Abstract
Rationale
A robust epidemiological literature suggests an association between chronic stress and the development of affective disorders. However, the precise biological underpinnings of this relationship remain elusive. Central to the human response and adaptation to stress, activation and inhibition of the hypothalamic pituitary adrenal (HPA) axis involves a multi-level, multi-system, neurobiological stress response which is as comprehensive in its complexity as it is precarious. Dysregulation in this complex system has implications for human stress related illness.
Objectives
The pioneering research of Robert Purdy and colleagues has laid the groundwork for advancing our understanding of HPA-axis regulation by stress-derived steroid hormones and their neuroactive metabolites (termed neurosteroids), which are potent allosteric modulators of GABAA receptor function in the central nervous system. This review will describe what is known about neurosteroid modulation of the HPA-axis in response to both acute and chronic stress, particularly with respect to the current state of our knowledge of this process in humans.
Results
Implications of this research to the development of human stress related illness are discussed in the context of two human stress-related psychiatric disorders - major depressive disorder and premenstrual dysphoric disorder.
Conclusions
Neurosteroid-mediated HPA-axis dysregulation is a potential pathophysiologic mechanism which may cross traditional psychiatric diagnostic classifications. Future research directions are identified.
Keywords: allopregnanolone, allotetrahydrodeoxycorticosterone, cortisol, depression, DHEA, GABA, HPA-axis, neurosteroids, premenstrual dysphoric disorder, stress
Introduction
In industrialized societies, it is estimated that stress contributes to a $150 billion dollar loss of revenue each year due to diminished productivity, absenteeism, stress-related mental illness, and substance abuse (Kalia 2002). However, the precise mechanisms by which stress “gets under the skin” to promote illness are still under investigation. Both experimental and epidemiological evidence suggest that dysregulation within integrated neuroendocrine, neurotransmitter and central networks governing the physiologic response to stress plays a role (Bifulco et al. 1998; Binder and Nemeroff 2010; Kendler et al. 1999).
Centrally important to the human response and adaptation to stress are the actions of the hypothalamic pituitary adrenal (HPA) axis (McEwen and Getz 2013). The pioneering research of Robert Purdy and colleagues has laid the groundwork for advancing our understanding of HPA-axis regulation by stress-derived steroid hormones and their neuroactive metabolites (termed neurosteroids). What is clear from animal studies is that the molecular underpinnings of neurosteroid regulation of the HPA-axis activity are complex and context-specific. Behavioral, hormonal, developmental, and environmental events play crucial and determinant roles in shaping physiological mechanisms underlying neurosteroid regulation of the HPA-axis.
The concept of allostatic load provides a framework by which to understand how regulation of the HPA-axis in response to stress may contribute to disease development. Allostasis is the process of achieving stability through change and, consequently, the process by which homeostasis in physiologic systems is maintained (Sterling and Eyer 1988). The body responds to stress by setting into motion a coordinated physiologic response involving hormonal and neurotransmitter mediators that determine physiological responses of cells and tissues in order to meet the organism’s metabolic needs (McEwen 2008). Glucocorticoids, the primary end products of the HPA axis, represent classic examples of allostatic mediators whose effects involve modulation of the HPA-axis to return the system to homeostasis following stress.
In the context of adaptation, activation of stress-responsive systems to an acute threat is appropriate. However, chronic activation of these systems, or failure to properly curtail activation following threat, while representing an ‘adaptation’ to environmental or historical context, may contribute to long-term physical or mental sequelae associated with stress. As stress responsive mediators are chronically mobilized to meet the homeostatic demands of stress (McEwen 2007), this causes some measure of wear on physiologic systems and this wear has been termed “allostatic load.” Allostatic load can manifest as: (1) repeated elevations of stress mediators (e.g., cortisol) over long periods; (2) a failure to adapt to a stressor; (3) a failure to shut off the normal stress response; or (4) an inadequate response to stress that may allow other systems that are normally counter-regulated to become overactive (McEwen and Seeman 1999).
We propose that the 3α, 5α – reduced metabolites of progesterone and deoxycorticosterone, allopregnanolone (ALLO) and allotetrahydrodeoxycorticosterone (ALLO-THDOC), respectively, represent allostatic mechanisms in the context of adaptation to stress by limiting the extent and duration of reduction of Gamma-Aminobutyric Acid (GABA)ergic inhibitory transmission and activation of the HPA-axis. In addition, dehydroepiandrosterone (DHEA), an androgenic hormone and precursor to androstenedione, along with its sulfate ester DHEA-S, which together comprise the most abundant steroid hormone(s) in the human body (Maninger et al. 2009), are associated with GABAA receptor (GABAAR) interactions and also exert profound antiglucocorticoid effects (Labrie et al. 2005; Maninger et al. 2009). In this way, DHEA may represent additional neurosteroid mechanisms in humans that are of relevance to the regulation of the HPA-axis response to stress. An overarching conceptual model guiding this review, therefore, is that there are both short-term adaptive actions in neurosteroid regulation of the HPA-axis (allostasis) that are protective, and long-term effects that can be damaging (allostatic load; McEwen 2007).
This review will describe what is known about neurosteroid modulation of the HPA-axis in response to stress in humans, drawing from animal research when relevant. We will describe the available evidence for alterations in neurosteroids and the HPA-axis in two forms of human stress-related illness: major depressive disorder and premenstrual dysphoric disorder. We conclude by proposing that neurosteroid-HPA-axis regulation is a fundamental pathophysiologic mechanism cutting across psychiatric diagnoses, and we will suggest future avenues of research.
Neurosteroid and GABAergic Regulation of the HPA-Axis
Background
Activation of the HPA-axis in reaction to, or in anticipation of, a stressor requires a coordinated neuroendocrine response (Herman et al. 2003; Ulrich-Lai and Herman 2009), involving inputs from multiple brain regions, neurotransmitters, and feedback systems [for a review see (Herman 2013)]. In brief, stress induces the release of corticotrophin releasing hormone (CRH) from the paraventricular nucleus (PVN) of the hypothalamus. CRH subsequently stimulates the synthesis and release of adrenocorticotropic hormone (ACTH) from the anterior pituitary which, in turn, stimulates the synthesis and release of glucocorticoids from the adrenal cortex (Pariante and Lightman 2008). In the context of stress, the most important glucocorticoid in humans is cortisol (Groeneweg et al. 2011). Cortisol plays a critical role in regulating cardiovascular, metabolic, and immunologic changes during stress (Buckingham 2006; Dhabhar 2009), and homeostatic mechanisms exist to limit the extent and duration of cortisol’s catabolic actions. The most obvious of these homeostatic mechanisms involves negative feedback regulation by glucocorticoids, via stimulating hippocampal, hypothalamic, and pituitary glucocorticoid receptors to inhibit further secretion of ACTH and CRH, thereby terminating the HPA-axis response to stress (Myers et al. 2012). Additionally, a role of GABA, the most prevalent inhibitory neurotransmitter in the mammalian central nervous system, in regulating the HPA-axis in response to stress is also well established (Cullinan et al. 2008; Decavel and Van den Pol 1990; Jones et al. 1984). Dysregulation in GABAergic transmission is emerging as a prominent theory underlying the etiology and perpetuation of stress-related psychiatric disorders (Luscher et al. 2011). While GABA exerts its central effects through activation of both GABAA (ligand-gated ion channels) and GABAB (G protein-coupled) receptors, current evidence suggests that GABAA receptors (GABAAR’s) are of greater relevance to stress-induced HPA-axis activation (Brickley and Mody 2012).
ALLO and ALLO-THDOC, the neuroactive steroid derivatives of progesterone, are stress responsive and among the most potent modulators of the GABAAR’s (Morrow et al. 1987). ALLO is synthesized not only in the ovaries and adrenals but also de novo in the brain (Paul and Purdy 1992), while ALLO-THDOC is derived primarily from adrenal sources (Reddy 2006). During stress, ACTH stimulates the conversion of cholesterol to pregnenolone in the adrenal cortex (Simpson and Waterman 1988), which is rapidly converted to progesterone and then to ALLO or ALLO-THDOC by 5α-reductase and 3αHSD reductase enzymes. Nonetheless, both ALLO and ALLO-THDOC readily cross into the brain, and the major proportion of CNS concentrations of these neuroactive steroids increased by stress comes from peripheral tissues (Purdy et al. 1991).
Both ALLO and ALLO-THDOC enhance GABAergic transmission by binding to “allosteric” sites (remote from the active site) on the GABAAR. In so doing, these neuroactive steroids increase the likelihood that GABA will bind to the active site, and thereby potentiate chloride channel opening and membrane currents (Belelli and Lambert 2005; Chisari et al. 2010). It is through this allosteric action that ALLO and ALLO-THDOC exert profound anxiolytic, anti-conflict, and analgesic effects (Bitran et al. 1995; Engin and Treit 2007; Frye and Rhodes 2006; Kavaliers and Wiebe 1987; Pibiri et al. 2006; Pinna et al. 2003), all consistent with an integrated and adaptive response to stress (Purdy et al. 1991). It is also through their modulation of GABAAR’s and alterations in GABAergic neurotransmission that these neuroactive steroids regulate the HPA-axis (Belelli and Lambert 2005; Morrow et al. 1995).
DHEA is also stress responsive, and is co-released with cortisol (Wolf and Kirschbaum 1999) via ACTH stimulation of adrenal DHEA synthesis (Hornsby 1995; Parker and Odell 1980). DHEA is widely considered to have anti-glucocorticoid effects (Browne et al. 1992; Kalimi et al. 1994), though the precise mechanisms underlying these effects are still unclear. Current evidence suggests that the anti-glucocorticoid effects of DHEA, or its metabolites, on cortisol may involve inhibition of the intermediary enzymes involved in the conversion of the inactive cortisol metabolite, cortisone, to cortisol (Hennebert et al. 2007; Maninger et al. 2009).
Whether DHEA/DHEA-S in humans is produced exclusively from the periphery, or may also be synthesized in the brain is still under debate (Maninger et al. 2009). DHEA and DHEA-S are generally considered non-competitive negative allosteric modulators of the GABAA receptor and positive modulators of NMDA and sigma-1 receptors (Amato et al. 2010; Baulieu 1998; Dubrovsky 2006; Imamura and Prasad 1998; Majewska 1992; Paul and Purdy 1992; Zheng 2009). However, the physiological significance, mechanism of action, and potential implications of DHEA and DHEA-S in human stress-related disease are, to date, not fully understood.
It has been established that complex relationships exist wherein both positive and negative modulators of the GABAAR have the capacity to modulate network excitability via GABAergic mechanisms (Majewska 1992; Park-Chung et al. 1999), thereby influencing the regulation of the HPA-axis. However, the relevance of DHEA and DHEA-S to human stress-related disease is complicated by the observed anxiolytic and antidepressant effects of exogenous DHEA administration (Bloch et al. 1999; Genazzani et al. 2003; Maayan et al. 2006; Schmidt et al. 2005; Strous et al. 2003; Wolf and Kirschbaum 1999; Wolkowitz et al. 1999), which appear counterintuitive to the hypothesized antagonistic effects of DHEA at the GABAA receptor. This apparent paradox may be explained by (1) the capacity of DHEA to influence the release of other GABAergic neurosteroids which are positive modulators of the GABAAR (Gartside et al. 2010; Genazzani et al. 2003; Majewska 1992); and/or (2) the anti-glucocorticoid properties of DHEA (Browne et al. 1992; Kalimi et al. 1994) which may serve to counteract the adverse effects of prolonged cortisol release (Wolkowitz et al. 2001).
Neurosteroid and GABAergic Regulation of the HPA-Axis: Acute Stress
Research in animal models has provided consistent evidence that stress-induced activation of the HPA-axis increases neurosteroid levels to concentrations which can functionally potentiate the effects of GABA on GABAAR’s (Akk et al. 2007; Purdy et al. 1991). Under the control of ACTH (Reddy 2006), DHEA (Maninger et al. 2010) and pregnenolone (Vallee et al. 2000), as well as their progesterone-derived and androstenedione-derived metabolites (Barbaccia et al. 1997; Paul and Purdy 1992), have been shown to increase in response to acute stress. Studies in rat models have shown that GABAergic transmission is rapidly decreased following acute stress (Barbaccia et al. 1996; Biggio et al. 2007; Sanna et al. 1992). Central nervous system increases in neurosteroids following stress are also rapid, though the time course of peripheral neurosteroid responses to acute stress in rats is delayed, peaking between 30 and 70 minutes as originally demonstrated by Purdy (Barbaccia et al. 1998; Paul and Purdy 1992; Purdy et al. 1991). Peripheral increases in neurosteroid concentrations have been correlated with restoration of GABAergic transmission in addition to anxiolytic actions (Barbaccia et al. 1998). Pretreatment of rats with ALLO, ALLO-THDOC, or progesterone attenuates stress-induced increases in plasma ACTH and cortisol (Owens et al. 1992; Patchev et al. 1996). These results implicate peripheral neurosteroids in curtailing the extent and duration of reduction in GABAergic inhibitory transmission and restoring HPA-axis homeostatic control following stress.
To date, relatively few studies have examined stress-induced ALLO or ALLO-THDOC in humans, and even fewer have examined concurrent indicators of HPA-axis activation. Genazzani and colleagues (1998) were the first to demonstrate, in healthy men and women, that both gonadotropin-releasing hormone (GnRH) and corticotropin releasing factor (CRF) administration increased serum progesterone and ALLO concentrations, with an ALLO time course consistent with that seen in rats, peaking 60 min post-challenge. Subsequent work by this group has yielded a similar time course for both peak plasma ALLO and cortisol responses to CRF challenge in healthy controls, though not in women with hypothalamic amenorrhea who fail to show any increase in ALLO (Meczekalski et al. 2000), or in obese subjects who show an exaggerated ALLO response with an earlier peak (Menozzi et al. 2002). In healthy volunteers, an experimental panic induction procedure using cholecystokinin-tetrapeptide (cck-4) produced an ACTH peak at 10 min following administration, while cortisol and ALLO-THDOC peaked at 20 min (Eser et al. 2005). The difference in time course of response for ACTH and ALLO-THDOC is consistent with the evidence that the formation of ALLO-THDOC requires availability of deoxycorticosterone from the adrenal cortex, the synthesis of which is under the control of ACTH (Simpson and Waterman 1988). Taken together, the results of these endocrine challenge studies suggest that neurosteroids increase in response to pharmacologically-induced stress in healthy humans with a time course similar to that seen in rodent models.
There have been only a handful of studies which have investigated progesterone-derived neurosteroid regulation of the HPA-axis following acute mental stress in humans, and they have focused almost exclusively on ALLO. Droogleever-Fortuyn and colleagues (2004), using a naturalistic stressor (PhD defense), observed, in a predominantly male sample, that both plasma ALLO and cortisol were significantly increased during the examination relative to concentrations four weeks earlier. In that study, stress-induced ALLO and cortisol concentrations were positively correlated, suggesting a coordinated HPA-axis and neurosteroid response to stress. However, in a study comparing postpartum women with healthy controls in the follicular phase of the menstrual cycle for cortisol and ALLO stress reactivity to the Trier Social Stress Test (TSST), a mental stressor battery that induces substantial HPA-axis activation (Kirschbaum et al. 1993), no effect of acute stress was seen for ALLO reactivity at a single 30 min post-stress time point in either group, though a significant cortisol response was observed (Altemus et al. 2001).
Childs and de Wit (2009) also used the TSST to examine progesterone and ALLO stress reactivity in healthy male smokers and non-smokers. The TSST induced significant increases in progesterone, but no changes in ALLO in either group when measured at 5, 10, 20 and 30 min post-stress. However, a negative association was observed between baseline concentrations of ALLO and the magnitude of the ALLO increase to the TSST, indicating that basal ALLO concentrations influence the degree of ALLO reactivity to acute psychosocial stress in humans. Subsequent work by this group (Childs et al. 2010) found that pretreatment with 50mg of progesterone 3hr prior to the TSST in healthy men attenuated the peak stress induced increase in cortisol, presumably reflecting the inhibitory action of progesterone-derived neurosteroids on the HPA-axis. This assumption is supported by their observations that progesterone also attenuated stress-induced increases in anger and frustration and promoted recovery from negative mood changes after stress. These data provide evidence in humans that progesterone (and possibly its neurosteroid metabolites) may play a role in recovery from stress (Childs et al. 2010).
While the studies summarized above are inconclusive regarding ALLO reactivity to acute psychological stress and HPA-axis modulation in humans, they relied on small samples, most were conducted in primarily or exclusively male samples (with substantially lower concentrations of progesterone-derived neurosteroids), and each used radioimmunoassay (RIA) techniques. While RIAs provide highly sensitive measurements of neuroactive steroids in brain, in serum samples neuroactive steroids are present at much lower concentrations. The use of gas chromatography and mass spectrometry (GC–MS), which allows the simultaneous detection of low amounts of neurosteroids in serum may advance our ability to study neurosteroid stress reactivity in humans as suggested by a prior study in which GC-MS detected increases in both ALLO and ALLO-THDOC in response to progesterone in women (Porcu et al. 2009).
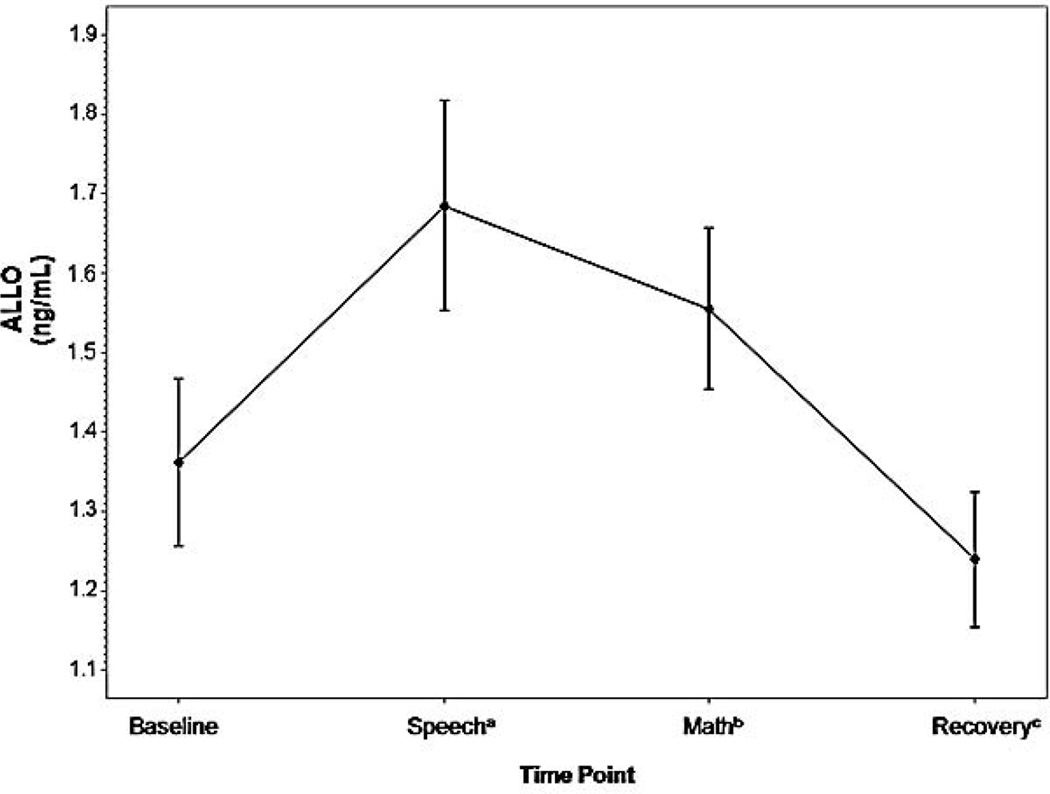
In addition, the possibility exists that the time course of the ALLO response to psychological stress in humans differs from that in rodent models. This was initially suggested to us by our observations for greater ALLO increases to psychological stress when ALLO was sampled 17 min after stress onset (Girdler et al. 2001) compared to the magnitude of reactivity when sampled 30 and 60 min post-stress onset (Klatzkin et al. 2006b) in healthy female subjects. We investigated this further, albeit in a post-hoc fashion, by analyzing ALLO in serum from 39 healthy premenopausal women tested in the luteal phase of their cycle after an extended rest and again during speech (12 minutes post-stress onset), mental arithmetic (17 minutes post-stress onset), and recovery following the TSST (30 min post-stress onset). As summarized in Figure 1 (unpublished data), although ALLO concentrations at both minute 12 (F (1, 38) = 13.02, p < .001) and minute 17 (F (1, 38) = 5.50, p < .05) were greater than baseline, ALLO concentrations at minute 12 were greater than those at minute 17 (F (1, 38) = 4.43, p < .05). While preliminary, these data suggest the possibility that ALLO reactivity to psychological stress may peak earlier than the time points selected for sampling in prior research summarized above, much of which failed to find significant increases in ALLO in response to the TSST in healthy samples (Altemus et al. 2001; Childs and de Wit 2009; Girdler et al. 2001; Klatzkin et al. 2006b). An alternative, though not mutually exclusive, explanation is that the nature of the stressor may influence the magnitude of neurosteroid responses in humans since speech stress elicits more robust physiologic responses than mental arithmetic (Dickerson and Kemeny 2004).
Fig. 1.
Time course of ALLO stress response in women, measured via radioimmunoassay. Data represent mean +/− SEM as a function of time across the task
aSample taken 12 minutes post-task onset
bSample taken 17 minutes post-task onset
cSample taken 30 minutes post-task onset
In addition to time course and stressor characteristics, inter-individual characteristics would be expected to influence the magnitude of neurosteroid reactivity to mental stress in humans. A prior study of ours illustrates this point (Girdler et al. 2006). In a sample of 85 healthy men and premenopausal women, half of each gender group was African American (AA) and the other half was non-Hispanic White (nHW), initial results revealed that in the entire sample, there was no significant change in ALLO in response to the TSST. However, about half the sample showed a significant stress-induced increase and half, a stress-induced decrease in ALLO. In women, the AA group had lower circulating ALLO concentrations, in general relative to nHW women, and were more likely to exhibit a stress-induced decrease in ALLO, while nHW women were more likely to exhibit a stress-induced increase in ALLO. Ethnicity did not predict ALLO reactivity in men. However, in the entire sample, both AA and nHW groups showed the expected negative correlation relating post-stress ALLO concentrations to post-stress cortisol.
To the extent that lower ALLO concentrations, especially following stress, are associated with diminished capacity to negatively modulate the HPA-axis and facilitate its recovery following stress exposure (Guo et al. 1995; Patchev and Almeida 1996), then the blunted ALLO stress response that was observed in AA women may be one mechanism increasing their vulnerability to stress and stress-related illness (Geronimus et al. 2006; Williams et al. 1997). Although the origin of the ALLO profile observed in AA women is unknown, it may reflect the greater chronic psychosocial stress experienced by AA women (Perry et al. 2012; Perry et al. 2013). While speculative at present, this hypothesis is consistent with an allostatic load model of blunted stress reactivity resulting from chronic stress, and data from animal models of chronic stress and diminished neurosteroid availability/synthesis (see below).
Acute psychosocial stress has also been shown to substantially increase DHEA and DHEA-S concentrations in humans (by as much as 60 – 75% above baseline concentrations) (Izawa et al. 2008; Lennartsson et al. 2012; Marceau et al. 2012), and most studies suggest a coordinated response with that of cortisol. For example, in 36 female university students, both DHEA and cortisol were significantly elevated and positively correlated 10 minutes after the cessation of a speech and mental arithmetic stressor (Pico-Alfonso et al. 2007). In studies using the standardized TSST, salivary DHEA has been shown to peak about 10 minutes before that of cortisol (Izawa et al. 2008; Lennartsson et al. 2012; Shirotsuki et al. 2009), and to correlate positively with the magnitude of the cortisol and ACTH response (Izawa et al. 2008; Lennartsson et al. 2012). DHEA-S reactivity to the TSST, on the other hand, may peak later than DHEA, and does not appear to correlate with HPA-axis reactivity (Lennartsson et al. 2012). Few studies have examined gender differences in DHEA or DHEA-S reactivity to acute stress. In a study of adolescents exposed to venipuncture stress, no gender differences existed in serum DHEA or DHEA-S reactivity, per se, but only for boys did DHEA stress reactivity correlate with cortisol reactivity (Marceau et al. 2012). In a study of adults (Lennartsson et al. 2012), no gender differences existed in the concentrations of DHEA or in DHEA stress reactivity to the TSST, but DHEA-S concentrations were significantly higher in men than women, though the comparable response pattern to the TSST was observed in both genders. The coordinated release of DHEA with cortisol in response to acute stress is thought to protect against the potentially damaging effects of excessive cortisol activity (Maninger et al. 2009), and also predicts less negative affect in response to stress (Izawa et al. 2008).
Neurosteroid and GABAergic Regulation of the HPA-Axis: Chronic Stress
Importantly, historical context plays a determinant role in the response of neuroendocrine stress mediators to challenge. Chronic social isolation in rats is associated with lower brain and plasma neuroactive steroid concentrations including ALLO and ALLO-THDOC (Dong et al. 2001; Guidotti et al. 2001; Serra et al. 2000; Serra et al. 2004). Findings that the expression of 5α-reductase, the rate-limiting enzyme in the conversion of progesterone to ALLO, is downregulated following chronic social isolation (Agis-Balboa et al. 2007; Reddy 2006) indicates the synthesis of ALLO and ALLO-THDOC may be diminished in the context of chronic stress. The observed reductions in endogenous brain and plasma ALLO concentrations in socially isolated rats has been associated with reduced feedback sensitivity of the HPA-axis to dexamethasone (DEX) challenge (Evans et al. 2012; Serra et al. 2005), suggesting that diminished neurosteroid availability may impair the normal negative feedback regulation of the HPA-axis and, thus, contribute to elevated HPA-axis activation. Since stress-induced increases in ACTH are involved in the biosynthesis of neurosteroids of adrenal origin, chronic HPA-axis activation could contribute to reduced ALLO and ALLO-THDOC in socially-isolated animals via a chronic “wear and tear” on adrenal functioning, eventually leading to a state of adrenal “exhaustion” and reduced neurosteroid synthesis – a prototypical expression of allostatic load.
Studies measuring both ALLO and HPA-axis activity in chronically stressed human samples are currently nonexistent. However, one recent study investigated chronic work-related stress and burnout and sensitivity of saccadic eye velocity (SEV) to exogenously administered ALLO and flumazenil, a GABAAR antagonist (Bäckström et al. 2013). Reductions in SEV are a measure of sedation strictly controlled by GABAergic transmission. Those with work related burn-out displayed an increased sensitivity in response to ALLO, as evidenced by larger decreases in the SEV/ALLO ratio, compared to controls. A greater reduction in SEV in response to flumazenil was also seen in the chronically stressed subjects. In animals, flumazenil changes its actions from a GABAAR antagonist (or inert compound) to a GABAAR agonist, but only in the presence of a conformational change in the GABAAR (Gangisetty and Reddy 2010; Smith et al. 1998). Although neurosteroid concentrations were not measured, the results suggest that in humans, chronic stress is associated with an increased GABAAR sensitivity, which may reflect compensatory changes in the GABAAR in response to low ALLO concentrations resulting from chronic stress, as shown in animals (Dong et al. 2001; Matsumoto et al. 2005; Serra et al. 2000).
Results from human studies which have investigated associations between chronic stress and peripheral DHEA or DHEA-S concentrations have been mixed. However, the majority of studies suggest reduced basal levels of DHEA or DHEA-S in association with chronic psychosocial stress (Bellingrath et al. 2009; Izawa et al. 2012; Jeckel et al. 2010; Lennartsson et al. 2013; Vedhara et al. 2002), though elevated levels have also been found (Lac et al. 2012; Mommersteeg et al. 2007). Because DHEA is a multifunctional steroid capable of a broad range of biological effects (Lac et al. 2012; Maninger et al. 2009; Oberbeck and Kobbe 2010), methodological differences within and across studies likely contribute to the heterogeneity of findings. For example, DHEA and DHEA-S concentrations in human studies have been shown to be highly influenced by age (Guazzo et al. 1996; Kraemer et al. 2001; Kushnir et al. 2010; Šulcová et al. 1997) and sex (Kushnir et al. 2010; Šulcová et al. 1997). Moreover, in light of the proposed antagonistic effects of DHEA and DHEA-S on cortisol, the cortisol/DHEA or cortisol/DHEA-S ratios may be more informative measures of allostatic load (and therefore risk for stress-related disease), than individual concentrations (McEwen 1998; Sapolsky et al. 1986; Wolkowitz et al. 2001).
In a recent prospective study of the effects of chronic stress (in the form of a two-week teaching practice) on salivary measures of the cortisol/DHEA ratio in 33 young adult women (Izawa et al. 2012), higher perceived stress following the chronic stressor was associated with an elevated cortisol/DHEA ratio at bedtime, and higher levels of negative mood were associated with elevated cortisol/DHEA ratio after awakening, though neither reached statistical significance. However, compared with pre-stressor concentrations, basal cortisol averaged over awakening, 30 min after awakening, and at bedtime significantly increased during the first and second week of the teaching practice, while DHEA concentrations significantly decreased. These findings are generally in agreement with other human studies of chronic stress, which have shown that DHEA and DHEA-S concentrations tend to decline with chronic or repeated stress, while cortisol levels tend to rise or remain unchanged, resulting in elevated cortisol to DHEA and/or DHEA-S ratios (Jeckel et al. 2010; Lennartsson et al. 2013; Wolkowitz et al. 2001).
In summary, studies using acute and chronic stress paradigms indicate that age, sex, and race/ethnicity are among the factors that may modify the neurosteroid response to psychological stress in humans. Indeed, data from both animal and human studies suggest that repeated and/or prolonged exposure to elevated levels of neurosteroids in response to chronic stress has the potential to result in long-term alterations in neurosteroid concentrations, and possible alterations in the plasticity and functioning of GABAAR’s, resulting in altered GABAergic transmission and potential dysregulation of the HPA-axis. If this is the case, impairment in neurosteroid-HPA-axis regulation could well play an etiopathological role in stress-related illness.
Implications of Neurosteroid – HPA-axis Regulation for Human Stress Related Illness
Major Depressive Disorder
Major depressive disorder (MDD) is one of the most extensively studied stress-related disorders in human psychopathology, and an apt model for investigation of neurosteroid-mediated HPA-axis dysregulation in the context of allostatic load. Epidemiological evidence suggests that depression is associated with increased daily stress (D'Angelo and Wierzbicki 2003; Hutchinson and Williams 2007; Wichers et al. 2009), and both new onset depression and risk of depression recurrence is increased by stressful life events (Bifulco et al. 1998; Brilman and Ormel 2001; Kendler et al. 2004). The olfactory bulbectomy (OB) model of depression (created by bilateral lesioning of the olfactory bulbs in animals) is considered one of the most valid animal models of human depression since the model produces behavioral and neuroendocrine alterations similar to those observed in depressed patients (Harkin et al. 2003; Kelly et al. 1997; Leonard and Tuite 1981; Leonard 1984; Song and Leonard 2005). In rats, OB significantly increases peripherally measured corticosterone (Harkin et al. 2003; Marcilhac et al. 1999) and significantly decreases centrally measured levels of ALLO (Uzunova et al. 2003). This decrease in ALLO has been hypothesized to reflect a distinct pathophysiological mechanism underlying the observed depressive-like behaviors associated with induced bulbectomy syndrome in rats (Uzunova et al. 2003), suggesting an etiopathological link among reduced neurosteroid function, dysregulation in HPA-axis activity, and depressive disorders.
In humans, both plasma and CSF ALLO concentrations are consistently decreased in patients with MDD (Eser et al. 2006; Nappi et al. 2001; Romeo et al. 1998; Strohle et al. 1999; Strohle et al. 2000; Uzunova et al. 1998; Uzunova et al. 2006), while basal cortisol levels are elevated (Young et al. 2004a; Young et al. 2004b; Young and Veldhuis 2006). In contrast, results from studies of DHEA and DHEA-S concentrations in individuals with depression, have shown them to be elevated (Hansen et al. 1982; Heuser et al. 1998; Takebayashi et al. 1998), decreased (Barrett-Connor et al. 1999; Michael et al. 2000; Morsink et al. 2007; Wong et al. 2011) or unchanged (Erdincler et al. 2004; Fabian et al. 2001), compared to controls. However, higher cortisol to DHEA ratios have been more consistently observed in individuals with depression vs. healthy controls (Markopoulou et al. 2009; Michael et al. 2000; Young et al. 2002). Higher cortisol/DHEA ratios are associated with longer duration of depressive illness (Young et al. 2002) and predictive of persistent MDD in first episode patients (Goodyer et al. 2003).
Individual differences in age, sex, or race may exert profound effects on the relationship of DHEA or DHEA-S concentration to depressive symptomatology (van Broekhoven and Verkes 2003). This is particularly well-illustrated in a cross-sectional study conducted by Morrison and colleagues (2001) which investigated the association between DHEA-S concentrations and depressive symptoms in adult women (ages 35–47y). In this study, the authors found that DHEA-S concentrations were inversely associated with depressive symptoms in women in the older half of their cohort, while DHEA-S concentrations were positively associated with depressive symptoms in the younger women. Interestingly, in AA women, the association between DHEA-S and depressive symptoms transitioned from a positive association to an inverse association at a younger age, compared to nHW women. Thus, in line with our prior work on ALLO reactivity to acute stress in AA women (described above), race may influence associations among neurosteroids, the HPA-axis, and risk for stress-related psychopathology.
The clinical relevance of neurosteroid concentrations to MDD is derived from studies showing inverse relationships between ALLO concentrations and the severity of depressive illness (Nappi et al. 2001; Uzunova et al. 1998), and from studies showing that clinically efficacious treatment for MDD is associated with increases in ALLO (Romeo et al. 1998; Strohle et al. 1999; Strohle et al. 2000; Uzunova et al. 1998). Hypercortisolemia resulting from chronic activation of the HPA-axis, including greater CRH concentrations and gene expression (Gillespie and Nemeroff 2005; Nemeroff 1998; Plotsky et al. 1998; Raadsheer et al. 1994), is implicated in the pathophysiology of depressive disorders (Nemeroff 1998). Given the role of neurosteroids as endogenous negative modulators of the HPA-axis, HPA-axis hyperactivity in many individuals with MDD may be reflective of an eventual depletion in neurosteroid synthesis resulting from chronically heightened HPA activation, as seen in animal models of neurosteroid depletion associated with chronic stress (Serra et al. 2000). Therefore, the ability of ALLO (or other neurosteroids) to limit the extent and duration of reduction in GABAergic inhibitory transmission is reduced, allowing the HPA-axis to become overactive.
It should be noted, however, that not all studies report hypercortisolemia in MDD (Ahrens et al. 2008; Appelhof et al. 2006; Barden 2004; Bockting et al. 2012; Gillespie and Nemeroff 2005; Zobel et al. 2001). Heterogeneity due to potential methodological differences in study design and/or cortisol sampling is beyond the scope of this review; however, viewed from an allostatic load perspective, differences in HPA-axis activation may also be representative of different disease (allostatic) states of the individual. This is supported by evidence that, in humans, initial HPA-axis hyperactivity associated with stress is reduced in response to prolonged chronic stress exposure, and may even manifest as blunted HPA-axis activation over time (Miller et al. 2007). Additionally, higher tonic activation of the HPA-axis following chronic stress may lead to blunted cortisol reactions to acute stress, due to a “ceiling effect,” and thereby inhibit the potential for appropriate HPA-axis stress reactivity (Richards et al. 2011). This phenomenon has been demonstrated in patients with MDD (Burke et al. 2005). Therefore, context of disease state (e.g., first episode depression vs. recurrent depression, or history of chronic life stress) may play an influential role in neurosteroid regulation of the HPA-axis, thereby influencing whether patients with depression exhibit hyper- or hypo-cortisolemia relative to controls.
Our laboratory has assessed neurosteroid stress reactivity in euthymic women with or without a history of depression, and some of that research has included HPA-axis assessment. In our first study (Klatzkin et al. 2006b), though there were no diagnosis-related differences in resting ALLO concentrations, women with a history of depression had a blunted ALLO response to mental stress relative to never depressed women. We also observed that, in contrast to never depressed controls, there was a lack of positive correlation between ALLO and progesterone concentrations in women with depression histories. This finding is consistent with a report in women with current postpartum depression who also failed to show the expected relationship between ALLO and progesterone (Nappi et al. 2001). We concluded at the time that these findings may reflect depression-related alterations in the conversion of progesterone to neuroactive steroids (Strohle et al. 1999; Strohle et al. 2000). Consequently, our subsequent study tested women in the early follicular phase of their menstrual cycle (low endogenous neurosteroids) following oral micronized progesterone (Klatzkin et al. 2006a). Histories of depression predicted lower plasma concentrations of both ALLO and progesterone over 255 minutes. However, contrary to expectations, our analyses of the ALLO/progesterone ratio as an index of metabolism revealed no evidence for depression-related differences and, consequently, did not suggest alterations in the conversion of progesterone to ALLO in women with histories of depression.
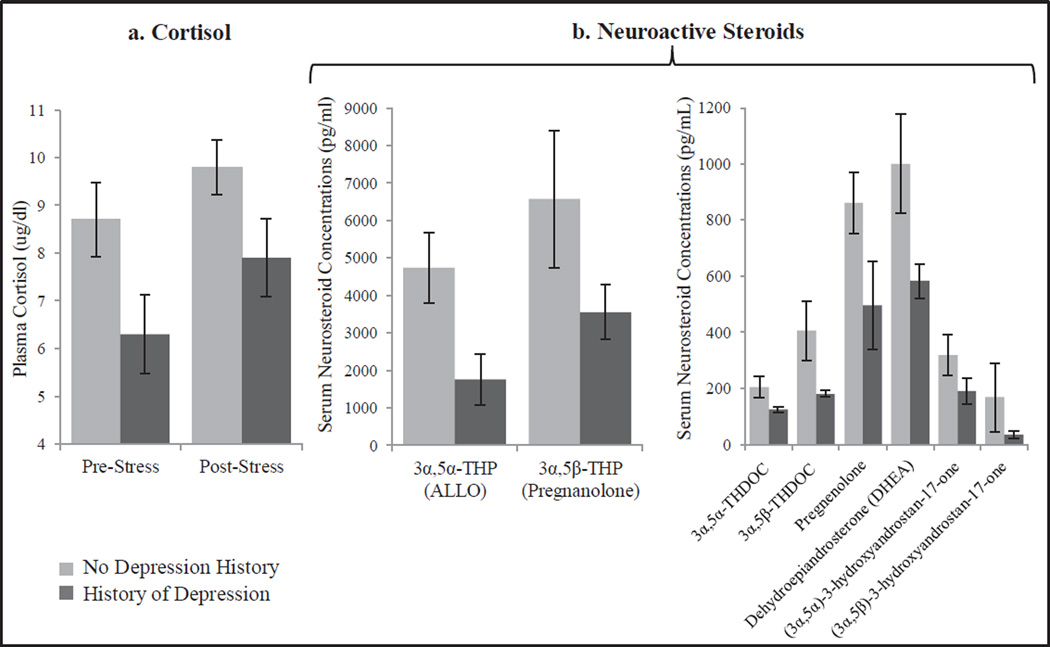
While we were unable to elucidate mechanisms contributing to the lower plasma ALLO following oral progesterone in that study, our subsequent report (Girdler et al. 2012), based on secondary analyses of the progesterone challenge study described above, assessed a broader array of GABAergic neuroactive steroids using GC-MS methods. The primary finding of those analyses was a generalized reduction in GABAergic neuroactive steroid concentrations (both before and after the oral progesterone challenge) in women with histories of depression relative to never depressed women. This was true not only for ALLO and ALLO-THDOC, but for other progesterone derived neuroactive steroids and their precursor, pregnenolone; as well as for androstenedione-derived neuroactive steroids and their precursor, DHEA (see Figure 2). We also observed that women with a depression history had lower cortisol at rest and in response to stress than never depressed controls. The most parsimonious explanation for the findings was one of adrenal suppression since the adrenals are the main secretory source of progesterone during the early follicular phase (the time of testing in this study). Adrenal suppression is also supported, in part, by the finding for lower rest and stress cortisol concentrations in those with a history of depression, and is consistent with other research in euthymic women with histories of depression who show lower resting cortisol and a blunted cortisol and ACTH response to mental stress relative to never depressed women (Ahrens et al. 2008). We hypothesized that the pattern of diminished GABAergic neuroactive steroid and cortisol concentrations at rest and in response to challenge reflects a physiologic adaptation resulting from depression and/or chronic stress, though the absence of measures of psychosocial stress limit that interpretation.
Fig. 2.
Mean (+/− SEM) plasma cortisol pre and post- laboratory mental stress challenge (a) and serum GABAergic neurosteroids following progesterone challenge (b) in women with (n=11) or without (n=17) a history of depression
Regardless of mechanism, a remarkable feature of these findings is for persistent disturbance in neurosteroid and HPA-axis function in women who were in remission for, on average, five years. If a history of depressive illness contributes to long-term alterations in neuroactive steroid concentrations, and potentially to neuroactive steroid regulation of the HPA-axis, this may represent one mechanism contributing to the increased risk of recurrent depression in those with a history of depression (Lewinsohn et al. 1989).
Premenstrual Dysphoric Disorder
Evidence from both animal and human studies suggest that periods of neurosteroid fluctuation (such as during the ovarian cycle) may be linked to alterations in the sensitivity of GABAAR’s to neurosteroids (Turkmen et al. 2011), resulting in mood instability in vulnerable women, such as those with premenstrual dysphoric disorder (PMDD). PMDD is a depressive disorder, characterized by the cyclic recurrence of emotional and physical symptoms during the luteal phase of the menstrual cycle that are of sufficient severity to interfere with function, but remit with the onset of menses. PMDD afflicts 5 – 10% of women in their reproductive years (Cohen et al. 2002). Since plasma levels of ALLO follow closely those of progesterone during the luteal phase, substantial interest has existed in investigating neurosteroid-related pathophysiology in PMDD. Overall, findings from studies comparing differences in basal concentrations of neurosteroids between women with PMDD and controls have been mixed (Girdler et al. 2001; Lombardi et al. 2004; Monteleone et al. 2000; Rapkin et al. 1997; Schmidt et al. 1994; Segebladh et al. 2013; Wang et al. 1996), and research which has included investigation of DHEA/DHEA-S in PMDD has been limited to two small studies (Eriksson et al. 1992; Lombardi et al. 2004). However, evidence does suggest that women with PMDD exhibit differences in GABAAR sensitivity to neurosteroids (Kask et al. 2008). In women with PMDD, greater symptom severity predicts reduced sensitivity to positive allosteric modulators of the GABAAR including pregnanolone, benzodiazepines, and alcohol (Sundstrom et al. 1997a; Sundstrom et al. 1997b; Sundstrom et al. 1998).
The research of Sundstrom and colleagues has demonstrated that women with PMDD show less of a reduction in SEV (a sensitive measure of benzodiazepine/GABAAR sensitivity) and report less sedation in response to benzodiazepines relative to healthy controls (Sundstrom et al. 1997a; Sundstrom et al. 1997b; Sundstrom et al. 1998). Since there is no evidence that PMDD women differ in the density or affinity of peripheral benzodiazepine receptors, at least as measured on lymphocytes (Daly et al. 2001), these results suggest a diminished functional sensitivity of GABAARs in PMDD. Moreover, the work of Le Melledo et al. (2000) has shown that exposure to flumazenil, a benzodiazepine receptor antagonist, in the luteal phase elicits marked increases in panic symptoms in PMDD women but not in non-PMDD controls. This effect is consistent with a shift in benzodiazepine sensitivity toward inverse agonism, as is seen with panic patients exposed to flumazenil (Nutt et al. 1990). Although speculative at present, the rodent animal model work of Smith and colleagues suggests that alterations in GABAAR function may reverse the anxiolytic effects of luteal phase increases in ALLO and trigger dysphoric mood states (Smith et al. 2006).
Since ALLO and ALLO-THDOC are stress responsive, and since women with PMDD report more stressful life events (Girdler et al. 1993; Woods et al. 1997) and have substantially higher rates of sexual and physical abuse histories than non-PMDD women (Girdler et al. 1998; Girdler et al. 2003; Girdler et al. 2007; Golding et al. 2000; Paddison et al. 1990), it became of interest to us to examine whether neurosteroid reactivity to stress is altered in PMDD. We assessed luteal phase ALLO following an extended rest and 17 minutes after the onset of a mental stress and found that PMDD women displayed significantly higher plasma ALLO and lower plasma cortisol concentrations at rest than controls (Girdler et al. 2001), the latter being consistent with the majority of the literature suggesting blunted HPA-axis function in PMDD (Girdler et al. 1998; Klatzkin et al. 2010; Rabin et al. 1990; Redei and Freeman 1993). Consistent with a negative modulatory role of ALLO on the HPA-axis, ALLO concentrations were negatively correlated with plasma cortisol concentrations both at rest and in response to stress in the entire sample. These results are consistent with a recent report on diurnal ALLO and cortisol concentrations in PMDD women tested in the late luteal phase (Segebladh et al. 2013) since PMDD patients with higher morning ALLO had blunted nocturnal cortisol concentrations relative to controls with low morning ALLO concentrations.
Regarding ALLO stress reactivity, we found that 83% of controls showed the expected stress-induced increase in ALLO, while only 42% of PMDD women showed an increase. Moreover, lack of ALLO responsiveness to stress in PMDD women was related to their greater baseline concentrations since ALLO reactivity and baseline concentrations were negatively correlated in the PMDD group but not in the controls. Our finding for blunted ALLO stress reactivity in PMDD is consistent with the work of Monteleone et al. (2000) and Lombardi et al. (2004). Despite the fact that those investigators reported lower, and not higher, baseline ALLO in PMDD, in response to an ACTH stimulation test following dexamethasone suppression, PMDD women had a blunted adrenal ALLO response (Lombardi et al. 2004); and blunted ovarian ALLO response to a GnRH challenge relative to controls (Monteleone et al. 2000).
Taken together, the results of the studies assessing both ALLO and HPA-axis measures in PMDD are consistent with a negative modulatory role of ALLO on the HPA-axis (Girdler et al. 2001; Segebladh et al. 2013). However, to the extent that ALLO reactivity to stress is blunted in women with PMDD, these results also suggest an impaired GABAAR mediated response to acute stress in PMDD. Subsequent work from our laboratory indicated that the pathophysiological role of altered ALLO reactivity to stress is especially apparent in PMDD women who also have a history of MDD (Klatzkin et al. 2006b). Only in PMDD women with a MDD history did ALLO stress reactivity predict the severity of premenstrual symptoms. However, since 45 – 60% of PMDD women have a history of MDD (Cohen et al. 2002; Pearlstein et al. 1990), MDD-related alterations in ALLO reactivity has special relevance to this population and is consistent with a heterogeneous etiology to the disorder (Halbreich 2003).
Conclusions and Future Directions
It is evident that neurosteroids play important roles in GABAergic, antiglucocorticoid, and other homeostatic mechanisms involved in the regulation of HPA-axis activity following stress. Indeed, the influence of neurosteroids on human stress-related illness comes from evidence that neurosteroids function as allostatic mediators in the homeostatic control of the HPA-axis, and that both neurosteroids and GABAAR’s are susceptible to long-term alterations in the context of stressful conditions, particularly if chronic.
The focus of this review centered on ALLO, ALLO-THDOC, DHEA and DHEA-S and their roles in the regulation of the HPA-axis response to stress because the human literature to date has almost exclusively focused on these neurosteroids with regards to HPA axis modulation. However, basic science research has demonstrated that a variety of other neuroactive steroids can exert both positive and negative modulation of GABAergic transmission. Therefore, these other neuroactive steroids are additional physiologic regulators of central nervous system excitability (Morrow 2007). GC – MS methods now allow for the simultaneous detection of multiple progesterone-derived neurosteroids and androstenedione-derived neuroactive steroids using small quantities of human serum (Porcu et al. 2009). Given the stress-responsiveness of neuroactive steroids (Purdy et al. 1991), their role in HPA-axis regulation (Morrow et al. 1995), and their effects on behavior (van Broekhoven and Verkes 2003), the field is poised to investigate the inter-relationships among other neuroactive steroids, and their relationship to HPA-axis function and the expression of dysphoric symptomology.
Based on the evidence that impairment in neurosteroid regulation of the HPA-axis may play a role in symptoms of anxiety, depression, as well as irritability, all common features to many psychiatric diagnostic categories, neurosteroid-HPA-axis regulation may represent a fundamental pathophysiological mechanism cutting across multiple psychopathologies. However, prior to informing our understanding of how neurosteroid-HPA-axis regulation contributes to dimensional aspects of psychopathology, more fundamental research must be done on this regulation in healthy humans. This would include establishing a reliable neurosteroid acute stress response profile, as has been done for the HPA-axis response to social stress (Kirschbaum et al. 1993; Kirschbaum et al. 1995a; Kirschbaum et al. 1995b; Young et al. 2000), and including concurrent measures of HPA-axis function as well as mood and behavior. Finally, prospective studies are needed which assess neurosteroid regulation of HPA-axis activity in the context of adaptation, in order to begin to untangle the complex relationships underlying the transition from adaptive stress mechanisms to stress response dysregulation in the etiology of human psychopathology.
Acknowledgments
This research was supported by NIH R01-MH081837 and T32-MH093315.
Footnotes
The authors declare that they have no conflicts of interest.
References
- Agis-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci U S A. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens T, Deuschle M, Krumm B, van der Pompe G, den Boer JA, Lederbogen F. Pituitary-adrenal and sympathetic nervous system responses to stress in women remitted from recurrent major depression. Psychosom Med. 2008;70:461–467. doi: 10.1097/PSY.0b013e31816b1aaa. [DOI] [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Mechanisms of neurosteroid interactions with GABA(A) receptors. Pharmacol Ther. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M, Redwine LS, Leong YM, Frye CA, Porges SW, Carter CS. Responses to laboratory psychosocial stress in postpartum women. Psychosom Med. 2001;63:814–821. doi: 10.1097/00006842-200109000-00015. [DOI] [PubMed] [Google Scholar]
- Amato RJ, Lewis PB, He H, Winsauer PJ. Effects of positive and negative modulators of the gamma-aminobutyric acid A receptor complex on responding under a differential-reinforcement-of-low-rate schedule of reinforcement in rats. Behav Pharmacol. 2010;8:8. doi: 10.1097/FBP.0b013e32833fa7c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhof BC, Huyser J, Verweij M, Brouwer JP, van Dyck R, Fliers E, Hoogendijk WJ, Tijssen JG, Wiersinga WM, Schene AH. Glucocorticoids and relapse of major depression (dexamethasone/corticotropin-releasing hormone test in relation to relapse of major depression) Biol Psychiatry. 2006;59:696–701. doi: 10.1016/j.biopsych.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Bixo M, Nyberg S, Savic I. Increased neurosteroid sensitivity--an explanation to symptoms associated with chronic work related stress in women? Psychoneuroendocrinology. 2013;38:1078–1089. doi: 10.1016/j.psyneuen.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Purdy RH, Mostallino MC, Concas A, Biggio G. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br J Pharmacol. 1997;120:1582–1588. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Concas A, Serra M, Biggio G. Stress and neurosteroids in adult and aged rats. Exp Gerontol. 1998;33:697–712. doi: 10.1016/s0531-5565(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci. 2004;29:185–193. [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E, von Muhlen D, Laughlin GA, Kripke A. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc. 1999;47:685–691. doi: 10.1111/j.1532-5415.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Baulieu E. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bellingrath S, Weigl T, Kudielka BM. Chronic work stress and exhaustion is associated with higher allostastic load in female school teachers: Original Research Report. Stress: The International Journal on the Biology of Stress. 2009;12:37–48. doi: 10.1080/10253890802042041. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown G, Moran P, Ball C, Campbell C. Predicting depression in women: the role of past and present vulnerability. Psychological Medicine. 1998;28:39–50. doi: 10.1017/s0033291797005953. [DOI] [PubMed] [Google Scholar]
- Biggio G, Concas A, Follesa P, Sanna E, Serra M. Stress, ethanol, and neuroactive steroids. Pharmacol Ther. 2007;116:140–171. doi: 10.1016/j.pharmthera.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau MA, Adams LF, Rubinow DR. Dehydroepiandrosterone treatment of midlife dysthymia. Biol Psychiatry. 1999;45:1533–1541. doi: 10.1016/s0006-3223(99)00066-9. [DOI] [PubMed] [Google Scholar]
- Bockting CL, Lok A, Visser I, Assies J, Koeter MW, Schene AH. Lower cortisol levels predict recurrence in remitted patients with recurrent depression: a 5.5 year prospective study. Psychiatry Res. 2012;200:281–287. doi: 10.1016/j.psychres.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilman EI, Ormel J. Life events, difficulties and onset of depressive episodes in later life. Psychol Med. 2001;31:859–869. doi: 10.1017/s0033291701004019. [DOI] [PubMed] [Google Scholar]
- Browne ES, Wright BE, Porter JR, Svec F. Dehydroepiandrosterone: antiglucocorticoid action in mice. Am J Med Sci. 1992;303:366–371. doi: 10.1097/00000441-199206000-00003. [DOI] [PubMed] [Google Scholar]
- Buckingham JC. Glucocorticoids: exemplars of multi-tasking. Br J Pharmacol. 2006;147:S258–S268. doi: 10.1038/sj.bjp.0706456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology. 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Van Dam NT, de Wit H. Effects of acute progesterone administration upon responses to acute psychosocial stress in men. Exp Clin Psychopharmacol. 2010;18:78–86. doi: 10.1037/a0018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABA(A) receptors. Trends Neurosci. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Otto MW, Sweeney BH, Liberman RF, Harlow BL. Prevalence and predictors of premenstrual dysphoric disorder (PMDD) in older premenopausal women. The Harvard Study of Moods and Cycles. J Affect Disord. 2002;70:125–132. doi: 10.1016/s0165-0327(01)00458-x. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. 2008;213:63–72. doi: 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- Daly RC, Schmidt PJ, Davis CL, Danaceau MA, Rubinow DR. Effects of gonadal steroids on peripheral benzodiazepine receptor density in women with PMS and controls. Psychoneuroendocrinology. 2001;26:539–549. doi: 10.1016/s0306-4530(01)00005-1. [DOI] [PubMed] [Google Scholar]
- D'Angelo B, Wierzbicki M. Relations of daily hassles with both anxious and depressed mood in students. Psychological reports. 2003;92:416–418. doi: 10.2466/pr0.2003.92.2.416. [DOI] [PubMed] [Google Scholar]
- Decavel C, Van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological bulletin. 2004;130:355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci U S A. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogleever Fortuyn HA, van Broekhoven F, Span PN, Backstrom T, Zitman FG, Verkes RJ. Effects of PhD examination stress on allopregnanolone and cortisol plasma levels and peripheral benzodiazepine receptor density. Psychoneuroendocrinology. 2004;29:1341–1344. doi: 10.1016/j.psyneuen.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Dubrovsky B. Neurosteroids, neuroactive steroids, and symptoms of affective disorders. Pharmacology Biochemistry and Behavior. 2006;84:644–655. doi: 10.1016/j.pbb.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behav Pharmacol. 2007;18:461–470. doi: 10.1097/FBP.0b013e3282d28f6f. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behav Pharmacol. 2007;18:461–470. doi: 10.1097/FBP.0b013e3282d28f6f. [DOI] [PubMed] [Google Scholar]
- Erdincler D, Bugay G, Ertan T, Eker E. Depression and sex hormones in elderly women. Arch Gerontol Geriatr. 2004;39:239–244. doi: 10.1016/j.archger.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Eriksson E, Sundblad C, Lisjo P, Modigh K, Andersch B. Serum levels of androgens are higher in women with premenstrual irritability and dysphoria than in controls. Psychoneuroendocrinology. 1992;17:195–204. doi: 10.1016/0306-4530(92)90058-f. [DOI] [PubMed] [Google Scholar]
- Eser D, di Michele F, Zwanzger P, Pasini A, Baghai TC, Schule C, Rupprecht R, Romeo E. Panic induction with cholecystokinin-tetrapeptide (CCK-4) Increases plasma concentrations of the neuroactive steroid 3alpha, 5alpha tetrahydrodeoxycorticosterone (3alpha, 5alpha-THDOC) in healthy volunteers. Neuropsychopharmacology. 2005;30:192–195. doi: 10.1038/sj.npp.1300572. [DOI] [PubMed] [Google Scholar]
- Eser D, Romeo E, Baghai TC, di Michele F, Schule C, Pasini A, Zwanzger P, Padberg F, Rupprecht R. Neuroactive steroids as modulators of depression and anxiety. Neuroscience. 2006;138:1041–1048. doi: 10.1016/j.neuroscience.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Evans J, Sun Y, McGregor A, Connor B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology. 2012;63:1315–1326. doi: 10.1016/j.neuropharm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Fabian TJ, Dew MA, Pollock BG, Reynolds CF, 3rd, Mulsant BH, Butters MA, Zmuda MD, Linares AM, Trottini M, Kroboth PD. Endogenous concentrations of DHEA and DHEA-S decrease with remission of depression in older adults. Biol Psychiatry. 2001;50:767–774. doi: 10.1016/s0006-3223(01)01198-2. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5alpha-pregnan-3alpha-ol-20-one (3alpha,5alpha-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3alpha,5alpha-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006;18:960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Gangisetty O, Reddy DS. Neurosteroid withdrawal regulates GABA-A receptor alpha4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience. 2010;170:865–880. doi: 10.1016/j.neuroscience.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside SE, Griffith NC, Kaura V, Ingram CD. The neurosteroid dehydroepiandrosterone (DHEA) and its metabolites alter 5-HT neuronal activity via modulation of GABAA receptors. J Psychopharmacol. 2010;24:1717–1724. doi: 10.1177/0269881109105836. [DOI] [PubMed] [Google Scholar]
- Genazzani AD, Stomati M, Bernardi F, Pieri M, Rovati L, Genazzani AR. Long-term low-dose dehydroepiandrosterone oral supplementation in early and late postmenopausal women modulates endocrine parameters and synthesis of neuroactive steroids. Fertil Steril. 2003;80:1495–1501. doi: 10.1016/j.fertnstert.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Journal Information. 2006;96 doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med. 2005;67:S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Pedersen CA, Stern RA, Light KC. Menstrual cycle and premenstrual syndrome: modifiers of cardiovascular reactivity in women. Health Psychol. 1993;12:180–192. doi: 10.1037//0278-6133.12.3.180. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Pedersen CA, Straneva PA, Leserman J, Stanwyck CL, Benjamin S, Light KC. Dysregulation of cardiovascular and neuroendocrine responses to stress in premenstrual dysphoric disorder. Psychiatry Res. 1998;81:163–178. doi: 10.1016/s0165-1781(98)00074-2. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biological Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Sherwood A, Hinderliter AL, Leserman J, Costello NL, Straneva PA, Pedersen CA, Light KC. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom Med. 2003;65:849–856. doi: 10.1097/01.psy.0000088593.38201.cd. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Beth Mechlin M, Light KC, Leslie Morrow A. Ethnic differences in allopregnanolone concentrations in women during rest and following mental stress. Psychophysiology. 2006;43:331–336. doi: 10.1111/j.1469-8986.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Leserman J, Bunevicius R, Klatzkin R, Pedersen CA, Light KC. Persistent alterations in biological profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health Psychol. 2007;26:201–213. doi: 10.1037/0278-6133.26.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Lindgren M, Porcu P, Rubinow DR, Johnson JL, Morrow AL. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology. 2012;37:543–553. doi: 10.1016/j.psyneuen.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding JM, Taylor DL, Menard L, King MJ. Prevalence of sexual abuse history in a sample of women seeking treatment for premenstrual syndrome. J Psychosom Obstet Gynaecol. 2000;21:69–80. doi: 10.3109/01674820009075612. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A. Psychoendocrine antecedents of persistent first-episode major depression in adolescents: a community-based longitudinal enquiry. Psychol Med. 2003;33:601–610. doi: 10.1017/s0033291702007286. [DOI] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joels M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J Endocrinol. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- Guazzo E, Kirkpatrick P, Goodyer I, Shiers H, Herbert J. Cortisol, dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: relation to blood levels and the effects of age. Journal of Clinical Endocrinology & Metabolism. 1996;81:3951–3960. doi: 10.1210/jcem.81.11.8923843. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5alpha-dihydroprogesterone in psychiatric disorders. Brain Res Brain Res Rev. 2001;37:110–115. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- Guo AL, Petraglia F, Criscuolo M, Ficarra G, Nappi RE, Palumbo MA, Trentini GP, Purdy RH, Genazzani AR. Evidence for a role of neurosteroids in modulation of diurnal changes and acute stress-induced corticosterone secretion in rats. Gynecol Endocrinol. 1995;9:1–7. doi: 10.3109/09513599509160184. [DOI] [PubMed] [Google Scholar]
- Halbreich U. The etiology, biology, and evolving pathology of premenstrual syndromes. Psychoneuroendocrinology. 2003;3:55–99. doi: 10.1016/s0306-4530(03)00097-0. [DOI] [PubMed] [Google Scholar]
- Hansen CR, Jr, Kroll J, Mackenzie TB. Dehydroepiandrosterone and affective disorders. 1982 doi: 10.1176/ajp.139.3.386a. [DOI] [PubMed] [Google Scholar]
- Harkin A, Kelly JP, Leonard BE. A review of the relevance and validity of olfactory bulbectomy as a model of depression. Clinical Neuroscience Research. 2003;3:253–262. [Google Scholar]
- Hennebert O, Chalbot S, Alran S, Morfin R. Dehydroepiandrosterone 7alpha-hydroxylation in human tissues: possible interference with type 1 11beta-hydroxysteroid dehydrogenase-mediated processes. J Steroid Biochem Mol Biol. 2007;104:326–333. doi: 10.1016/j.jsbmb.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors. Pharmacol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP. Neural control of chronic stress adaptation. Front Behav Neurosci. 2013;7:00061. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser I, Deuschle M, Luppa P, Schweiger U, Standhardt H, Weber B. Increased diurnal plasma concentrations of dehydroepiandrosterone in depressed patients. J Clin Endocrinol Metab. 1998;83:3130–3133. doi: 10.1210/jcem.83.9.5081. [DOI] [PubMed] [Google Scholar]
- Hornsby PJ. Biosynthesis of DHEAS by the Human Adrenal Cortex and Its Age-Related Decline. Annals of the New York Academy of Sciences. 1995;774:29–46. doi: 10.1111/j.1749-6632.1995.tb17370.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson JG, Williams PG. Neuroticism, daily hassles, and depressive symptoms: An examination of moderating and mediating effects. Personality and Individual Differences. 2007;42:1367–1378. [Google Scholar]
- Imamura M, Prasad C. Modulation of GABA-gated chloride ion influx in the brain by dehydroepiandrosterone and its metabolites. Biochem Biophys Res Commun. 1998;243:771–775. doi: 10.1006/bbrc.1998.8177. [DOI] [PubMed] [Google Scholar]
- Izawa S, Sugaya N, Shirotsuki K, Yamada KC, Ogawa N, Ouchi Y, Nagano Y, Suzuki K, Nomura S. Salivary dehydroepiandrosterone secretion in response to acute psychosocial stress and its correlations with biological and psychological changes. Biol Psychol. 2008;79:294–298. doi: 10.1016/j.biopsycho.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Izawa S, Saito K, Shirotsuki K, Sugaya N, Nomura S. Effects of prolonged stress on salivary cortisol and dehydroepiandrosterone: a study of a two-week teaching practice. Psychoneuroendocrinology. 2012;37:852–858. doi: 10.1016/j.psyneuen.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Jeckel CM, Lopes RP, Berleze MC, Luz C, Feix L, Argimon II, Stein LM, Bauer ME. Neuroendocrine and immunological correlates of chronic stress in 'strictly healthy' populations. Neuroimmunomodulation. 2010;17:9–18. doi: 10.1159/000243080. [DOI] [PubMed] [Google Scholar]
- Jones MT, Gillham B, Altaher AR, Nicholson SA, Campbell EA, Watts SM, Thody A. Clinical and experimental studies on the role of GABA in the regulation of ACTH secretion: a review. Psychoneuroendocrinology. 1984;9:107–123. doi: 10.1016/0306-4530(84)90030-1. [DOI] [PubMed] [Google Scholar]
- Kalia M. Assessing the economic impact of stress[mdash] The modern day hidden epidemic. Metabolism. 2002;51:49–53. doi: 10.1053/meta.2002.33193. [DOI] [PubMed] [Google Scholar]
- Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA) Mol Cell Biochem. 1994;131:99–104. doi: 10.1007/BF00925945. [DOI] [PubMed] [Google Scholar]
- Kask K, Gulinello M, Backstrom T, Geyer MA, Sundstrom-Poromaa I. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33:2283–2290. doi: 10.1038/sj.npp.1301599. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Wiebe JP. Analgesic effects of the progesterone metabolite, 3 alpha-hydroxy-5 alpha-pregnan-20-one, and possible modes of action in mice. Brain Res. 1987;415:393–398. doi: 10.1016/0006-8993(87)90228-9. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom Med. 1995a;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology. 1995b;20:509–514. doi: 10.1016/0306-4530(94)00078-o. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006a;31:1208–1219. doi: 10.1016/j.psyneuen.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Histories of depression, allopregnanolone responses to stress, and premenstrual symptoms in women. Biol Psychol. 2006b;71:2–11. doi: 10.1016/j.biopsycho.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Lindgren ME, Forneris CA, Girdler SS. Histories of major depression and premenstrual dysphoric disorder: Evidence for phenotypic differences. Biol Psychol. 2010;84:235–247. doi: 10.1016/j.biopsycho.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer RR, Synovitz LB, Gimpel T, Kraemer GR, Johnson LG, Castracane VD. Effect of estrogen on serum DHEA in younger and older women and the relationship of DHEA to adiposity and gender. Metabolism. 2001;50:488–493. doi: 10.1053/meta.2001.21036. [DOI] [PubMed] [Google Scholar]
- Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999;39:327–348. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, Bunker AM, Meikle AW. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56:1138–1147. doi: 10.1373/clinchem.2010.143222. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Belanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- Lac G, Dutheil F, Brousse G, Triboulet-Kelly C, Chamoux A. Saliva DHEAS changes in patients suffering from psychopathological disorders arising from bullying at work. Brain Cogn. 2012;80:277–281. doi: 10.1016/j.bandc.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Le Melledo JM, Van Driel M, Coupland NJ, Lott P, Jhangri GS. Response to flumazenil in women with premenstrual dysphoric disorder. Am J Psychiatry. 2000;157:821–823. doi: 10.1176/appi.ajp.157.5.821. [DOI] [PubMed] [Google Scholar]
- Lennartsson AK, Kushnir MM, Bergquist J, Jonsdottir IH. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biol Psychol. 2012;90:143–149. doi: 10.1016/j.biopsycho.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Lennartsson AK, Theorell T, Kushnir MM, Bergquist J, Jonsdottir IH. Perceived stress at work is associated with attenuated DHEA-S response during acute psychosocial stress. Psychoneuroendocrinology. 2013;38:1650–1657. doi: 10.1016/j.psyneuen.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Leonard BE, Tuite M. Anatomical, physiological, and behavioral aspects of olfactory bulbectomy in the rat. Int Rev Neurobiol. 1981;22:251–286. doi: 10.1016/s0074-7742(08)60295-0. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The olfactory bulbectomized rat as a model of depression. Pol J Pharmacol Pharm. 1984;36:561–569. [PubMed] [Google Scholar]
- Lewinsohn PM, Zeiss AM, Duncan EM. Probability of relapse after recovery from an episode of depression. J Abnorm Psychol. 1989;98:107–116. doi: 10.1037//0021-843x.98.2.107. [DOI] [PubMed] [Google Scholar]
- Lombardi I, Luisi S, Quirici B, Monteleone P, Bernardi F, Liut M, Casarosa E, Palumbo M, Petraglia F, Genazzani AR. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gynecol Endocrinol. 2004;18:79–87. doi: 10.1080/09513590310001652955. [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan R, Touati-Werner D, Ram E, Strous R, Keren O, Weizman A. The protective effect of frontal cortex dehydroepiandrosterone in anxiety and depressive models in mice. Pharmacology Biochemistry and Behavior. 2006;85:415–421. doi: 10.1016/j.pbb.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maninger N, Capitanio JP, Mason WA, Ruys JD, Mendoza SP. Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology. 2010;35:1055–1062. doi: 10.1016/j.psyneuen.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Dorn LD, Susman EJ. Stress and puberty-related hormone reactivity, negative emotionality, and parent--adolescent relationships. Psychoneuroendocrinology. 2012;37:1286–1298. doi: 10.1016/j.psyneuen.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Marcilhac A, Anglade G, Hery F, Siaud P. Olfactory bulbectomy increases vasopressin, but not corticotropin-releasing hormone, content in the external layer of the median eminence of male rats. Neurosci Lett. 1999;262:89–92. doi: 10.1016/s0304-3940(98)00981-1. [DOI] [PubMed] [Google Scholar]
- Markopoulou K, Papadopoulos A, Juruena MF, Poon L, Pariante CM, Cleare AJ. The ratio of cortisol/DHEA in treatment resistant depression. Psychoneuroendocrinology. 2009;34:19–26. doi: 10.1016/j.psyneuen.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Pinna G, Puia G, Guidotti A, Costa E. Social isolation stress-induced aggression in mice: a model to study the pharmacology of neurosteroidogenesis. Stress. 2005;8:85–93. doi: 10.1080/10253890500159022. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Getz L. Lifetime experiences, the brain and personalized medicine: An integrative perspective. Metabolism. 2013;62(Supplement 1):S20–S26. doi: 10.1016/j.metabol.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Meczekalski B, Tonetti A, Monteleone P, Bernardi F, Luisi S, Stomati M, Luisi M, Petraglia F, Genazzani AR. Hypothalamic amenorrhea with normal body weight: ACTH, allopregnanolone and cortisol responses to corticotropin-releasing hormone test. European journal of endocrinology. 2000;142:280–285. doi: 10.1530/eje.0.1420280. [DOI] [PubMed] [Google Scholar]
- Menozzi R, Florio P, Bondi M, Luisi S, Cobellis L, Genazzani AR, Del Rio G, Petraglia F. Increased response of plasma allopregnanolone to corticotropin-releasing hormone in obese patients. Neuroendocrinology. 2002;75:124–129. doi: 10.1159/000048228. [DOI] [PubMed] [Google Scholar]
- Michael A, Jenaway A, Paykel ES, Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol Psychiatry. 2000;48:989–995. doi: 10.1016/s0006-3223(00)00955-0. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mommersteeg P, Heijnen CJ, Kavelaars A, van Doornen LJ. The HPA-axis and immune function in burnout. Progress in brain research. 2007;167:281–285. doi: 10.1016/S0079-6123(07)67024-1. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, Petraglia F, Genazzani AR. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142:269–273. doi: 10.1530/eje.0.1420269. [DOI] [PubMed] [Google Scholar]
- Morrison MF, Ten Have T, Freeman EW, Sammel MD, Grisso JA. DHEA-S levels and depressive symptoms in a cohort of African American and Caucasian women in the late reproductive years. Biol Psychiatry. 2001;50:705–711. doi: 10.1016/s0006-3223(01)01169-6. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Devaud LL, Purdy RH, Paul SM. Neuroactive steroid modulators of the stress response. Ann N Y Acad Sci. 1995;771:257–272. doi: 10.1111/j.1749-6632.1995.tb44687.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL. Recent developments in the significance and therapeutic relevance of neuroactive steroids--Introduction to the special issue. Pharmacol Ther. 2007;116:1–6. doi: 10.1016/j.pharmthera.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsink LF, Vogelzangs N, Nicklas BJ, Beekman AT, Satterfield S, Rubin SM, Yaffe K, Simonsick E, Newman AB, Kritchevsky SB, Penninx BW. Associations between sex steroid hormone levels and depressive symptoms in elderly men and women: results from the Health ABC study. Psychoneuroendocrinology. 2007;32:874–883. doi: 10.1016/j.psyneuen.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP. Neural regulation of the stress response: the many faces of feedback. Cellular and molecular neurobiology. 2012;32:683–694. doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum "blues". Obstet Gynecol. 2001;97:77–80. doi: 10.1016/s0029-7844(00)01112-1. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The neurobiology of depression. SCIENTIFIC AMERICAN-AMERICAN EDITION. 1998;278:42–49. doi: 10.1038/scientificamerican0698-42. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Glue P, Lawson C, Wilson S. Flumazenil provocation of panic attacks. Evidence for altered benzodiazepine receptor sensitivity in panic disorder. Arch Gen Psychiatry. 1990;47:917–925. doi: 10.1001/archpsyc.1990.01810220033004. [DOI] [PubMed] [Google Scholar]
- Oberbeck R, Kobbe P. Dehydroepiandrosterone (DHEA): a steroid with multiple effects. Is there any possible option in the treatment of critical illness? Curr Med Chem. 2010;17:1039–1047. doi: 10.2174/092986710790820570. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Ritchie JC, Nemeroff CB. 5 alpha-pregnane-3 alpha, 21-diol-20-one (THDOC) attenuates mild stress-induced increases in plasma corticosterone via a non-glucocorticoid mechanism: comparison with alprazolam. Brain Res. 1992;573:353–355. doi: 10.1016/0006-8993(92)90788-b. [DOI] [PubMed] [Google Scholar]
- Paddison PL, Gise LH, Lebovits A, Strain JJ, Cirasole DM, Levine JP. Sexual abuse and premenstrual syndrome: comparison between a lower and higher socioeconomic group. Psychosomatics. 1990;31:265–272. doi: 10.1016/S0033-3182(90)72162-7. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Parker LN, Odell WD. Control of adrenal androgen secretion. Endocr Rev. 1980;1:392–410. doi: 10.1210/edrv-1-4-392. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Almeida OF. Gonadal steroids exert facilitating and "buffering" effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J Neurosci. 1996;16:7077–7084. doi: 10.1523/JNEUROSCI.16-21-07077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. Faseb J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pearlstein TB, Frank E, Rivera-Tovar A, Thoft JS, Jacobs E, Mieczkowski TA. Prevalence of axis I and axis II disorders in women with late luteal phase dysphoric disorder. J Affect Disord. 1990;20:129–134. doi: 10.1016/0165-0327(90)90126-s. [DOI] [PubMed] [Google Scholar]
- Perry BL, Pullen E, Oser CB. Too Much of a Good Thing? Psychosocial Resources, Gendered Racism, and Suicidal Ideation Among Low-SES African American Women. Soc Psychol Q. 2012;75:334–359. doi: 10.1177/0190272512455932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry BL, Stevens-Watkins D, Oser CB. The moderating effects of skin color and ethnic identity affirmation on suicide risk among low-SES African American women. Race Soc Probl. 2013;5:1–14. doi: 10.1007/s12552-012-9080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Carboni G, Pinna G. Neurosteroids regulate mouse aggression induced by anabolic androgenic steroids. Neuroreport. 2006;17:1537–1541. doi: 10.1097/01.wnr.0000234752.03808.b2. [DOI] [PubMed] [Google Scholar]
- Pico-Alfonso MA, Mastorci F, Ceresini G, Ceda GP, Manghi M, Pino O, Troisi A, Sgoifo A. Acute psychosocial challenge and cardiac autonomic response in women: the role of estrogens, corticosteroids, and behavioral coping styles. Psychoneuroendocrinology. 2007;32:451–463. doi: 10.1016/j.psyneuen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci U S A. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL. Simultaneous quantification of GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in human and rat serum. Steroids. 2009;74:463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raadsheer F, Hoogendijk WJ, Stam F, Tilders F, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Rabin DS, Schmidt PJ, Campbell G, Gold PW, Jensvold M, Rubinow DR, Chrousos GP. Hypothalamic-pituitary-adrenal function in patients with the premenstrual syndrome. J Clin Endocrinol Metab. 1990;71:1158–1162. doi: 10.1210/jcem-71-5-1158. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstetrics & Gynecology. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Physiological role of adrenal deoxycorticosterone-derived neuroactive steroids in stress-sensitive conditions. Neuroscience. 2006;138:911–920. doi: 10.1016/j.neuroscience.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Redei E, Freeman EW. Preliminary evidence for plasma adrenocorticotropin levels as biological correlates of premenstrual symptoms. Acta Endocrinol. 1993;128:536–542. doi: 10.1530/acta.0.1280536. [DOI] [PubMed] [Google Scholar]
- Richards JM, Stipelman BA, Bornovalova MA, Daughters SB, Sinha R, Lejuez CW. Biological mechanisms underlying the relationship between stress and smoking: state of the science and directions for future work. Biol Psychol. 2011;88:1–12. doi: 10.1016/j.biopsycho.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Sanna E, Cuccheddu T, Serra M, Concas A, Biggio G. Carbon dioxide inhalation reduces the function of GABAA receptors in the rat brain. Eur J Pharmacol. 1992;216:457–458. doi: 10.1016/0014-2999(92)90447-c. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Purdy RH, Moore PH, Jr, Paul SM, Rubinow DR. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–1260. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, St Clair LS, Murphy JH, Haq N, Rubinow DR. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62:154–162. doi: 10.1001/archpsyc.62.2.154. [DOI] [PubMed] [Google Scholar]
- Segebladh B, Bannbers E, Moby L, Nyberg S, Bixo M, Bäckström T, Poromaa IS. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Archives of women's mental health. 2013:1–7. doi: 10.1007/s00737-013-0327-1. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Floris I, Floris S, Cannas E, Mossa A, Trapani G, Latrofa A, Purdy RH, Biggio G. Social isolation increases the response of peripheral benzodiazepine receptors in the rat. Neurochem Int. 2004;45:141–148. doi: 10.1016/j.neuint.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu M, Floris I, Biggio G. Social isolation-induced changes in the hypothalamic-pituitary-adrenal axis in the rat. Stress: The International Journal on the Biology of Stress. 2005;8:259–264. doi: 10.1080/10253890500495244. [DOI] [PubMed] [Google Scholar]
- Shirotsuki K, Izawa S, Sugaya N, Yamada KC, Ogawa N, Ouchi Y, Nagano Y, Nomura S. Salivary cortisol and DHEA reactivity to psychosocial stress in socially anxious males. Int J Psychophysiol. 2009;72:198–203. doi: 10.1016/j.ijpsycho.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Waterman MR. Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu Rev Physiol. 1988;50:427–440. doi: 10.1146/annurev.ph.50.030188.002235. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha,5beta-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology. 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. 1988 [Google Scholar]
- Strohle A, Romeo E, Hermann B, Pasini A, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Concentrations of 3 alpha-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry. 1999;45:274–277. doi: 10.1016/s0006-3223(98)00328-x. [DOI] [PubMed] [Google Scholar]
- Strohle A, Pasini A, Romeo E, Hermann B, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Fluoxetine decreases concentrations of 3 alpha, 5 alpha-tetrahydrodeoxycorticosterone (THDOC) in major depression. J Psychiatr Res. 2000;34:183–186. doi: 10.1016/s0022-3956(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, Weizman A. Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry. 2003;60:133–141. doi: 10.1001/archpsyc.60.2.133. [DOI] [PubMed] [Google Scholar]
- Šulcová J, Hill M, Hampl R, Starka L. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. Journal of Endocrinology. 1997;154:57–62. doi: 10.1677/joe.0.1540057. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Ashbrook D, Backstrom T. Reduced benzodiazepine sensitivity in patients with premenstrual syndrome: a pilot study. Psychoneuroendocrinology. 1997a;22:25–38. doi: 10.1016/s0306-4530(96)00035-2. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Nyberg S, Backstrom T. Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology. 1997b;17:370–381. doi: 10.1016/S0893-133X(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Andersson A, Nyberg S, Ashbrook D, Purdy RH, Backstrom T. Patients with premenstrual syndrome have a different sensitivity to a neuroactive steroid during the menstrual cycle compared to control subjects. Neuroendocrinology. 1998;67:126–138. doi: 10.1159/000054307. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Kagaya A, Uchitomi Y, Kugaya A, Muraoka M, Yokota N, Horiguchi J, Yamawaki S. Plasma dehydroepiandrosterone sulfate in unipolar major depression. Short communication. J Neural Transm. 1998;105:537–542. doi: 10.1007/s007020050077. [DOI] [PubMed] [Google Scholar]
- Turkmen S, Backstrom T, Wahlstrom G, Andreen L, Johansson IM. Tolerance to allopregnanolone with focus on the GABA-A receptor. Br J Pharmacol. 2011;162:311–327. doi: 10.1111/j.1476-5381.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Ceci M, Kohler C, Uzunov DP, Wrynn AS. Region-specific dysregulation of allopregnanolone brain content in the olfactory bulbectomized rat model of depression. Brain Res. 2003;976:1–8. doi: 10.1016/s0006-8993(03)02577-0. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3alpha-reduced neurosteroids to depression and antidepressant action. Psychopharmacology. 2006;186:351–361. doi: 10.1007/s00213-005-0201-6. [DOI] [PubMed] [Google Scholar]
- Vallee M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal Biochem. 2000;287:153–166. doi: 10.1006/abio.2000.4841. [DOI] [PubMed] [Google Scholar]
- van Broekhoven F, Verkes RJ. Neurosteroids in depression: a review. Psychopharmacology. 2003;165:97–110. doi: 10.1007/s00213-002-1257-1. [DOI] [PubMed] [Google Scholar]
- Vedhara K, McDermott MP, Evans TG, Treanor JJ, Plummer S, Tallon D, Cruttenden KA, Schifitto G. Chronic stress in nonelderly caregivers: psychological, endocrine and immune implications. J Psychosom Res. 2002;53:1153–1161. doi: 10.1016/s0022-3999(02)00343-4. [DOI] [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy RH, Backstrom T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. J Clin Endocrinol Metab. 1996;81:1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- Wichers M, Geschwind N, Jacobs N, Kenis G, Peeters F, Derom C, Thiery E, Delespaul P, van Os J. Transition from stress sensitivity to a depressive state: longitudinal twin study. Br J Psychiatry. 2009;195:498–503. doi: 10.1192/bjp.bp.108.056853. [DOI] [PubMed] [Google Scholar]
- Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health socio-economic status, stress and discrimination. Journal of health psychology. 1997;2:335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Res Brain Res Rev. 1999;30:264–288. doi: 10.1016/s0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Keebler A, Nelson N, Friedland M, Brizendine L, Roberts E. Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry. 1999;156:646–649. doi: 10.1176/ajp.156.4.646. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2:115–143. doi: 10.3109/15622970109026799. [DOI] [PubMed] [Google Scholar]
- Wong SY, Leung JC, Kwok T, Ohlsson C, Vandenput L, Leung PC, Woo J. Low DHEAS levels are associated with depressive symptoms in elderly Chinese men: results from a large study. Asian J Androl. 2011;13:898–902. doi: 10.1038/aja.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NF, Lentz M, Mitchell ES, Heitkemper M, Shaver J. PMS after 40: persistence of a stress-related symptom pattern. Res Nurs Health. 1997;20:329–340. doi: 10.1002/(sici)1098-240x(199708)20:4<329::aid-nur6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Young AH, Gallagher P, Porter RJ. Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. American Journal of Psychiatry. 2002;159:1237–1239. doi: 10.1176/appi.ajp.159.7.1237. [DOI] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology. 2000;23:411–418. doi: 10.1016/S0893-133X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Cameron OG. Effect of comorbid anxiety disorders on the hypothalamic-pituitary-adrenal axis response to a social stressor in major depression. Biol Psychiatry. 2004a;56:113–120. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M, Lopez JF, Kocsis JH, Schatzberg AF, DeBattista C, Zubieta JK. HPA axis activation in major depression and response to fluoxetine: a pilot study. Psychoneuroendocrinology. 2004b;29:1198–1204. doi: 10.1016/j.psyneuen.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Young EA, Veldhuis JD. Disordered adrenocorticotropin secretion in women with major depression. J Clin Endocrinol Metab. 2006;91:1924–1928. doi: 10.1210/jc.2005-2397. [DOI] [PubMed] [Google Scholar]
- Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol. 2009;89:134–152. doi: 10.1016/j.pneurobio.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Sonntag A, Uhr M, Holsboer F, Ising M. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression. a prospective study. J Psychiatr Res. 2001;35:83–94. doi: 10.1016/s0022-3956(01)00013-9. [DOI] [PubMed] [Google Scholar]