Summary
Objective
Obesity is a major health problem associated with high morbidity and mortality. NSAID activated gene, (NAG-1) is a TGF-β superfamily member reported to alter adipose tissue levels in mice. We investigated whether hNAG-1 acts as a regulator of adiposity and energy metabolism.
Design/Subjects
hNAG-1 mice, ubiquitously expressing hNAG-1, were placed on a control or high fat diet (HFD) for 12 weeks. hNAG-1 expressing B16/F10 melanoma cells were used in a xenograft model to deliver hNAG-1 to obese C57BL/6 mice.
Results
As compared to wild-type littermates, transgenic hNAG-1 mice have less white fat and brown fat despite equivalent food intake, improved glucose tolerance, lower insulin levels and are resistant to dietary- and genetic-induced obesity. hNAG-1 mice are more metabolically active with higher energy expenditure. Obese C57BL/6 mice treated with hNAG-1 expressing xenografts show decreases in adipose tissue and serum insulin levels. hNAG-1 mice and obese mice treated with hNAG-1 expressing xenografts show increased thermogenic gene expression (UCP1, PGC1α, ECH1, Cox8b, Dio2, Cyc1, PGC1β, PPARα, Elvol3) in brown adipose tissue (BAT) and increased expression of lipolytic genes (Adrb3, ATGL, HSL) in both white adipose tissue (WAT) and BAT, consistent with higher energy metabolism
Conclusion
hNAG-1 modulates metabolic activity by increasing the expression of key thermogenic and lipolytic genes in BAT and WAT. hNAG-1 appears to be a novel therapeutic target in preventing and treating obesity and insulin resistance.
Keywords: NAG-1/GDF-15, insulin resistance, BAT, thermogenesis, lipolysis
Introduction
Obesity has reached near epidemic proportions, with an estimated 36% of the adult population considered obese or overweight (1). This rapid rise in obesity has also led to a concurrent rise in type-2 diabetes, cardiovascular disease, and some forms of cancer (2–3). A suggested link between these diseases and obesity is due to the development of underlying chronic inflammation (4). White adipose tissue (WAT) is the major storage site for lipids and the source of inflammatory adipokines. Brown adipose tissue (BAT) controls energy expenditure and thermogenesis through fatty acid oxidative metabolism. Stored triglycerides (TGs) are converted to free fatty acids (FFAs) through lipolysis directed by adipose triglyceride lipase (ATGL) and hormone sensitive lipase (HSL) and subsequently utilized for the production of energy (5–7). Increasing circulating FFAs levels leads to decreases in both glucose uptake and insulin sensitivity (8–9). BAT metabolism is controlled by the expression of uncoupling protein-1, UCP1, and relies on FFAs for heat production and to induce activation of UCP1 (6,10). Disruptions in the balance between lipid storage and metabolism are critical for the development of obesity.
TGF-β super-family members modulate adipogenesis, adipocyte differentiation and function (11), and can induce or inhibit adipogenesis. Growth differentiation factor 3 (GDF3), administered to mice on a HFD, increases body weight, WAT, serum leptin levels, and PPAR-γ expression compared to control mice (12). In contrast, GDF3 knockout mice do not gain weight or show an increase in WAT when fed a HFD (13). No difference in energy expenditure in liver, muscle, and BAT is observed between wild type (WT) and knockout (KO) mice (14). GDF8 (myostatin), administered systemically, results in a near total loss of WAT in mice, and GDF8 transgenic mice have less WAT and on HFD, do not gain as much body fat, despite similar food intake and activity as WT littermates (15–16). Bone morphogenic protein 7 (BMP7) induces weight loss by promoting BAT development and increasing energy expenditure (17). Recently, BMP8B is reported to increase BAT thermogenesis (18).
NAG-1 (nonsteroidal anti-inflammatory drug-activated gene), also known as GDF-15 (growth differentiation factor 15) (19) and MIC-1 (macrophage inhibitory cytokine-1) (20), is also a TGF-β super-family member (21). In 2006, this laboratory created a transgenic mouse that ubiquitously over-expresses the human NAG-1 gene (hNAG-1 mouse) (22) to examine the biological activity of this protein. We found the transgenic mouse is resistant to chemical- and genetic-induced intestinal cancer and has a decreased systemic inflammatory response (22–23). Furthermore, we reported the transgenic mice weigh less and have less fat (22) despite similar food intake as wild type (WT) littermates, suggesting NAG-1 may act to alter metabolism. Subsequently, Johnen et al. reported that elevated levels of NAG-1 observed in cancer patients appear to be related to cachexia and suggested this protein acts to suppress appetite (24). In xenograft mouse models, nude mice with tumors expressing hNAG-1 show a decrease in body weight and fat (24–25). Thus, the mechanism for the reduction of adipose tissue in mice by NAG-1 is not clear and needs to be resolved. In this study, we use hNAG-1 mice and xenografts expressing hNAG-1 to explore how hNAG-1 reduces adipose tissue. Here we report that hNAG-1 mice have increased expression of key genes in adipose tissue that regulate thermogenesis, lipolysis, and hence increased oxidative metabolism. As a result, hNAG-1 mice have lower adipose tissue and are resistant to obesity. Furthermore, increasing circulating hNAG-1 in obese mice increases expression of key metabolism genes and reduces adipose tissue and body weight. These findings suggest hNAG-1 is a novel regulator of metabolism with potential therapeutic uses in metabolic diseases like obesity.
Materials and Methods
Animals
Previously, transgenic mice were generated to ubiquitously express human NAG-1 (22). Two independent transgenic lines, 1377 and 1398, were chosen and expanded because of their strong expression of the transgene. We focused on mice from the 1377 line for all studies. All mouse experiments were performed under approval from the Animal Care and Use Committee from each institution (NIEHS, University of Tennessee, and ILS). All mice were handled and cared for according to the NIH Guide for the Care and Use of Laboratory Animals.
Food intake and fat measurements
The food intake of sixteen 13 week-old mice was measured daily for 10 days. Body weights were measured twice weekly. The male and female mice were euthanized and total fat was removed and weighed. In some experiments, WAT was separated into gonadal (GOD), retroperitoneal (RP), and inguinal (ING) fat.
High fat diet study
20-week-old mice were placed on control and high fat diets (10% and 60% kcal fat from Research Diets, New Brunswick, NJ) for 12 weeks. Body weights were measured once a week. At the end of study, the mice were euthanized and tissue was collected for analysis. Serum was collected and stored at −80°C until analysis.
Ay/NAG-1 experiments
hNAG-1 mice were bred with Ay mice (Jackson Laboratories, Bar Harbor, ME) to generate Ay/NAG-1 mice. Body weights of male and female Ay and Ay/NAG-1 mice were measured from 3 weeks of age to 15 weeks of age. At the end of 15 weeks, the mice were euthanized, fat tissue was weighed, and serum was collected for analysis.
Metabolic activity
Sixteen-week-old male and female mice were placed in metabolic chambers for 3 days (Labmaster calorimetry unit/TSE systems (Chesterfield, MO)). VO2, VCO2, and heat were measured.
Glucose tolerance test
Sixteen- to eighteen-week old male and female mice were fasted for 16 h and D-glucose (Sigma, St. Louis, MO) was intraperitoneally injected into mice at dose of 1 g/kg BW. Blood was collected from the tail at 0, 15, 30, 60, 90, and 120 min after injection and glucose concentration was analyzed using a glucose meter.
B16/F10 xenograft
18-week-old diet-induced obese C57BL/6 mice were purchased from Jackson Laboratory and maintained on the 60% kcal/fat diet from Research Diets for the duration of the study. Stably transduced B16/F10 cells were grown in culture, trypsinized, washed, and resuspended in PBS. At 20 weeks of age, mice were randomized by weight into 3 groups: no injection, control (vector), and hNAG-1 expressing cells. B16/F10 cells carrying vector and hNAG-1 were subcutaneously injected into the right flanks of the obese C57BL/6 mice. All mice were fed and given water ad libitum. Body weights were measured twice weekly and food consumption measured twice a week. Palpable tumors were measured once a week using digital vernier calipers. 4 weeks after injection, all mice were euthanized. Serum, WAT, BAT, liver, muscle, and tumor tissue was snap frozen in liquid nitrogen and stored at −80°C for analysis.
Mitochondrial DNA content
Relative mitochondrial DNA content of brown adipose tissue was determined using a modified protocol by Humble M et al. 2012 (new reference 26). Briefly, BAT was lysed in 200μl of 25mM NaOH per 15mg of tissue for 2 hours at 98°C, then neutralized with 20 μl of 1M Tris pH 8. Tissue lysates were subjected to PCR using the following cycling protocol: 95°C for 10 min, and 40 cycles at 95°C for 15s and 60°C for 1 min. All reactions were performed in duplicate and 2 μl of a diluted (1:100) lysate was used in a 25 μl reaction with TaqMan 2X Universal Mix (Applied Biosystems, Foster City, CA). FAM-labeled probes targeting the mitochondrial-specific gene Nd1(Applied Biosystems) and nuclear Actin (Applied Biosystems) were added to separate reactions. PCR was carried out on a BioRad MyQ iCycler to generate Ct values for each reaction. Relative mitochondrial DNA content was determined by the ratio of Nd1 to Actin Ct values for a given lysate.
Real-time PCR
Adipose tissue was isolated using QIAzol from Qiagen (Valencia, CA) and RNA isolated following RNeasy kit instructions from Qiagen. All other tissues were handled according to the RNeasy kit protocol for tissue lysis and RNA isolation. 1 microgram of RNA was reverse transcribed using iScript cDNA synthesis kit from BioRad. Real-time PCR assays were performed using Taqman master mix and primers (Applied Biosystems,) by MyiQ PCR detection system (BioRad) for semi-quatitative real-time PCR analysis. Relative fold changes were calculated using the delta delta Ct method, with β-actin or GAPDH serving as control genes.
Western blots
20mg of brown and white adipose tissue was homogenized with a tissue homogenizer (Omni) in a small volume of RIPA buffer containing protease inhibitors. Lysates were cleared by spinning in a refrigerated centrifuge. Protein concentration was determined by performing a BCA Assay (Pierce, Rockford, IL). 1μg of each protein lysate was boiled under reducing conditions and subjected to SDS-PAGE. Protein was transferred onto PVDF membrane and then blocked in 5% nonfat milk in TBST (Tris-buffered saline with 0.5% Tween 20) for 1hr at room temperature, then blotted overnight at 4°C with the following antibodies at concentrations suggested by the manufacturer: UCP-1 (Abcam, Cambridge, MA ), PGC1-α (Abcam), PGC1-β (Abcam), and GAPDH (Fitzgerald, Acton, MA). Membranes were probed with HRP-conjugated secondary antibodies at a 1:5000 dilution for 1hr at room temperature (Cell Signaling, Danvers, MA), washed, submersed in WesternBright ECL kit solution (Advansta, Menlo Park, CA) and exposed to autoradiography film.
ELISA Analysis
Terminal bleeds from mice were incubated at room temperature for 1 hour in serum separator tubes (Sarstedt, Nümbrecht, Germany) and then spun at 10,000 × rpm for 5 minutes. Serum was collected and stored at −80°C until analysis. Mouse leptin, human NAG-1, mouse IGF-1 (R&D, Minneapolis, MN), mouse insulin (Alpco, Salem, NH), and mouse glycerol (Cayman, Detroit, MI) ELISA kits was used according to manufacturer’s instructions. Cholesterol and triglycerides kit was purchased from Beckman Coulter (Melvill, NY) and non-esterified free fatty acid kit was purchased from Sekisui Diagnostics (Exton, PA). Analysis was done using an Olympus AU400e clinical analyzer by Beckman Coulter.
Statistical Analysis
Dr. Grace Kissling of the NIEHS Biostatistics Branch analyzed and determined the appropriate statistical method (Student’s t- test and Mann-Whitney test). The data are presented as mean ± S. E. and p value at 0.05 for statistical significance.
Results
hNAG-1 mice are leaner, with less body weight and fat
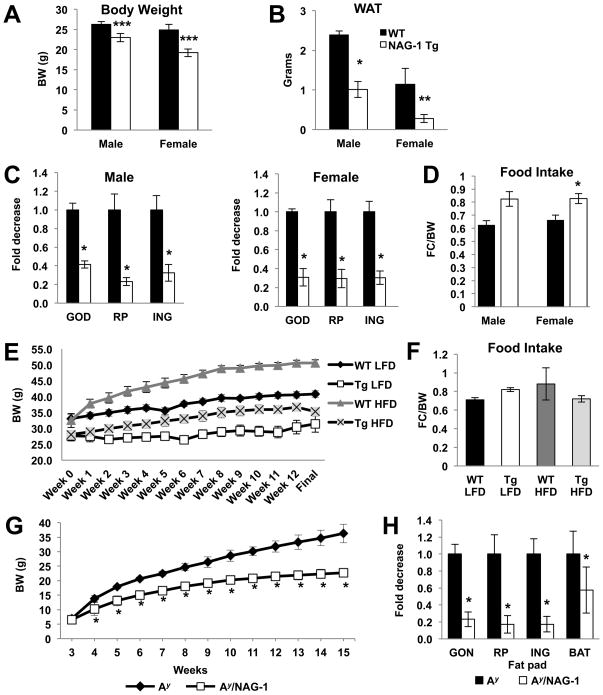
hNAG-1 mice have lower mean body weights compared to WT littermates (Figure 1A). Total WAT for hNAG-1 mice is about 75% and 58% lower in females and males, respectively, compared to WT littermates (Figure 1B). This difference in body weight and WAT is seen in both transgenic lines maintained by the laboratory and is independent of genetic background (data not shown). Furthermore, retroperitoneal, gonadal, and inguinal fat depots, normalized to per gram body weight, are all significantly reduced in hNAG-1 mice compared to WT mice (Figure 1C).
Figure 1. hNAG-1 mice are leaner, have less fat compared, and are resistant to diet- and genetic-induced obesity.
(A) Body weights of 13 week old WT and hNAG-1 mice (n=4 mice per group). (B) Total WAT is reduced in hNAG-1 mice. Relative weights of WAT components: gonadal, retroperitoneal, and inguinal (C) are individually reduced in hNAG-1 mice. (D) Food intake is similar or higher in hNAG-1 mice compared to WT littermates. The solid bars are the wild-type littermates and open bars the hNAG-1 mice (n=4 mice per group). WT and hNAG-1 mice placed on a control (10% kcal fat) or high fat diet (60% kcal fat) for 12 weeks (n=5–7 mice per group). (E) Body weights over time of WT control (10% diet- black diamond), hNAG-1 control (10% diet- open square), WT HFD (60% diet- gray triangle), and hNAG-1 HFD (60% diet- black x mark; weights for hNAG-1 mice on both diets is significant at all time points. (F) Food intake of WT and hNAG-1 mice, regardless of diet, is similar. hNAG-1 mice were crossed to Ay mice to create Ay/NAG-1 mice (n=5–7 mice per group). (G) Lower body weights of Ay/NAG-1 mice (white squares) compared to Ay littermates (black diamonds). (H) Levels of BAT and the components of WAT (inguinal, retroperitoneal, and gonadal) are reduced in Ay/NAG-1 mice compared to Ay littermates. Data are presented as ± SE. *p<0.05, **p<0.01, and ***p<0.001 as determined by Student’s t-test.
A possible explanation for the reduction in WAT and body weights is reduced food consumption in hNAG-1 mice. Food consumption of hNAG-1 and WT mice was monitored for 10 days. No differences were observed in total food consumed per day (Supplemental Figure 1A) and food intake (food consumption/body weight (gram)/day) was similar or even higher as compared to WT littermates (Figure 1D). Lower WAT and body weights were observed in both the 1398 line on hNAG-1 mice on the FVB/N background (data not shown). These data are consistent with our previous findings (22) and suggests that reduced food intake does not explain why the mice have less body weight and fat.
hNAG-1 protects against diet- and genetic-induced obesity
To determine if hNAG-1 would protect against diet-induced obesity, hNAG-1 and WT mice were placed on a HFD for 12 weeks. hNAG-1 mice on a HFD do not gain as much weight compared to WT mice (Figure 1E; Supplemental Figure 1B–C; female mice data are shown in Supplemental Figure 2). Further, this reduced weight gain occurs despite similar food intake (Figure 1F). A possible explanation for the lack of body weight gain in hNAG-1 mice could be decreased fat absorption during digestion. Steatocrit analysis of feces reveals no difference in fat absorption between WT and hNAG-1 mice (data not shown). Lipid analysis of blood from WT and hNAG-1 mice shows that hNAG-1 mice on the control diet have lower total cholesterol and LDL content compared to control diet WT mice, while hNAG-1 mice on the HFD have lower LDL and TGs compared to HFD WT mice (Supplemental Tables 1A–B). Free fatty acid levels are similar for the WT and hNAG-1 mice on either diet.
To determine if the expression of NAG-1 would provide similar protection in a genetic model of obesity, hNAG-1 mice were bred to Ay mice to produce Ay/NAG-1 mice. Ay mice have a mutation in the agouti or Ay gene, exhibit yellow skin color, become obese upon maturity, and develop hyperglycemia and insulin resistance (27–29). Ay mice gain more weight than their littermates on either a regular or high fat diet. As shown in Figure 1G and Supplemental Figure 3A, Ay/NAG-1 mice from 3 weeks of age to 15 weeks of age do not significantly gain as much weight as their Ay littermates. Furthermore, there is approximately 25% less WAT in the Ay/NAG-1 mice as compared to Ay mice (Figure 1H; Supplemental Figure 3B). Thus, the expression of NAG-1 in a genetic mouse model of obesity protects the mice from body weight and WAT gain.
HFD is a contributor to the development of hepatic steatosis, the accumulation of TGs in the liver (30). Livers from hNAG-1 mice on control or HFD show very little or no macrovesicular or microvesicular lipid accumulation (Supplemental Figure 4A and C). In contrast, WT mice on both control and HFD show varying degrees of steatosis, with the HFD livers showing the greatest degree of lipid accumulation. HFD leads to fat accumulation in adipocytes and can contribute to the development of crown-like structures or ‘rosettes’ (CLR), areas of inflammation where macrophages gather around ‘dead’ cells, in the fat (31–32). WT mice on the HFD display a higher grade of CLR compared to WT mice on the control diet. hNAG-1 mice on both the control and HFD have significantly less CLR (Supplemental Figure 4B–C) than WT mice. Analysis of the mRNA gene expression of F4/80 confirms lower macrophage accumulation in hNAG-1 mice on HFD as compared to WT mice (Supplemental Figure 4D) and is consistent with lower inflammatory cytokine levels observed in the NAG-1 mice as recently reported (23). Thus, hNAG-1 mice are resistant to dietary induced obesity and its deleterious effects.
hNAG-1 mice have higher glucose tolerance
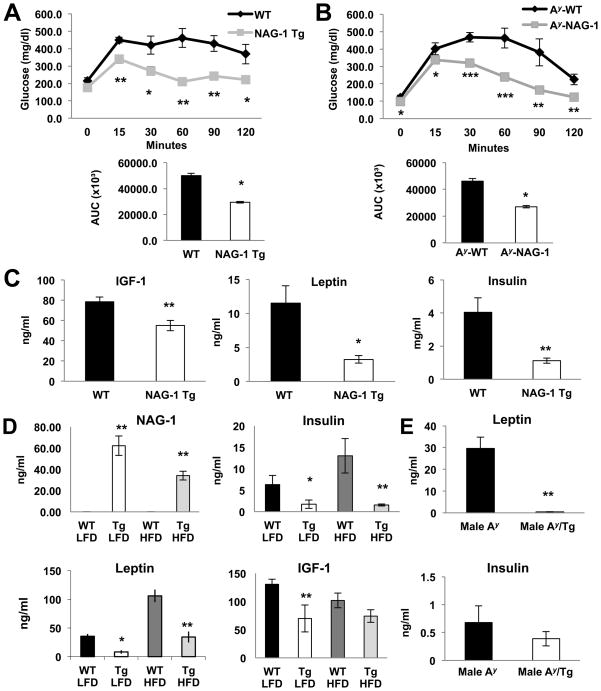
hNAG-1 mice have improved glucose utilization compared to littermate WT mice (Figure 2A; Supplemental Figure 5A). hNAG-1 mice have significantly lower levels of insulin indicating increased insulin sensitivity (Figure 2C). In addition, hNAG-1 mice have lower leptin and insulin levels in both LFD and HFD compared to the wild-type mice (Figure 2D). Furthermore, glucose tolerance tests indicate improved glucose utilization (Figure 2B, Supplemental Figure 5B) in the Ay/NAG-1 mice compared to Ay littermates. Analysis of serum from the Ay/NAG-1 and Ay mice also reveal the Ay/NAG-1 mice have lower leptin and insulin levels compared to Ay mice (Figure 2E, Supplemental Figure 5C).
Figure 2. hNAG-1 mice have higher glucose tolerance.
Glucose tolerance test (A). hNAG-1 mice utilize glucose more efficiently than WT littermates over time (n=4 mice per group). (B) Ay/NAG-1 mice utilize glucose more efficiently compared to Ay mice (n=5–7 mice per group). (C–D) hNAG-1 mice have lower serum levels of insulin, leptin, and IGF-1 (n= 4–6 mice per group). (E) Serum leptin and insulin levels of Ay and Ay/NAG-1 mice (n=5–7 mice per group). Data are presented as ± SE. *p<0.05, **p<0.01, and ***p<0.001 as determined by Student’s t-test.
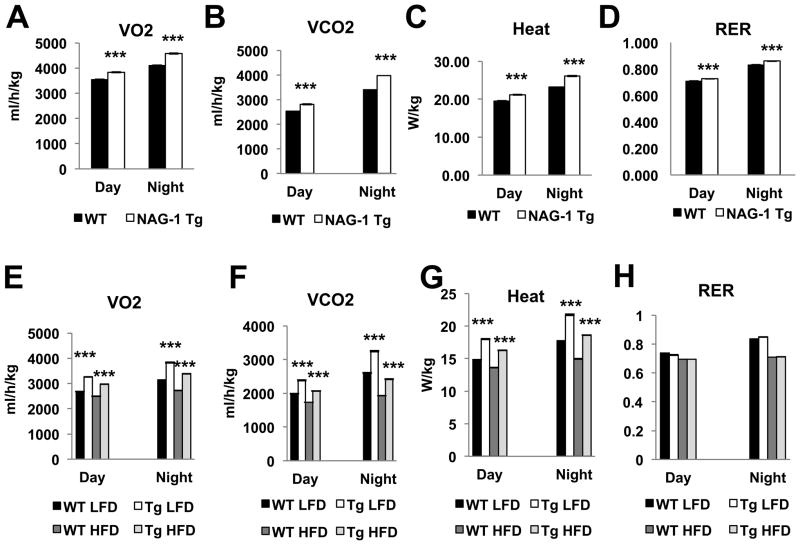
hNAG-1 mice have higher metabolic activity and energy expenditure
Using indirect calorimetry, oxygen consumption (VO2), carbon dioxide production (VCO2), and heat production (Heat) were measured during both day and night cycles (12 hr day cycle and 12 hr night cycle) for 3 days. VO2, oxygen utilization, and VCO2, CO2 production, were all significantly higher while the RER was the same, regardless of day or night cycle, in hNAG-1 mice compared to WT littermates (Figure 3A–B, 3D). Heat production is higher in hNAG-1 mice (Figure 3C) in both day and night cycles. Similarly, measurements for VO2, VCO2, and heat are all significantly increased while the RER was same in the hNAG-1 mice (Figure 3D–H) as compared to WT mice on a HFD. The higher oxygen utilization and heat production indicate that the hNAG-1 mice are more metabolically active and have higher energy expenditure.
Figure 3. hNAG-1 mice are more metabolically active.
Indirect calorimetry measurements of oxygen consumption (A), carbon dioxide production (B), heat production (C), and RER (D) for both WT and hNAG-1 mice. hNAG-1 mice are more metabolically active during both diurnal and nocturnal cycles. (E–H) Indirect calorimetry measurements of oxygen consumption (E), carbon dioxide production (F), heat production (G), and RER (H) for hNAG-1 and WT mice on HFD. Data are presented as ± SE. *p<0.05, **p<0.01, and ***p<0.001 as determined by Student’s t-test.
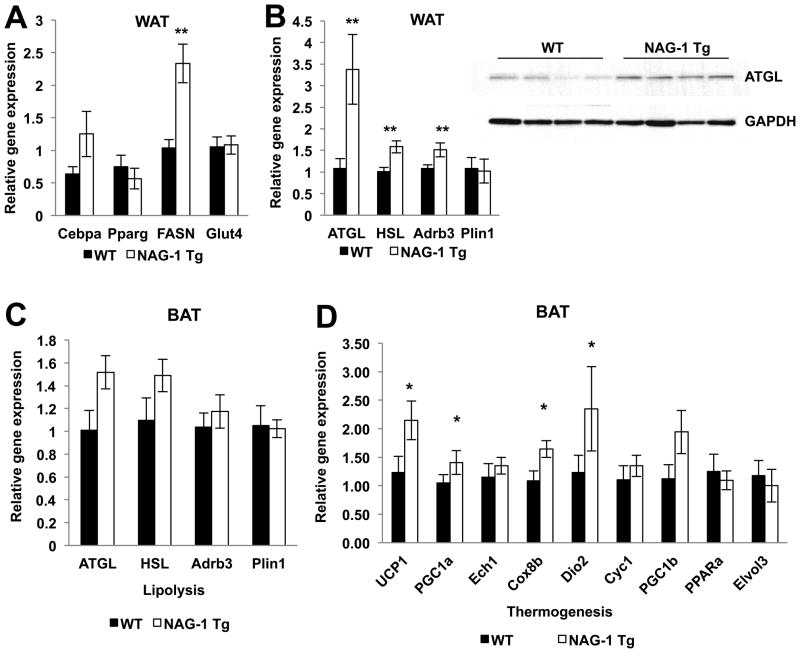
Smaller adipocyte size is frequently linked to higher metabolic activity (16,33) because smaller adipocytes suggest greater utilization of fat storage for metabolism. Alternatively, smaller adipocytes may also be a sign of decreased differentiation (34). WT and hNAG-1 mouse WAT H&E sections appear similar, with no obvious histological differences (Supplemental Figure 6A). Closer examination reveals that hNAG-1 mice have smaller adipocytes compared to WT littermates (Supplemental Figure 6B–C) (WT mice 61.6μm, hNAG-1 mice 45.3μm, p value < 0.001). To determine if hNAG-1 mice have reduced adipogenesis, we measured the expression of markers of adipocyte differentiation Cepbα, PPARγ, Glut4 and Fasn by real-time PCR. Except for Fasn, no differences in relative gene expression of adipogenesis markers are detected between WT and transgenic mice (Figure 4A) suggesting adipocyte differentiation is likely unaltered in the hNAG-1 mice. The increase in Fasn and ACC (data not shown) suggests lipogenesis may be altered in hNAG-1 mice. We next examined if relative gene expression of lipolysis genes Adrb3, ATGL, HSL, and Perilipin (Plin1) genes are altered in the WAT of hNAG-1 mice. Expression levels of Adrb3, ATGL, and HSL genes are increased in hNAG-1 mice, suggesting increased lipolysis occurs in the WAT of the hNAG-1 mice (Figure 4B). Protein expression of ATGL appears to be higher in the WAT of hNAG-1 mice as compared to WT littermates, suggesting increased lipolysis occurs in the WAT of the hNAG-1 mice (Figure 4B). Increased lipolysis is consistent with lower WAT observed in hNAG-1 mice.
Figure 4. hNAG-1 mice have increased gene expression of lipolysis and thermogenesis.
hNAG-1 mice, compared to WT littermates, have no difference in relative gene expression in markers of adipocyte differentiation (Cebpα, PPAR-γ, and Glut4), except for FASN, in the WAT (A). (B) Relative gene expressions of lipolysis gene markers (ATGL, HSL, and Adrb3) are higher in hNAG-1 mice compared to WT mice in WAT (n=3–6 mice per group). Western blot analysis confirms higher expression of ATGL in hNAG-1 mice (n=4 mice per group). Data are presented as ± SE. *p<0.05, **p<0.01, and ***p<0.001 as determined by Student’s t-test.
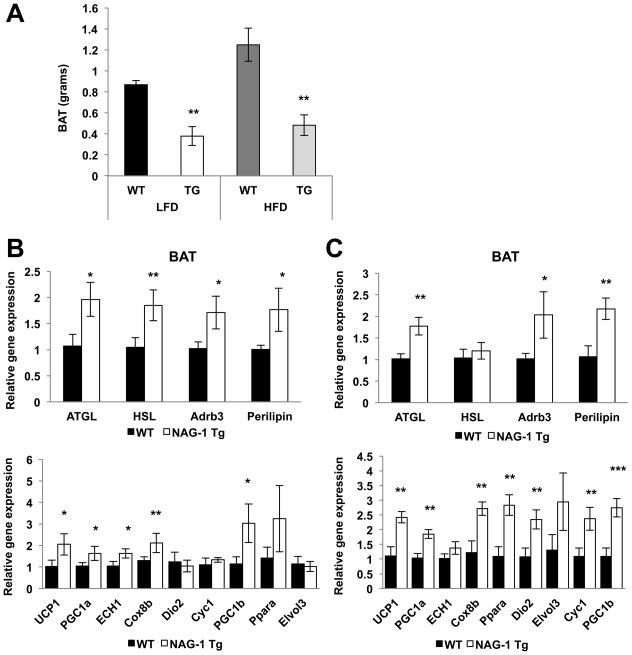
Higher heat production is observed in hNAG-1 mice on both regular and high fat diets (Figure 3C and 3G). Heat production is regulated by enzymes in BAT through the uncoupling of oxidative phosphorylation of ATP by uncoupling protein 1 (UCP-1). “Browning” of WAT is also associated with increased UCP-1 expression in the WAT and protection from HFD-induced obesity (35). BAT weight is lower in hNAG-1 mice as compared to WT littermates, while HFD increases the BAT weight of WT, but not hNAG-1 mice (Figure 5A). The expression of UCP-1 is very low in the WAT and is not higher in hNAG-1 mice (data not shown), indicating “browning” of the WAT is not responsible for the higher heat production observed. Increased expression of the markers of lipolysis in the BAT of hNAG-1 mice and in hNAG-1 LFD and HFD mice (Figure 4C; Figure 5B–C, upper panels) suggests higher lipolysis in BAT. The expression genes involved in heat formation and oxidative metabolism (UCP1, PGC1α, ECH1, Cox8b, Dio2, Cyc1, PGC1β, PPARα, and Elvol3) were measured. Increased relative gene expression of thermogenic genes is observed in the BAT of hNAG-1 mice on regular (Figure 4D) or on either the LFD (Figure 5B) or HFD (Figure 5C). Next, the expression of several key proteins of thermogenesis was examined by Western blot analysis. Although the results confirmed the protein expression of UCP-1 and PGC1α/β in the BAT from mice on a regular diet or on a LFD, the increased expression predicted from measurement of RNA expression in the NAG-1 mice could not be fully confirmed due, in part, to the variability between the individual mice tissues (Supplemental Figure 7A–B). Overall the results support the hypothesis for hNAG-1 increasing thermogenesis in BAT and lipolysis in WAT and BAT, which may eventually lower WAT (36) and provide an explanation for the resistance to genetic or dietary induced obesity.
Figure 5. hNAG-1 HFD mice have decreased BAT but increased lipolytic and thermorgenic gene expression.
(A) BAT weights are significantly lower in hNAG-1 mice compared to WT mice (n= 5 mice per group). (B) BAT from hNAG-1 or WT mice on low fat diet (LFD). Upper graph shows that hNAG-1 mice have higher lipolytic gene expression compared to WT mice in the BAT. Lower graph shows that hNAG-1 mice have higher thermogenic gene expression compared to WT littermates in the BAT (n=4–6 mice per group). (C) BAT from hNAG-1 or WT mice on HFD. Upper graph shows that hNAG-1 mice have higher lipolytic gene expression compared to WT mice in the BAT. (F–G) Lower graph shows that HFD hNAG-1 have higher thermogenic gene expression compared to WT littermates in the BAT (n=4–6 mice per group). Data are presented as ± SE. *p<0.05, **p<0.01, and ***p<0.001 as determined by Student’s t-test.
NAG-1 reverses obesity in mice
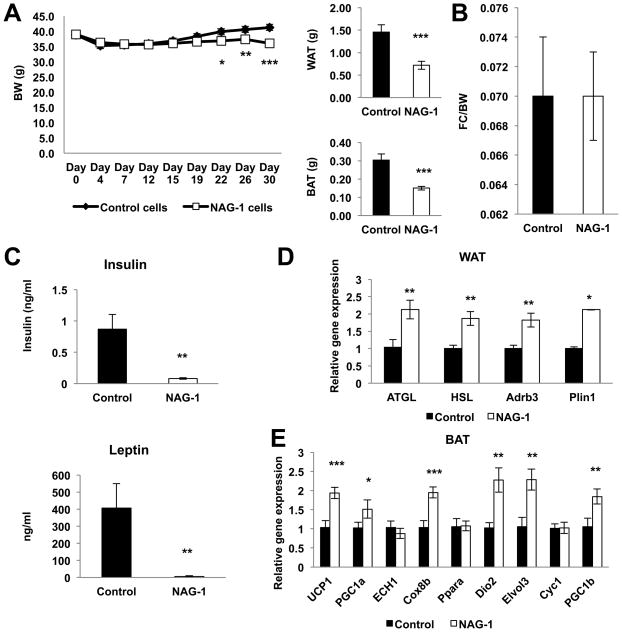
To test the potential therapeutic value of hNAG-1 and to determine if secreted hNAG-1 alters gene expression we investigated if increasing serum levels of hNAG-1 would decrease the body weight, WAT, and BAT of diet-induced obese mice. Because sufficient hNAG-1 protein is not available, tumor xenografts with stably transduced B16–F10 melanoma cells expressing hNAG-1 was used as a tool to increase serum levels of hNAG-1 in obese C57BL/6 mice as previously reported with nude mice (24–25). B16–F10 melanoma cells expressing only vector served as the control. B16–F10 melanoma cells were selected because they derive from C57BL/6 mice. In obese mice bearing hNAG-1-expressing tumors, a significant reduction in body weight and adipose tissue is observed, without a difference in food intake (Figure 6A–B). Xenograft size or weight was the same for vector and hNAG-1 expressing xenografts (data not shown). The serum concentration of hNAG-1 from hNAG-1 expressing xenografts ranges from 20–30 ng/ml as compared to 50–80ng/ml for hNAG-1 mice (data not shown). Serum levels of leptin and insulin are reduced in obese hNAG-1 tumor-bearing mice compared to vector tumor-bearing obese mice (Figure 6C), while thyroid hormones T3 and T4 are similar (data not shown). Relative gene expressions for markers of thermogenesis, adipogenesis, and lipolysis were examined in the WAT and BAT tissue from vector and hNAG-1 tumor-bearing mice. Increased lipolytic relative gene expression is observed in the WAT of obese mice with hNAG-1 expressing tumors (Figure 6D). Markers for thermogenesis show increased relative gene expression in the BAT of obese mice with hNAG-1 tumor (Figure 6E). Although the increase in the relative gene expression of lipolysis markers in BAT was observed, the difference was not statistically significant (Supplemental Figure 8). These data indicate that hNAG-1 circulating in the blood lower BAT and WAT and increases the gene expression of thermogenesis genes in BAT and lipolysis genes in WAT. Thus, NAG-1 expressed in the transgenic mouse and present in the circulation enhances adipocyte lipolysis, thermogenesis and oxidative metabolism. The transgenic mice exhibit higher oxygen consumption and energy expenditure (heat) without a change in food intake or RER. As a result the transgenic mice have lower adipose tissue (WAT/BAT) and a diminished body weight.
Figure 6. hNAG-1 acts to reduce WAT and weight in obesity through increased lipolysis and thermogenesis.
Obese C57BL/6 mice xenografted with stably transduced vector (Control) or hNAG-1 B16/F10 melanoma cells (n=9–11 mice per group). (A) hNAG-1 tumor bearing obese mice significantly lose weight over time and have decreased amounts of WAT and BAT compared to Control tumor bearing obese mice. (B) Food intake is similar between Control and hNAG-1 tumor bearing obese mice. (C) ELISA analysis of serum leptin and insulin levels from Control and hNAG-1 tumor bearing obese mice (n=6 mice per group). (D) Relative gene expression of lipolysis markers in the WAT and (E) thermogenesis markers in the BAT (n=9–11 mice per group). Data are presented as ± SE. *p<0.05, **p<0.01, and ***p<0.001 as determined by Student’s t-test.
Discussion
This laboratory is the first to identify hNAG-1 as a regulator of oxidative metabolism by increasing the gene expressions of lipolysis and thermogenesis genes in adipose tissue. Here, we show that hNAG-1 mice are leaner and resistant to dietary- and genetic-induced obesity despite equivalent food intake as compared to their WT littermates. Furthermore, in obese C57BL/6 mice, increasing levels of hNAG-1, delivered through tumor xenografts, induces the expression of key thermogenic and lipolytic genes, resulting in rapid loss of adipose tissue without a decrease in food consumption. Thus, NAG-1 may have therapeutic potentials in the treatment of obesity.
hNAG-1 mice have higher oxygen utilization, carbon dioxide production, and heat expenditure consistent with higher metabolic activity. The expressions of lipolytic genes ATGL, HSL, and Adrb3 are higher in WAT and BAT of hNAG-1 and hNAG-1 tumor-bearing obese mice. Furthermore, key thermogenic gene expressions of UCP1, PGC1α, ECH1, Cox8b, Dio2, Cyc1, PGC1β, PPARα, and Elvol3, are elevated in the BAT of hNAG-1 and obese hNAG-1 tumor-bearing mice, consistent with higher metabolic activity. Combined with indirect calorimetry data, the gene expression results provide an explanation for the significantly lower WAT and BAT in both hNAG-1 and hNAG-1 tumor-bearing mice. With the exception of FASN, whose inhibition of expression leads to decreased adipocyte differentiation (37), the expression of adipogenesis genes in the WAT of hNAG-1 mice is equivalent to WT mice, suggesting that the decreased levels of WAT in hNAG-1 mice are not due to inhibition of differentiation. Lower leptin and insulin levels in hNAG-1 and obese xenograft mice are consistent with the reduction in WAT and increased metabolic activity. The decrease in leptin levels with increased thermogenic gene expression in BAT is consistent with previously published work (6,38). Decreased basal insulin levels lead to increased HSL, resulting in a reduction in adipose tissue (39). Both hNAG-1 and hNAG-1 tumor-bearing obese mice have decreased insulin levels and increased expression of HSL, and reduced adipose tissue. Increased expression of lipolytic genes in both WAT and BAT, and increased thermogenic gene expression in BAT, without concurrent increase in serum FFAs, suggests increased use of lipids in situ for energy production. Further, glycerol concentration in the sera from wild type and NAG-1 mice is essentially the same (data not shown), similar to our data on FFAs and TGs, despite an increase in the expression lipolytic protein in NAG-1 mice. Other studies report (34,40) similar findings in mice with higher lipolysis, thermogenesis and oxidative metabolism and is also consistent with the lower inflammation in liver and adipose tissue. Thus, we propose in the transgenic and obese xenograft bearing mice, hNAG-1 acts to increase metabolism through an increase in lipolysis and thermogenesis, reducing adipose tissue and leptin levels. Increased thermogenic and lipolytic gene expressions in hNAG-1 mice are likely responsible for improved glucose utilization, increased insulin sensitivity, lower adipose tissue and resistance to obesity. In a report by Stanford et al., BAT improves glucose homeostasis and insulin sensitivity (41), which is consistent with our findings.
Obesity is associated with increased inflammation and macrophage infiltration in the hypertrophied WAT. Adipocytes are enlarged in obesity, increasing the production of pro-inflammatory cytokines such as TNF-α contributing to insulin resistance (8–9,42). Small adipocyte size, however, is related to increased insulin sensitivity, reduced serum leptin levels, and higher metabolic activity (16,33,43–44). In the absence of adipocyte hypertrophy, the WAT of hNAG-1 mice show little or no CLRs and decreased presence of macrophages. hNAG-1 HFD mice, despite equivalent food intake, have decreased WAT and TG levels, implying that FFAs are used in situ, and explaining their lack of hepatic steatosis and more efficient utilization of glucose. From this phenotype, we expect hNAG-1 mice to exhibit increased insulin sensitivity and resistance to the development of type-2 diabetes and non-alcoholic fatty liver disease.
The effect of NAG-1 on human obesity is unclear, with some studies showing a positive correlation between obesity and serum NAG-1 levels, while others report a negative correlation (45–48). From the results of this study we conclude that NAG-1 increases metabolism by increasing the expression of key thermogenic and lipolytic genes. hNAG-1 mice are leaner and are resistant to dietary- and genetic-induced obesity through increased metabolic activity. Other investigators report that NAG-1 does not alter energy metabolism, but instead reduces food intake, thereby lowering adipose tissue and is responsible for the resistance to diet-induced obesity (49). However, transgenic mice used in these experiments express mouse NAG-1 under macrophage specific colony stimulating factor-1 receptor promoter (24,49) and the serum levels of secreted mNAG-1 are not reported. The conflicting findings from these investigations may be due to the differences in experimental mouse models and/or expressed protein. Our laboratory and other investigators have used xenografts to increase serum hNAG-1 levels in mice (24,25). Wang et al., report that nude mice with xenografts expressing either wild-type or the H6D hNAG-1 variant, have lower body weight and abdominal fat, and reduced serum levels of leptin as compared to mice with control vector xenografts (25). As hNAG-1 serum levels increase with xenograft growth, body weight increase of wild-type hNAG-1 and H6D xenograft nude mice slowed compared to control mice (25). However, food intake was not measured. Johnen et al., also report a similar approach with nude mice, with decreases in body weight and adipose tissue, but also a reduction in food intake (24). Using stably transduced B16/F10 melanoma cells expressing hNAG-1, we find that obese C57BL/6 mice rapidly lose adipose tissue, without a concurrent change in food intake. Circulating hNAG-1 alters lipolytic and thermogenic gene expression and thus reduces adipose tissue. Thus, similar results are obtained from the hNAG-1 mouse and the B16/F10 xenograft model. Other members of the TGF-β family, BMP7, BMP8B, and GDF8, alter energy metabolism without changes in food consumption (17–18), findings consistent with the results reported here. Thus, multiple pathways are involved in the regulation of metabolism by NAG-1 and further studies are needed to fully elucidate the mechanism(s) by which NAG-1 alters adipose tissue levels and prevents obesity.
The reduction in adipose tissue and increased metabolism mediated by changes in gene expression by circulating hNAG-1 suggests that NAG-1 is acting as a hormone and raises the possibility that NAG-1 could be used as therapeutic tool to treat obesity. From our studies, hNAG-1 mice are resistant to obesity, thereby preventing the development of associated inflammation, and thus mitigating insulin resistance. Elevating NAG-1 in the serum should produce similar affects, thus improving insulin sensitivity. In clinical studies, inducing BAT activation increases energy expenditure (50–52), which is consistent with our observations. The up-regulation of lipolytic and thermogenic gene expression by NAG-1, which increase then increases metabolic activity, is a novel finding that could potentially be used in as a target for the future development of drugs for reducing obesity.
In summary, hNAG-1 appears to be a mediator of fat lipolysis, thermogenesis, and oxidative metabolism and its expression prevents dietary- and genetic-induced obesity and insulin resistance. NAG-1 is a potential new therapeutic target for counteracting obesity and insulin resistance
Supplementary Material
Acknowledgments
We thank David Goulding, Page Myers, and the Necropsy core for their technical help. We thank Drs. Paul Wade and Xiaoling Li for critical reading of this manuscript. This research was supported by NIH, NIEHS Intramural Research Program (Eling) Z01- ES010016-14, and in part, the Center of Excellence in Livestock Diseases and Human Health, University of Tennessee (Baek).
Footnotes
Supplementary information is available at the journal’s website.
There are no conflicts of interests.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- 2.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 5.Harwood HJ., Jr The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2012;63:57–75. doi: 10.1016/j.neuropharm.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Heeren J, Münzberg H. Novel aspects of brown adipose tissue biology. Endocrinol Metab Clin. 2013;42:89–107. doi: 10.1016/j.ecl.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcelin G, Chua S., Jr Contributions of adipocyte lipid metabolism to body fat content and implications for the treatment of obesity. Curr Opin Pharmacol. 2010;10:588–593. doi: 10.1016/j.coph.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-α. Cytokine Growth Factor Rev. 2003;14:447–455. doi: 10.1016/s1359-6101(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 9.Ruan H, Lodish HF. Regulation of insulin sensitivity by adipose tissue-derived hormones and inflammatory cytokines. Curr Opin Lipidol. 2004;15:297–302. doi: 10.1097/00041433-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 11.Zamani N, Brown CW. Emerging roles for the transforming growth factor-β superfamily in regulating adiposity and energy expenditure. Endocr Rev. 2011;32:387–403. doi: 10.1210/er.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Yang Y, Meng Y, Shi Y. GDF-3 is an adipogenic cytokine under high fat dietary condition. Biochem Biophys Res Comm. 2004;321:1024–1031. doi: 10.1016/j.bbrc.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 13.Shen JJ, Huang L, Li L, Jorgez C, Matzuk MM, Brown CW. Deficiency of growth differentiation factor 3 protects against diet-induced obesity by selectively acting on white adipose. Mol Endocrinol. 2009;23:113–123. doi: 10.1210/me.2007-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson O, Korach-Andre M, Reissmann E, Ibáñez CF, Bertolino P. Growth differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc Natl Acad Sci USA. 2008;105:7252–7256. doi: 10.1073/pnas.0800272105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 16.Feldman BJ, Streeper RS, Farese RV, Jr, Yamamoto KR. Myostatin modulates adiogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci USA. 2006;103:15675–15680. doi: 10.1073/pnas.0607501103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, et al. New role of bone morphogenic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez MJ, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Böttner M, Suter-Crazzolara C, Schober A, Unsicker K. Expression of a novel member of the TGF-beta superfamily, growth/differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell Tissue Res. 1999;297:103–110. doi: 10.1007/s004410051337. [DOI] [PubMed] [Google Scholar]
- 20.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001;59:901–908. [PubMed] [Google Scholar]
- 22.Baek SJ, Okazaki R, Lee SH, Martinez J, Kim JS, Yamaguchi K, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology. 2006;131:1553–1560. doi: 10.1053/j.gastro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Kim JM, Kosak JP, Kim JK, Kissling G, Germolec DR, Zeldin DR, et al. NAG-1/GDF15 transgenic mouse has less white adipose tissue and a reduced inflammatory response. Mediators of Inflammation 2013. doi: 10.1155/2013/641851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-β superfamily cytokine MIC-1. Nat Med. 2007;13:1333–1340. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Chrysovergis K, Bienstock RJ, Shim M, Eling TE. The H6D variant of NAG-1/GDF15 inhibits prostate xenograft growth in vivo. Prostate. 2012;72:677–689. doi: 10.1002/pros.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Humble MM, Young MJ, Foley JF, Pandiri AR, Travlos GS, Copeland WC. Hum Mol Genet. 2012;22:1017–1025. doi: 10.1093/hmg/dds506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpenter KJ, Mayer J. Physiologic observations on yellow obesity in the mouse. Am J Physiol. 1958;193:499–504. doi: 10.1152/ajplegacy.1958.193.3.499. [DOI] [PubMed] [Google Scholar]
- 28.Dickerson GE, Gowan JW. Hereditary obesity and efficient food utilization in mice. Science. 1947;105:496–498. doi: 10.1126/science.105.2732.496-a. [DOI] [PubMed] [Google Scholar]
- 29.Friedman JM, Leibel RL. Tackling a weighty problem. Cell. 1992;69:217–220. doi: 10.1016/0092-8674(92)90402-x. [DOI] [PubMed] [Google Scholar]
- 30.Reddy JK, Rao MS. Lipid metabolism and lipid inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G852–G858. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- 31.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stienstra R, Joosten LAB, Koenen T, van Tits B, van Diepen JA, van den Berg SAA, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metabol. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liew CW, Boucher J, Cheong JK, Vernochet C, Koh HJ, Mallol C, et al. Ablation of TRIP-Br2, a regulator of fat lipolysis, thermogenesis and oxidative metabolism, prevents diet-induced obesity and insulin resistance. Nat Med. 2013;19:217–226. doi: 10.1038/nm.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes. 2010;59:17–25. doi: 10.2337/db09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Wang X, Hu Y, Kang J, Wang L, Li S. Effects of a fatty acid synthase inhibitor on adipocyte differentiation of mouse 3T3-L1 cells. Acta Pharmacol Sin. 2004;25:1052–1057. [PubMed] [Google Scholar]
- 38.Ahima RS. Adipose tissue as an endocrine organ. Obesity. 2006;14:242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 39.Hajer GR, van Haeften TW, Visseren FLJ. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 40.Ahmadian M, Duncan RE, Varady KA, Frasson D, Hellerstein MK, Birkenfeld AL, Samuel VT, et al. Adipose overexpression of desnutrin promotes faty acid use and attenuates diet-induced obesity. Diabetes. 2009;58:855–866. doi: 10.2337/db08-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type II diabetes: The role of cytokines. Ann NY Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 43.Lundgren M, Svensson M, Lindmark S, Renström F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia. 2007;50:625–633. doi: 10.1007/s00125-006-0572-1. [DOI] [PubMed] [Google Scholar]
- 44.Roberts R, Hodson L, Dennis AL, Neville MJ, Humphreys SM, Harnden KE, et al. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52:882–890. doi: 10.1007/s00125-009-1300-4. [DOI] [PubMed] [Google Scholar]
- 45.Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, et al. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology. 2009;150:1688–1696. doi: 10.1210/en.2008-0952. [DOI] [PubMed] [Google Scholar]
- 46.Dostálová I, Roubícek T, Bártlová M, Mráz M, Lacinová Z, Haluzíková D, et al. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur J Endocrinol. 2009;161:397–404. doi: 10.1530/EJE-09-0417. [DOI] [PubMed] [Google Scholar]
- 47.Karczewska-Kupczewska M, Kowalska I, Nikolajuk A, Adamska A, Otziomek E, Gorska M, et al. Hyperinsulinemia acutely increases serum macrophage inhibitory cytokine-1 concentration in anorexia nervosa and obesity. Clin Endocrin. 2012;76:46–50. doi: 10.1111/j.1365-2265.2011.04139.x. [DOI] [PubMed] [Google Scholar]
- 48.Vila G, Riedl M, Anderwald C, Resl M, Handisurya A, Clodi M, et al. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin Chem. 2011;57:309–316. doi: 10.1373/clinchem.2010.153726. [DOI] [PubMed] [Google Scholar]
- 49.Macia L, Tsai VW, Nguyen AD, Johnen H, Kuffner T, Shi YC, et al. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. PLoS One. 2012;7:e34868. doi: 10.1371/journal.pone.0034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 52.Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci USA. 2012;109:10001–10005. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.