Abstract
Herpes simplex virus type 1 (HSV-1) is an important human pathogen which requires activation of nuclear factor–kappa B (NFκB) during its replication cycle. The persistent nature of HSV-1 infection, and the emergence of drug-resistant strains, highlights the importance of research to develop new antiviral agents. Toll-like receptors (TLR) play a prominent role during the early antiviral response by recognizing viral nucleic acid and gene products, activating NFκB, and stimulating the production of inflammatory cytokines. We demonstrate a significant effect on HSV-1 replication in ARPE-19 and Vero cells when oligonucleotides designed to inhibit TLR9 are added 2 hours prior to infection. A greater than 90% reduction in the yield of infectious virus was achieved at oligonucleotide concentrations of 10 to 20 micromolar. TLR9 inhibitory oligonucleotides prevented expression of essential immediate early herpes gene products as determined by immunofluorescence microscopy and Western blotting. TLR9 oligonucleotides also interfered with viral attachment and entry. A TLR9 inhibitory oligonucleotide containing five adjacent guanosine residues (G-ODN) exhibited virucidal activity and inhibited HSV-1 replication when added post-infection. The antiviral effect of the TLR9 inhibitory oligonucleotides did not depend on the presence of TLR9 protein, suggesting a mechanism of inhibition that is not TLR9 specific. TLR9 inhibitory oligonucleotides also reduced NFκB activity in nuclear extracts. Studies using these TLR inhibitors in the context of viral infection should be interpreted with caution.
Keywords: Herpes simplex virus, ICP4, TLR9, NFκB, Retinal Pigment Epithelium, ARPE-19
1.0 Introduction
Herpes simplex virus type 1 (HSV-1) is a significant human pathogen infecting approximately 70% of the world’s population (Fatahzadeh and Schwartz, 2007). Infection with HSV-1 commonly causes ulcerative lesions on the skin or mucosal membranes (Whitley, 1996) and HSV-1 infection of the eye can result in conjunctivitis and keratitis, which may lead to blindness due to an immunopathological response (Streilein et al., 1997). A high percentage of primary infections with HSV-1 are asymptomatic and the virus can establish latent infection in the innervating sensory ganglia (Whitley, 1996).
Currently, there is no cure for persistent HSV-1 infection and prolonged therapy with anti-herpetic drugs can lead to the emergence of drug resistant strains especially in immunocompromised individuals. Acyclovir resistant strains of HSV-1 have also been isolated from patients with herpetic keratitis and uveitis (Duan et al., 2008; van Velzen et al., 2013). Research into anti-herpetic agents, with a mechanism of action different than that of the currently available nucleoside analogs, is therefore crucial. Oligomeric nucleic acids are intriguing candidates for antiviral therapy as they have high affinity for a given target. They also have high inhibitory potential, can be selected against almost any target, and exhibit limited toxicity or immunogenicity (Hagedorn et al., 2013; Mescalchin and Restle, 2011). Studies have indicated antisense oligonucleotides may function as antiviral agents for viruses including HIV and HBV (Galderisi et al., 1999; Mescalchin and Restle, 2011), HCV (Janssen et al., 2013) and Influenza (Wong et al., 2010; Wong et al., 2007). Vitravene, an antisense drug used for the treatment of CMV retinitis in AIDs patients, consists of a phosphorothioate oligonucleotide designed to inhibit human CMV replication (Galderisi et al., 1999).
The antiviral properties of amphipathic phosphorothioated oligonucleotides (PS-ONs), regardless of sequence specificity, were demonstrated some time ago against EBV and HSV (Fennewald et al., 1995 ; Gao et al., 1990; Yao et al., 1993). The antiviral activity of PS-ONs is dependent on the length and hydrophobicity of the oligonucleotide as demonstrated by studies with HIV-1 (Vaillant et al., 2006), LCMV (Cardin et al., 2009; Lee et al., 2008), HCMV (Luganini et al., 2008), HCV (Counihan and Lindenbach, 2009), DHBV (Noordeen et al., 2013a; Noordeen et al., 2013b) and HSV (Bernstein et al., 2008; Guzman et al., 2007; Shogan et al., 2006). A GT rich 20 base PS-ON (ISIS 5652) was shown to have potent antiviral activity against HSV which is thought to be mediated by a conformational change in the viral glycoprotein gB (Shogan et al., 2006).
Innate recognition of viruses by the mammalian immune system is crucial in inducing interferon and other immune responses. The most highly studied family of pattern recognition receptors (PRRs) is the Toll-like receptors (TLRs) (Huang and Yang, 2009). Multiple human TLRs have been identified to date, each recognizing a unique set of pathogen-associated molecular patterns (PAMPs) (Akira and Takeda, 2004; Imler and Zheng, 2004). TLR signaling pathways culminate in activation of the transcription factor NFκB which controls the expression of many inflammatory cytokines and other genes (Kawai and Akira, 2006). Herpes viruses contain several PAMPs that are recognized by TLRs and this interaction is thought to play a crucial role in viral pathogenesis (Martinez-Martin and Viejo-Borbolla, 2010). The HSV-1 glycoproteins gB and gH/gL are recognized by cell surface TLR2 (Cai et al., 2013; Kurt-Jones et al., 2004; Leoni et al., 2012), TLR3 recognizes HSV-1 dsRNA (Zhang et al., 2007), and TLR9 recognizes unmethylated CpG dinucleotides in the genome of Herpes simplex virus (Lund et al., 2003). This recognition of HSV DNA by TLR9 activates NFκB and results in the production of type 1 interferons and cytokines, including IL-6 (Huang and Yang, 2009; Krug et al., 2004; Rasmussen et al., 2007). Purified HSV-1 DNA up-regulates IL-6 in human corneal epithelial cells (HCEs) and primary human corneal fibroblasts in part via their interaction with TLR9 and TLR3 (Hayashi et al., 2006). Retinal pigment epithelial (RPE) cells express TLRs 1-7, 9 and 10 (Kumar et al., 2004), suggesting a role for these molecules in retinal immune responses.
Because some TLRs recognize nucleic acids, oligonucleotides have been developed that inhibit TLR signaling and function as competitive agonists (Barrat and Coffman, 2008; Latz et al., 2007; Peter et al., 2007). Administration of a TLR9 inhibitory oligonucleotide after HSV-1 infection of mice decreased mortality by controlling the inflammatory response in the brain (Boivin et al., 2012). A TLR9 inhibitory oligonucleotide significantly reduced HSV-1 replication in human corneal endothelial cells without affecting viral entry (Takeda et al., 2011), but this study did not determine whether the antiviral effect of the TLR9 oligonucleotide was dependent on the presence of the TLR9 protein.
In this study we investigated the effects of two commercially available TLR9 inhibitory oligonucleotides on HSV-1 infection of ARPE-19 cells. The inhibitory oligonucleotides reduced viral replication and prevented synthesis of essential herpes proteins. TLR9 oligonucleotides interfered with early stages of the viral life cycle, including attachment and entry, as well as post-entry events. The TLR9 inhibitory oligonucleotide decreased NFκB activity in infected cells. Surprisingly, this antiviral effect was not dependent on the presence of TLR9 protein, suggesting a mechanism of inhibition that is not TLR9 specific.
2.0 Materials and Methods
2.1 Cell culture and virus
Human adherent retinal pigmented epithelial cells (ARPE-19) (ATCC, CRL-2302) were maintained in DMEM/F-12 (Mediatech, Herndon, VA, 10-092-CV) supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine at 37°C in 5% CO2. Vero cells (ATCC, CCL-81) were maintained in DMEM (Mediatech, 10-017-CV) supplemented with 5% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine at 37°C in 5% CO2. 293XL/null (#293xl-null) and 293XL-hTLR9-HA cells (#293xl-htlr9ha) were obtained from Invivogen (San Diego, CA). 293XL cells were maintained in DMEM supplemented with 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin, 100 μg/ml Normocin (Invivogen, #anti-nr-1) and 10 μg/ml Blasticidin (Invivogen,# ant-bl-1). High titer, sucrose gradient purified stocks of HSV-1 KOS were prepared as described previously (Visalli and Brandt, 1993). Viral titers were determined by plaque assay in Vero cells as described previously (Grau et al., 1989). The plaquing efficiency of HSV-1 KOS was equivalent on Vero and ARPE-19 cells.
2.2 TLR oligonucleotides
The TLR9 inhibitory oligonucleotide (#tlrl-hinhodn, 5′-TTT AGG GTT AGG GTT AGG GTT AGG G-3′), G-ODN oligonucleotide (#tlrl-godn, 5-CTC CTA TTG GGG GTT TCC TAT-3,) and TLR9 control oligonucleotide (#tlrl-hinhodnc, 5-GCT AGA TGT TAG CGT-3′) were purchased from Invivogen. The oligos were resuspended in endotoxin-free water prior to use.
2.3 Antibodies and Immunofluorescence
The following primary antibodies were utilized in this study: mouse anti-ICP4 (Virusys Corp. Taneytown, MD; H1A021), mouse anti-ICP8 (Santa Cruz, Dallas, TX, sc-53329), mouse anti-ICP27 (Virusys, H1142), mouse anti-gD (Virusys, HA025), and goat anti-actin (Santa Cruz, sc1616). The following secondary antibodies were utilized: donkey anti-mouse IgG Alexafluor 488 (Invitrogen, Grand Island, NY, A21202), donkey anti-mouse IgG-HRP (Santa Cruz, sc-2314) donkey anti-goat IgG-HRP (Santa Cruz, sc-2020), and goat anti-mouse IgG-HRP (Santa Cruz sc-2031).
For immunofluorescence assays, ARPE-19 cells were seeded in 8-well chamber slides (Lab-Tek, Rochester, NY). At various times post infection (PI) the supernatants were removed, the cells were rinsed with PBS, and fixed in 4% paraformaldehyde/PBS. Cells were permeabilized with 0.1%Triton X-100 (Sigma-Aldrich, St. Louis, MO) in PBS, washed in PBS, and blocked in 5% normal donkey serum/PBS (Jackson Immunoresearch Laboratories, West Grove, PA). Anti-ICP4 and anti-gD antibodies were diluted 1:1000 and applied for 1 hour at RT in PBS containing 2.5% normal donkey serum. Slides were then washed with PBS containing 0.1% Tween-20 (Fisher, Fairlawn, NJ). Secondary antibody was applied at a dilution of 1:400 for 1 hour at RT in PBS containing 2.5% normal donkey serum. Slides were washed in PBS/0.1% Tween-20 before incubation with 1.0 μg/ml Hoechst (33342, Invitrogen) in PBS for 5 minutes. Slides were washed in PBS prior to mounting with Immu-mount (Richard Allen Scientific, Kalamazoo, MI). Fluorescence was observed with a Zeiss Axioplan 2 microscope equipped with an Axiocam HR camera with Axiovision 4.8 software (Carl Zeiss Microimaging Inc., Thornwood, NY). Photographs were taken at 200x magnification.
2.3 Oligonucleotide and Acyclovir Inhibition Assays
ARPE-19, Vero, 293XLnull, or 293XLhTLR9HA cells grown in 8-well chamber slides were pretreated with culture media, various concentrations of inhibitory or control oligonucleotides, or increasing concentrations of acyclovir (SIGMA, A4669) for 2 hours at 37°C. Cells were then infected with HSV-1 KOS at a multiplicity of infection (MOI) of 1 to 2 and incubated ON at 37°C. Supernatants were collected and stored at −80°C for titering in Vero cells (Grau et al., 1989). Titers were calculated as plaque forming units/ml (pfu/ml). The half maximal effective concentration (EC50) was defined as the concentration of oligonucleotide or acyclovir needed to reduce viral titer to 50% of media control in ARPE-19 cells.
2.4 Western Blotting
ARPE-19 cells grown to confluence in 10 cm culture dishes were pretreated with media only (DMEM/F-12/10% FBS), 20 μM TLR9 control oligonucleotide in media, or 20 μM TLR9 inhibitory oligonucleotide in media for 2 hours at 37°C. Cells were then infected with HSV-1 KOS at a MOI of 2 for 6 or 15 hours. At the designated time points, the cells were washed, collected, and pelleted for 10 minutes at 1000 rpm in a Beckman Model TJ-6 table top centrifuge. Cell pellets were resuspended in Laemmli sample buffer with 5% 2-mercaptoethanol, sonicated, and boiled prior to loading equal amounts onto a 10% (ICP4, ICP8) or 12% (ICP27, gD) denaturing polyacrylamide gel. Protein was electrophorectically transferred to nitrocellulose and the membrane blocked overnight at 4°C with 5% non-fat dry milk in Genius Buffer I (100mM maleic acid, 150mM NaCl, pH 7.5) containing 0.3% v/v Tween-20. The primary antibodies were applied for 1 hour at RT, followed by washing in Genius Buffer I containing 0.3% v/v Tween-20. Secondary antibodies were applied for 1 hour at RT. After washing, the blots were developed using an enhanced chemilluminescence system (GE Healthcare, Piscataway, NJ, PRN2232). For the actin blot, the membrane was incubated for 1 hour at 50°C in stripping buffer (80mM Tris-HCl, pH 6.7, 100mM β-mercaptoethanol, 2% SDS), washed in PBS, blocked overnight as above, and blotted with the actin antibody.
Mouse anti-ICP4, which recognizes a 120kDa protein, was used at a 1:1000 dilution. Mouse-anti ICP8, which recognizes a 130 kDa protein, was used at a 1:200 dilution. Mouse anti-gD, which recognizes a 55 kDa protein, was used at a 1:1000 dilution. Mouse anti-ICP27, which recognizes a 63kDa protein, was used at a 1:100 dilution. The secondary antibody, donkey anti-mouse IgG HRP, was diluted 1:5000. Goat anti-actin, which recognizes a 42kDa protein, was diluted 1:200. Donkey anti-goat IgG HRP was diluted 1:4000.
2.5 Cellular Toxicity Assay
ARPE-19 cells were seeded in 96 well plates until confluent. Triplicate wells were incubated with media or increasing concentrations of TLR9 control, TLR9 inhibitory, or G-ODN oligonucleotides overnight at 37°C. As a positive control for cell lysis, triplicate wells were incubated with 0.5% IGEPAL in media. A CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, TB245) was performed to determine the number of viable cells per well. The mean viability of the triplicate media wells was set at 100%, and results were graphed as percent media control.
2.6 Virucidal Assay
5 × 106 plaque forming units of HSV-1 KOS were incubated with media or increasing concentrations of TLR9 control, TLR9 inhibitory, or G-ODN oligonucleotide for 1 hour at 37°C. Samples were diluted, to reduce ODN concentrations well below EC50 values, and titered on Vero cells (Grau et al., 1989). Mean titers of triplicate samples were graphed with error bars representing standard error of the mean.
2.7 Viral Attachment Assay
ARPE-19, 293XLnull, and 293XLhTLR9-HA cells were plated in 6 well plates until confluent. Duplicate wells were treated with media, or 20 μM TLR9 control, TLR9 inhibitory, or G-ODN oligonucleotides for 2 hours at 37°C. Cells were chilled on ice and infected with HSV-1 KOS at an MOI of 5 for 1 hour at 4°C. Cells were washed with cold PBS, and collected via scraping and centrifugation prior to lysis in 20mM Tris, 100mM NaCl, 1mM EDTA, 0.5% Triton-X-100, and a 1:100 dilution of protease inhibitor cocktail (SIGMA, P8340). Protein concentration of lysates was assayed by the Pierce BCA protein assay kit (Pierce, 23225). Twenty micrograms of each ARPE-19 lysate or 40 μg of each 293XLnull and 293XLhTLR9HA lysate were run on 4-15% Mini-PROTEAN TGX Precast gels (BIO-RAD). Protein was electrophorectically transferred to nitrocellulose, and the membranes blocked overnight with 5% non-fat dry milk in Genius Buffer I (100mM maleic acid, 150mM NaCl, pH 7.5) containing 0.3% v/v Tween-20. The membranes were incubated with a 1:1000 dilution of mouse anti-gD primary antibody (Virusys, HA025) for 1 hour at RT in blocking buffer containing 1% FBS. After incubation, the membranes were washed in Genius Buffer I with 0.3% Tween-20. A 1:5000 dilution of goat anti-mouse IgG HRP (Santa Cruz, sc-2031) in blocking buffer was applied to the membranes for one hour at RT. After washing, the blots were developed using an enhanced chemilluminescence system (GE, PRN2232).
2.8 Viral Entry Assay
ARPE-19, 293XLnull, or 293XLhTLR9HA cells were plated in 6 well plates until confluent. Cells were chilled on ice prior to addition of 150 pfu of HSV-1 KOS for 1 hour at 4°C to allow for viral attachment. 150 pfu of virus was chosen to allow for adequate spacing of plaques to facilitate counting of wells. Media or oligonucleotides (20 μM) were added to triplicate wells for 15 minutes at 4°C prior to increasing the temperature to 37°C for 1 hour to allow for viral entry. Cells were rinsed with PBS, treated for 1 minute with pH 3 citrate buffer (40mM Citric acid, 10mM KCl, 135mM NaCl, pH 3.0), rinsed with PBS, then overlayed with methylcellulose (2% in DMEM supplemented with 2% FBS) and media (DMEM/2% FBS). Cells were incubated at 37°C for 3 days. ARPE-19 wells were stained for plaques as described previously (Grau et al., 1989), and the number of plaques per well were counted. To visualize 293XL plaques, cells were fixed in 3.7% formaldehyde/PBS, rinsed with PBS, and blocked for 1 hour in 5%milk/0.1% Tween-20/PBS. Mouse anti-gD (1:1000 dilution in block solution) was then applied to wells for 1 hour at RT. Wells were washed with PBS/0.1% Tween before incubating with a 1:2500 dilution of goat anti-mouse alkaline phosphatase (SIGMA, A3562) in block solution for 1 hour. After washing with PBS/0.1% Tween, the wells were exposed to SIGMAFAST BCIP/NBT reagent (SIGMA, B5655) until plaques were detectable. The data are represented as mean plaque number per well with error bars representing standard error of the mean.
2.9 NFκB Transcription Factor Binding ELISA
ARPE-19 cells grown to confluency in 10 cm plates were pretreated with media (DMEM/F-12/10% FBS), 20 μM TLR9 control oligonucleotide in media, or 20 μM TLR9 inhibitory oligonucleotide in media for 2 hours at 37°C. Plates, except uninfected control plate, were infected with HSV-1 KOS at a MOI of 5 for 6 hours at 37°C. Cells were washed twice with cold PBS, collected by scraping, and pelleted at 1000 rpm for 10 minutes at 4°C in a Beckman Model TJ-6 table top centrifuge. Nuclear and cytoplasmic extracts were prepared with a SIGMA CelLytic NuCLEAR Extraction Kit, utilizing hypotonic lysis buffer and IGEPAL (NXTRACT, SIGMA). The protein concentration of each extract was determined by BCA assay (Pierce, Rockford, IL, 23225). The equivalent of 14 μg of nuclear extract was diluted in complete transcription factor binding assay buffer and loaded, in duplicate, into wells of a NFκB (p65) Transcription Factor Assay Kit plate (Cayman Chemical Company, Ann Arbor, MI, 100007889). To assay p65 binding in the cytoplasmic fractions, the equivalent of 91 μg of cytoplasmic extract was loaded into wells of the transcription factor assay plate. The positive control cell lysate (PC) was diluted in hypotonic lysis buffer during the cytoplasmic fraction ELISA. Plates were read at OD 450nm on a Safire2 TECAN plate reader. The OD value of blank well(s) was subtracted from the OD value of each sample. Error bars represent standard error of the mean.
3.0 Results
3.1 Effect of TLR Oligonucleotides on HSV-1 Replication
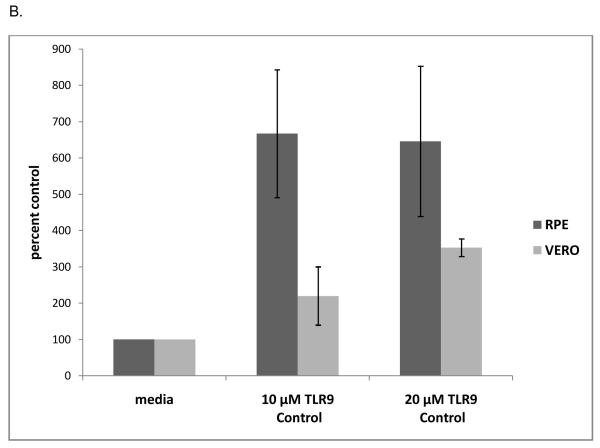
As shown in Figure 1A, pretreatment of ARPE-19 cells with 10 or 20 μM TLR9 inhibitory oligonucleotide reduced viral titers by approximately 50% and 99%, respectively, compared to the media control. The G-ODN oligonucleotide, which contains a stretch of five guanosines, had an even greater effect on viral titer, reducing titers to less than 1% of control at concentrations of 10 μM and 20 μM (Figure 1A). To determine if this effect was cell type specific, Vero cells were pre-incubated with TLR9 inhibitory oligonucleotide prior to HSV-1 infection. The results in Vero cells correlated with those of the ARPE-19 cells, as 10 μM TLR9 inhibitory oligonucleotide reduced viral titers by approximately 89% and 20 μM inhibitory oligonucleotide reduced viral titers by 96% of the media control (Figure 1A). The G-ODN oligonucleotide reduced viral titers in Vero cells to less than 1% of control at 10 and 20 μm (Figure 1A). Pre-incubation with the TLR9 control oligonucleotide resulted in an enhancement of viral replication, in that viral titers were increased approximately 6-fold over the media control in ARPE-19 cells and 3 to 4-fold in Vero cells (Figure 1B). The positive effect on viral replication by the TLR9 control oligonucleotide was not concentration dependent, suggesting this was a nonspecific effect.
Figure 1. TLR9 Inhibitory oligonucleotides decrease KOS titer in ARPE-19 and Vero cells.
ARPE-19 (RPE) or Vero cells were incubated with 10 or 20 μM of the TLR9 inhibitory oligos TLR9I or G-ODN for 2 hours at 37°C (A). ARPE-19 or VERO cells were incubated with 10 or 20 μM of the TLR9 control oligo for 2 hours at 37°C (B). HSV-1 KOS virus was added to the wells at an MOI of 1 to 2 and cells were cultured overnight in the continued presence of the oligo and viral titers were determined. Mean titers of triplicate media controls were set at 100% and titers from triplicate experiments were averaged and graphed as percent of control. Error bars represent standard error of the mean. RPE titers (pfu/ml): Media 7.5 ×107; 10 μM TLR9 Control 3.8 × 108; 20 μM TLR9 Control 2.2 × 108; 10 μM TLR9 Inhibitory 3.8 × 107; 20 μM TLR9 Inhibitory 1.1 × 106; 10 μM G-ODN 1.9 × 105; 20 μM G-ODN 2.5 × 101. Vero titers (pfu/ml): Media 2.0 ×107; 10 μM TLR9 Control 4.4 × 107; 20 μM TLR9 Control 7.1 × 107; 10 μM TLR9 Inhibitory 2.3 × 106; 20 μM TLR9 Inhibitory 7.8 × 105; 10 μM G-ODN 2.2 × 104; 20 μM G-ODN 2.6 × 102. Student’s t-test confirmed all treatments were significantly different from the media control (p <0.05) except for RPE 20 μM TLR9C (p= 0.058) and Vero 10 μM TLR9C (p=0.209).
The EC50 for the TLR9 inhibitory oligonucleotide in ARPE-19 cells was 10 μM, while the EC50 for the G-ODN oligonucleotide was 5.5 μM. These values correspond to the data in Figure 1A, which indicates that the G-ODN oligonucleotide is approximately two-fold more effective at reducing viral titers than the TLR9 inhibitory oligonucleotide. We determined that the EC50 value of acyclovir for KOS infection of ARPE-19 cells was 2.25 μM (data not shown), indicating that the TLR9 inhibitory oligonucleotides require a 2.4 to 4.4-fold higher concentration compared to acyclovir.
3.2 Synthesis of HSV-1 Proteins in the Presence of TLR9 Oligonucleotides
To better understand the effect of the TLR9 oligonucleotides on HSV-1 replication, ARPE-19 cells were pretreated with media or 10 μM TLR9 oligonucleotide prior to infection, and the expression of viral α (ICP4 and ICP27), β (ICP8), and γ (gD, and VP5) proteins was assayed by immunofluorescence (IF). IF on uninfected cells confirmed that antibodies to these herpes proteins did not cross react with cellular proteins (data not shown). Note that similar results were obtained when using the G-ODN oligonucleotide or the TLR9 inhibitory oligonucleotide.
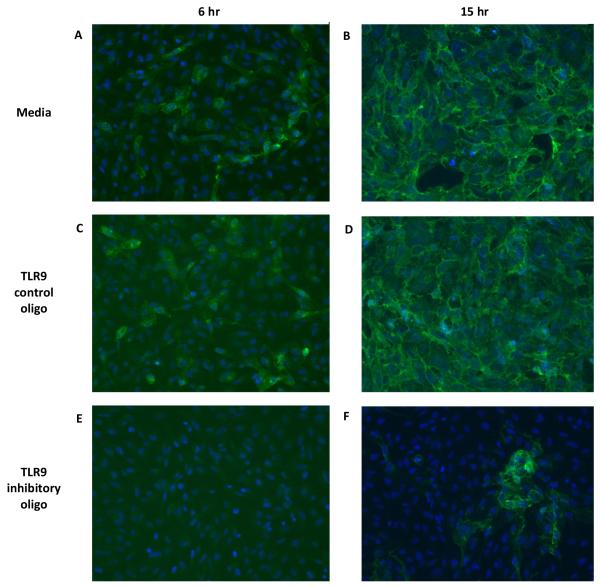
In media treated ARPE-19 cells, ICP4 localized to 30-40% of cell nuclei at 6 hours (2A) and 90-100% of cell nuclei at 15 hours (2B) post infection (PI). In ARPE-19 cells treated with the TLR9 control oligonucleotide, the distribution of ICP4 at 6 hours (2C) or 15 hours (2D) PI was similar to the media control at 6 and 15 hours, respectively. Treatment with the TLR9 inhibitory oligonucleotide, however, resulted in very few (3%) ICP4 positive nuclei at 6 hours PI (2E). At 15 hours PI, small foci of cells expressed ICP4 in both the nucleus and cytoplasm (8%), but the majority of cells did not express ICP4 (2F). Similar results were obtained when staining for the HSV-1 immediate early protein ICP27 or the major DNA binding protein ICP8 (data not shown).
To determine the effect of the TLR9 inhibitory oligonucleotide on viral γ-proteins, cells were stained for the HSV-1 glycoprotein gD and the major capsid protein VP5. Figure 3 shows that at 6 hours PI, about 20% ARPE-19 cells stained positive for gD (3A), and by 15 hours every cell was gD positive (3B). Treatment with theTLR9 control oligonucleotide had no effect on gD expression or distribution at 6 hours (3C) or 15 hours (3D) PI compared to the media control. In contrast, pretreatment with the TLR9 inhibitory oligonucleotide eliminated all gD positive cells at 6 hours PI (3E), and reduced the number of gD positive cells at 15 hours PI to roughly 17% (3F). In media or control oligonucleotide treated cells, the VP5 antibody stained the cytoplasm of some ARPE-19 cells at 6 hours PI, and by 15 hours PI, VP5 was localized in punctate nuclear clusters in all ARPE-19 cells (data not shown). Pretreatment with the TLR9 inhibitory oligonucleotide reduced the number of VP5 positive cells at 6 hours PI, and reduced the number of cell nuclei staining positive for VP5 at 15 hours PI (data not shown).
Figure 3. Expression of HSV-1 gD protein after treatment with TLR9 oligonucleotides.
ARPE-19 cells were pretreated with media, 10 μM TLR9 control oligo, or 10 μM TLR9 inhibitory oligo for 2 hours at 37°C. Cells were then infected with HSV-1 KOS at an MOI of 2. At 6 or 15 hours post-infection, cells were fixed and stained with a mouse anti-gD primary antibody followed by a donkey anti-mouse IgG Alexafluor 488 secondary antibody. Nuclei were visualized by staining with Hoechst. Cells photographed at 200x magnification.
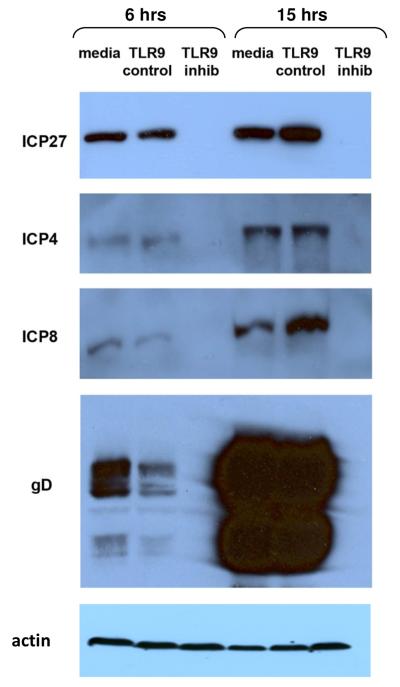
To confirm the effect of TLR9 oligonucleotides on the synthesis of HSV-1 proteins, Western blotting was performed on infected ARPE-19 lysates. A concentration of 20 μM was chosen because at this concentration the TLR9 inhibitory oligonucleotide decreased viral titers by greater than 90% in ARPE-19 cells (Fig 1A). Cell lysates were prepared at 6 and 15 hours PI and samples were analyzed by gel electrophoresis and Western blotting. Media treated, infected ARPE-19 cells expressed ICP27, ICP4, ICP8 and gD at 6 hours PI, with increased amounts of proteins evident at 15 hours PI (Figure 4). ARPE-19 cells pretreated with theTLR9 control oligonucleotide expressed viral proteins in approximately the same amounts as the media control at both 6 and 15 hours PI (Figure 4). In contrast, in cells pretreated with the TLR9 inhibitory oligonucleotide, there was no detectable ICP27, ICP4, ICP8 or gD at 6 or 15 hours PI. To confirm equal loading of cell lysates, the ICP4-blotted membrane was stripped and blotted with an anti-actin antibody (Figure 4). These data confirm that the TLR9 inhibitory oligonucleotide blocks HSV-1 protein synthesis in ARPE-19 cells.
Figure 4. TLR9 inhibitory oligonucleotides block synthesis of HSV-1 proteins.
ARPE-19 cells were pretreated with media, 20 μM TLR9 control oligo, or 20 μM TLR9 inhibitory oligo for 2 hours at 37°C. Cells were infected with HSV-1 KOS at a MOI of 2 for 6 or 15 hours. Cell lysates were prepared and loaded onto 10% (ICP4, ICP8) or 12% (ICP27, gD) denaturing polyacrylamide gels. Proteins were transferred to nitrocellulose and Western blotting was performed with HSV-1 antibodies. The ICP4 blotted membrane was stripped and re-blotted with goat-anti actin to demonstrate equivalent loading of cell lysates. Mouse anti-ICP4 recognized a 120kDa protein. Mouse anti-ICP8 recognized a 130 kDa protein. Mouse anti-gD recognized a 55 kDa protein. Mouse anti-ICP27 recognized a 63kDa protein. Goat anti-actin recognized a 42 kDa protein
3.3 Cellular Toxicity of TLR9 Oligonucleotides
To determine whether TLR9 oligonucleotides exhibit cellular toxicity, ARPE-19 cells were incubated overnight with increasing concentrations of TLR9 control, TLR9 inhibitory, or G-ODN oligonucleotides before determining cell viability. Results in Figure 5 indicate that the TLR9 control (TLR9C), TLR9 inhibitory (TLR9I), and G-ODN oligonucleotides are not toxic to ARPE-19 cells, as cells treated with 5, 10, or 20 μM oligonucleotide concentrations had greater viability than cells treated with media alone. Note that cells treated with 0.5% IGEPAL had a mean viability of 13.7% compared to the media control. Overall, the negative effect of the TLR9 inhibitory and G-ODN oligonucleotides on HSV-1 replication cannot be explained by cellular toxicity.
Figure 5. Do TLR9 oligonucleotides exhibit cellular toxicity?
ARPE-19 cells, plated in triplicate wells, were incubated with media or increasing concentrations of TLR9 control (TLR9C), TLR9 inhibitory (TLR9I) or G-ODN oligos overnight. Cell viability was determined via the CellTiter 96 Aqueous One Solution Cell Proliferation Assay. The mean cell viability for each oligonucleotide concentration is graphed as percent of the media control. Mean viability of cells treated with 0.5% IGEPAL was 13.7%. Error bars represent standard error of the mean. Student’s t-test confirmed that all treatments were significantly different from the media control (p<0.05).
3.4 Antiviral Mechanism of TLR9 Oligonucleotides
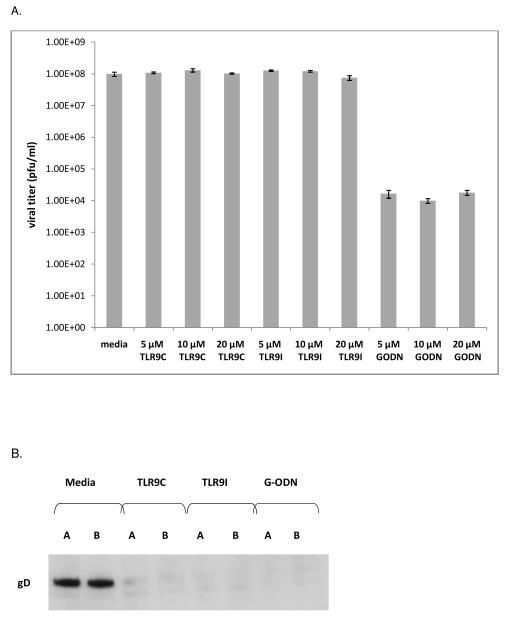
To determine whether TLR9 oligonucleotides were virucidal, we incubated increasing concentrations of oligonucleotides with HSV-1 KOS for 1 hour at 37°C. After incubation, the samples were diluted to reduce oligonucleotide concentrations to below active levels, and titered on Vero cells. Figure 6A demonstrates no effect on viral titer for the TLR9 control or TLR9 inhibitory oligonucleotides at 5, 10, or 20μM. The G-ODN oligonucleotide, however, decreased viral titers by 4 logs at 5, 10, or 20 μM. These data indicate that the G-ODN oligonucleotide exhibits virucidal activity against HSV-1 KOS, which may explain why it is more effective at inhibiting HSV-1 replication, compared to the TLR9 inhibitory oligonucleotide.
Figure 6. Antiviral mechanism of TLR9 oligonucleotides.
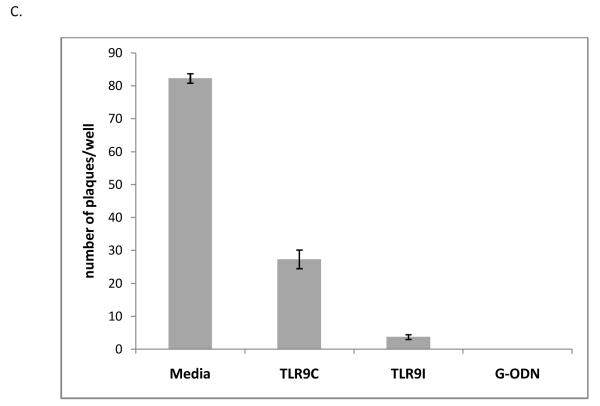
Virucidal activity (A): 5 × 106 pfu of HSV-1 KOS was incubated for 1 hour at 37°C with media or increasing concentrations of TLR9 control (TLR9C), TLR9 inhibitory (TLR9I), or G-ODN oligonucleotides. Triplicate samples were diluted and titered on Vero cells to determine pfu/ml. Error bars represent standard error of the mean. Student’s t-test confirmed a statistically significant difference between all G-ODN treatments and media control (p<0.05). Results for TLR9C and TLR9I treatment were not significant compared to the media control (p>0.05). Viral attachment (B): Duplicate wells of ARPE-19 were treated with media, or 20μM TLR9 control, TLR9 inhibitory, or G-ODN oligonucleotides for 2 hours at 37°C. Cells were chilled on ice, infected with HSV-1 KOS, washed with cold PBS, and lysates were prepared. Twenty μg of each lysate was run on a 4-15% SDS-PAGE gel. Immunoblotting was performed with mouse anti-gD primary antibody followed by goat anti-mouse IgG HRP secondary antibody. Viral entry (C): ARPE-19 cells were chilled on ice prior to addition of HSV-1 KOS at 4°C to allow for viral attachment. Media or oligonucleotides (20μM) were added to triplicate wells at 4°C prior to increasing the temperature to 37°C to allow for entry. Graph represents mean plaque number per well with error bars representing standard error of the mean. Student’s t-test indicated a statistically significant difference between TLR9C, TLR9I, and G-ODN treatment compared to the media control (p<0.001).
To assess whether TLR9 oligonucleotides affected HSV-1 attachment, ARPE-19 cells were incubated with media, TLR9 control, TLR9 inhibitory, or G-ODN oligonucleotide for 2 hours at 37°C, then transferred to 4°C prior to virus infection. Immunoblotting with an antibody to the viral glycoprotein gD indicated that viral attachment had occurred (Figure 6B, Media lanes). The TLR9 control, the TLR9 inhibitory, and the G-ODN oligonucleotides significantly reduced the level of detectable gD, indicating that all TLR9 oligonucleotides reduced the ability of HSV-1 to bind to cells. Since the TLR9 control oligonucleotide had a positive effect on viral replication in ARPE-19 cells (Figure 1B) and did not alter the expression or distribution of HSV-1 proteins during the viral life cycle (Figures 2, 3, 4), this data suggests that the inhibition seen with our pre-addition studies was not due to inhibition of viral attachment.
Figure 2. Expression of HSV-1 ICP4 protein after treatment with TLR9 oligonucleotides.
ARPE-19 cells were pretreated with media, 10 μM TLR9 control oligo, or 10 μM TLR9 inhibitory oligo for 2 hours at 37°C. Cells were then infected with HSV-1 KOS at an MOI of 2. At 6 or 15 hours post infection, cells were fixed and stained with a mouse anti-ICP4 primary antibody followed by a donkey anti-mouse IgG Alexafluor 488 secondary antibody. Nuclei were visualized by Hoechst staining. Cells were photographed at 200x magnification.
Viral entry assays were also performed to determine whether TLR9 oligonucleotides were affecting entry of HSV-1 into ARPE-19 cells. Figure 6C shows that all oligonucleotides decreased viral entry in ARPE-19 cells compared to the media control. The TLR9 control oligonucleotide decreased plaque number to about one third of the media control for ARPE-19 cells. The TLR9 inhibitory and G-ODN oligonucleotides had a greater effect, with a 10-fold (TLR9 inhibitory) or 80-fold (G-ODN) decrease in plaque number per well for ARPE-19 cells.
3.5 Post-infection Treatment with TLR9 Oligonucleotides
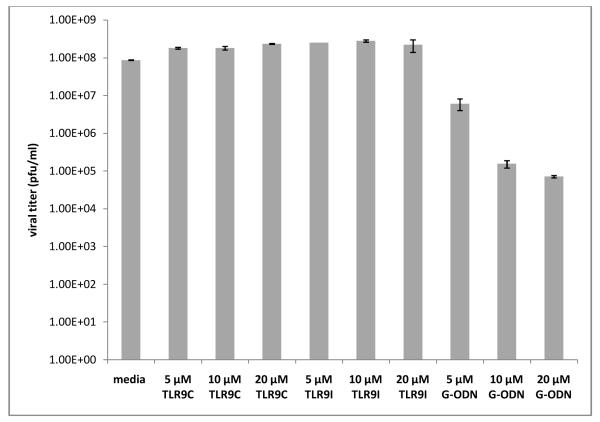
To determine whether TLR9 oligonucleotides inhibited HSV-1 replication when added post-infection, ARPE-19 cells were infected with HSV-1 KOS for 1 hour prior to addition of increasing concentrations of TLR9 control, TLR9 inhibitory, or G-ODN oligonucleotides. Figure 7 indicates that no reduction of viral titer was achieved when up to 20 μM of the TLR9 control or TLR9 inhibitory oligonucleotides were added to ARPE-19 cells 1 hour after infection. In contrast, 5 μM of the G-ODN oligonucleotide reduced viral titers by about 1 log compared to the media control, and higher concentrations (10 and 20 μM) reduced titers by up to 3 logs. These titers correspond to a 93% reduction at 5 μM G-ODN compared to the media control and a greater than 99% reduction in viral titers at concentrations of 10 and 20 μM G-ODN. These results indicate a difference in the mechanism of action of the TLR9 inhibitory versus G-ODN oligonucleotides that may be the result of sequence or charge differences.
Figure 7. Post-infection treatment with TLR9 oligonucleotides.
ARPE-19 cells were infected with HSV-1 KOS at a MOI of 2 for 1 hour at 37°C. TLR9 control, TLR9 inhibitory, or G-ODN oligos were then added to a final concentration of 5, 10 or 20 μM and cells were incubated overnight at 37°C. Supernatants were collected for titering on Vero cells. Graph shows mean viral titer of duplicate wells with error bars representing the standard error of the mean. Student’s t-test indicated a statistically significant difference between all treatments and media control (p<0.05) except for 20 μM TLR9I (p=0.237).
3.6 TLR9 is not Essential for Antiviral Activity of the TLR9 Inhibitory Oligonucleotides
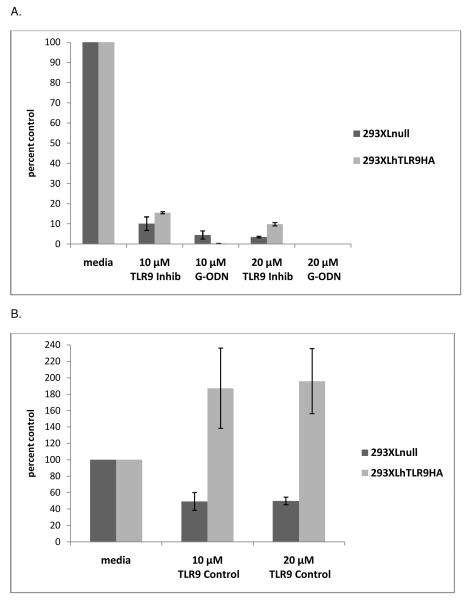
To determine whether the antiviral effect of the TLR9 inhibitory oligonucleotides was dependent on the expression of the TLR9 protein, the TLR9 negative cell line, 293XLnull, and its TLR9 expressing counterpart, 293XLhTLR9HA, were employed. 293XLnull and 293XLhTLR9HA cells were treated with TLR9 oligonucleotides for 2 hours at 37°C, prior to overnight infection with HSV-1 strain KOS. The TLR9 inhibitory oligonucleotide decreased viral titers in 293XLnull cells to roughly 10% of media control at 10 μM and 3.5% of media control at 20 μM (Figure 8A). Similar reductions were seen in the 293XLhTLR9HA cells where the TLR9 inhibitory oligonucleotide reduced titers to 15% of media control at 10 μM and about 10% of media control at 20 μM. The G-ODN inhibitory oligonucleotide had a profound effect on viral titer in 293XLnull cells, with decreases in viral titer to 5% of media control at 10 μM and less than 1% of media control at 20 μM (Figure 8A). Viral titers in 293XLhTLR9HA cells were reduced to less than 1% of media control at the 10 and 20 μM concentrations of G-ODN (Figure 8A). The presence or absence of TLR9 protein did not influence the antiviral activity of these oligonucleotides, indicating that the antiviral effect was not due to inhibition of TLR9 signaling and suggesting that another target is involved. The TLR9 control oligonucleotide decreased viral titers to approximately 50% of the media control in 293XLnull cells at both 10 and 20μM (Figure 8B). Similar to what was seen with ARPE-19 and Vero cells, the TLR9 control oligonucleotide increased viral titers in 293XLhTLR9HA cells to about 190% of media control at 10 and 20 μM and the effect was not dose dependent (Figure 8B).
Figure 8. TLR9 inhibitory oligonucleotides decrease HSV-1 replication independent of TLR9.
Triplicate wells of 239XLnull and 293XLhTLR9HA cells were incubated with media or two concentrations of the TLR9 control, the TLR9 inhibitory, or the G-ODN oligonucleotide for 2 hours at 37°C. Cells were infected with HSV-1 KOS and cultured overnight in the continued presence of the oligonucleotide. Supernatants were collected and viral titer determined by plaque assay. Mean titers of media controls from two experiments were set at 100%, and titers from two experiments were averaged and graphed as percent of control. Error bars represent standard error of the mean. 293XLnull titers (pfu/ml): Media 6.8 ×107; 10 μM TLR9 Control 1.4 × 107; 20 μM TLR9 Control 2.5 × 107; 10 μM TLR9 Inhibitory 4.3 × 106; 20 μM TLR9 Inhibitory 9.6 × 105; 10 μM G-ODN 1.1 × 106; 20 μM G-ODN 4.9 × 101. 293XLhTLR9HA titers (pfu/ml): Media 1.0 ×107; 10 μM TLR9 Control 7.4 × 107; 20 μM TLR9 Control 2.0 × 107; 10 μM TLR9 Inhibitory 1.6 × 106; 20 μM TLR9 Inhibitory 1.0 × 106; 10 μM G-ODN 2.7 × 104; 20 μM G-ODN 6.5 × 101. Student’s t-test indicated that all oligonucleotide treatments were statistically significant compared to the corresponding media control (p<0.04).
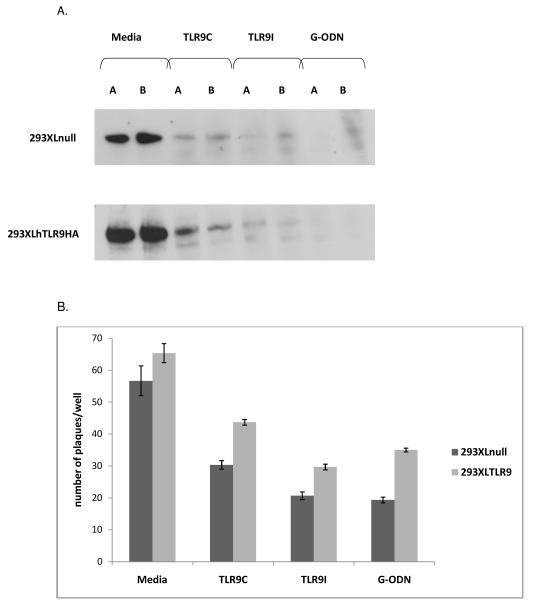
We then assessed whether the TLR9 oligonucleotides affected HSV-1 attachment to 293XLnull and 293XLhTLR9HA cells. Cells were incubated with media, TLR9 control, TLR9 inhibitory, or G-ODN oligonucleotide for 2 hours at 37°C, then transferred to 4°C prior to virus infection. Immunoblotting with an antibody to the viral glycoprotein gD showed that the gD protein was detected in the media lanes of both 293XLnull and 293hTLR9HA cells, indicating viral attachment had occurred (Figure 9B). The TLR9 control, the TLR9 inhibitory, and the G-ODN oligonucleotides, significantly reduced the level of detectable gD in both cell types, indicating that all TLR9 oligonucleotides reduced the ability of HSV-1 to bind to both TLR9 negative and TLR9 positive cells.
Figure 9. Antiviral mechanism of TLR9 oligonucleotides on 293XLnull and 293XLTLR9 cells.
Viral attachment (A): Duplicate wells of 293XLnull, or 293XLhTLR9HA cells were treated with media, or 20μM TLR9 control, TLR9 inhibitory, or G-ODN oligonucleotides for 2 hours at 37°C. Cells were chilled on ice, infected with HSV-1 KOS for 1 hour, washed with cold PBS, and lysed. Fourty μg of each lysate was run on a 4-15% SDS-PAGE gel. Immunoblotting was performed with mouse anti-gD primary antibody followed by a goat anti-mouse IgG HRP secondary antibody. Viral entry (B): 293XLnull and 293XLhTLR9HA cells were chilled on ice prior to addition of HSV-1 KOS to allow for viral attachment. Media or oligonucleotides (20μM) were added to triplicate wells at 4°C prior to increasing the temperature to 37°C to allow for entry. Graph represents mean plaque number per well with error bars representing standard error of the mean. Student’s t-test indicated all oligonucleotide treatments were statistically significant compared to media controls (p<0.007). Post-infection treatment with TLR9 oligonucleotides (C): 293XLnull and 293XLhTLR9HA cells were infected with HSV-1 KOS for 1 hour at 37°C. TLR9 control, TLR9 inhibitory, or G-ODN oligonucleotides were then added to a final concentration of 5, 10 or 20 μM and cells were incubated overnight at 37°C. Supernatants were collected for titering on Vero cells. Graph shows mean viral titer of duplicate wells with error bars representing the standard error of the mean. For 293XLnull cells, Student’s t-test indicated no statistically significant difference vs. media control for TLR9C treatments and 10 and 20 μM TLRI treatments (p>0.05). 5 μM TLR9I treatment and G-ODN treatments were significantly different compared to the media (p<0.05). For 293XLTLR9 cells, Student’s t-test indicated no statistically significant difference between media and any of the oligonucleotide treatments (p>0.05).
Viral entry assays were performed to determine whether the effect of TLR9 oligonucleotides on HSV-1 entry in ARPE-19 cells (Figure 6C) also occurred with the 293XL cells. Figure 9B shows that all oligonucleotides decreased viral entry in 293XLnull and 293XLhTLR9HA cells compared to the media control. The TLR9 control oligonucleotide decreased plaque number to about one half of the media control for 293XLnull, and two thirds of the media control for 293XLhTLR9HA cells. The TLR9 inhibitory and G-ODN oligonucleotides decreased plaque number in 293XLnull cells to roughly one third of the media control. In 293XLhTLR9HA cells, the TLR9 inhibitory and G-ODN oligonucleotides decreased plaque number to about one half of the media control. Thus the oligonucleotides are acting in a similar manner in all of the three cell types.
To determine whether the post-infection inhibitory effect of the G-ODN oligonucleotide observed in ARPE-19 cells (Figure 7) was dependent on the TLR9 protein, 293XLnull and 293XLhTLR9HA cells were infected with HSV-1 KOS for 1 hour prior to addition of increasing concentrations of TLR9 control, TLR9 inhibitory, or G-ODN oligonucleotides. Figure 9C indicates that no reduction of viral titer was achieved when up to 20 μM of the TLR9 control or TLR9 inhibitory oligonucleotides were added to 293XLnull and 293XLhTLR9HA cells 1 hour after infection. In contrast, 5 μM of the G-ODN oligonucleotide reduced viral titers by about 2.5 logs compared to the media control, and higher concentrations (10 and 20 μM) reduced titers by up to 4 logs. These titers correspond to a greater than 99% reduction in viral titer compared to the media control at concentrations of 5, 10, and 20 μM G-ODN. 293XL cells grow in clusters and cannot be trypsinized, making efforts to accurately count cells difficult. A large standard of deviation in the media treated 293XLhTLR9HA wells resulted in no significant difference between G-ODN and media treatments (p>0.05). Despite these challenges, this experiment confirmed that the G-ODN oligonucleotide functioned post-infection to inhibit HSV-1 replication, and that this inhibition was not dependent on TLR9.
3.7 The Effect of TLR9 Oligonucleotides on NFκB Binding in Cellular Extracts
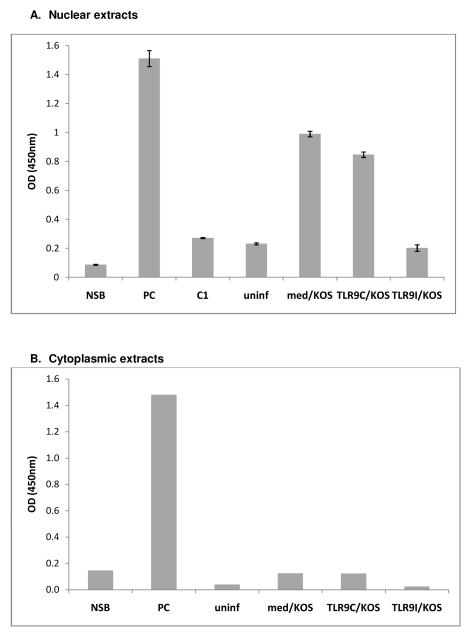
To investigate the effect of TLR9 oligonucleotides on the ability of NFκB to bind to its response element, ARPE-19 cells were treated with media, TLR9 control oligonucleotide, or TLR9 inhibitory oligonucleotide prior to infection with HSV-1 KOS. At 6 hours PI, cells were collected and nuclear and cytoplasmic extracts were prepared. The uninfected nuclear extract contained low levels of NFκB binding activity (Figure 10A, uninf). KOS infection increased the level of NFκB binding activity in the extract approximately 5-fold, consistent with NFκB activation and nuclear translocation during HSV-1 infection (10A, med/KOS). Pretreatment with the TLR9 control oligonucleotide had little effect on the amount of NFκB binding activity in the nuclear extract (10A, TLR9C/KOS). Pretreatment with the TLR9 inhibitory oligonucleotide, however, reduced NFκB binding activity to levels similar to the uninfected extract (10A, TLR9I/KOS). These results indicate that pretreatment with the TLR9 inhibitory oligonucleotide significantly reduced the level of NFκB activation during HSV-1 infection.
Figure 10. TLR9 Inhibitory oligonucleotides decrease NFκB binding activity in nuclear extracts.
ARPE-19 cells were pretreated with media, 20 μM TLR9 control oligo, or 20 μM TLR9 inhibitory oligo for 2 hours at 37°C prior to infection with HSV-1 KOS for 6 hours. A) The equivalent of 14 μg of nuclear extract was loaded, in duplicate, into wells of a Cayman NFκB (p65) Transcription Factor Assay Kit plate. The average OD value of duplicate blank wells was subtracted from the average OD of each duplicate sample. Error bars represent standard error of the mean. Student’s t-test indicated all OD values for nuclear extracts were statistically significant compared to NSB (p<0.03). B) The equivalent of 91 μg of ARPE-19 cytoplasmic extract was loaded into wells of a Cayman NFκB (p65) Transcription Factor Assay Kit plate. The OD value of the blank well was subtracted from the OD value of each sample. NSB: Non-specific binding, PC: positive control extract, C1: competitor dsDNA added to well prior to positive control, uninf: uninfected nuclear extract, med/KOS: media treated and KOS infected extract, TLR9C/KOS: TLR9C treated and KOS infected extract, TLR9I/KOS: TLR9I treated and KOS infected extract.
Since the TLR9 inhibitory oligonucleotide blocked activation of NFκB during HSV-1 infection, we wanted to examine whether the cytoplasmic NFκB in TLR9 inhibitory oligonucleotide treated cells was in an inactive state, or was active but not able to translocate to the nucleus. No significant NFκB binding was detected in any of the ARPE-19 cytoplasmic extracts, including KOS infected extracts, compared to the nonspecific binding control (Figure 10B). This data indicates that cytoplasmic NFκB remains inactive, even when nuclear translocation is inhibited by the TLR9 inhibitory oligonucleotide.
4.0 Discussion
In summary, we have found that commercially available TLR9 inhibitory oligonucleotides are potent antivirals that result in a significant reduction in HSV-1 replication in several cell types. In the presence of TLR9 inhibitory oligonucleotides, expression of the essential viral α protein ICP4 was significantly inhibited, as was subsequent expression of viral β and γ proteins. All the TLR9 oligonucleotides, including the control, decreased viral attachment and entry, while the G-ODN oligonucleotide also exhibited virucidal activity and decreased viral titers when added post infection. The antiviral effect of the TLR9 inhibitory oligonucleotides was not dependent on TLR9, as similar reductions in viral titer were observed in a TLR9 null cell, and its TLR9 expressing counterpart. The TLR9 inhibitory oligonucleotides also decreased the ability of HSV-1 to activate NFκB during infection of ARPE-19 cells. These results indicate a potential use for TLR9 inhibitory oligonucleotides as antiviral agents during HSV-1 infection and emphasize that care must be taken when using these agents to study the role of TLRs in viral infection.
In the present study we infected ARPE-19, Vero, 293XLnull, and 293XLhTLR9HA cells with different multiplicity of infections (MOIs) in separate experiments. Titers of HSV-1 stocks were determined in Vero cells, and the plaquing efficiency of HSV-1 KOS was equivalent in ARPE-19 cells. Higher MOIs could potentially decrease the amount of oligonucleotide available at a given concentration to interact with each virion. For studies involving titrations, we infected overnight with an MOI of 1 to 2 to allow for adequate infection of all cells. Immunofluroescence and Western blot assays, which were performed at 6 or 15 hours post-infection, also utilized an MOI of 1 to 2. During the attachment assay, we infected at an MOI of 5 for 1 hour at 4°C to allow for detection of viral glycoprotein bound to cells. In the entry assay, 150 pfu/well was chosen to generate a reasonable number of plaques/well to count. A high MOI of 5 was chosen for the p65 ELISA to get enough virus to activate p65 within the 6 hour time frame. Regardless of the MOI chosen, the oligonucleotides (TLR9C, TLR9I, G-ODN) performed consistently across different experiments indicating that the MOIs chosen did not influence the results. In addition, the antiviral properties of the TLR9 oligonucleotides were similar in 3 different cell types (ARPE-19, Vero, 293XL) from two different species.
The cellular toxicity assay indicated that all oligonucleotides had a mitogenic effect on ARPE-19 cells. Previous studies have indicated mitogenic properites of bacterial and protozoan DNA, which is largely un-methylated and contains CpG sequences, for B cells (Brown et al., 1998; Krieg et al., 1995; Sun et al., 1997). It is thus possible that the TLR9 oligonucleotides have mitogenic potential, however it is unlikely that this effect is relevant, as all oligonucleotides are mitogenic in ARPE-19 cells, yet only the TLR9 control oligonucleotide increased viral titers.
The TLR9 control oligonucleotide increased HSV-1 titers in ARPE-19 and Vero cells, but this increase was not concentration dependent and was not accompanied by a change in the expression or localization of HSV-1 alpha (ICO4, ICP27), beta (ICP8), or gamma (gD, VP5) proteins. Interestingly, the TLR9 control oligonucleotide also increased viral titers in 293XLhTLR9HA cells, but not in the TLR9 negative cell line 293XLnull, implying that the stimulatory effect may depend on the presence of the TLR9 protein. The stimulatory effect of the TLR9 control oligonucleotide on viral replication in these 3 cell lines was most likely an off-target effect, since it wasn’t dose dependent, but further studies would be needed to determine its mechanism.
When added 2 hours prior to infection, the G-ODN oligonucleotide, which contains a stretch of 5 guanosines, was approximately two-fold more effective at reducing viral titers in several cell types, compared to the TLR9 inhibitory oligonucleotide. This data is consistent with studies which have indicated that G-ODN oligonucleotides are more effective at inhibiting TLR9 activation compared to TLR9 inhibitory oligonucleotides lacking the guanosine rich repeat (Peter et al., 2007). In addition, the G-ODN oligonucleotide was the only oligonucleotide to exhibit virucidal activity against HSV-1. The virucidal activity of the G-ODN oligonucleotide may be due to interaction with HSV-1 glycoproteins yielding conformational changes that affect their functionality. ISI 5652, a 20 base PS-ON, which contains 3 stretches of 4 guanosines, has been shown to exhibit virucidal activity against HSV-1 KOS (Shogan et al., 2006). The G-ODN oligonucleotide decreased viral titers significantly when added to ARPE-19 post infection, while the control and TLR9 inhibitory oligonucleotides did not. Similar results were obtained with the guanosine repeat containing ISIS 5652 oligonucleotide versus the ISIS 2922, which lacks the guanosine repeat, in plaque reduction assays on Vero cells (Shogan et al., 2006). These data suggest a distinct mechanism of antiviral action for oligonucleotides containing guanosine repeats.
PS-ONs can prevent viral attachment via interaction with the α helices of viral glycoproteins such as the HIV-1 fusion protein gp41 (Vaillant et al., 2006) or the HSV-1 viral glycoprotein gB (Shogan et al., 2006). Our studies into the mechanism of TLR9 oligonucleotide antiviral activity revealed that all oligonucleotides interfered with HSV-1 attachment and entry into ARPE-19, 293XLnull, and 293XLhTLr9HA cells. These results conflict with the Takeda study (Takeda et al., 2011) in which TLR9 control and inhibitory oligonucleotides were not shown to interfere with attachment to human corneal endothelial cells (HCEn). It is possible that HCEn cells express different cell surface receptors for viral attachment, as HSV-1 is known to bind to several cellular proteins (Eisenberg et al., 2012), and these interactions may be affected differently by the TLR9 oligonucleotides. TLR9 oligonucleotides could also be triggering internalization of cell surface receptor proteins and thereby decreasing HSV-1 attachment and entry.
The inhibitory effect of the TLR9 control oligonucleotide on viral attachment and entry was surprising, as this oligonucleotide enhanced viral replication by roughly 6 fold when added to APRE-19 cells prior to infection. A PS-ON lacking guanosine stretches (ISIS 2922) was a potent inhibitor of HSV-1 attachment, but did not inhibit viral titers in plaque reduction assays (Shogan et al., 2006). These results mirror our data with the TLR9 control oligonucleotide, which does not inhibit HSV-1 replication in plaque assays, but does decrease viral attachment probably by acting as a polyanion. The observation that the control oligonucleotide inhibited attachment and entry, but enhanced replication when added to cells prior to infection, suggests that inhibition of attachment and entry was not involved in pre-infection treatment.
Our data lead us to believe that the TLR9 oligonucleotides are working on at least two and perhaps three levels i) intracellularly to block TLR9 signaling and NFκB activation as they were designed to do, ii) intracellularly to inhibit some process critical for infection, and iii) externally to interfere with early events in the viral life cycle such as attachment and entry. Thus, the order in which the oligonucleotide and virus is added during experiments is crucial for determining which mechanism of inhibition will predominate. We postulate that when oligonucleotides are added 2 hours prior to infection, the majority are internalized and not available on the cell surface to interfere with viral attachment or entry, thus allowing for robust infection during these assays. Phosphorothioation of nucleci acids can lead to off-target effects, which in the case of oblimersen, a phosphorothioate antisense oligonucleotide directed against bcl-2, results in useful therapeutic properties (Anderson et al., 2006; Gjertsen et al., 2007; Winkler et al., 2010). This may be the case for TLR9 oligonucleotides, which are potent antivirals even in TLR9 negative cells. The antiviral activity of oligonucleotides may also be dependent on temperature, as demonstrated by ISIS 5652, which loses its antiviral activity at 4°C, probably because low temperatures inhibit oligonucleotide-induced conformational changes in the HSV-1 gB protein (Shogan et al., 2006).
Recently, the importance of several classes of DNA sensors in antiviral immunity has been elucidated (Rathinam and Fitzgerald, 2011). During viral infection, the cytosol accumulates viral RNAs or DNAs that originate from incoming viral genomes, viral transcripts, or transcription and replication intermediates (Rathinam and Fitzgerald, 2011). Several classes of Interferon-inducing DNA receptors including DNA-dependent activator of interferon regulatory factors (IRFs) (Takaoka et al., 2007), the mostly nuclear interferon inducible protein IFI16 (Horan et al., 2013; Unterholzner et al., 2010), and several DExD/H-box helicases (Kim et al., 2010; Zhang et al., 2011) have been linked to an antiviral response during HSV-1 infection. Notably, the DExD/H proteins 9 and 36 are known to bind to CpG rich DNA in the cytosol and have been shown to be important in dendritic cell cytokine response, including NFκB activation, to DNA viruses (Kim et al., 2010). It is possible that the TLR9 inhibitory oligonucleotides, which do not require TLR9 for their antiviral effect, are targeting DExD/H box proteins or other DNA sensing cytosolic receptors. Further studies are needed to identify the target of the TLR9 inhibitory oligonucleotides in order to determine their mechanism of action.
The interaction of HSV with NFκB is complicated in that the virus activates NFκB to facilitate its replication but concomitantly blocks NFκB activation to limit production of antiviral and inflammatory cytokines (Liu et al., 2008). HSV-1 activation of NFκB occurs in two waves (Amici et al., 2006). The first wave of NFκB activation requires virus binding and entry and the action of several viral proteins including glycoprotein gD, which binds to HVEM to activate NFκB and suppress apoptosis (Aubert and Blaho, 1999; Cheung et al., 2009; Goodkin et al., 2003; Medici et al., 2003; Sciortino et al., 2008a; Sciortino et al., 2008b), and the UL31 or UL37 tegument proteins (Liu et al., 2008; Roberts and Baines, 2011). Recently, it has been demonstrated that HSV-1 utilizes the recognition of its glycoproteins gB and gH/g/L by the pattern recognition receptor TLR2 to activate NFκB early in infection (Leoni et al., 2012). We detected decreased activation of NFκB by HSV-1 when ARPE-19 cells were incubated with the TLR9 inhibitory oligonucleotide prior to infection. This decrease in NFκB activation is not likely caused by decreased viral attachment or entry as the TLR9 control oligonucleotide did not decrease NFκB activation in nuclear extracts, yet was able to decrease both viral attachment and entry.
The second wave of NFκB activation requires viral replication (Amici et al., 2006; Cai and Brandt, 2008) and is required for sustained HSV-1 gene expression and efficient replication. It is likely that multiple HSV-1 proteins including ICP27 and ICP0 (Gregory et al., 2004; Hargett et al., 2006), and possibly multiple pathways, are involved in NFκB activation, providing redundancy to optimize infection. Studies in human corneal endothelial cells suggest that HSV-1 is using TLR9-mediated NFκB activation for its own replication, yet these authors did not determine whether the oligonucleotide was acting in a TLR9-dependent manner (Takeda et al., 2011). Further studies will be needed to address any link between decreased NFκB activation and the antiviral mechanism of TLR9 oligonucleotides.
In summary, we have shown that TLR9 inhibitory oligonucleotides exhibit a profound antiviral effect on HSV-1 replication in both TLR9 positive and TLR9 negative cell lines. The phosphorothioated oligonucleotides appear to act both externally and intracellularly to inhibit several stages of the viral life cycle and the most potent oligonucleotides contain stretches of guanosine residues. Caution must be used when utilizing these oligonucleotides to study interactions between the TLR system and viruses.
Highlights.
Oligonucleotides Designed to Inhibit TLR9 Block Herpes Simplex Virus type 1 Infection at Multiple Steps by Monica M. Sauter, Joshua J. L. Gauger, and Curtis R. Brandt
TLR9 inhibitory oligonucleotides inhibit HSV-1 replication in several cell types
TLR9 inhibitory oligonucleotides prevent expression of essential herpes proteins
Multiples stages of infection are targeted by the TLR9 inhibitory oligonucleotides
The antiviral effect of the TLR9 inhibitory oligonucleotides is not TLR9 specific
NFκB activation is decreased in the presence of TLR9 inhibitory oligonucleotides
Acknowledgements
This work was supported by a grant from The Retina Research Foundation, Houston TX, a Core Grant for Vision Research from the NIH P30 EY016665 (CRB), and an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.0 References
- Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Amici C, Rossi A, Costanzo A, Ciafre S, Marinari B, Balsamo M, Levrero M, Santoro MG. Herpes simplex virus disrupts NF-kappaB regulation by blocking its recruitment on the IkappaBalpha promoter and directing the factor on viral genes. J. Biol. Chem. 2006;281:7110–7117. doi: 10.1074/jbc.M512366200. [DOI] [PubMed] [Google Scholar]
- Anderson EM, Miller P, Ilsley D, Marshall W, Khvorova A, Stein CA, Benimetskaya L. Gene profiling study of G3139- and Bcl-2-targeting siRNAs identifies a unique G3139 molucular signature. Cancer Gene Ther. 2006;13:406–414. doi: 10.1038/sj.cgt.7700901. [DOI] [PubMed] [Google Scholar]
- Aubert M, Blaho J. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 1999;73:2803–2813. doi: 10.1128/jvi.73.4.2803-2813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol. Rev. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- Bernstein DI, Goyette N, Cardin R, Kern ER, Boivin G, Ireland J, Juteau JM, Vaillant A. Amphipathic DNA polymers exhibit antiherpetic activity in vitro and in vivo. Antimicrob. Agents Chemother. 2008;52:2727–2733. doi: 10.1128/AAC.00279-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin N, Menasria R, Piret J, Boivin G. Modulation of TLR9 response in a mouse model of herpes simplex virus encephalitis. Antiviral Res. 2012;96:414–421. doi: 10.1016/j.antiviral.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Brown WC, Estes DM, Chantler SE, Kegerreis KA, Suarez CE. DNA and a CpG oligonucleotide derived from Babesia bovis are mitogenic for bovine B cells. Infect. Immun. 1998;66:5423–5432. doi: 10.1128/iai.66.11.5423-5432.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Li M, Wang K, Wang S, Lu Q, Yan J, Mossman KL, Lin R, Zheng C. The herpes simplex virus 1-encoded envelope glycoprotein B activates NF-κB through the Toll-like receptor 2 and MyD88/TRAF6-dependent signaling pathway. PLoS One. 2013;8:e54586. doi: 10.1371/journal.pone.0054586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Brandt CR. Induction of interleukin-6 in human retinal epithelial cells by an attenuated Herpes simplex virus vector requires viral replication and NFkappaB activation. Exp. Eye Res. 2008;86:178–188. doi: 10.1016/j.exer.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin RD, Bravo FJ, Sewell AP, Cummins J, Flamand L, Juteau JM, Bernstein DI, Vaillant A. Amphipathic DNA polymers exhibit antiviral activity against systemic murine Cytomegalovirus infection. Virol. J. 2009;6:214. doi: 10.1186/1743-422X-6-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TC, Steinberg MW, Oborne LM, Macauley MG, Fukuyama S, Sanjo H, D’Souza C, Norris PS, Pfeffer K, Murphy KM, Kronenberg KM, Spear PG, Ware CF. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6244–6249. doi: 10.1073/pnas.0902115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counihan NA, Lindenbach BD. Gumming up the works: DNA polymers as HCV entry inhibitors. Gastroenterology. 2009;137:427–430. doi: 10.1053/j.gastro.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R, de Vries RD, Osterhaus ADME, Rameijer L, Verjans GMGM. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J. Infect. Dis. 2008;198:659–663. doi: 10.1086/590668. [DOI] [PubMed] [Google Scholar]
- Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. Herpes virus fusion and entry: a story with many characters. Viruses. 2012;4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 2007;57:737–763. doi: 10.1016/j.jaad.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Fennewald SM, Mustain S, Ojwang J, Rando RF. Inhibition of herpes simplex virus in culture by oligonucleotides composed entirely of deoxyguanosine and thymidine. Antiviral Res. 1995;26:37–54. doi: 10.1016/0166-3542(94)00064-f. [DOI] [PubMed] [Google Scholar]
- Galderisi U, Cascino A, Giordano A. Antisense oligonucleotides as therapeutic agents. J. Cell. Physiol. 1999;181:251–257. doi: 10.1002/(SICI)1097-4652(199911)181:2<251::AID-JCP7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Gao WY, Hanes RN, Vazquez-Padua MA, Stein CA, Cohen JS, Cheng YC. Inhibition of herpes simplex virus type 2 growth by phosphorothioate oligodeoxynucleotides. Antimicrob. Agents Chemother. 1990;34:808–812. doi: 10.1128/aac.34.5.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjertsen BT, Bredholt T, Anensen N, Vintermyr OK. Bcl-2 antisense in the treatment of human malignancies: a delusion in targeted therapy. Curr. Pharm. Biotechnol. 2007;8:373–381. doi: 10.2174/138920107783018381. [DOI] [PubMed] [Google Scholar]
- Goodkin ML, Ting AT, Blaho JA. NF-kappaB is required for apoptosis prevention during herpes simplex virus type 1 infection. J. Virol. 2003;77:7261–7280. doi: 10.1128/JVI.77.13.7261-7280.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau DR, Visalli RJ, Brandt CR. Herpes simplex virus stromal keratitis is not titer-dependent and does not correlate with neurovirulence. Invest. Ophthalmol. Vis. Sci. 1989;30:2474–2480. [PubMed] [Google Scholar]
- Gregory D, Hargett D, Holmes D, Money E, Bachenheimer SL. Efficient replication by herpes simplex virus type 1 involves activation of the IkappaB kinase-IkappaB-p65 pathway. J. Virol. 2004;78:13582–13590. doi: 10.1128/JVI.78.24.13582-13590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman EM, Cheshenko N, Shende V, Keller MJ, Goyette N, Juteau JM, Boivin G, Vaillant A, Herold BC. Amphipathic DNA polymers are candidate vaginal microbicides and block herpes simplex virus binding, entry and viral gene expression. Antivir. Ther. 2007;12:1147–1156. [PubMed] [Google Scholar]
- Hagedorn PH, Yakimov V, Ottosen S, Kammler S, Nielsen NF, Høg AM, Hedtjärn M, Meldgaard M, Møller MR, Orum H, Koch T, Lindow M. Hepatotoxic potential of therapeutic oligonucleotides can be predicted from their sequence and modification pattern. Nucleic Acid Ther. 2013;23:302–310. doi: 10.1089/nat.2013.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargett D, Rice S, Bachenheimer SL. Herpes simplex virus type 1 ICP27-dependent activation of NF-kappaB. J. Virol. 2006;80:10565–10578. doi: 10.1128/JVI.01119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Hooper LC, Chin MS, Nagineni CN, Detrick B, Hooks JJ. Herpes simplex virus 1 (HSV-1) DNA and immune complex (HSV-1-human IgG) elicit vigorous interleukin 6 release from infected corneal cells via Toll-like receptors. J. Gen. Virol. 2006;87:2161–2169. doi: 10.1099/vir.0.81772-0. [DOI] [PubMed] [Google Scholar]
- Horan KA, Hansen K, Jakobsen MR, Holm CK, Søby S, Unterholzner L, Thompson M, West JA, Iversen MB, Rasmussen SB, Ellermann-Eriksen S, Kurt-Jones E, Landolfo S, Damania B, Melchjorsen J, Bowie AG, Fitzgerald KA, Paludan SR. Proteasomal degradation of herpes simplex virus capsids in macrophages releases DNA to the cytosol for recognition by DNA sensors. J. Immunol. 2013;190:2311–2319. doi: 10.4049/jimmunol.1202749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Yang Y. Innate immune recognition of viruses and viral vectors. Hum. Gene Ther. 2009;20:293–301. doi: 10.1089/hum.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler JL, Zheng L. Biology of Toll receptors: lessons from insects and mammals. J. Leukoc. Biol. 2004;75:18–26. doi: 10.1189/jlb.0403160. [DOI] [PubMed] [Google Scholar]
- Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patic k.A.K., Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. New Engl. J. Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- Kim T, Pazhoor S, Bao M, Zhang Z, Hanabuchi S, Facchinetti V, Bover L, Plumas J, Chaperot L, Qin J, Liu Y-J. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15181–15186. doi: 10.1073/pnas.1006539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J. Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DCG, Monks BG, McKnight CJ, Lamphier MS, Duprex WP, Espevik T, Golenbock DT. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat. Immunol. 2007;8:772–779. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- Lee AM, Rojek JM, Gundersen A, Ströher U, Juteau JM, Vaillant A, Kunz S. Inhibition of cellular entry of lymphocytic choriomeningitis virus by amphipathic DNA polymers. Virology. 2008;372:107–117. doi: 10.1016/j.virol.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni V, Gianni T, Salvioli S, Campadelli-Fiume G. Herpes simplex virus glycoproteins gH/gL and gB bind Toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-κB. J. Virol. 2012;86:6555–6562. doi: 10.1128/JVI.00295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XQ, Fitzgerald K, Kurt-Jones E, Fingerg R, Knipe DM. Herpesvirus tegument protein activates NF-kB signaling through the TRAF6 adaptor protein. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11335–11339. doi: 10.1073/pnas.0801617105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luganini A, Caposio F, Landolfo S, Gribaudo G. Phosphorothioate-modified oligodeoxynucleotides inhibit human cytomegalovirus replication by blocking virus entry. Antimicrob. Agents Chemother. 2008;52:1111–1120. doi: 10.1128/AAC.00987-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Martin N, Viejo-Borbolla A. Toll-like receptor-mediated recognition of herpes simplex virus. Front. Biosci. (Schol Ed) 2010;S2:718–729. doi: 10.2741/s96. [DOI] [PubMed] [Google Scholar]
- Medici MA, Sciortino MT, Perri D, Amici C, Avitabile E, Ciotti M, Balestrieri E, De Smaele E, Franzoso G, Mastino A. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: role of nuclear factor kappaB. J. Biol. Chem. 2003;278:36059–36067. doi: 10.1074/jbc.M306198200. [DOI] [PubMed] [Google Scholar]
- Mescalchin A, Restle T. Oligomeric nucleic acids as antivirals. Molecules. 2011;16:1271–1296. doi: 10.3390/molecules16021271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers inhibit duck hepatitis B virus infection in vitro. Antimicrob. Agents Chemother. 2013a;57:5291–5298. doi: 10.1128/AAC.01003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers prevent the establishment of duck hepatitis B virus infection in vivo. Antimicrob. Agents Chemother. 2013b;57:5299–5306. doi: 10.1128/AAC.01005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M, Bode K, Lipford GB, Eberle F, Heeg K, Dalpke AH. Characterization of suppressive oligodeoxynucleotides that inhibit Toll-like receptor-9-mediated activation of innate immunity. Immunology. 2007;123:118–128. doi: 10.1111/j.1365-2567.2007.02718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SB, Sørensen LN, Malmgaard L, Ank N, Baines JD, Chen ZJ, Paludan SR. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 2007;81:13315–13324. doi: 10.1128/JVI.01167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VAK, Fitzgerald KA. Cytosolic surveillance and antiviral immunity. Curr. Opin. Virol. 2011;1:455–462. doi: 10.1016/j.coviro.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KL, Baines JD. UL31 of Herpes simplex virus 1 is necessary for optimal NF-{kappa}B activation and expression of viral gene products. J. Virol. 2011;85:4947–4953. doi: 10.1128/JVI.00068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino MT, Medici MA, Marino-Merlo F, Zaccaria D, Giuffrè-Cuculletto M, Venuti A, Grelli S, Mastino A. Involvement of HVEM receptor in activation of nuclear factor kappaB by herpes simplex virus 1 glycoprotein D. Cell Microbiol. 2008a;10:2297–2311. doi: 10.1111/j.1462-5822.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- Sciortino MT, Medici MA, Marino-Merlo F, Zaccaria D, Giuffrè-Cuculletto M, Venuti A, Grelli S, Bramanti P, Mastino A. Involvement of gD/HVEM interaction in NF-kB-dependent inhibition of apoptosis by HSV-1 gD. Biochem. Pharmacol. 2008b;76:1522–1532. doi: 10.1016/j.bcp.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Shogan B, Kruse L, Mulamba GB, Hu A, Coen DM. Virucidal activity of a GT-rich oligonucleotide against herpes simplex virus mediated by glycoprotein B. J. Virol. 2006;80:4740–4747. doi: 10.1128/JVI.80.10.4740-4747.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW, Dana MR, Ksander BR. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol. Today. 1997;18:443–449. doi: 10.1016/s0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- Sun S, Beard C, Jaenisch R, Jones P, Sprent J. Mitogenicity of DNA from different organisms for murine B cells. J. Immunol. 1997;159:3119–3125. [PubMed] [Google Scholar]
- Takaoka A, Wang Z, Choi MK, Yana i.H., Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- Takeda S, Miyazaki D, Sasaki S.-i., Yamamoto Y, Terasaka Y, Yakura K, Yamagami S, Ebihara N, Inoue Y. Roles played by toll-like receptor-9 in corneal endothelial cells after herpes simplex virus type 1 infection. Invest. Ophthalmol. Vis. Sci. 2011;52:6729–6736. doi: 10.1167/iovs.11-7805. [DOI] [PubMed] [Google Scholar]
- Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant A, Juteau J, Lu H, Liu S, Lackman-Smith C, Ptak R, Jiang S. Phosphorothioate oligonucleotides inhibit human immunodeficiency virus type 1 fusion by blocking gp41 core formation. Antimicrob. Agents Chemother. 2006;50:1393–1401. doi: 10.1128/AAC.50.4.1393-1401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velzen M, Missotten T, van Loenen FB, Meesters RJ, Luider TM, Baarsma GS, Osterhaus AD, Verjans GM. Acyclovir-resistant herpes simplex virus type 1 in intra-ocular fluid samples of herpetic uveitis patients. J. Clin. Virol. 2013;57:215–221. doi: 10.1016/j.jcv.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Visalli RJ, Brandt CR. The HSV-1 UL45 18 kDa gene product is a true late protein and a component of the virion. Virus Res. 1993;29:167–178. doi: 10.1016/0168-1702(93)90057-t. [DOI] [PubMed] [Google Scholar]
- Whitley RJ. Herpes simplex viruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Lippincott-Raven; Philadelphia: 1996. pp. 2297–2342. [Google Scholar]
- Winkler J, Stessl M, Amartey J, Noe CR. Off-target effects related to the phosphorothioate modification of nucleic acids. ChemMedChem. 2010;5:1344–1352. doi: 10.1002/cmdc.201000156. [DOI] [PubMed] [Google Scholar]
- Wong JP, Christopher ME, Salaza r.A.M., Sun LQ, Viswanathan S, Wang M, Saravolac EG, Cairns MJ. Broad-spectrum and virus-specific nucleic acid-based antivirals against influenza. Front. Biosci. (Schol Ed) 2010;2:791–800. doi: 10.2741/s102. [DOI] [PubMed] [Google Scholar]
- Wong JP, Christopher ME, Salazar AM, Dale RM, Sun LQ, Wang M. Nucleic acid-based antiviral drugs against seasonal and avian influenza viruses. Vaccine Res. 2007;25:3175–3178. doi: 10.1016/j.vaccine.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Yao G-Q, Grill S, Egan W, Gheng Y-C. Potent inhibition of Epstein-Barr Virus by phosphorothiate oligodeoxyneuleotides without sequence specification. Antimicrob. Agents Chemother. 1993;37:1420–1425. doi: 10.1128/aac.37.7.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. TLR3 deficiency in patients with Herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu Y-J. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat. Immunol. 2011;12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]