1111/j.1749-6632.1995.tb44687.x. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Fraser D, Wisner KL, et al. Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol Psychiatry. 2011;70:395–9. doi: 10.1016/j.biopsych.2011.02.021. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, et al. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167:1373–80. doi: 10.1176/appi.ajp.2010.09081235. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara MW, Swain AM. Rates and risk of postpartum depression-A meta-analysis. Int Rev Psychiatry. 1996;8:37–54. doi: 10.3109/09540269609037816. [DOI] [Google Scholar]
- Patchev VK, Hassan AHS, Holsboer F, Almeida OFX. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X9600096-6. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OFX. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–271. doi: 10.1016/0306-45229490330-1. [DOI] [PubMed] [Google Scholar]
- Pearlstein T, Yonkers KA, Fayyad R, Gillespie JA. Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. J Affect Disord. 2005;85:275–282. doi: 10.1016/j.jad.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Russo M, Santoro AN, et al. Steroid hormones and BDNF. Neuroscience. 2013;239:271–279. doi: 10.1016/j.neuroscience.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Altemus M, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci U S A. 2005;102:16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108:87–94. doi: 10.1016/j.jad.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Berman SM, Mandelkern MA, et al. Neuroimaging evidence of cerebellar involvement in premenstrual dysphoric disorder. Biol Psychiatry. 2011;69:374–380. doi: 10.1016/j.biopsych.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–714. doi: 10.1016/S0029-78449700417-1. [DOI] [PubMed] [Google Scholar]
- Romeo E, Ströhle A, Spalletta G, et al. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Girdler SS. Hormones, heart disease, and health: individualized medicine versus throwing the baby out with the bathwater. Depress Anxiety. 2011;28:282–296. doi: 10.1002/da.20810. [DOI] [PubMed] [Google Scholar]
- Sampson GA. Premenstrual syndrome: a double-blind controlled trial of progesterone and placebo. Br J Psychiatry. 1979;135:209–215. doi: 10.1192/bjp.135.3.209. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Parvez S, Wali B, et al. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism for better neuroprotective effects of allopregnanolone over progesterone. Brain Res. 2009;1263:165–173. doi: 10.1016/j.brainres.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential Behavioral Effects of Gonadal Steroids in Women with and in Those without Premenstrual Syndrome. N Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Purdy RH, Moore PH, et al. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–1260. doi: 10.1210/jc.79.5.1256. [DOI] [PubMed] [Google Scholar]
- Schüle C, Baghai TC, di Michele F, et al. Effects of combination treatment with mood stabilizers and mirtazapine on plasma concentrations of neuroactive steroids in depressed patients. Psychoneuroendocrinology. 2007;32:669–680. doi: 10.1016/j.psyneuen.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Schüle C, Eser D, Baghai TC, et al. Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs? Neuroscience. 2011;191:55–77. doi: 10.1016/j.neuroscience.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Schüle C, Romeo E, Uzunov DP, et al. Influence of mirtazapine on plasma concentrations of neuroactive steroids in major depression and on 3α-hydroxysteroid dehydrogenase activity. Mol Psychiatry. 2005;11:261–272. doi: 10.1038/sj.mp.4001782. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2011;16:751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, et al. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/S0006-32230300181-1. [DOI] [PubMed] [Google Scholar]
- Silverman ME, Loudon H, Safier M, et al. Neural dysfunction in postpartum depression: an fMRI pilot study. CNS Spectr. 2007;12:853–62. doi: 10.1017/s1092852900015595. Research Support, N.I.H., Extramural. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, et al. Effects of ovarian hormones on human cortical excitability. Ann Neurol. 2002;51:599–603. doi: 10.1002/ana.10180. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, et al. Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychiatry. 2003;54:757–762. doi: 10.1016/S0006-32230201924-8. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, et al. GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–30. doi: 10.1038/31948. Research Support, U.S. Gov’t, P.H.S. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, et al. Withdrawal from 3α-OH-5α-Pregnan-20-One Using a Pseudopregnancy Model Alters the Kinetics of Hippocampal GABAA-Gated Current and Increases the GABAAReceptor α4 Subunit in Association with Increased Anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Frye C, et al. Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha,5beta-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology (Berl) 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Greene LA, Miranda RC, Toran-Allerand CD. Reciprocal regulation of estrogen and NGF receptors by their ligands in PC12 cells. J Neurobiol. 1994a;25:974–988. doi: 10.1002/neu.480250807. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci Off J Soc Neurosci. 1994b;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorwell KG, Kohama SG, Urbanski HF. Perimenopausal regulation of steroidogenesis in the nonhuman primate. Neurobiol Aging. 2012;33:1487.e1–1487.e13. doi: 10.1016/j.neurobiolaging.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ströhle A, Romeo E, Hermann B, et al. Concentrations of 3α-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry. 1999;45:274–277. doi: 10.1016/S0006-32239800328-X. [DOI] [PubMed] [Google Scholar]
- Sundström I, Andersson A, Nyberg S, et al. Patients with Premenstrual Syndrome Have a Different Sensitivity to a Neuroactive Steroid during the Menstrual Cycle Compared to Control Subjects. Neuroendocrinology. 1998;67:126–138. doi: 10.1159/000054307. [DOI] [PubMed] [Google Scholar]
- Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: An open trial. Psychoneuroendocrinology. 1998;23:73–88. doi: 10.1016/S0306-45309700064-4. [DOI] [PubMed] [Google Scholar]
- Sundström I, Nyberg S, Bäckström T. Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology. 1997;17:370–381. doi: 10.1016/S0893-133X9700086-9. [DOI] [PubMed] [Google Scholar]
- Sundström Poromaa I, Smith S, Gulinello M. GABA receptors, progesterone and premenstrual dysphoric disorder. Arch Womens Ment Health. 2003;6:23–41. doi: 10.1007/s00737-002-0147-1. [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Couse JF, Rodriguez KF, et al. Estrogen receptor-α mediates an intraovarian negative feedback loop on thecal cell steroidogenesis via modulation of Cyp17a1 (cytochrome P450, steroid 17α-hydroxylase/17,20 lyase) expression. FASEB J. 2007;21:586–595. doi: 10.1096/fj.06-6681com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemstra JD, Patel K. Hormonal Therapy in the Management of Premenstrual Syndrome. J Am Board Fam Pract. 1998;11:378–381. doi: 10.3122/15572625-11-5-378. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Dichter GS, Garber J, Simien C. Resting frontal brain activity: linkages to maternal depression and socio-economic status among adolescents. Biol Psychol. 2004;67:77–102. doi: 10.1016/j.biopsycho.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Tuohy A, McVey C. Subscales measuring symptoms of non-specific depression, anhedonia, and anxiety in the Edinburgh Postnatal Depression Scale. Br J Clin Psychol. 2008;47:153–169. doi: 10.1111/j.2044-8260.2008.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy RH, Bãckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. J Clin Endocrinol Metab. 1996;81:1076–1082. doi: 10.1210/jc.81.3.1076. [DOI] [PubMed] [Google Scholar]
- Wieland S, Lan NC, Mirasedeghi S, Gee KW. Anxiolytic activity of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Brain Res. 1991;565:263–268. doi: 10.1016/0006-8993(91)91658-N. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/en.137.5.2163. [DOI] [PubMed] [Google Scholar]


