Abstract
Rationale
T-type calcium channels (T-channels) play an important role in controlling excitability of nociceptors. We have previously shown that a synthetic series of 5β-reduced steroids induce voltage-dependent blockade of T-currents in rat dorsal root ganglia (DRG) cells in vitro and induce potent analgesia to thermal stimuli in rats in vivo (Todorovic et al., 2004). Objectives: Here we investigated the effects of the endogenous 5β-reduced neuroactive steroid molecule, epipregnanolone (3β,5β)-3-hydroxypregnan-20-one), on peripheral nociception. Methods: We used acutely dissociated DRG cells in vitro from adult rats, as well as in vivo pain studies in mice and rats to investigate effects of epipregnanolone on DRG T-channels.
Results
We found that epipregnanolone reversibly blocked DRG T-currents with an IC50 of 2 μM and stabilized the channel in the inactive state. However, sodium, potassium and GABA-gated ionic currents were not sensitive to the blocking effects of epipregnanolone even at 10 μM. In ensuing in vivo studies, we found that intraplantar (i.pl.) injections of epipregnanolone directly into peripheral receptive fields reduced responses to nociceptive heat stimuli in rats in a dose-dependent fashion. Furthermore, i.pl. epipregnanolone injections effectively reduced responses to peripheral nociceptive thermal and mechanical stimuli in wild type mice, but had no effect on the responses of CaV3.2 knock-out mice.
Conclusions
We conclude that inhibition of peripheral CaV3.2 T-channels contributes to the potent analgesic effect of the endogenous steroid epipregnanolone.
Keywords: Low-voltage-activated, Ca2+, pain, dorsal root, hyperalgesia
INTRODUCTION
The neuroactive steroids are potent modulators of neuronal activity in the peripheral and central nervous system by causing a variety of behavioral and neuroendocrine changes in humans and animals (e.g. general anesthesia, analgesia, cognitive and mood disturbances) (reviewed in Jevtovic-Todorovic and Todorovic 2009; Zorumski et al. 2013). It is believed that effects on neurosensory processing and neuronal excitability are primarily mediated by actions at various ligand-gated ion channels, with much attention focused on the modulation of γ-aminobutyric acid (GABAA) receptors by steroids such as alphaxalone (3α,5α)-3-hydroxypregnane-11,20-dione) (Zorumski et al. 2013). Furthermore, we have shown that analgesic potency of alphaxalone and related 5α-reduced steroids is correlated to their ability to potentiate GABAA-gated currents and/or inhibit T-currents in peripheral sensory neurons (Pathirathna et al. 2005). We have also previously identified several synthetic 5β-reduced steroid analogues that lack any direct effect on GABAA receptors but potently inhibit T-currents in DRG cells and exhibit potent analgesic potency in vivo when locally injected into peripheral receptive fields in rats (Todorovic et al. 2004). One of the most potent steroid analogues in this group, 3β5βCN ((3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile) is a voltage-dependent and selective blocker of T-currents in acutely dissociated dorsal root ganglion (DRG) cells (IC50 3 μM) (Todorovic et al. 2004). We found that at these concentrations, 3β5βCN had negligible effects on other voltage-gated currents in acutely dissociated DRG cells (Todorovic et al. 2004). However, effects of endogenous 5β-reduced steroid molecules that lack GABA-mimetic activity upon T-channels in peripheral nociceptors and their possible effects upon pain transmission in vivo are not well studied. Epipregnanolone (3β,5β)-3-hydroxypregnan-20-one) (Fig. 1A) is one such molecule that is synthesized endogenously in brain tissues from cholesterol (Liu et al. 2003) and, unlike most other endogenous neuroactive steroids, has no significant activity upon neuronal GABAA-gated ion currents in native cells (Poisebeau et al. 1997; Weir et al. 2004). In the present study we build on our previous work on the role of 5β-reduced steroids in analgesia using epipregnanolone as a prototypical endogenous molecule. We studied the effects of epipregnanolone on voltage-gated T-type calcium currents and other voltage-gated currents using in vitro patch-clamp recording from the putative nociceptive sensory neurons, as well as in vivo pain studies using wild type rats, wild type mice and mice lacking the CaV3.2 isoform of T-channels.
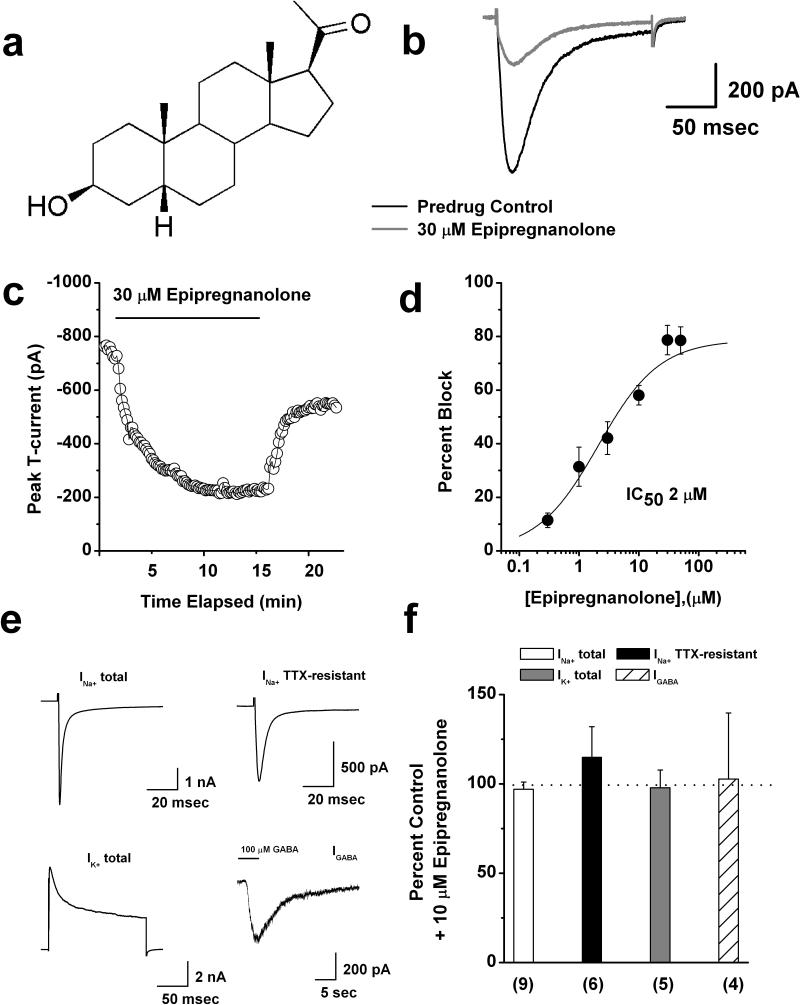
Figure 1. Concentration-dependent inhibition of rat DRG T-currents by epipregnanolone.
a: Scheme represents chemical structure of epipregnanonolone. b: Original traces show DRG T-currents in predrug control conditions (black trace) and after application of 30 μM epipregnanolone (gray trace). c: Time course of T-current inhibition by 30 μM epipregnanolone in the same representative DRG cell presented on panel b. d: Concentration-response relationship for epipregnanolone inhibition of T-current in rat DRG cells (n = 3-18 per data point). Solid line is the best fit (equation # 1, see Materials and Methods) yielding IC50 of 2.1 ± 0.5 μM, slope coefficient 0.8 ± 0.2, and maximal inhibition of 79 % of the peak of T-current. e: Original control current traces from different representative DRG cells show total Na+ current, TTX-resistant Na+ current, total K+ current, and GABA-gated currents. f: Bar graphs show average effects of 10 μM epipregnanolone upon different ionic currents in DRG cells as depicted on panel e of this figure. Dashed line indicates control predrug levels of currents. Number of cells per each experiment is indicated in parenthesis.
MATERIALS AND METHODS
Acutely isolated DRG neurons
DRG cells from adolescent rats were prepared as previously described (Todorovic et al. 1998; Nelson et al. 2005; Choe et al. 2011). For recording, cells were plated onto uncoated glass coverslips, placed in a culture dish, and perfused with external solution. All in vitro experiments were done at room temperature.
Electrophysiology
Recording electrodes were pulled from borosilicate glass microcapillary tubes (Drummond Scientific, Broomall, PA); when filled with solution, they had resistances between 1-4 MΩ. We made recordings using an Axopatch 200B patch-clamp amplifier (Molecular Devices, Foster City, CA). Digitization of membrane voltages and currents was controlled using a Digidata 1322A interfaced with Clampex 8.2 or 9.0 (Molecular Devices). We analyzed data using Clampfit 10.3 (Molecular Devices) and Origin 7.0 (Microcal Software, Northampton, MA). Currents were low-pass filtered at 2 kHz. Multiple independently controlled glass syringes served as reservoirs for a gravity-driven perfusion system.
Recording solutions
The external solution for voltage-clamp experiments involving T-currents contained (in mM), 152 TEA-Cl, 2 CaCl2, and 10 HEPES, adjusted to pH 7.4 with TEA-OH. To allow studies of well-isolated T-currents in acutely isolated DRG cells, we used only fluoride (F−)-based internal solution in order to facilitate high voltage-activated (HVA) Ca2+ current rundown (Todorovic et al. 1998). This internal solution for voltage-clamp experiments with DRG neurons contained (in mM) 135 TMA-OH, 40 HEPES, 10 EGTA, and 2 MgCl2, adjusted to pH 7.2 with hydrogen fluoride (HF). Typically, T-currents are evoked from the holding potential (Vh) of −90 mV and depolarization to test potential (Vt) of −30 mV. The amplitude of the T-current at any given potential was measured from the end of the pulse to its peak. For recordings of voltage-gated sodium currents in DRG cells, we used the same fluoride-based internal solution as for recordings of T-currents. The internal solution for recordings of voltage-gated potassium currents and GABA-gated currents contained (in mM), 130 KCl, 40 HEPES, 5 MgCl2, 2 Mg-ATP, 1 EGTA, and 0.1 Na3GTP, adjusted to pH 7.3 with KOH. The external solution for recordings of voltage-gated sodium currents, voltage-gated potassium currents and GABA-gated currents contained (in mM), 140 NaCl, 4 KCl, 2 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, adjusted to pH 7.4. In some experiments, this solution was supplemented with 1 μM tetrodotoxin (TTX) and 0.5 mM CdCl2.
All drugs were prepared as stocks and freshly diluted to the final concentrations in the external solution at the time of experiments. Epipregnanolone was prepared as a 10 mM stock in dimethylsulfoxide (DMSO). Most drugs were obtained from Sigma-Aldrich (St. Louis, MO), except epipregnanolone which was obtained from Steraloids, Inc. (Newport, RI).
Analysis
Statistical comparisons in our in vitro experiments were made using paired t-test. All data are expressed as mean ± standard error of the mean (SEM); p values are reported only when statistically significant (<0.05). The percent reductions in peak current at various concentrations of epipregnanolone were used to generate concentration-response curve. Mean values were fit to the following Hill-Langmuir function:
| (1) |
where PImax is the maximal percent inhibition of peak current by epipregnanolone, IC50 is the concentration that produces 50% inhibition, and h is the apparent Hill-Langmuir coefficient for inhibition. The fitted values are reported with > 95% linear confidence limits.
To study steady-state inactivation of T-channels currents are evoked by test steps to −30 mV after 3.5-sec prepulses to potentials ranging from −110 mV to −45 mV in 5-mV increments. The voltage dependence of steady-state inactivation was described with a single Boltzmann distribution of the following form:
| (2) |
where Imax is the maximal current, V50 is the voltage where half the current is inactivated, and k is the voltage-dependence (slope) of the distribution.
To study T-current deactivation, the cells were held at −90 mV, then subjected to a 14 ms-long activating pulse to −30 mV, followed by 10-mV incremental deactivating steps from −160 to −60 mV. Deactivating currents were fit using single exponential function.
Double-pulse protocol with variable duration was used to measure recovery from inactivation at −90 mV after 500 msec-long inactivating pulse (Vh −90 mV, Vt −30 mV). Time course of current recovery was fitted using single exponential function.
Behavioral studies
For our behavioral studies we used adult female rats, as well as adult male and female wild type (WT) and the CaV3.2 knock-out (KO) mice (CaV3.2 −/−). The CaV3.2−/− mice were generated as described previously (Chen et al. 2003). Mice and rats were maintained in a 12-h light/dark cycle and given free access to food and water. Experiments were done in accordance with institutional and federal guidelines, including the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 2002). Every effort was made to minimize animal suffering and the number of animals used.
Assessment of mechanical sensitivity
The withdrawal response to mechanical stimulation was measured by our standard method using von Frey filaments (Lee et al. 2009). Mice were placed in a clear plastic cage with a wire-mesh bottom divided into four compartments, permitting mice freedom of movement while allowing access to their paws. Von Frey filaments (Stoelting, Wood Dale, IL), which are designated as the log10 (milligram weight required to cause bending X10), were used to assess the mechanical threshold for paw withdrawal. We have found that applying the filament # 4.08 to the plantar surface of the foot causes a response in mice that results in an average of 5-6 paw withdrawal responses (PWRs) in 10 trials. Baseline PWRs were determined in both paws immediately before (marked as 0 on Figure 7) intradermal administration of either epipregnanolone or vehicle and then at 10, 20 and 60 minutes thereafter.
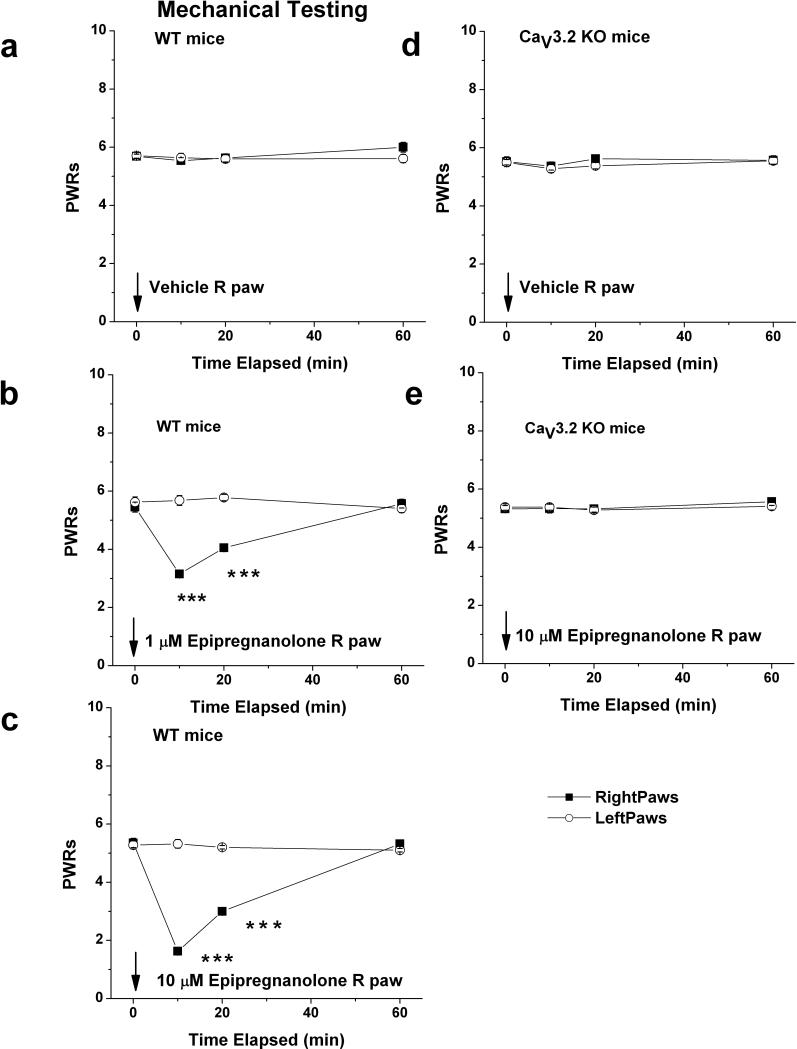
Figure 7. Local application of epipregnanolone induces potent dose-dependent analgesia to mechanical stimuli in WT mice but is ineffective in CaV3.2 KO mice.
a: Injection of 10 l of saline containing vehicle (0.1 % DMSO) into right paws (■) of WT (CaV3.2 +/+) mice had very little effects on mechanical PWRs. Note that PWRs in uninjected, left paws (○) also remained stable during the course of experiment. b,c: Dose dependent analgesia with 1 μM (b) and 10 μM (c) epipregnanolone is evidenced by significant prolongation of mechanical PWRs in injected (right paws) at 10 and 20 minutes following i.pl. injection. d,e: Injection of 10 μl of saline containing vehicle (d) or 10 μM epipregnanolone (e) into right paws (■) of KO (CaV3.2 −/−) mice had very little effects on mechanical PWRs.
Solid arrow indicates times of injection in all panels. Symbol *** indicates p < 0.001 for right versus left paw. We used 6-9 mice per experiment.
Assessment of thermal sensitivity
We used our previously described custom-built plantar test device (Pathirathna et al. 2005) adapted for rat and mouse testing, to measure hind paw thermal sensitivity. During this commonly used test of peripheral nociceptive responses, animals moved freely within an open-topped transparent plastic chamber. Mice and rats were accommodated on the glass floor for 60 min before testing. A movable radiant heat source was placed under the glass floor and focused on either hind paw. Paw withdrawal latency (PWL) times were measured with a cutoff time of 15 sec (mice) and 20 sec (rats) to prevent thermal injury to the skin. Baseline PWLs were determined in both paws of rats and mice a day before (marked as point B on Fig. 5) and immediately before (marked as point 0 on Figs. 5 and 6) intradermal administration of either epipregnanolone or vehicle and then at 10, 20 and 60 minutes thereafter.
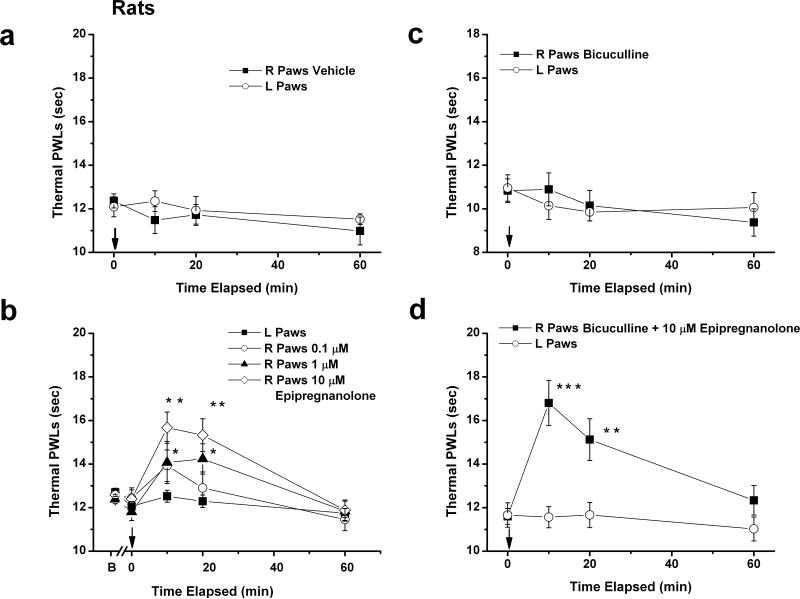
Figure 5. Local application of epipregnanolone induces potent dose-dependent decrease in heat nociception in healthy rats.
a: Injection of 100 μl of solution containing vehicle into right paws (■) had very little effects on thermal PWLs. Note that PWL in uninjected, left paws (□) also remained stable during the course of experiment. Data points are averages from 6 rats. b: Injection of 1 μM (▲) and 10 μM (◊) but not 0.1 μM (○) epipregnanolone into right paws significantly increased thermal PWLs at 10 and 20 minutes time points when we compared right and left paws (*p<0.05; ** p<0.01; n = 8-9 rats per group). c: Injection of 100 μl of solution containing 60 μM bicuculline into right paws (■) had very little effects on thermal PWLs. Note that PWL in uninjected, left paws (□) also remained stable during the course of experiment (n=8 rats). d: Injection of 10 μM epipregnanolone with 60 μM bicuculline (■) into right paws significantly increased thermal PWLs at 10 and 20 minutes time points when we compared right and left paws (**p<0.01; *** p<0.001; n = 8 rats). Note that PWLs in uninjected left paws (□) remained stable. Solid arrows on all panels indicate time of injection.
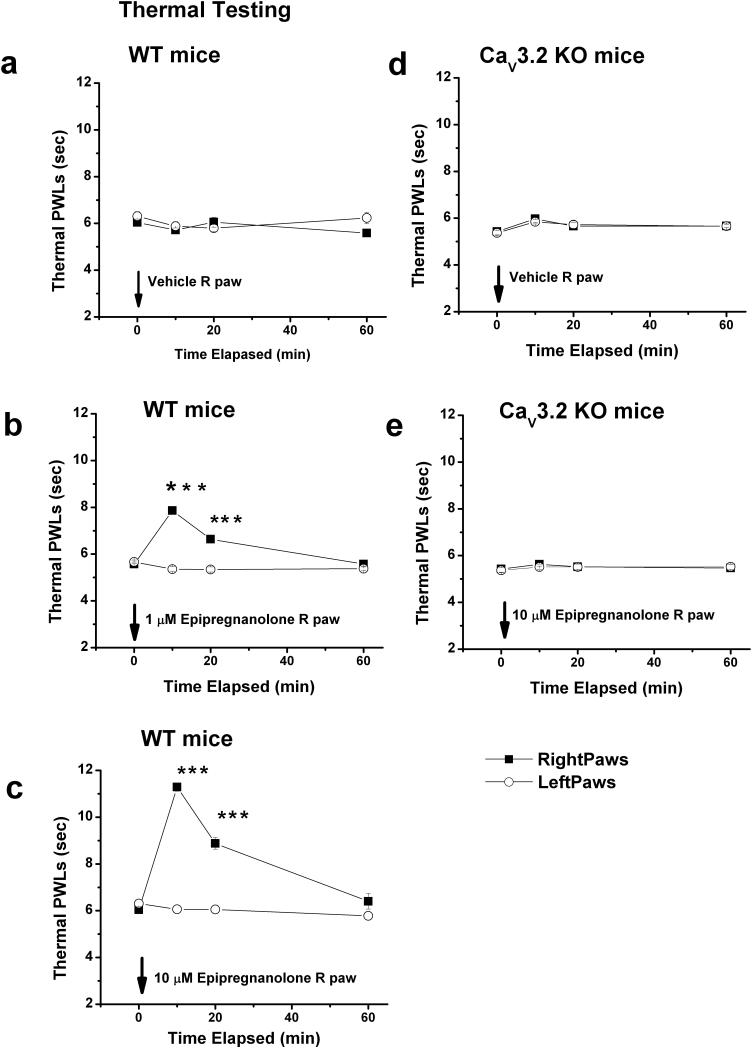
Figure 6. Local application of epipregnanolone induces potent dose-dependent analgesia to heat in WT mice but is ineffective in CaV3.2 KO mice.
a: Injection of 10 μl of saline containing vehicle (0.1 % DMSO) into right paws (■) of WT (CaV3.2 +/+) mice had very little effects on thermal PWLs. Note that PWL in uninjected, left paws (○) also remained stable during the course of experiment. b,c: Dose dependent analgesia with 1 μM (b) and 10 μM (c) epipregnanolone is evidenced by significant prolongation of thermal PWLs in injected (right paws) at 10 and 20 minutes following i.pl. injection. d,e: Injection of 10 μl of saline containing vehicle (d) or 10 μM epipregnanolone (e) into right paws (■) of KO (CaV3.2 −/−) mice had very little effects on thermal PWLs.
Solid arrow indicates times of injection in all panels. Symbol *** indicates p < 0.001 for right versus left paw. We used 6-9 mice per experiment.
Local intraplantar injections
To test the behavioral effects of epipregnanolone, we intradermally injected into the ventral side of the right hind paw solutions containing concentrations of 0.1, 1 or 10 μM of epipregnanolone or vehicle (DMSO) in 10 μl (mice) or 100 μL (rats) of saline. All solutions were pH balanced to 7.4 to avoid skin irritation. No signs of skin inflammation, discoloration or irritation were noted at the sites of injection with test compounds. For all behavioral experiments, statistical comparisons were made using one-way repeated ANOVAs followed by Holm-Sidak multiple comparison with statistical significance accepted if p < 0.05.
RESULTS
Concentration-dependent inhibition of T-currents in DRG neurons by epipregnanolone
Dorsal root ganglia contain the soma of nociceptive small-diameter primary afferent sensory fibers that originate as pain endings in the periphery and terminate in the dorsal horn of the spinal cord. Here we used whole-cell recordings from acutely dissociated DRG neurons of adolescent rats to study peripheral nociceptive mechanisms because the small size of peripheral nerve endings precludes direct measurement of currents from sensory endings. We limited our experiments to smaller (<35 μm average diameter) acutely dissociated neurons because the majority of these cells are likely to be involved in nociceptive processing in vivo and are rich in T-currents (Nelson et al. 2005; Jagodic et al. 2007).
We began our study by testing the effects of epipregnanolone (Fig. 1a) on well-isolated T-currents in rat sensory neurons. Traces (Fig. 1b) and time course (Fig. 1c) from the same representative DRG cell indicate that at 30 μM, epipregnanolone inhibited about 75% of the T-current (Vh of −90 mV, and Vt of −30 mV). Figure 1c shows that the inhibitory effect of epipregnanolone had a fast onset, but was slowly and only partially reversible. To compare the potency of epipregnanolone in inhibiting T-currents in DRG cells with synthetic 5β-reduced steroids we obtained multiple points on concentration-response relationships and generated a best fit using equation 1 (solid line, Fig. 1d). These experiments indicated that epipregnanolone was similar to 3β5βCN (IC50 = 3 μM; Todorovic et al. 2004), very potent in inhibiting DRG T-currents with an IC50 about 2 μM. We next tested the ability of epipregnanolone to inhibit voltage-gated sodium currents and voltage-gated potassium currents, which are also critical regulators of the excitability of nociceptive DRG neurons (Campbell and Meyer 2006). Furthermore, we also examined the effects of epipregnanolone on GABA-gated currents in DRG cells since one previous study has well documented that many 5β-reduced steroids, including epipregnanolone, inhibit recombinant GABAA-gated currents (Wang et al., 2002). Original traces of these currents in control conditions are depicted on Fig. 1e. We found that that 10 μM epipregnanolone had little effect on the amplitude of total voltage-gated sodium currents (INa+ total), the tetrodoxin-resistant component of voltage-gated sodium currents (INa+TTX-resistant), total voltage-gated potassium currents (IK+ total), and currents evoked by brief (3-5 seconds) applications of 100 μM GABA. The average effects of 10 μM epipregnanolone on the amplitude of these currents are presented on bar graphs of Fig. 1f as follows: total INa+ 3 ± 4% change (open bar, p > 0.05, n = 9), 15 ± 17% change of INa+TTX-resistant (filled black bar, p > 0.05, n = 6), 2 ± 10% change of IK+ total (filled gray bar, p>0.05, n=5) and 3 ± 37% change of IGABA (stripe bar, p>0.05, n=4). In separate experiments we determined that near-maximal IGABA in DRG cells were obtained during applications of 1 mM GABA alone (data not shown).
Mechanisms of inhibition of T-channels in rat DRG neurons by epipregnanolone
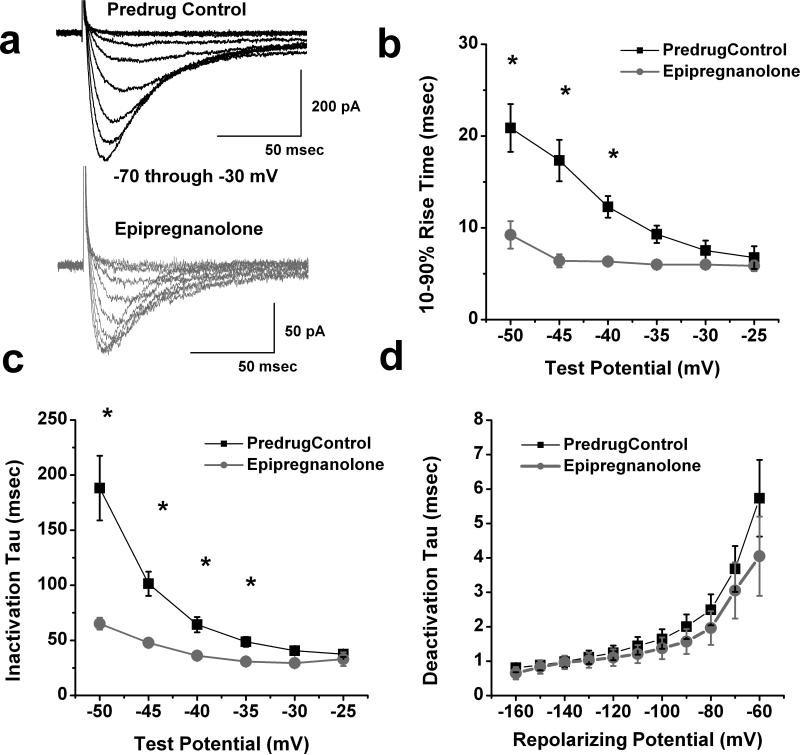
We also investigated the biophysical mechanisms of T-current inhibition by epipregnanolone. To determine the effects epipregnanolone on the kinetic properties of DRG T-currents, we evoked families of inward currents at different Vt ranging from −70 mV to −30 mV in the presence (gray traces) and absence (black traces) of 10 μM epipregnanolone in the same cells, finding that epipregnanolone reduced T-current amplitudes over the range of tested potentials (Fig. 2a). We also found that epipregnanolone (●) significantly increased (up to two-fold) the kinetics of macroscopic current activation and inactivation when compared to predrug controls (■), the effects were more prominent at hyperpolarized test potentials (Fig. 2b and 2c, respectively; n = 8). In contrast, epipregnanolone did not have significant effect on the rate of channel closure after repolarization, as demonstrated by similar deactivation time constants (τs) from −160 to −60 mV (Fig. 2d, n = 8).
Figure 2. Effects of epipregnanolone of macroscopic T-current kinetics and deactivation in rat DRG cells.
a: Traces represent families of T-currents evoked in a representative DRG cell in predrug control conditions (black traces on top panel) and during application of 10 μM epipregnanolone (gray traces on lower panel) by voltage steps from Vh of -90 mV to Vt from −70 through −30 mV in 5-mV increments. Bars indicate calibration. b,c: We measured time-dependent activation (10%-90% rise time, panel b) and inactivation τ(single exponential fit of decaying portion of the current waveforms, panel c) in 8 DRG cells over the range of test potentials from −50 mV to −25 mV before (■) and after application of 10 μM epipregnanolone (●). Note that epipregnanolone speeded T-current kinetics at more negative Vt. Symbol * indicates significance of p < 0.05.
d: Deactivating tail currents in control predrug conditions (■) and after application of 10 μM epipregnanolone (●) were fit with a single exponential function. The resulting tau values are plotted (n = 6). All points are not statistically significant between two groups (p > 0.05).
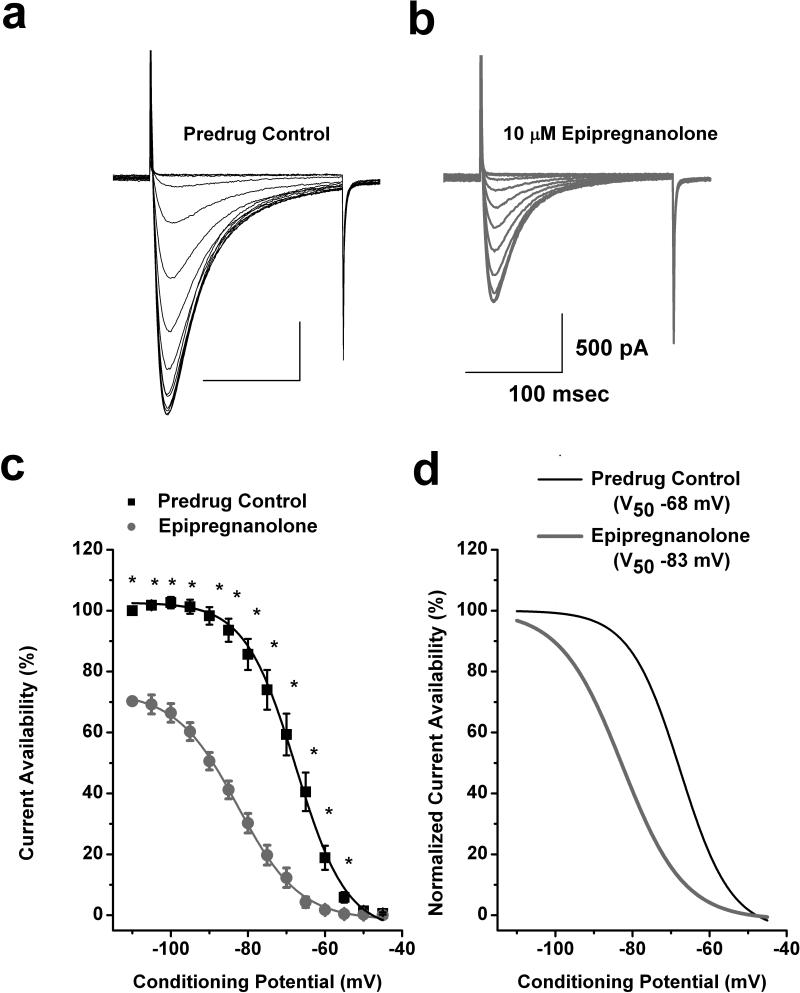
Drug binding to inactivated states of ion channels is an important property since it allows for tissue selectivity based on differences in membrane potentials, where more depolarized membranes will have T-channels which cycle through the inactivated state more often versus less excitable tissue. Transitions from closed to inactivated states can be measured using long prepulses at different potentials, producing what are commonly referred to as steady-state inactivation curves. We assessed steady-state inactivation curves and resulting current availability in 14 DRG cells using a standard double-pulse protocol with 3.6 s-long prepulses to variable voltages (from −110 to −45 mV) and Vt to −30 mV. As shown with original current traces from a representative DRG cell in Figures 3a and 3b, 10 μM epipregnanolone (gray traces), as compared to control predrug conditions (black traces), decreased T-current amplitudes over all tested conditioning potentials. Furthermore, when compared to control (■) predrug conditions, epipregnanolone (●) had a great effect on the voltage-dependent kinetics of channel inactivation, as determined by a hyperpolarizing shift in steady-state inactivation curves of about 15 mV (Figs. 3c and 3d). These data suggest that epipregnanolone binds to and stabilizes inactive states of the T-channel and thus is a more potent blocker at depolarized membrane potentials. For example, in Fig. 3c 10 μM epipregnanolone inhibits about 30% of maximal T-current at −110 mV, while the same concentration inhibits about 90% T-current at conditioning potentials of −65 and −60 mV.
Figure 3. Epipregnanolone stabilizes inactive states of T-channels in rat DRG cells.
a,b: Representative original current traces of a T-rich DRG cell in control conditions (panel a) and after 5 minutes of bath application of 10 μM epipregnanolone (panel b). Calibration bars pertain to both panels. c: The average T-current steady-state inactivation curves from similar experiments shown in the upper panels of this figure (n = 14 cells). Black filled squares represent the control conditions; gray filled circles represent the conditions after bath applications of epipregnanolone in the same DRG cells. All points are normalized to maximal current at −110 mV in predrug control conditions. Solid lines are fitted using equation #2 (see Materials and Methods), giving half-maximal availability (V50), which occurred at −68 ± 1 mV with a slope k of 7 ± 1 mV in control conditions. V50 was −83 ± 1 mV with a slope k of 8 ± 1 mV in the conditions after epipregnanolone was applied. Symbol * indicates significance of p < 0.05. d: The same steady state-inactivation curves as depicted on panel c of this figure are normalized to its own maximal current. Solid black curve represent control conditions and gray solid curve reflects the hyperpolarizing shift of steady-state inactivation by 15 mV induced by epipregnanolone.
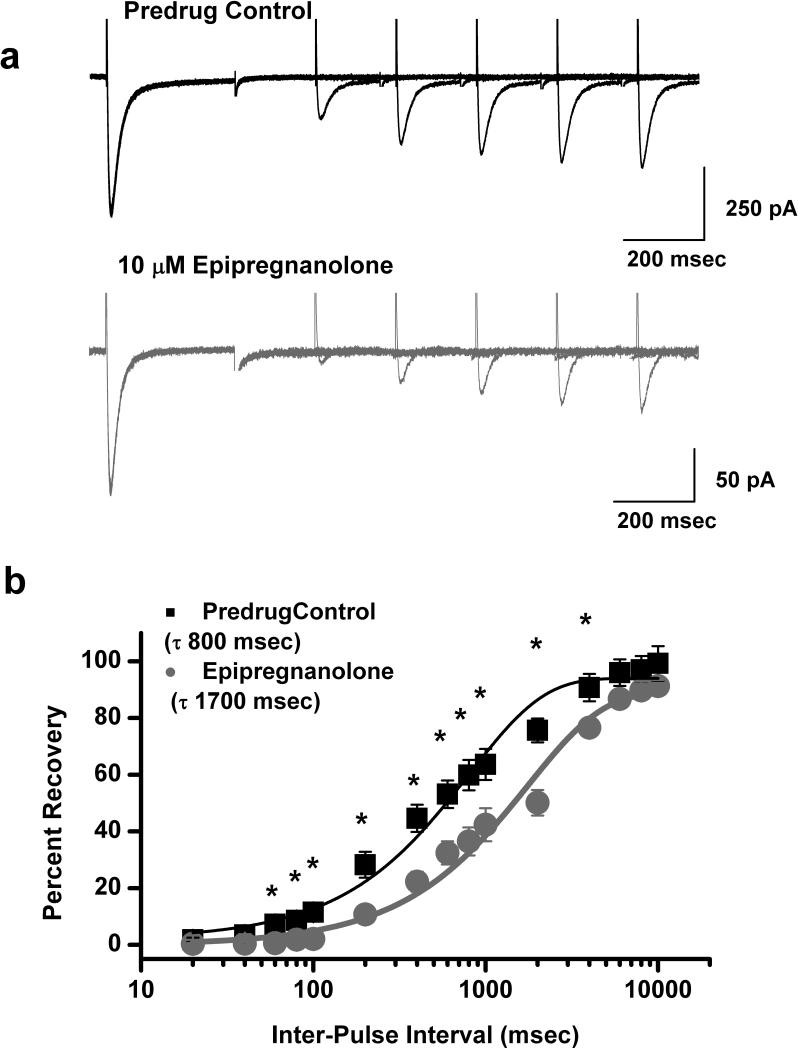
T-channels can recover from inactivation during sufficiently long hyperpolarizations of the neuronal membrane (Nelson et al. 2005). This can significantly influence firing properties of DRG cells that express T-channels. Thus, we studied the effects of 10 μM epipregnanolone on recovery from inactivation using our standard double-pulse protocol with variable inter-pulse duration at −90 mV (Fig. 4) after a 500 msec-long inactivating pulse (Vh −90 mV, Vt −30 mV). Figure 4a shows original current traces in predrug control conditions (top black trace) and after applications of epipregnanolone for 5 minutes (bottom gray traces). Epipregnanolone decreased recovery from inactivation in this cell as evident by about 70% maximal recovery after a 1 sec interval in control conditions, vs. only about 40% recovery in the presence of this steroid. Figure 4b depicts the normalized average data (n = 8 cells) indicating that in the presence of epipregnanolone (●, τ of 1700 msec) T-currents recover about 2-fold slower than in predrug control values (■, τ of 800 msec).
Figure 4. Epipregnanolone slows recovery from inactivation of T-currents in rat DRG cells.
a: Representative original current traces of a DRG cell in control conditions (top panel) and after 5 minutes of bath application of 10 μM epipregnanolone (bottom panel). b: Symbols indicate averaged data from multiple DRG cells (n = 8) that were fitted with a single exponential equation (solid lines). Recovery in control predrug conditions (black symbols and black solid line) was best described with τ of 800 ± 80 msec. After application of epipregnanolone in the same DRG cells (gray symbols and gray solid line) τ was about 2-fold slower: 1700 ± 200 msec. Symbol * indicates significance of p < 0.05.
Analgesic effects of epipregnanolone in rats
The hyperpolarizing shift in steady-state inactivation and slower recovery from inactivation are potentially useful properties for a channel inhibitor like epipregnanolone. When applied in vivo, epipregnanolone may affect actively firing neurons more potently than neurons at rest. Thus, we tested the efficacy of epipregnanolone in a commonly-used rat model of peripheral heat nociception. We first injected 100 μL of vehicle i.pl. into right hind paw of adult rats and showed that thermal PWLs remained stable in both right and left paws for up to 60 minutes (Fig. 5a). We next performed the same experiment in by injecting 100 μl of epipregnanolone at concentrations 0.1, 1 and 10 μM (Fig. 5b), the similar range as tested in our in vitro experiments and shown in Fig. 1d. We found that epipregnanolone induced dose-dependent analgesia as evident by about 20% prolongation of thermal PWLs in the right hind paws of rats 10 and 20 minutes after injection (point 0 on Fig. 5b). In contrast, thermal PWLs remained stable in uninjected, left paws throughout the experiment, indicating lack of systemic effects. We next injected into rat right hind paws 100 μL of solutions containing competitive GABAA receptor antagonist bicuculline to probe for possible involvement of GABAA receptors in peripheral analgesic effects of epipregnanolone. Figure 5c shows that bicuculline at a concentration of 60 μM given alone had minimal effect on baseline thermal PWLs, nor did it affect analgesic effect of 10 μM epipregnanolone when given in combination (Fig. 5d). As depicted on Fig. 5d, thermal PWLs in the presence of combined bicuculline and epipregnalone are increased after 10 and 20 minutes post injection to a similar degree as when the same concentration of epipregnanolone was injected alone (Fig. 5b). We have previously shown, using the same experimental paradigm of heat nociception that at this concentration bicuculline inhibited analgesia induced by injections of 5β-reduced steroids with GABAA-mimetic properties (Pathirathna et al. 2005).
Thus, our data indicate that epipregnanolone is a potent and dose-dependent modulator of peripheral heat nociception, and that this effect does not involve peripheral GABAA receptors. However, based on these data it is not possible to conclude whether analgesic properties of epipregnanolone could be related to the inhibition of T-channels alone in sensory neurons. CaV3.2 is the main isoform of T-channels expressed in sensory neurons (Chen et al. 2003). We have also recently demonstrated that immunoreactivity for CaV3.2 protein is largely confined to the smaller diameter pain-processing unmyelinated axons of peripheral nerves of rat and mouse (Rose et al. 2013). Hence, we used wild-type (WT, CaV3.2 +/+) and CaV3.2 knock-out (KO, CaV3.2 −/−) mice to determine if peripheral analgesic effects of epipregnanolone are indeed mediated by inhibition of CaV3.2 channels.
The CaV 3.2 channel in peripheral sensory neurons is required for epipregnanolone-induced modulation of thermal and mechanical sensation in vivo
We first examined whether epipregnanolone modifies in vivo sensitivity to noxious thermal (heat) stimuli. In these studies, we injected 10 μl of the steroid or vehicle directly into the peripheral receptive fields of sensory neurons in the hind paws of adult WT and CaV3.2 KO mice, and then measured the latency to paw withdrawal in the presence of a radiant heat stimulus (Fig. 6). DMSO (0.1%), the vehicle used to dissolve epipregnanolone, had no effect on thermal PWLs neither in WT littermates (Fig. 6a), nor CaV3.2 −/− mice (Fig. 6d). As reported previously (Choi et al., 2007; Barbara et al., 2009) and as shown in Figures 6a and 6d, CaV3.2−/− mice have similar baseline heat sensitivities to their WT littermate counterparts. However, i.pl. injection of 1 μM and 10 μM epipregnanolone produced a robust dose-dependent decrease in sensitivity to heat stimuli in CaV3.2+/+ mice at 10 min and 20 min after injection. This was manifested by the transient prolongation of PWLs in injected (right, R) hindpaws by about 30% with 1 μM (Fig. 6b) and about 80% with 10 μM epipregnanolone (Fig. 6c) at 10 minutes after injection. It is important to note that PWLs in uninjected (left, L) paws remained stable throughout the testing period, indicating a lack of systemic effect. In control experiments, higher concentration of epipregnanolone (10 μM) had no effect on PWLs in CaV3.2−/− mice (Fig. 6e).
The majority of small-size DRG neurons are polymodal nociceptors that respond to a variety of noxious stimuli. Thus, we also studied the effects of i.pl. injection of epipregnanolone on mechanical sensation using von Frey filament # 4.08 which allows measure of allodynia. We measured baseline mechanical PWRs before injections (0 time) and 10, 20 and 60 minutes following injection. DMSO (0.1%), the vehicle used to dissolve epipregnanolone, had no effect on PWRs neither in WT littermates (Fig. 7a), nor CaV3.2 −/− mice (Fig. 7d). As reported previously (Choi et al., 2007; Barbara et al., 2009) and as shown in Figs. 7A and 7D, CaV3.2−/− mice have similar baseline mechanical sensitivities to their WT littermate counterparts. We found that locally injected epipregnanolone induced dose-dependent decrease in mechanical sensitivity at 10 minutes following injections only in WT mice. For example, after injections of 1 μM epipregnanolone into right (R) paws, PWRs decreased by 47% (Fig. 7b), and injections of 10 μM epipregnanolone decreased PWRs by 66% (Fig. 7c). Note that PWRs in uninjected (left, L) paws remained stable throughout the testing period, indicating a lack of systemic effect. In control experiments, the higher concentrations of epipregnanolone (10 μM) had no effect on PWRs in CaV3.2−/− mice (Fig. 7e). These data indicate that CaV3.2 channels in peripheral sensory neurons are required for epipregnanolone-induced modulation of mechanical and heat sensitivity in vivo.
DISCUSSION
Here we report for the first time that naturally occurring 5β-reduced neurosteroid epipregnanolone is a potent blocker of T-currents in putative nociceptive DRG neurons in vitro and an effective analgesic in vivo. Robust analgesic effect in CaV3.2 +/+ mice and complete absence of effect in CaV3.2 −/− mice strongly suggest that epipregnanolone's effect is at least in part mediated via CaV3.2 isoform of T-channels. It is interesting that our study, as well as studies of others (Choi et al., 2003; Barbara et al., 2009) have documented that CaV3.2 KO mice exhibit normal baseline levels of heat and mechanical sensitivities, possibly as a results of compensatory alterations of other proteins in peripheral nociceptors. However, in contrast to complete insensitivity of CaV3.2 KO mice to analgesic effects of T-channel blockers such as epipregnanolone (our study) and lipoamino acids (Barbara et al., 2009), previous study has demonstrated that CaV3.2 KO mice exhibit unaltered responses to analgesic effects of opioid agonists such as morphine when compared to WT littermates (Barbara et al., 2009). This argues that CaV3.2 KO mice are a good model for validating pharmacological specificity of agents targeting CaV3.2 channels.
Acute pain can provide useful information where it alerts the organism to harmful events in the peripheral tissues. This form of pain generally responds well to traditional pain killers like opioids and non-steroidal anti-inflammatory drugs. However, chronic pain caused by mechanical injury, diabetes or chemotherapy responds poorly to conventional pain therapies. It is well established that the CaV3.2 isoform expressed in nociceptive DRG cells, as well as dorsal horn (DH) cells, contribute to neuronal hyperexcitability in peripheral and central pain pathways, respectively (Nelson et al. 2005; Jacus et al. 2012). Furthermore, the link between neuronal hyperexcitability and two frequent symptoms of neuropathic pain such as hyperalgesia (intensified pain sensation) and allodynia (painful experience with normally nonnoxious stimuli) has long been recognized (Meyer and Campbell 2006). Hence, drugs that inhibit function of CaV3.2 channels can be useful for the treatment of conditions associated with intractable neuropathic pain (reviewed in Todorovic and Jevtovic-Todorovic 2013).
Here, we used in vitro and in vivo methods to describe analgesic properties of the potent, voltage-dependent blocker of T-channels, epipregnanolone. We found that the potency of epipregnanolone in inhibiting DRG T-currents (IC50 of 2 μM at Vh of −90 mV) and voltage-dependent mechanisms of block are similar to those previously described for 3β5βCN (IC50 of 3 μM at Vh −90 mV, Todorovic et al. 2004). However, given the strong voltage-dependent aspect of the current inhibition it is very likely that the potency of epipregnanolone in inhibiting DRG T-currents in vivo is higher than reported here. This is supported by the fact that the analgesic effects are achieved by injecting small amounts of steroid into the peripheral receptive fields of hind paws where nociceptive nerve fibers terminate within the epidermis. Furthermore, potency of epipregnanolone in vitro in blocking isolated T-currents is mirrored in our in vivo pain experiments. Lastly, we used KO mice to validate that CaV3.2 channels are required for the analgesic effects of epipregnanolone in vivo. We also investigated the selectivity of epipregnanolone and found that at 10 μM epipregnanolone had no significant effect on total sodium currents, TTX-resistant sodium currents and total voltage-gated potassium currents in DRG cells. This suggests that epipregnanolone could be used as a local analgesic to provide comfort without inducing motor weakness or complete numbness that is invariably observed with clinically-used local anesthetics that commonly target voltage-gated sodium currents. In our previous study we reported that the prototypical synthetic 5β-reduced neuroactive steroid, 3β5βCN (structure differs from that of epipregnanolone by having a 17β CN group instead of a 17β acetyl group), at 10 μM also had very little effect on voltage-gated sodium currents, as well as voltage-gated potassium currents and high-voltage-activated (HVA) calcium currents in DRG cells (Todorovic et al. 2004). It is possible that epipregnanolone like 3β5βCN also has a less potent effect on HVA calcium currents in DRG cells, although this notion remains to be confirmed in future studies.
Our results showing insensitivity of IGABA in acutely dissociated DRG cells to epipregnanolone and lack of effects of bicuculline on epipregnanolone-induced analgesia in vivo are consistent with other reports on native neuronal GABAA receptors in CNS (Poisebeau et al. 1997; Weir et al. 2004). However, an elegant study using recombinant GABAA channels has described that epipregnanolone and other 5β-reduced steroids act as noncompetitive, likely state-dependent blockers of GABAA receptors (Wang et al., 2002). The discrepancy for this is not completely understood but may be due to different experimental conditions, differences in recombinant and native GABAA channels and/or tissue conditions of DRG cells and peripheral nociceptive endings not favoring state-dependent effects of epipregnanolone. This represents an important area for future investigations.
Epipregnanolone and other endogenous neuroactive steroids are actively synthesized in the brain tissue from cholesterol (Liu et al. 2003). Previously we have reported that other endogenous steroid molecules with the 5α-ring configuration at the steroid A, B ring fusion such as allopregnanolone, which activates GABAA receptors and blocks DRG T-currents, exhibit strong analgesic effects in the rat neuropathic pain model of loose sciatic nerve ligation (Pathirathna et al. 2005a). Interestingly, a recent study by Patte-Mensah and colleagues (2010) reports that allopregnanolone can be synthesized in DRG neurons. Furthermore, they found that the function of the key enzyme involved in the production of allopregnanolone in DRG, 3α-hydroxysteroid oxidoreductase is upregulated during development of chronic neuropathic pain and that thermal and mechanical hyperalgesia are worsened when the activity of this enzyme is knocked-down (Patte-Mensah et al. 2010). The results of the above study strongly support the idea that endogenous synthesis of 5α-reduced steroids may be protective in some forms of neuropathic pain. It remains to be determined if a similar endogenous mechanism operates in DRG cells for production of 5β-reduced steroid molecules like epipregnanolone that potently inhibit DRG T-currents. The concentration of epipregnanolone at peripheral nociceptors is not known but studies have determined that plasma levels of this steroid in humans are in the low nanomolar and subnanomolar range (Bicikova et al., 2013). In contrast, we found that T-currents in vitro and pain responses in vivo in rats and mice are affected with low micromolar concentrations of this steroid. This suggests that peripheral T-channels are likely not saturated by endogenously present epipregnanolone and/or other 5β-reduced steroid. Nevertheless, it appears that manipulating levels of endogenously-synthesized neuroactive steroids and/or exogenous applications of steroids may represent novel therapeutic approach to diminish pathological hyperexcitability of DRG neurons that contribute to peripheral nociception and neuropathic pain development. Further preclinical and clinical studies are needed to investigate this possibility.
ACKNOWLEDGMENTS
This work was supported in part by grants from NIH DA 029342 to SMT and VJ-T, funds from the Department of Anesthesiology at UVA to SMT, funds from Hallym University to SJH and SMH, funds from Korea University to J-YP, and the Harold Carron Endowment to VJ-T.
Abbreviations
- DRG
dorsal root ganglion)
- LVA
low-voltage-activated)
- TEA-OH
tetraethylammonium hydroxide)
- PWL
paw withdrawal latency)
- PWR
paw withdrawal responses)
- TMA-OH
tetramethylammonium hydroxide)
- TTX
tetrodotoxin)
- DMSO
dimethylsulfoxide)
- BAPTA
1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid)
- EGTA
ethylene glycol tetraacetic acid)
- ECN
[(3β, 5α, 17β)-17-hydroxyestrane-3-carbonitrile]
- 3β5βCN
[(3β, 5β, 17β)-3-hydroxyandrostane-17-carbonitrile]
- Epipregnanolone
3β,5β)-3-hydroxypregnan-20-one)
Footnotes
The authors declare no conflict of interest.
REFERENCES
- Barbara G, Alloui A, Nargeot J, Lory P, Eschalier A, Bourinet E, Chemin J. T-type calcium channel inhibition underlies the analgesic effects of the endogenous lipoamino acids. J Neurosci. 2009;29:13106–14. doi: 10.1523/JNEUROSCI.2919-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicikova M, Hill M, Ripova D, Mohr P, Hampl R. Determination of steroid metabolome as a possible tool for laboratory diagnosis of schizophrenia. J Steroid Biochem Mol Biol. 2013;133:77–83. doi: 10.1016/j.jsbmb.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52(1):77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell CP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science. 2003;302:1416–8. doi: 10.1126/science.1089268. [DOI] [PubMed] [Google Scholar]
- Choe WJ, Messinger RB, Leach E, Eckle V-S, Obradovic A, Salajegheh R, Jevtovic-Todorovic V, Todorovic SM. TTA-P2 is a potent and selective blocker of T-type calcium channels in rat sensory neurons and a novel antinociceptive agent. Molecular Pharmacology. 2011;80(5):900–10. doi: 10.1124/mol.111.073205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Na HS, Kim J, Lee J, Lee S, Kim D, Park J, Chen CC, Campbell KP, Shin HS. Attenuated pain responses in mice lacking CaV3.2 T-type channels. Genes Brain Behav. 2007;6(5):425–431. doi: 10.1111/j.1601-183X.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- Jacus MO, Uebele VN, Renger JJ, Todorovic SM. Presynaptic CaV3.2 channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. J Neurosci. 2012;32(27):9374–82. doi: 10.1523/JNEUROSCI.0068-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagodic MM, Pathirathna S, Nelson MT, Mancuso S, Joksovic PM, Rosenberg ER, Bayliss DA, Jevtovic-Todorovic V, Todorovic SM. Cell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. J Neurosci. 2007;27(12):3305–16. doi: 10.1523/JNEUROSCI.4866-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Covey DF, Todorovic SM. Are neuroactive steroids promising therapeutic agents in the management of acute and chronic pain? Psychoneuroendocrinology. 2009;34(Suppl 1):S178–85. doi: 10.1016/j.psyneuen.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham JR, Pathirathna S, Jagodic MM, Choe WJ, Levin ME, Nelson MT, Lee WY, Krishnan K, Covey D, Todorovic SM, Jevtovic-Todorovic V. Selective T-type calcium channel blockade alleviates hyperalgesia in ob/ob mice. Diabetes. 2009;58(11):2656–2665. doi: 10.2337/db08-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Orestes P, Latham J, Naik AK, Nelson MT, Vitko I, Perez-Reyes E, Jevtovic-Todorovic V, Todorovic SM. Molecular mechanisms of lipoic acid modulation of T-type calcium channels in pain pathway. J Neurosci. 2009;29(30):9500–9509. doi: 10.1523/JNEUROSCI.5803-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Sjövall J, Griffiths WJ. Neurosteroids in rat brain: extraction, isolation, and analysis by nanoscale liquid chromatography-electrospray mass spectrometry. Anal Chem. 2003;75(21):5835–46. doi: 10.1021/ac0346297. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Joksovic PM, Perez-Reyes E, Todorovic SM. The endogenous redox agent L-cysteine induces T-type Ca2+ channel-dependent sensitization of a novel subpopulation of rat peripheral nociceptors. J Neurosci. 2005;25(38):8766–75. doi: 10.1523/JNEUROSCI.2527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patte-Mensah C, Meyer L, Schaeffer V, Mensah-Nyagan AG. Selective regulation of 3 alpha-hydroxysteroid oxido-reductase expression in dorsal root ganglion neurons: a possible mechanism to cope with peripheral nerve injury-induced chronic pain. Pain. 2010;150(3):522–34. doi: 10.1016/j.pain.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Pathirathna S, Brimelow BC, Jagodic MM, Kathiresan K, Jiang X, Zorumski CF, Mennerick S, Covey DF, Todorovic SM, Jevtovic-Todorovic V. New evidence that both T-type Ca2+ channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5α-reduced neuroactive steroids. Pain. 2005;114:429–443. doi: 10.1016/j.pain.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Pathirathna S, Todorovic SM, Covey DF, Jevtovic-Todorovic V. 5α-reduced neuroactive steroids alleviate thermal and mechanical hyperalgesia in rats with neuropathic pain. Pain. 2005a;117:326–39. doi: 10.1016/j.pain.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Feltz P, Schlichter R. Modulation of GABAA receptor-mediated IPSCs by neuroactive steroids in a rat hypothalamo-hypophyseal coculture model. J Physiol. 1997;500(Pt 2):475–85. doi: 10.1113/jphysiol.1997.sp022034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose KE, Lunardi N, Boscolo A, Dong X, Erisir A, Jevtovic-Todorovic V, Todorovic SM. Immunohistological demonstration of CaV3.2 T-type voltage-gated calcium channel expression in soma of dorsal root ganglion neurons and peripheral axons of rat and mouse. Neuroscience. 2013;250:263–274. doi: 10.1016/j.neuroscience.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic SM, Prakriya M, Nakashima YM, Nillson KR, Han M, Zorumski CF, Covey DF, Lingle CJ. Enantioselective blockade of T-type Ca2+ current in adult rat sensory neurons by a steroid that lacks GABA-modulatory activity. Mol Pharmacol. 1998;54:918–927. doi: 10.1124/mol.54.5.918. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Pathirathna S, Brimelow BC, Jagodic MM, Ko S-H, Jiang X, Nilsson KR, Mennerick S, Zorumski CF, Covey DF, Jevtovic-Todorovic V. 5β–reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol Pharmacol. 2004;66(5):1223–1235. doi: 10.1124/mol.104.002402. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Jevtovic-Todorovic V. Neuropathic pain: Role of presynaptic T-type channels in nociceptive signaling. Pflugers Archiv - European Journal of Physiology. 2013;465(7):921–7. doi: 10.1007/s00424-012-1211-y. [DOI] [PubMed] [Google Scholar]
- Weir CJ, Ling AT, Belelli D, Wildsmith JA, Peters JA, Lambert JJ. The interaction of anaesthetic steroids with recombinant glycine and GABAA receptors. Br J Anaesth. 2004;92(5):704–11. doi: 10.1093/bja/aeh125. [DOI] [PubMed] [Google Scholar]
- Wang M, He Y, Eisenman LN, Fields C, Zeng CM, Mathews J, Benz A, Fu T, Zorumski E, Steinbach JH, Covey DF, Zorumski CF, Mennerick S. 3beta-hydoxypregnane steroids are pregananolone sulfate-like GABA(A) receptor antagonists. J Neurosci. 2002;22(9):3366–75. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109–22. doi: 10.1016/j.neubiorev.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]