Abstract
At least 10% of adults and nearly all children who receive renal-replacement therapy have an inherited kidney disease. These patients rarely die when their disease progresses and can remain alive for many years because of advances in organ-replacement therapy. However, these disorders substantially decrease their quality of life and have a large effect on health-care systems. Since the kidneys regulate essential homoeostatic processes, inherited kidney disorders have multisystem complications, which add to the usual challenges for rare disorders. In this review, we discuss the nature of rare inherited kidney diseases, the challenges they pose, and opportunities from technological advances, which are well suited to target the kidney. Mechanistic insights from rare disorders are relevant for common disorders such as hypertension, kidney stones, cardiovascular disease, and progression of chronic kidney disease.
Introduction
In the USA a rare disease is defined as a disease that affects fewer than 200 000 people in the country, whereas this designation is given to diseases that affect fewer than one in 2000 people in Europe,1 fewer than one in 2500 people in Japan,2 and fewer than one in 500 000 people in China.3 Rare diseases are often categorised as orphan diseases to stress their severity, insufficient resources and knowledge available, and the specific conditions to develop or make drugs for them. They represent a group of 6000 to 8000 highly heterogeneous disorders that affect roughly 30 million patients in Europe.1 About 80% of rare diseases have an identified genetic origin. The incidence of a rare disease can vary substantially between regions or ethnic groups. For example, congenital nephrotic syndrome of the Finnish type occurs more frequently in Finland (incidence of one in 8200 people) than in other parts of the world.
Rare kidney diseases constitute at least 150 different disorders and they have an overall prevalence of about 60–80 cases per 100 000 in Europe and the USA.4–6 At least 10% of adults and nearly all children who progress to renal-replacement therapy have an inherited kidney disease, the fifth most common cause of end-stage renal disease after diabetes, hypertension, glomerulonephritis, and pyelonephritis. Because of progress in renalreplacement therapy, patients with inherited kidney disorders rarely die when their disease progresses and can live for many years. However, these patients often have compromised health with a poor quality of life. For instance, children with severe congenital nephropathies, who can be dialysed from neonatal age onwards, face many decades of life with end-stage renal disease and have a high likelihood of changes in physical, cognitive, and psychosocial development. Inherited kidney disorders have multisystem complications that add to the typical challenges for rare disorders—ie, variable phenotypes, fragmented clinical and biological data, an absence of standardisation for diagnostic procedures, and poor knowledge for disease mechanisms and natural history.7
In this review, we discuss the epidemiology, range, and specific nature of rare inherited kidney diseases of genetic origin and note challenges that arise in their management. We then address opportunities from technological advances and high-throughput screening approaches, which are particularly well suited to target the kidney. We particularly focus on the link between these technologies and the innovative clinical research programmes and initiatives. We show how these collaborative studies could affect the clinical management of rare kidney diseases and beyond, with mention of insights about effects of sex and ageing, the progression of chronic kidney disease, and understanding for more common disorders.
Rare inherited kidney diseases: why they are different
The kidney is a complex organ, composed of many specialised cell types, with highly regulated functions that are essential for homoeostasis.8 The kidneys are exposed to and affect the extracellular environment more than any other organ—regulating water and electrolyte balance, acid-base homoeostasis, tissue oxygen supply, hormone and vitamin metabolism, and innate and adaptive immunity. The kidneys are also essential for metabolic clearance and secretion of drug metabolites. These functions have large quantitative effects that can directly affect body composition. Primary kidney disorders can substantially affect blood pressure, plasma composition, electrolyte and acid-base homoeostasis, cardiac excitability, growth dynamics and puberty, and CNS and cognitive functions. Various aspects of renal function can also be affected in extrarenal rare disorders or polymalformative syndromes, including mitochondrial cytopathies.9–12
Genetics were first used in nephrology in the 1980s with the mapping of autosomal dominant polycystic kidney disease in 198513 and the first identification of a causal mutation for a monogenic kidney disorder (Alport’s syndrome) in 1990.14 These breakthroughs were followed by identification of genes involved in classic disorders such as nephrogenic diabetes insipidus,15 autosomal dominant polycystic kidney disease type 1,16 Liddle’s syndrome,17 Dent’s disease,18 Bartter’s and Gitelman’s syndromes,19,20 nephropathic cystinosis,21 and steroid-resistant nephrotic syndrome (panel).22 With the increased use of high-throughput and next-generation sequencing technologies, investigators have now defined the genetic basis of more than 160 rare kidney diseases (table 1, table 2). These disorders are caused by mutations in genes coding for a wide range of proteins including receptors, channels and transporters, enzymes, transcription factors, and structural components that might also have a role in extrarenal organs (bone, eye, brain, skin, etc). Figure 1 shows a functional classification of rare inherited disorders of the kidney. In addition to monogenic diseases, the combination of variants in the same genes or in genes involved in common pathways that operate in the kidney might cause variable effect sizes that cannot be explained by conventional genotype– phenotype correlations.8,28 Careful phenotype assessments of recessively inherited kidney disorders have substantiated the effect of carrier states. For example, Gitelman’s syndrome is caused by loss-of-function mutations in SLC12A3, which encodes the thiazide-sensitive sodium–chloride cotransporter in the distal convoluted tubule. About 1% of the general population are heterozygous carriers of SLC12A3 mutations; such carriers have a lower blood pressure and a lower risk of hypertension than have the general population.29
Table 1.
List and classification of genetic disorders of renal growth and structure
| Transmission | Affected proteins* | Protein function | Phenotype MIM entry |
|
|---|---|---|---|---|
|
Congenital abnormalities of the kidney and urinary tract | ||||
| Renal hypodysplasia or aplasia | All autosomal recessive | RET; PAX2; UPK3A | Tyrosine-kinase receptor; transcription factor; membrane protein | 191830 |
| Vesicoureteral reflux | All autosomal dominant | ROBO2; SOX17; TNXB | Transmembrane receptor; transcription factor; extracellular matrix glycoprotein |
610878; 613674 |
| Renal coloboma syndrome | Autosomal dominant | PAX2 | Transcription factor | 120330 |
| Renal cysts and diabetes syndrome | Autosomal dominant | HNF1B | Transcription factor | 137920 |
| Branchio-otorenal syndrome | All autosomal dominant | EYA1; SIX1; SIX5 | Transcriptional coactivator; transcription factor; transcription factor | 113650 |
| Fraser’s syndrome | All autosomal recessive | FRAS1; GRIP1; FREM2 | Extracellular matrix protein; receptor interacting protein; extracellular matrix protein |
219000 |
| Urofacial (Ochoa) syndrome | Both autosomal recessive | HPSE2; LRIG2 | Matrix enzyme; membrane protein | 236730; 615112 |
| Hypoparathyroidism, deafness, renal disease syndrome |
Autosomal dominant | GATA3 | Transcription factor | 146255 |
| Kallmann’s syndrome | KAL1 is X-chromosome | KAL1; FGFR1 | Adhesion-like protein, protease inhibitor; tyrosine-kinase receptor | 308700; |
| (subtypes with renal phenotype) | linked and FGFR1 is autosomal recessive |
147950 | ||
| Split-hand–split-foot malformation | Autosomal dominant | Duplication of 10q24 | ·· | 246560 |
| Townes-Brocks syndrome | Autosomal dominant | SALL1 | Transcription factor | 107480 |
| Perlman’s syndrome (nephroblastomatosis, gigantism) Simpson-Golabi-Behmel syndrome (gigantism, enlarged dysplastic kidneys) |
Autosomal recessive | DIS3L2 | Ribonuclease | 267000 |
| Type 1 | X-chromosome linked | GPC3 | Heparin sulphate proteoglycan | 312870 |
| Type 2 | X-chromosome linked | OFD1 | Centrosome protein involved in ciliogenesis | 300209 |
| Renal tubular dysgenesis | All autosomal recessive | REN; AGT; AGTR1; ACE | Endopeptidase (angiotensinogenase); secreted peptide; G-protein coupled receptor; carboxypeptidase |
267430 |
| Ciliopathies | ||||
| Autosomal dominant polycystic kidney disease, type 1 and type 2† |
Both autosomal dominant | PKD1; PKD2 | Both ciliary proteins, involved in mechanosensation and cell signalling | 173900; 613095 |
| Autosomal recessive polycystic kidney disease |
Autosomal recessive | PKHD1 | Receptor-like cilium and cytoskeleton protein (centrosome regulator) | 263200 |
| Medullary cystic kidney disease and familial juvenile hyperuricaemic nephropathy |
All autosomal dominant | UMOD; REN; MUC1 | Surface-bound and secreted glycoprotein (Tamm-Horsfall protein); endopeptidase (angiotensinogenase); surface glycoprotein |
603860; 613092; 174000 |
| Nephronophthisis | ||||
| Type 1 | Autosomal recessive | NPHP1 | Ciliary protein, involved in organisation of apical junctions | 256100 |
| Type 2 | Autosomal recessive | INVS | Ciliary protein, associates with microtubules, inhibits WNT signalling | 602088 |
| Type 3 | Autosomal recessive | NPHP3 | Ciliary protein, inhibits WNT signalling | 604387 |
| Type 4 | Autosomal recessive | NPHP4 | Ciliary protein, involved in organisation of apical junctions | 606966 |
| Type 5 (Senior-Løken syndrome 5) | Autosomal recessive | IQCB1 | Centrosome protein, involved in ciliogenesis | 609254 |
| Type 6 (Joubert’s syndrome 5) | Autosomal recessive | CEP290 | Centrosome protein, involved in ciliogenesis | 610188 |
| Type 7 | Autosomal recessive | GLIS2 | Transcription factor | 611498 |
| Type 8 (Joubert’s syndrome 7) | Autosomal recessive | RPGRIP1L | Centrosome protein, regulates TXA2 receptor signalling | 611560 |
| Type 9 | Autosomal recessive | NEK8 | Serine–threonine protein kinase, targets proteins to cilia | 613824 |
| Type 10 (Senior-Løken syndrome 7) | Autosomal recessive | SDCCAG8 | Centrosome-associated protein, might be involved in ciliogenesis | 613615 |
| Type 11 | Autosomal recessive | TMEM67 | Ciliary protein, involved in centrosome migration | 613550 |
| Type 12 | Autosomal recessive | TTC21B | Ciliary protein, involved in retrograde ciliary transport | 613820 |
| Type 13 | Autosomal recessive | WDR19 | Ciliary protein, involved in retrograde ciliary transport | 614377 |
| Type 14 | Autosomal recessive | ZNF423 | Centrosome protein, involved in DNA damage response | 614844 |
| Type 15 | Autosomal recessive | CEP164 | Centrosome protein, involved in DNA damage response | 614845 |
| Type 16 | Autosomal recessive | ANKS6 | Ciliary protein | 615382 |
| Joubert’s syndrome (subtypes with renal phenotype) | ||||
| Type 1 | Autosomal recessive | INPP5E | Inositol trisphosphate phosphatase | 213300 |
| Type 2 | Autosomal recessive | TMEM216 | Ciliary protein, might be involved in ciliogenesis | 608091 |
| Type 3 | Autosomal recessive | AHI1 | Basal body protein, might be involved in ciliary signalling | 608629 |
| Type 4 | Autosomal recessive | NPHP1 | Ciliary protein, involved in organisation of apical junctions | 609583 |
| Type 5 | Autosomal recessive | CEP290 | Centrosome protein, involved in ciliogenesis | 610188 |
| Type 6 | Autosomal recessive | TMEM67 | Ciliary protein, involved in centrosome migration | 610688 |
| Type 7 | Autosomal recessive | RPGRIP1L | Centrosome protein, regulates thromboxane-A2 receptor signalling | 611560 |
| Type 9 | X-chromosome linked | CC2D2A | Ciliary protein, involved in ciliogenesis and SHH signalling | 612285 |
| Type 10 | Autosomal recessive | OFD1 | Centrosome protein, involved in ciliogenesis | 300804 |
| Type 11 | Autosomal recessive | TTC21B | Ciliary protein, involved in retrograde ciliary transport | 613820 |
| Type 14 | Autosomal recessive | TMEM237 | Ciliary protein, involved in ciliogenesis | 614424 |
| Type 15 | Autosomal recessive | CEP41 | Centrosome protein, required during ciliogenesis | 614464 |
| Type 16 | Autosomal recessive | TMEM138 | Multipass transmembrane protein required for ciliogenesis | 614465 |
| Type 18 | Autosomal recessive | TCTN3 | Membrane protein, required for ciliogenesis and SHH signalling | 614815 |
| Type 19 | Autosomal recessive | ZNF423 | Centrosome protein, involved in DNA damage response | 614844 |
| Type 20 | Autosomal recessive | TMEM231 | Ciliary protein, required for ciliogenesis and SHH signalling | 614970 |
| Type 21 | Autosomal recessive | CSPP1 | Centrosome protein, involved in spindle organisation | 615636 |
| Type 22 | Autosomal recessive | PDE6D | Phosphodiesterase, involved in ciliogenesis | 615665 |
| Meckel-Gruber syndrome | ||||
| Type 1 | Autosomal recessive | MKS1 | Ciliary protein, regulates cilia structure and function | 249000 |
| Type 2 | Autosomal recessive | TMEM216 | Ciliary protein, might be involved in ciliogenesis | 603194 |
| Type 3 | Autosomal recessive | TMEM67 | Ciliary protein, involved in centrosome migration | 607361 |
| Type 4 | Autosomal recessive | CEP290 | Centrosome protein, involved in ciliogenesis | 611134 |
| Type 5 | Autosomal recessive | RPGRIP1L | Centrosome protein, regulates thromboxane-A2 receptor signalling | 611561 |
| Type 6 | Autosomal recessive | CC2D2A | Ciliary protein, involved in ciliogenesis and SHH signalling | 612284 |
| Type 7 | Autosomal recessive | NPHP3 | Ciliary protein, inhibits WNT signalling | 267010 |
| Type 8 | Autosomal recessive | TCTN2 | Ciliary protein, involved in ciliogenesis | 613885 |
| Type 9 | Autosomal recessive | B9D1 | Ciliary protein, involved in ciliogenesis | 614209 |
| Type 10 | Autosomal recessive | B9D2 | Ciliary protein, involved in ciliogenesis | 614175 |
| Type 11 | Autosomal recessive | TMEM231 | Ciliary protein, required for ciliogenesis and SHH signalling | 615397 |
| Short rib-polydactyly syndrome (Jeune’s syndrome) | ||||
| Type 1 | Autosomal recessive | Unknown | ·· | 208500 |
| Type 2 | Autosomal recessive | IFT80 | Ciliary protein, involved in anterograde ciliary transport | 611263 |
| Type 3 | Autosomal recessive | DYNC2H1 | Ciliary motor protein, involved in retrograde ciliary transport | 613091 |
| Type 4 | Autosomal recessive | TTC21B | Ciliary protein, involved in retrograde ciliary transport | 613819 |
| Type 5 | Autosomal recessive | WDR19 | Ciliary protein, involved in retrograde ciliary transport | 615633 |
| Type 6 | Autosomal recessive | NEK1 | Centrosomal serine–threonine protein kinase, involved in ciliogenesis | 263520 |
| Type 7 | Autosomal recessive | WDR35 | Ciliary protein, involved in retrograde ciliary transport | 614091 |
| Type 8 | Autosomal recessive | WDR60 | Ciliary base protein, involved in ciliogenesis | 615503 |
| Type 9 | Autosomal recessive | IFT140 | Ciliary protein, involved in retrograde ciliary transport | 266920 |
| Type 10 | Autosomal recessive | IFT172 | Ciliary protein, involved in anterograde ciliary transport | 615630 |
| Type 11 | Autosomal recessive | WDR34 | Ciliary protein, involved in retrograde ciliary transport | 615633 |
| Bardet-Biedl syndrome | ||||
| Type 1 | Autosomal recessive | BBS1 | BBSome complex protein, required for ciliogenesis | 209900 |
| Type 2 | Autosomal recessive | BBS2 | BBSome complex protein, required for ciliogenesis | 209900 |
| Type 3 | Autosomal recessive | ARL6 | Cilium base protein, targets BBSome to plasma membrane | 209900 |
| Type 4 | Autosomal recessive | BBS4 | BBSome complex protein, required for ciliogenesis | 209900 |
| Type 5 | Autosomal recessive | BBS5 | BBSome complex protein, required for ciliogenesis | 209900 |
| Type 6 | Autosomal recessive | MKKS | Chaperone, may assist folding of BBSome proteins | 209900 |
| Type 7 | Autosomal recessive | BBS7 | BBSome complex protein, required for ciliogenesis | 209900 |
| Type 8 | Autosomal recessive | TTC8 | BBSome complex protein, required for ciliogenesis | 209900 |
| Type 9 | Autosomal recessive | PTHB1 | BBSome complex protein, required for ciliogenesis | 209900 |
| Type 10 | Autosomal recessive | BBS10 | Chaperone, affects folding and stability of ciliary and basal body proteins |
209900 |
| Type 11 | Autosomal recessive | TRIM32 | E3 ubiquitin ligase activity | 209900 |
| Type 12 | Autosomal recessive | BBS12 | Chaperone, assists folding of BBSome proteins | 209900 |
| Type 13 | Autosomal recessive | MKS1 | Ciliary protein, regulates cilia structure and function | 209900 |
| Type 14 | Autosomal recessive | CEP290 | Centrosome protein, involved in ciliogenesis | 209900 |
| Type 15 | Autosomal recessive | Human fritz (WDPCP; C2orf86) | Controls ciliogenesis by regulating septin cytoskeleton | 209900 |
| Type 17 | Autosomal recessive | LZTFL1 | BBSome regulator, involved in ciliogenesis | 209900 |
| Alström’s syndrome | Autosomal recessive | ALMS1 | Centrosome protein, required for cilia formation and maintenance | 203800 |
| Cranioectodermal dysplasia | ||||
| Type 1 | Autosomal recessive | IFT122 | Ciliary proteins, involved in retrograde ciliary transport | 218330 |
| Type 2 | Autosomal recessive | WDR35 | Ciliary proteins, involved in retrograde ciliary transport | 613610 |
| Type 3 | Autosomal recessive | IFT43 | Ciliary proteins, involved in retrograde ciliary transport | 614099 |
| Type 4 (Sensenbrenner syndrome) | Autosomal recessive | WDR19 | Ciliary proteins, involved in retrograde ciliary transport | 614378 |
| Oral-facial-digital syndrome type 1 | X-chromosome linked | OFD1 | Centrosome protein, involved in ciliogenesis | 311200 |
| Renal-hepatic-pancreatic dysplasia | Autosomal recessive | NPHP3 (nephrocystin-3); NEK8 (nephrocystin-9) | Ciliary protein, inhibits WNT signalling; serine–threonine protein kinase, might target proteins to cilia |
208540; 615415 |
MIM=Mendelian Inheritance in Man.
HUGO Gene Nomenclature Committee symbol. †Not classified as a rare disease.
Table 2.
List and classification of genetic disorders of renal function
| Transmission | Affected proteins* | Protein function | Phenotype MIM entry |
|
|---|---|---|---|---|
| Glomerular diseases | ||||
| Autosomal recessive steroid-resistant nephrotic syndrome |
All autosomal recessive | NPHS1; NPHS2; PLCE1; MYO1E; PTPRO; DGKE; ARHGDIA |
Podocyte adhesion receptor, component of slit diaphragm; podocyte membrane protein, links slit diaphragm to cytoskeleton; phospholipase, regulates protein kinase C pathway and small GTPases; cytoplasmic protein, regulates actin cytoskeleton functions; receptor-type tyrosine phosphatase; enzyme involved in cell signalling, activates protein kinase C pathway; cytoplasmic protein, involved in Rho protein signalling |
256300; 600995; 610725; 614131; 614196; 615008; 615244 |
| Autosomal dominant steroid-resistant nephrotic syndrome |
All autosomal dominant | WT1; INF2; ACTN4; TRPC6 |
Transcription factor; cytoplasmic protein, severs actin filaments; F-actin cross-linking cytoplasmic protein; receptor-activated calcium channel |
256370; 613237; 603278; 603965; |
| Denys-Drash syndrome, Frasier’s syndrome | Autosomal dominant | WT1 | Transcription factor | 194080; 136680 |
| WAGR (Wilms’ tumour, aniridia, genitourinary anomalies, retardation) syndrome |
Autosomal dominant | WT1 and PAX6 | Transcription factors | 194072 |
| Pierson’s syndrome | Autosomal recessive | LAMB2 | Extracellular matrix glycoprotein | 609049 |
| Nail-patella syndrome | Autosomal dominant | LMX1B | Transcription factor | 161200 |
| Schimke immuno-osseous dystrophy | Autosomal recessive | SMARCAL1 | Annealing helicase, catalyses rewinding of unwound DNA | 242900 |
| Mitochondrial disorders with steroid- resistant nephrotic syndrome: primary coenzyme Q10 deficiency, types 1 and 6 |
All autosomal recessive | COQ2; COQ6; ADCK4 | Enzyme involved in coenzyme Q10 biosynthesis; enzyme involved in coenzyme Q10 biosynthesis; mitochondrial protein involved in coenzyme Q10 biosynthesis |
607426; 614650; 615573 |
| Fabry’s disease | X-chromosome linked | GLA | Lysosomal enzyme, catalyses galactosyl–glycolipid moieties | 301500 |
| Alport’s syndrome | X-chromosome linked; autosomal recessive |
COL4A5; COL4A4; COL4A3 |
α5-chain of type IV collagen; α4-chain of type IV collagen; α3-chain of type IV collagen |
301050; 203780; 615573 |
| Benign familial haematuria (thin basement membane nephropathy) |
Autosomal dominant | COL4A3 | α3-chain of type IV collagen | 141200 |
| Fechtner’s syndrome (Alport’s syndrome with macrothrombocytopenia) |
Autosomal dominant | MYH9 | Non-muscle myosin, involved in cell shape and movement | 153640 |
| Alport’s syndrome with leiomyomatosis | X-chromosome linked | COL4A5 and COL4A6 (contiguous gene deletion) |
α5-chains and α6-chain of type IV collagen | 308940 |
| Familial amyloidosis | All autosomal dominant | FGA; LYZ; APOA1; B2M | Secreted protein; secreted enzyme; secreted lipoprotein; secreted protein | 105200 |
| Renal tubular diseases and metabolic diseases | ||||
| Renal glucosuria | Autosomal recessive and autosomal dominant |
SLC5A2 | Sodium–glucose cotransporter | 233100 |
| Dicarboylic aminoaciduria | Autosomal recessive | SLC1A1 | Glutamate transporter | 222730 |
| Lysinuric protein intolerance | Autosomal recessive | SLC7A7 | Cationic aminoacid transporter | 222700 |
| Proximal renal tubular acidosis | Autosomal recessive | SLC4A4 | Sodium bicarbonate cotransporter | 604278 |
| Distal renal tubular acidosis | Autosomal dominant | SLC4A1 | Inorganic anion transmembrane transport protein | 179800 |
| Renal tubular acidosis with osteopetrosis | Autosomal recessive | CA2 | Enzyme involved in bicarbonate transport | 259730 |
| Hypophosphataemic rickets | X-chromosome linked; autosomal dominant; autosomal recessive; autosomal recessive; |
PHEX; FGF23; ENPP1; DMP1 |
Endopeptidase, degrades FGF23; osteocyte hormone, inhibits tubular phosphate reabsorption; pyrophosphatase, regulates mineralisation; osteoblast transcriptional activator or osteocyte matrix regulator |
307800; 193100; 613312; 241520 |
| Nephropathic cystinosis | Autosomal recessive | CTNS | Lysosomal membrane cystine transporter | 219800 |
| Primary renal Fanconi’s syndrome, types 1 and 2 |
Autosomal dominant; autosomal recessive |
15q15.3; SLC34A1 | Affected genes unknown; sodium–phosphate cotransporter | 134600; 613388 |
| Fanconi-Bickel syndrome (hepatorenal glycogenosis) |
Autosomal recessive | SLC2A2 | Facilitated glucose transporter | 227810 |
| Dent’s disease | ||||
| Type 1 | X-chromosome linked | CLCN5 | Chlorid-proton exchanger | 300009 |
| Type 2 | X-chromosome linked | OCRL | 5-phosphatase, regulates early endosomes | 300555 |
| Lowe oculocerebrorenal syndrome | X-chromosome linked | OCRL | 5-phosphatase, regulates early endosomes | 309000 |
| Hereditary renal hypouricaemia | Autosomal recessive | SLC22A12 | Urate transporter | 220150 |
| Familial juvenile hyperuricaemic nephropathy; medullary cystic kidney disease type 2 |
All autosomal dominant | UMOD; REN; MUC1 | Surface-bound and secreted glycoprotein (Tamm–Horsfall protein); endopeptidase (angiotensinogenase); surface glycoprotein |
603860 and 162000; 613092; 174000 |
| Bartter’s syndrome, types 1–4 | SLC12A1, KCNJ1, and BSND are autosomal recessive; CLCNKB is autosomal recessive or digenic; BSND is digenic |
SLC12A1; KCNJ1; CLCNKB; CLNCKA; BSND |
Sodium–potassium–chloride cotransporter; potassium channel; chloride channel; chloride channel; β-subunit of CLCNKA and CLCNKB chloride channels |
601678; 241200; 607364; 613090; 602522 |
| Gitelman’s syndrome | Both autosomal recessive | SLC12A3; CLCNKB | Thiazide-sensitive sodium–chloride cotransporter; chloride channel | 263800 |
| Familial hypocalciuric hypercalcaemia, type 1; neonatal severe hyperparathyroidism; utosomal dominant hypocalcaemia (including with Bartter’s syndrome) |
Autosomal dominant; autosomal recessive; autosomal dominant |
CASR | Calcium-sensing receptor (loss of function); calcium-sensing receptor (loss of function); calcium-sensing receptor (gain of function) |
145980; 239200; 601198 |
| Hypomagnesaemia | ||||
| Type 1 (intestinal) | Autosomal recessive | TRPM6 | Magnesium channel | 602014 |
| Type 2 (renal) | Autosomal dominant | FXYD2 | Gamma subunit of sodium–potassium–ATPase | 154020 |
| Type 3 (renal) | Autosomal recessive | CLDN16 | Paracellular protein, component of tight junctions | 248250 |
| Type 4 (renal) | Autosomal recessive | EGF | Epidermal growth factor | 611718 |
| Type 5 (renal, with ocular involvement) | Autosomal recessive | CLDN19 | Paracellular protein, component of tight junctions | 248190 |
| Type 6 (renal) | Autosomal dominant | CNNM2 | Membrane protein of unknown function | 613882 |
| Renal (associated with myokymia) | Autosomal dominant | KCNA1 | Potassium channel | 160120 |
| Liddle’s syndrome | Both autosomal dominant | SCNN1G; SCNN1B |
γ-subunit of amiloride-sensitive sodium channel (gain of function); β-subunit of amiloride-sensitive sodium channel (gain of function) |
177200 |
| Pseudohypoaldosteronism type 1 | Both autosomal recessive | SCNN1A; SCNN1G; SCNN1B |
α-subunit of amiloride-sensitive sodium channel; γ-subunit of amiloride- sensitive sodium channel; β-subunit of amiloride-sensitive sodium channel |
264350 |
| Pseudohypoaldosteronism type 2 (Gordon’s syndrome) |
All autosomal dominant | WNK1; WNK4; KLHL3; CUL3 |
Serine–threonine kinase modulating sodium and potassium-coupled chloride transporters; serine–threonine kinase modulating sodium and potassium-coupled chloride transporters; structural protein mediating ubiquitination of SLC12A3; component of ubiquitin E3 ligase complex |
614492; 614491; 614495; 614496 |
| SeSAME syndrome (EAST; epilepsy, ataxia, sensorineural deafness, salt-wasting renal tubulopathy) | Autosomal recessive | KCNJ10 | Potassium channel | 612780 |
| Distal renal tubular acidosis, isolated; distal renal tubular acidosis, with haemolytic anaemia; distal renal tubular acidosis, with progressive nerve deafness |
All autosomal recessive | ATP6V0A4; SLC4A1; ATP6V1B1 |
Subunit of the vacuolar proton ATPase; anion exchanger (erythroid band 3); subunit of the vacuolar proton ATPase |
602722; 611590; 267300 |
| Nephrogenic syndrome of inappropriate antidiuresis |
X-chromosome linked | AVPR2 | G-protein coupled receptor for arginine-vasopressin (gain of function) | 300539 |
| Nephrogenic diabetes insipidus type 1; | X-chromosome linked; | AVPR2; AQP2 | G-protein coupled receptor for arginine-vasopressin (loss of function); | 304800; |
| nephrogenic diabetes insipidus type 2 | autosomal dominant or autosomal recessive |
water channel | 125800 | |
| Nephrolithiasis | ||||
| Cystinuria, types 1–3 | Autosomal recessive; autosomal dominant |
SLC3A1; SLC7A9 | Activator of cystine transporter SLC7A9; cysteine transporter | 220100 |
| Dent’s disease type 1; Dent’s disease type 2, Lowe’s oculocerebrorenal syndrome |
X-chromosome linked | CLCN5; OCRL | Chloride-proton exchanger; 5-phosphatase, regulates early endosomes | 300009; 300555; 309000 |
| Primary hyperoxaluria | ||||
| Type 1 | Autosomal recessive | AGXT | Vitamin B6-dependent peroxisomal enzyme | 259900 |
| Type 2 | Autosomal recessive | GRHPR | Peroxisomal enzyme | 260000 |
| Type 3 | Autosomal recessive | HOGA1 | Mitochondrial enzyme (hydroxyproline metabolic pathway) | 613616 |
| Adenine-phosphoribosyl-transferase deficiency | Autosomal recessive | APRT | Cytoplasmic enzyme forming AMP from adenine | 614723 |
| Xanthinuria type 1 | Autosomal recessive | XDH | Key enzyme in purine degradation | 278300 |
MIM=Mendelian Inheritance in Man.
HUGO Gene Nomenclature Committee symbol.
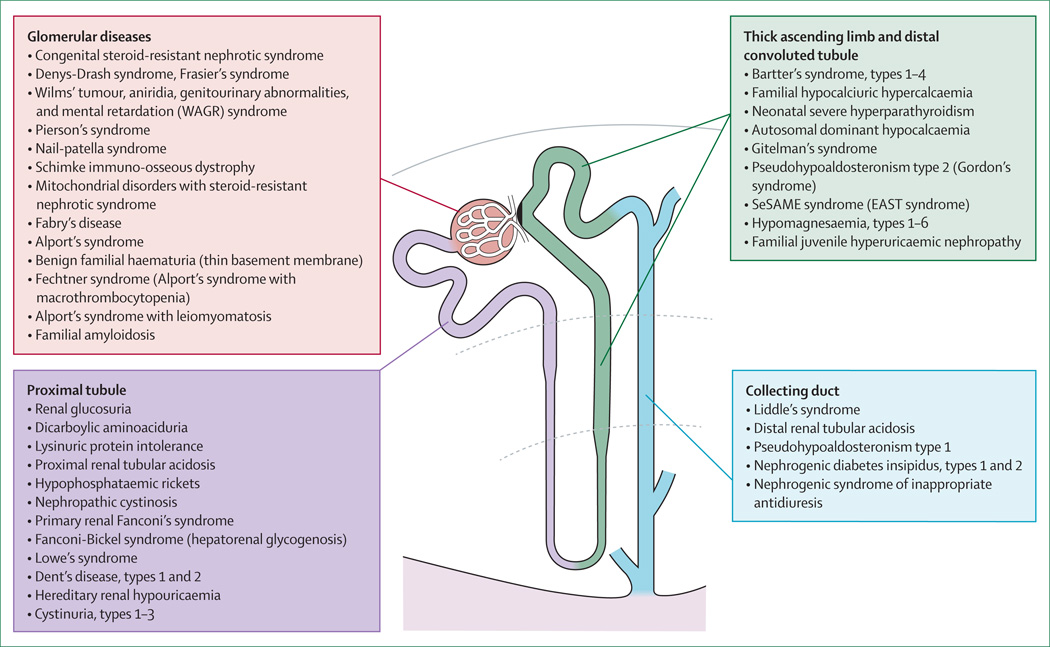
Figure 1. Inherited kidney disorders linked to nephron segments.
Shows the segmental distribution of rare inherited diseases of the kidney (does not include cystic and developmental disorders). Urinalysis might point to the segmental origin of some kidney disorders. For example, glomerular diseases are usually characterised by albuminuria and dysmorphic red blood cells in urine; disorders of the proximal tubule by inappropriate urinary loss of low-molecular-weight proteins (eg, Clara Cell protein, β2-microglobulin, and vitamin D-binding protein), aminoacids, glucose, phosphate, uric acid, and calcium; disorders of the thick ascending limb by hypercalciuria and urinary concentrating defects; disorders of the distal convoluted tubule by inappropriate urinary loss of magnesium; and disorders of the collecting duct by inappropriate urinary concentration or dilution and defective potassium handling.
Specific challenges
Unknown genetic cause
Despite progress in understanding of molecular causes of rare kidney diseases, the pathways for most inherited nephropathies still need identification. Known monogenic causes explain only 30–40% of cases of familial steroid-resistant nephrotic syndrome, 40–50% of cases of congenital tubulopathy, and 50–60% of cases of atypical haemolytic uraemic syndrome. Poor appreciation of genetic studies by health-care providers is of concern. Even for well defined disorders such as Alport’s, Bartter’s, and Gitelman’s syndromes, use of genetic testing remains rare, mainly because of high cost and long turnaround times for conventional genetic screening, the preconception that a genetic diagnosis will not affect clinical management, insufficient genetic literacy, and differences in access to genetic testing and insurance coverage.30,31
Absence of biomarkers
Even though routine analysis of urine samples can be helpful to indicate the origin of some disorders (figure 1), the assessment of kidney disease activity and progression is still mainly based on crude markers such as serum creatinine and proteinuria. The descriptive assessment of kidney biopsy specimens with use of light and electron microscopy, supplemented by a small set of immunological marker proteins, is still the diagnostic gold standard.32
Clinical heterogeneity
Many rare mendelian kidney diseases have a different prevalence in different populations and have substantial clinical heterogeneity in presence, age of onset, severity, and progression of symptoms. Different incidence rates in populations lend support to a role for genetics, and potentially the environment, in the pathogenesis of disease. Phenotypical differences can be the result of genetic (locus) heterogeneity—eg, in Bartter’s syndrome, mutations in SLC12A1 or KCNJ1 are associated with a severe neonatal onset of disease, whereas mutations in CLCNKB usually result in milder and later-onset disease symptoms and mutations in BSND cause Bartter’s syndrome plus sensorineural deafness.33 Allelic heterogeneity might also explain disease variability. For instance, in autosomal recessive polycystic kidney disease, the presence of two truncating mutations in the disease gene, PKHD1, is associated with a lethal disease; at least one missense mutation is necessary for survival after neonatal age. However, the absence of two truncating mutations cannot be regarded as synonymous with a favourable prognosis.34 Therefore, prediction of the clinical outcome for children with autosomal recessive polycystic kidney disease with one or two missense mutations remains difficult. In 2014, Tory and colleagues25 described mutation-dependent recessive inheritance of NPHS2-associated steroid-resistant nephrotic syndrome. In most rare inherited kidney diseases, the mutational diversity is large and genotype–phenotype correlations are loose or absent, showing the insufficient study populations sizes and poor access to genetic testing.
Effects of modifier genes, epigenetic changes, or other modifying factors also contribute to intrafamilial variability. Sex might modify the phenotype, as it does in Gitelman’s syndrome.35 Oligogenic modifier effects, by which a second gene can modify the action of a dominant gene, play a part in genetic ciliary diseases such as nephronophthisis. For instance, in patients with homozygous NPHP1 deletions, the presence of an additional heterozygous NPHP6 or NPHP8 mutation might cause additional eye or cerebellar involvement.36,37 However evidence is in early stages for genetic and epigenetic modifiers in rare inherited kidney diseases.
Insufficient ontology
An increasing number of rare kidney diseases that were previously considered to be single disorders have been shown to be aetiologically heterogeneous. Different abnormalities can affect the same biological pathways and give rise to similar clinical, biochemical, and histopathological features. The imperfect prognostic value of traditional diagnostic nomenclatures is largely explained by its inability to differentiate underlying disease mechanisms. For instance, membranoproliferative glomerulonephritis can be caused by glomerular deposition of circulating immunoglobulins or immune complexes, by mutations in complement proteins regulating C3 convertase, and by acquired autoantibodies directed against these proteins or C3 itself.38 Another example of heterogeneity is the generalised dysfunction of the proximal tubule (renal Fanconi’s syndrome).39 Emerging disease ontologies based on molecular pathophysiology will need prognostic validation with long-term outcomes.
Carrier state
Information on an individual’s carrier status for a genetic renal disorder is not only important for genetic counselling, but might also have clinical implications for the carriers themselves. Heterozygous carriers of X-chromosome-linked disorders are usually asymptomatic or mildly affected, but in some heterozygotes, a severe disease outcome is noted. Disease severity in women with X-linked renal disorders such as Alport’s syndrome and Fabry’s disease is not related to the genotype, and is most probably a result of skewed X-chromosome inactivation. Therefore, women carrying an X-chromosome-linked Alport’s syndrome mutation (COL4A5 mutation), should be considered at risk to develop disease and be observed similarly to men to assess for early signs or progression to renal insufficiency. Likewise, women carrying heterozygous GLA mutations can be as severely affected by Fabry’s disease as can hemizygous men, with progressive, multiorgan involvement, particularly nephropathy. Further research is needed to determine methods to predict the individual outcome for female carriers of these rare X-chromosome-linked renal disorders.
Carrier status might also have implications for living-related kidney transplantation. Unaffected individuals carrying a heterozygous recessive gene mutation are theoretically expected not to develop disease. Therefore, living related transplantation is usually considered suitable from patients with rare autosomal recessive renal diseases such as podocytopathies, autosomal recessive polycystic kidney disease, cystinosis, and nephronophthisis. However, whether unilateral nephrectomy in heterozygous individuals affects long-term renal function has not been determined, and studies from large registries are needed.
Insufficient model organisms
Knockout and transgenic mouse models are highly informative about the effects of genetic variation on renal phenotypes.40 Limitations of these models include long generation time, strain effects, adaptation, and species differences in development, growth, physiology, metabolism, and adaptation to chronic kidney disease.41,42 These obstacles mean that mouse models are of little use for rapid testing of candidate genes arising from next-generation sequencing and for drug development.
Opportunities
Omics technologies
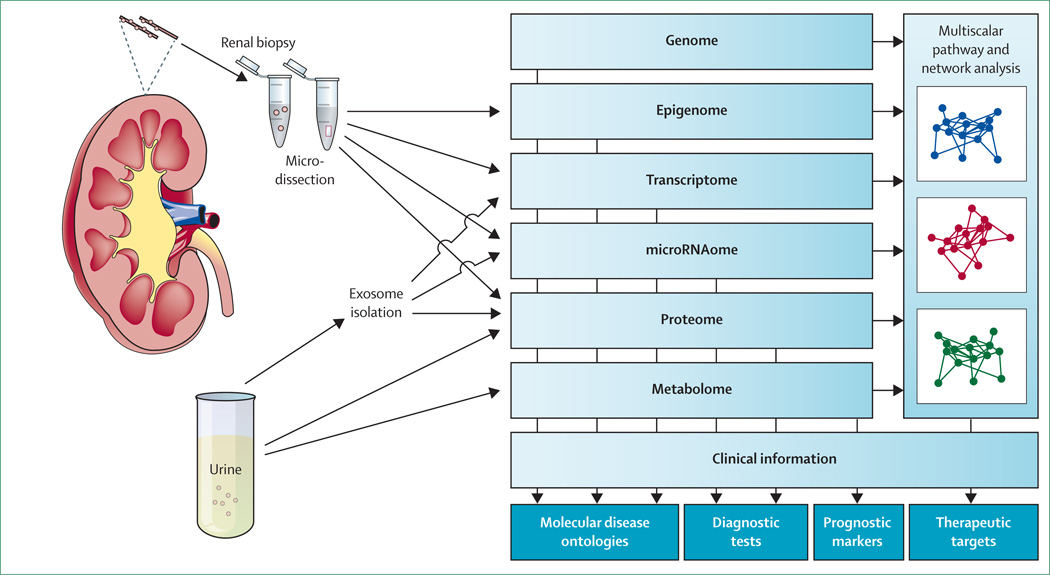
Omics technologies provide great opportunity in research for rare renal diseases because they can probe the diseased organ directly (figure 2). Kidney biopsy samples allow investigators to study intrarenal processes ex vivo with use of transcriptomic and proteomic approaches for compartment-specific profiling of mRNA transcripts and non-coding regulatory RNA species. The European Renal cDNA Bank project has provided a reference database for gene expression profiles of microdissected kidney specimens from patients with various renal disorders and from healthy individuals.43 Urine is a non-invasive resource to study biochemical and molecular readouts directly from the kidney. Amniotic fluid is available prenatally for studies of renal development or transport defects.44 Exosome isolation from urine and amniotic fluid allows study of membrane and cytoplasmatic proteins and RNAs that derive from epithelial cells facing the urinary space. Although exosome isolation remains technically challenging, studies of podocyte and cystic kidney disorders indicate great potential of this analysis in the study of hereditary kidney diseases.45–47 The possibility that application of omics approaches to such samples could identify molecular signatures and prognostic biomarkers was suggested by findings from studies in common kidney disorders such as diabetic nephropathy,48 allograft rejection,49 and vesicoureteral reflux.50 Changes in urinary miRNA profile have been detected in disorders such as lupus nephritis51 and renal fibrosis.52 The study of the urine metabolome by nuclear magnetic resonance spectroscopy and mass spectrometry is another emerging technology that can generate molecular fingerprints of diagnostic or prognostic value,53 as already shown in patients with Fanconi’s syndrome.54
Figure 2. Application of omics technologies in rare kidney diseases.
Next-generation sequencing techniques and omics technologies, which can directly probe the kidney, will improve diagnostic efficiency for genetic renal diseases. Genomic studies and molecular profiling of kidney tissues, plain and exosome-enriched urine, and multiscalar bioinformatic analysis of crucial disease pathways, will allow the development of mechanistic renal disease ontologies, diagnostic tests, biomarkers, and novel therapeutic targets.
Next-generation sequencing
Next-generation sequencing techniques will improve diagnostic efficiency for genetic renal diseases through simultaneous investigation of all relevant genes for a given phenotype at much reduced costs and turn-around times.24,55,56 Successful application of next-generation sequencing in diagnostic mutation screening with use of multigene panels has been shown for Alport’s syndrome,57 steroid-resistant nephrotic syndrome,58 and nephronophthisis.59 Beyond disease-specific next-generation sequencing panels, exome sequencing (and potentially even whole-genome sequencing) will soon become part of routine molecular diagnostics, further improving diagnostic yield. Sequencing-based technologies are also increasingly applied to individual cells, with the aim to integrate genomics, transcriptomics, epigenomics, and proteomics for multilevel analysis of cellular mechanisms. These analyses will need robust single-cell isolation, a potentially challenging task for a heterogeneous tissue such as kidney.60
The abundance of genetic and molecular information generated by next-generation sequencing poses a new challenge because bioinformatic capacities and analysis methods need development. The characterisation of candidate disease genes and individual mutations needs efficient model systems. Innovative strategies are also needed to integrate multilevel omics information with clinical phenotypes.61 The European Consortium for High-Throughput Research in Rare Kidney Diseases (EURenOmics) is now working on a cohesive bioinformatic platform for rare nephropathies. A renal phenome database is being created, using the Human Phenotype Ontology website.62 The phenotype information will be linked to genomic, transcriptomic, proteomic, and metabolomic studies, omics datasets, and the public domain knowledge-base in a systems biology approach to identify molecular pathways associated with phenotypic features.
Model organisms
The mouse is still the major organism used to model rare kidney disorders. Cell-specific and time-specific gene-targeting methods and RNA-based technologies can manipulate gene function, which can be paralleled by targeted embryonic stem-cell clones and large-scale mutagenesis programmes.40 The precision and number of phenotypic traits that can be tested in mice has largely increased.42,63–65 Advances in rat genetics and genome editing, combined with robust phenotype analyses in more than 500 rat strains, pave the way for use of the rat as an alternative model organism for human diseases.66,67
By contrast with rodents, simple model organisms provide opportunity for higher-throughput gene manipulation and phenotype quantification. The zebrafish (Danio rerio) is now routinely used for study of kidney diseases and renal regeneration, based on conserved genomic organisation and nephron structure.68 Zebrafish larvae are used to investigate kidney developmental disorders and ciliopathies,69 glomerular disorders,70 and tubulopathies.71 The fruit fly (Drosophila melanogaster) nephrocyte combines filtration with protein reabsorption and can therefore be used as a model for podocytes and proximal tubule cells.72,73 Although the nematode Caenorhabditis elegans does not possess an excretory system comparable with the mammalian kidney, conserved genes involved in formation of the primary cilium, kidney filtration barrier, or vasopressin response do exist in this organism.74,75 Because few laboratories engage in such model studies, it is a challenge to integrate functional annotations into clinically relevant information.
Research programmes, cohorts, biorepositories
Fragmentation of patient-related information is a major obstacle for research into rare disease. Networks, registries, databases, and biorepositories have been created to overcome this issue. The European Platform for Rare Disease Registries provides instruments to develop exchange between individual registries, whereas the Patient Registries Initiative will promote interoperable patient registries. The RD-CONNECT platform will integrate rare disease projects devoted to next-generation sequencing and high-throughput approaches, and is linked with the International Rare Diseases Research Consortium (IRDiRC) which aims to deliver 200 new therapies by 2020. EURenOmics is one of the first clinical research projects of IRDiRC and RD-CONNECT. It is a consortium for omics research that integrates registries and biobanks with detailed phenotype information and biomaterials from more than 13 000 patients with rare kidney diseases. These efforts are paralleled by initiatives launched by professional and scientific societies. For instance, European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) has implemented a working group on inherited kidney diseases to promote research, care, and dissemination of knowledge.76 Nationally, centres of excellence are being established by health authorities to improve health-care access and to help to transition patients from paediatric to adult care.77
Non-rare inherited kidney disorders
Rare kidney diseases exist alongside autosomal dominant polycystic kidney disease, one of the most common inherited disorders, with a prevalence of one in 1000 people (about 750 000 patients in Europe). Because of its autosomal dominant transmission and the slow progression of disease, patients with autosomal dominant polycystic kidney disease form an important pressure group that is able to drive attention to rarer inherited kidney diseases and to influence other organisations, as well as trends in research funding and drug development.
Opportunities for common diseases
Study of rare kidney diseases provides insights into more common disorders. The UMOD gene, in which dominantly inherited mutations can cause familial juvenile hyperuricaemic nephropathy, is an example.23 Genome-wide association studies showed that common variants in the UMOD promoter were strongly associated with the risk of chronic kidney disease and hypertension in the general population.78 Further studies showed the biological activity of these variants and how they cause hypertension.79 The elucidation of this mechanism was helped by previous studies of genes that cause Bartter’s syndrome.20 Likewise, variants in the genes encoding the megalin (LRP2) and cubilin (CUBN) receptors that mediate tubular endocytosis of ultrafiltered proteins and are defective in rare disorders were shown by genome-wide association studies to affect renal function and risk of chronic kidney disease.80,81 Conversely, genome studies might incidentally point to candidate genes for rare diseases. For example, the identification of CNNM2 as the causative gene for a rare genetic disorder of renal magnesium wasting was based, among other findings, on the association between common variants in CNNM2 and serum Mg²+ concentrations.82
Perspectives
Diagnostics
The use of next-generation sequencing is expected to increase diagnostic efficiency for rare kidney diseases. Accurate genetic counselling and carrier testing will become available for an increased number of families, with potential for early prenatal or preimplantation diagnostic testing in severe cases. A definite genetic diagnosis could have important prognostic value in some diseases. For instance, the efficacy of plasmapheresis and outcome of renal transplantation in atypical haemolytic uraemic syndrome is correlated with the type of mutation in complement genes. Patients with mutations in complement genes that encode circulating proteins (CFH and CFI) have worse outcomes than have patients with mutations in MCP (CD46), encoding a cell-associated protein.83 Likewise, genetic testing in patients with steroid-resistant nephrotic syndrome helps to predict the response to immunosuppressive therapies and the risk of post-transplant disease recurrence.84 In primary hyperoxaluria type 1, the Gly170Arg and Phe152Ile aminoacid changes caused by mutations in AGXT have been associated with responsiveness to pyridoxine supplementation.85 As we discuss, policies to promote clinically relevant genetic testing and the adequate delivery and integration of genetic information should be implemented.
Treatment
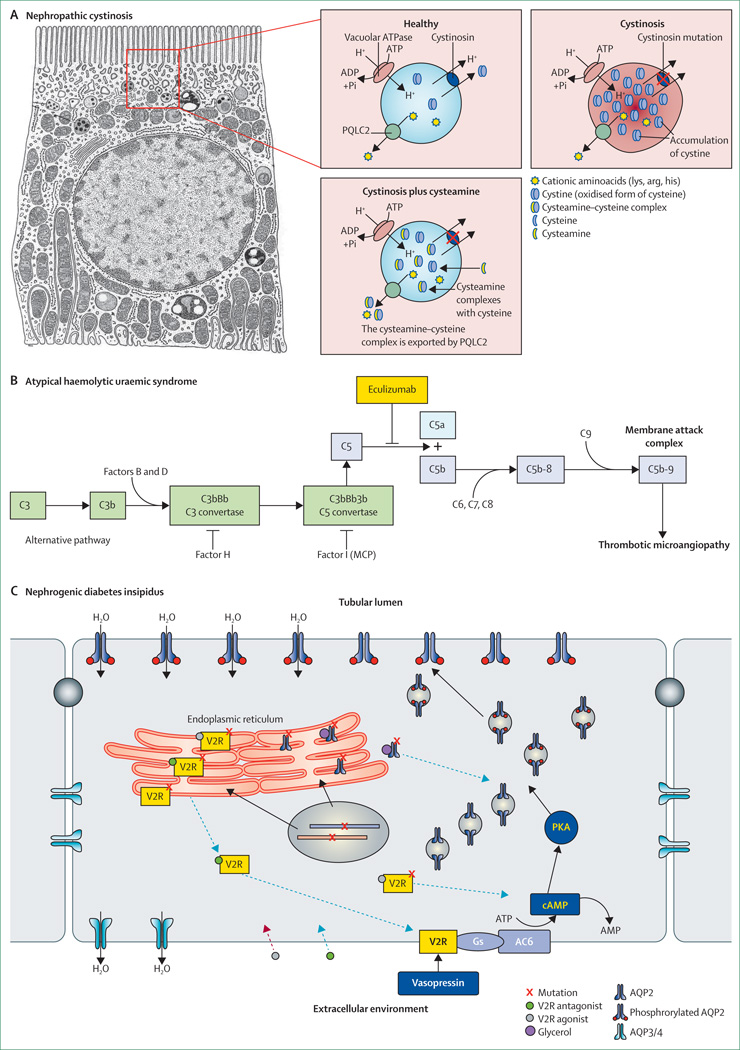
Effective therapeutic approaches exist for some rare kidney diseases (panel). Genetic and mechanistic insights could improve existing therapies or help to develop new ones (figure 3). Cystinosis is a lysosomal storage disease caused by mutations in the CTNS gene resulting in intralysosomal accumulation of cystine crystals, which damage several organs, including kidney.21 Oral administration of cysteamine, which reverses cystine accumulation via a newly described PQLC2 heptahelical protein (figure 3),86 substantially delays renal disease progression and other complications.89 Findings from a mouse study of cystinosis suggest that stem-cell gene therapy might also improve the multisystem complications of cystinosis.27
Figure 3. Examples of molecular targets in rare inherited kidney diseases.
(A) Cystinosis is caused by defective cystinosin, a ubiquitous lysosomal proton-driven cystine transporter working in parallel with the vacuolar H+–ATPase. In patients with cystinosis, the loss of function of cystinosin causes cystine to accumulate in lysosomes (shown here in a proximal tubule cell). Cysteamine reduces the accumulation of cysteine by entering lysosomes and forming a cysteamine–cysteine complex, which resembles lysine and can be exported by PQLC2. Modified from Jézégou and colleagues.86 (B) Patients with haemolytic uraemic syndrome have uncontrolled complement activation due to deficiency of natural complement regulatory factors. Eculizumab is a humanised monoclonal antibody that inhibits the cleavage of the complement protein C5, blocking complement activation and complement-mediated thrombotic microangiopathy in patients with haemolytic uraemic syndrome. Patients with haemolytic uraemic syndrome due to recessive mutations in DGKE, which is not associated with activation of the complement system, do not respond to eculizumab or plasma exchange. Modified from Noris and colleagues.87 (C) In the principal cells that line the collecting ducts, stimulation of the vasopressin-2 receptor (V2R) by vasopressin leads to an increase in cAMP, causing a protein kinase A (PKA)-mediated phosphorylation of AQP2 and their insertion into the apical plasma membrane. The resulting increase in transcellular water permeability mediates concentration of urine. Most mutations in AVPR2 (X-chromosome-linked nephrogenic diabetes insipidus) and AQP2 (autosomal recessive nephrogenic diabetes insipidus) result in misfolded V2R and AQP2 mutations in the endoplasmic reticulum (class 2 mutations). Pharmacological chaperones (eg, glycerol) can rescue such class 2 mutant proteins from the endoplasmic reticulum. Cell-permeable V2R antagonists stabilise the structure of mutant V2R and allow them to exit the endoplasmic reticulum and translocate to the basolateral plasma membrane. At the membrane, vasopressin will displace the antagonist and allow restoration of the cAMP cascade. This action of V2R antagonists can depend on V2R mutation. V2R agonists function similarly and might also stimulate misfolded V2R in the endoplasmic reticulum without inducing maturation. Mutant AQP2 can be rescued from the endoplasmic reticulum by glycerol. Modified from Robben and colleagues.88
The elucidation of molecular mechanisms for disease might create opportunities for drug repositioning (ie, application of known drugs to new indications). For instance, recurrence of proteinuria after kidney transplantation in some patients with focal-segmental glomerulosclerosis has been linked to an upregulation of B7-1 (CD80), a costimulatory molecule normally expressed in T-lymphocytes, which changes foot process anchoring in podocytes.90 The B7-1 inhibitor abatacept, approved for patients with rheumatoid arthritis, induces remission of proteinuria in both patients with post-transplant focal-segmental glomerulosclerosis and those with primary focal-segmental glomerulosclerosis.91 Rare glomerulopathies involving podocyte integrins might also benefit from this new indication for abatacept.
Monoclonal antibodies have shown remarkable efficacy in several rare renal diseases. Eculizumab is a potent inhibitor of the terminal complement cascade (figure 3) and was first approved for paroxysmal nocturnal haemoglobinuria. Mendelian inherited forms of atypical haemolytic uraemic syndrome have been associated with changes in proteins that regulate the alternative complement pathway. The resulting excessive complement activation leads to renal and systemic thrombotic microangiopathy, which leads to high mortality, rapid progression to end-stage renal disease, and a high risk of recurrence after kidney transplantation.87 In studies, eculizumab caused complete disease remission in most patients and investigators reported notable recovery of renal function.92 Its highly selective mechanism of action and positive benefit–risk ratio also make eculizumab attractive for other complement-mediated diseases. Unfortunately, the prohibitive cost of eculizumab is demonstrative of the challenges that face drug development in rare diseases. The discovery that recessive loss-of-function mutations in DGKE causes atypical haemolytic uraemic syndrome without activation of the complement system suggests that eculizumab will not be effective for this subset of patients.93
Most AVPR2 and AQP2 mutations in nephrogenic diabetes insipidus result in retention of a normal protein within the endoplasmic reticulum (figure 3). Promising drugs include cell-permeable vasopressin-2 (V2) receptor antagonists and agonists that prevent the intracellular retention of mutated receptors in vitro.26,88 Of note, V2 receptor antagonists might produce different effects depending on the various mutations.94 In individuals with missense AVPR2 mutations, a non-peptide vasopressin-1a receptor antagonist had beneficial effects on urine volume and osmolality within hours of administration.95 Although the long-term efficacy of this drug could not be tested (its clinical development was discontinued because of cytochrome P450 interaction) other pharmacological chaperones await further in-vivo testing. Chaperones might also become attractive for other diseases in which point mutations lead to defective folding and cellular trafficking of otherwise intact membrane proteins (eg, uromodulin-associated kidney diseases).78 Developments in this specialty rely on high-throughput compound screening systems to reproduce mutations in individual renal-cell types.
Health policies
A major objective for the inherited kidney disorders community is to design methods so that approaches developed at highly specialised and resourced tertiary care centres (which have access to patient cohorts and diagnostic and genetic information) can be adopted in less-resource-intensive settings (which cover most of the population). Investigators should devise practical ways to promote the adoption and implementation of clinically relevant genetic testing. Changes to the medical model in many countries—increased patient empowerment, more active roles for patient organisations, possibilities of crowd-sourcing, and creation of online communities— will probably favour genetic interpretation on a personal level. Best practice guidelines for diagnosis and treatment of rare inherited kidney disorders are being established.96 Equally important will be measures to ensure delivery and to support the effect of the genetic information on physicians, patients, and society.30,31 These efforts are supported by numerous network and initiatives, and by organisations such as National Organisations of Rare Disorders (NORD) in the USA and Orphanet in Europe.
Research for rare diseases is expected to have important repercussions on public health policies. Measures to translate research insights into clinical benefit include creation of centres of excellence with adequate diagnostic and therapeutic capabilities, genetic counselling, early detection by global or targeted public screening programmes, and facilitated approval of novel orphan drugs.97 Insights from research into rare diseases could also be used to modify established public health measures through identification of patients at particular risk. For instance, results of a study of children with idiopathic hypercalciuria identified mutations in CYP24A1 gene coding for the vitamin D metabolising enzyme as the underlying pathology.98 Subsequently, investigators also detected mutations in this gene in patients who developed severe hypercalcaemia after prophylactic bolus vitamin D administration, thereby identifying a subset of individuals intolerant to this public health measure.
Finally, we stress that patient organisations have a crucial role in closing of the gap between mechanistic understanding and the development of drugs for rare diseases.99,100 Patients work with physicians and researchers to share personal insights, provide biological samples, contact family members, and participate in clinical trials. Patient organisations can foster these activities and provide support to the community. Examples in rare kidney diseases include associations for cystic kidney disorders, primary hyperoxaluria, cystinosis, Lowe’s syndrome, metabolic disorders, and so on. Coalitions of patient organisations have been important stakeholders in health policies, helping to pass the US Orphan Drug Act in 1983 and to establish the Framework Programme 7 research agenda in Europe.
Search strategy and selection criteria.
We searched PubMed and Medline for articles published in English with search terms that included, but were not restricted to, “inherited kidney disease”, “orphan disease”, “rare disease”, “nephrogenetics”, “congenital abnormalities”, in combination with “kidney” or “urinary tract”, “ciliopathies”, “tubulopathies”, “nephrolithiasis”, “glomerular diseases”, “cystic diseases”, “glomerulus”, “proximal tubule”, “thick ascending limb”, “distal tubule”, “collecting duct”, “-omics”, and “model organisms”. We identified further reports from our own experience and from references cited in relevant articles and the Online Mendelian Inheritance in Man (OMIM) database. We did not use date restrictions for searches. We did our last search in March, 2014. We modified our reference list on the basis of comments from peer reviewers.
Panel: Milestones in research of inherited kidney diseases.
Milestones in nephrogenetics
1985 Mapping the first gene location for an inherited kidney disorder (autosomal dominant polycystic kidney disease, on chromosome 16)13
1990 First detection of a point mutation at a specific locus single-gene disorder, COL4A514
1992 Molecular basis of nephrogenic diabetes insipidus described15
1993 Identification of the tuberous sclerosis gene (TSC2)
1994 Cloning of the PKD1 gene, responsible for about 85% of autosomal dominant polycystic kidney disease cases; challenging due to the size (46 exons) and complex organisation (presence of six highly homologous sequences of exons 1–33) of the gene on chromosome 16p13·316
1994 Liddle’s syndrome reported to be due to activating mutation of the sodium channel ENaC17
1996 Molecular basis for inherited kidney stone diseases identified18
1996 Molecular basis of Bartter’s and Gitelman’s syndromes described19,20
1996 Cloning of PKD2, the second gene involved in autosomal dominant polycystic kidney disease
1997 First nephronophthisis gene reported on
1998 Mutations in factor H reported to cause atypical haemolytic uraemic syndrome
1998 Molecular basis of cystinosis described21
1999 Mutations in a paracellular protein (claudin-16) causes familial hypomagnesaemia with hypercalciuria
2000 Podocin (NPHS2) described as the major gene for steroid-resistant nephrotic syndrome22
2001 Mutations in different genes shown to cause Bardet–Biedl syndrome (digenic inheritance)
2001 Mutations in WNK kinases shown to change regulation of sodium, potassium, and blood pressure
2002 Mutations in UMOD (Tamm-Horsfall protein) shown to cause familial juvenile hyperuricaemic nephropathy, an autosomal dominantly inherited form of interstitial nephritis23
2005 Mutations in a cation channel (TRPC6) described to cause glomerular disease
2010 First success of exome sequencing in rare renal diseases (SDCCA8 in Senior-Løken syndrome; retinal-renal ciliopathy)24
2011 Broad spectrum and clinical heterogeneity of HNF1B gene mutations shown
2013 Description of MUC1 as the cause of medullary cystic kidney disease type 1; the gene was missed by massive parallel sequencing, showing the need for refinement of analysis methods and assessment of clinical use of whole-exome sequencing for autosomal dominant heterogeneous disorders
2014 First description of mutation-dependent recessive inheritance in the case of NPHS2-associated steroid-resistant nephrotic syndrome25
Milestones in treatment
1981 Oral cysteamine given for cystinosis
2000 Enzyme replacement therapy for Fabry’s disease
2000 First in-vitro evidence that pharmacological chaperones can rescue cell-surface expression and function of misfolded vasopressin 2 receptors in nephrogenic diabetes insipidus26
2005 First open-label, randomised, crossover, placebo-controlled trial for the effect of somatostatin analogue octreotide longacting release in autosomal dominant polycystic kidney disease
2008 Development of mTOR inhibitors for tuberous sclerosis
2009 Eculizumab for atypical haemolytic uraemic syndrome
2009 Proof-of-principle for use of bone marrow transplantation for treatment of mouse model with cystinosis27
2009 Randomised, double-blind, placebo-controlled trial of the effect of somatostatin analogue lanreotide in polycystic liver disease associated with autosomal dominant polycystic kidney disease
2012 Global, randomised, double-blinded, placebo-controlled trial of the vasopressin 2 receptor antagonist tolvaptan in autosomal dominant polycystic kidney disease
2013 First randomised, single-blind, placebo-controlled, multicentre trial of octreotide longacting release for autosomal dominant polycystic kidney disease
Complete reference list included in the appendix.
Acknowledgments
We thank Renaud Beauwens, Daniel Bichet, Pierre Cochat, Rosanna Coppo, Karin Dahan, Francesco Emma, Ali Gharavi, Yves Pirson, Bernard Rossier, Arrigo Schieppati, and Roser Torra for fruitful discussions and their help in selection of milestones in inherited kidney diseases.
The authors’ research on rare inherited kidney diseases is supported by the European Union 7th Framework Programme (FP7; 2007–13) under grant agreement number 305608 (EURenOmics, to all authors); the Gebert-Rüf Stiftung (GRS-038/12; to OD), the Cystinosis Research Foundation (to OD), the Swiss National Science Foundation (Project Grant 310030_146490; to OD), the Klinischer Forschungsschwerpunkt (KFSP) radiz - Rare Disease Initiative Zurich (to OD), the National Centre of Competence in Research (NCCR) Kidney.CH (to OD); the Dutch Kidney Foundation (CP11.18 Project Kouncil; to NK); the KfH Foundation for Preventive Medicine (4C Study; to FS), and the National Institutes of Health (5R01DK082394–03, PediGFR; to FS).
Footnotes
See Online for appendix
For more on EURenOmics see http://www.eurenomics.eu
For more on Human Phenotype Ontology website http://www.human-phenotype-ontology.org/
For the website of the European Platform for Rare Disease Registries see http://www.epirare.eu
For the website of the Patient Registries Initiative see http://www.patientregistries.eu For more on RD-CONNECT see http://www.rd-connect.eu
For the International Rare Diseases Research Consortium see http://www.irdirc.org/
For the website of the Working Group on Inherited Kidney Diseases see http://www.era-edta.org/wgikd/ERA-EDTA_working_group_on_ Inherited_kidney_disorders.htm
For website of NORD see http://www.rarediseases.org
For website of Orphanet see http://www.orpha.net/
Contributors
All authors discussed the overall concept and plan for the review, contributed to specific sections, and reviewed and approved the final version. OD and FS integrated and edited the contributions from all authors.
Members of the Board of the Working Group for Inherited Kidney Diseases of the European Renal Association and the European Dialysis and Transplant Association
Corinne Antignac, René Bindels, Dominique Chauveau, Olivier Devuyst (President), Francesco Emma, Ron T Gansevoort, Patrick H Maxwell, Albert Ong, Giuseppe Remuzzi, Pierre Ronco, and Franz Schaefer (Secretary).
Declaration of interests
We declare no competing interests.
References
- 1.Schieppati A, Henter JI, Daina E, Aperia A. Why rare diseases are an important medical and social issue. Lancet. 2008;371:2039–2041. doi: 10.1016/S0140-6736(08)60872-7. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi S, Umeda T. 35 years of Japanese policy on rare diseases. Lancet. 2008;372:889–890. doi: 10.1016/S0140-6736(08)61393-8. [DOI] [PubMed] [Google Scholar]
- 3.Liu BC, He L, He G, He Y. A cross-national comparative study of orphan drug policies in the United States, the European Union, and Japan: towards a made-in-China orphan drug policy. J Public Health Policy. 2010;31:407–420. doi: 10.1057/jphp.2010.30. [DOI] [PubMed] [Google Scholar]
- 4.Soliman NA. Orphan Kidney Diseases. Nephron Clin Pract. 2012;120:c194–c199. doi: 10.1159/000339785. [DOI] [PubMed] [Google Scholar]
- 5. [accessed March 31, 2014];Orphanet Report Series: Prevalence of rare diseases. 2013 Nov; http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_decreasing_prevalence_or_cases.pdf.
- 6.Institute of Medicine (US) Committee on Accelerating Rare Diseases Research and Orphan Product Development. Profile of rare diseases. In: Field MJ, Boat TF, editors. Rare diseases and orphan products: accelerating research and development. Washington, DC: National Academies Press; 2010. [accessed March 31, 2014]. http://www.ncbi.nlm.nih.gov/books/NBK56184/ [PubMed] [Google Scholar]
- 7.Devuyst O, Meij I, Jeunemaitre X, et al. for the EUNEFRON consortium. EUNEFRON, the European Network for the Study of Orphan Nephropathies. Nephrol Dial Transplant. 2009;24:2011–2015. doi: 10.1093/ndt/gfp095. [DOI] [PubMed] [Google Scholar]
- 8.Eckardt KU, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 9.Jouret F, Bernard A, Hermans C, et al. Cystic fibrosis is associated with a defect in apical receptor-mediated endocytosis in mouse and human kidney. J Am Soc Nephrol. 2007;18:707–718. doi: 10.1681/ASN.2006030269. [DOI] [PubMed] [Google Scholar]
- 10.Boyer O, Nevo F, Plaisier E, et al. INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N Engl J Med. 2011;365:2377–2388. doi: 10.1056/NEJMoa1109122. [DOI] [PubMed] [Google Scholar]
- 11.Kantarci S, Al-Gazali L, Hill RS, et al. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat Genet. 2007;39:957–959. doi: 10.1038/ng2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emma F, Bertini E, Salviati L, Montini G. Renal involvement in mitochondrial cytopathies. Pediatr Nephrol. 2012;27:539–550. doi: 10.1007/s00467-011-1926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeders ST, Breuning MH, Davies KE, et al. A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. Nature. 1985;317:542–544. doi: 10.1038/317542a0. [DOI] [PubMed] [Google Scholar]
- 14.Barker DF, Hostikka SL, Zhou J, et al. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248:1224–1227. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal W, Seibold A, Antaramian A, et al. Molecular identification of the gene responsible for congenital nephrogenic diabetes insipidus. Nature. 1992;359:233–235. doi: 10.1038/359233a0. [DOI] [PubMed] [Google Scholar]
- 16.The European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77:881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 17.Shimkets RA, Warnock DG, Bositis CM, et al. Liddle’s syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell. 1994;79:407–414. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd SE, Pearce SH, Fisher SE, et al. A common molecular basis for three inherited kidney stone diseases. Nature. 1996;379:445–449. doi: 10.1038/379445a0. [DOI] [PubMed] [Google Scholar]
- 19.Simon DB, Nelson-Williams C, Bia MJ, et al. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 20.Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP. Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet. 1996;13:183–188. doi: 10.1038/ng0696-183. [DOI] [PubMed] [Google Scholar]
- 21.Town M, Jean G, Cherqui S, et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18:319–324. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- 22.Boute N, Gribouval O, Roselli S, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 23.Hart TC, Gorry MC, Hart PS, et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002;39:882–892. doi: 10.1136/jmg.39.12.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otto EA, Hurd TW, Airik R, et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet. 2010;42:840–850. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tory K, Menyhárd DK, Woerner S, et al. Mutation-dependent recessive inheritance of NPHS2-associated steroid-resistant nephrotic syndrome. Nat Genet. 2014;46:299–304. doi: 10.1038/ng.2898. [DOI] [PubMed] [Google Scholar]
- 26.Morello JP, Salahpour A, Laperrière A, et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest. 2000;105:887–895. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Syres K, Harrison F, Tadlock M, et al. Successful treatment of the murine model of cystinosis using bone marrow cell transplantation. Blood. 2009;114:2542–2552. doi: 10.1182/blood-2009-03-213934. [DOI] [PubMed] [Google Scholar]
- 28.Hildebrandt F. Genetic kidney diseases. Lancet. 2010;375:1287–1295. doi: 10.1016/S0140-6736(10)60236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji W, Foo JN, O’Roak BJ, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogowski WH, Grosse SD, Khoury MJ. Challenges of translating genetic tests into clinical and public health practice. Nat Rev Genet. 2009;10:489–495. doi: 10.1038/nrg2606. [DOI] [PubMed] [Google Scholar]
- 31.Scott J, Trotter T. Primary care and genetics and genomics. Pediatrics. 2013;132(suppl 3):S231–S237. doi: 10.1542/peds.2013-1032H. [DOI] [PubMed] [Google Scholar]
- 32.Gadegbeku CA, Gipson DS, Holzman LB, et al. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 2013;83:749–756. doi: 10.1038/ki.2012.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seyberth HW, Schlingmann KP. Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects. Pediatr Nephrol. 2011;26:1789–1802. doi: 10.1007/s00467-011-1871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denamur E, Delezoide AL, Alberti C, et al. for the Société Française de Foetopathologie. Genotype-phenotype correlations in fetuses and neonates with autosomal recessive polycystic kidney disease. Kidney Int. 2010;77:350–358. doi: 10.1038/ki.2009.440. [DOI] [PubMed] [Google Scholar]
- 35.Riveira-Munoz E, Chang Q, Godefroid N, et al. for the Belgian Network for Study of Gitelman Syndrome. Transcriptional and functional analyses of SLC12A3 mutations: new clues for the pathogenesis of Gitelman syndrome. J Am Soc Nephrol. 2007;18:1271–1283. doi: 10.1681/ASN.2006101095. [DOI] [PubMed] [Google Scholar]
- 36.Tory K, Lacoste T, Burglen L, et al. High PHP1 and NPHP6 mutation rate in patients with Joubert syndrome and nephronophthisis: potential epistatic effect of NPHP6 and AHI1 mutations in patients with NPHP1 mutations. J Am Soc Nephrol. 2007;18:1566–1575. doi: 10.1681/ASN.2006101164. [DOI] [PubMed] [Google Scholar]
- 37.Hoefele J, Wolf MT, O’Toole JF, et al. Evidence of oligogenic inheritance in nephronophthisis. J Am Soc Nephrol. 2007;18:2789–2795. doi: 10.1681/ASN.2007020243. [DOI] [PubMed] [Google Scholar]
- 38.Bomback AS, Appel GB. Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat Rev Nephrol. 2012;8:634–642. doi: 10.1038/nrneph.2012.213. [DOI] [PubMed] [Google Scholar]
- 39.Devuyst O, Thakker RV. Dent’s disease. Orphanet J Rare Dis. 2010;5:28. doi: 10.1186/1750-1172-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ly JP, Onay T, Quaggin SE. Mouse models to study kidney development, function and disease. Curr Opin Nephrol Hypertens. 2011;20:382–390. doi: 10.1097/MNH.0b013e328347cd4a. [DOI] [PubMed] [Google Scholar]
- 41.Eddy AA, López-Guisa JM, Okamura DM, Yamaguchi I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr Nephrol. 2012;27:1233–1247. doi: 10.1007/s00467-011-1938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meneton P, Ichikawa I, Inagami T, Schnermann J. Renal physiology of the mouse. Am J Physiol Renal Physiol. 2000;278:F339–F351. doi: 10.1152/ajprenal.2000.278.3.F339. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda Y, Cohen CD, Henger A, Kretzler M for the European Renal cDNA Bank (ERCB) Consortium. Gene expression profiling analysis in nephrology: towards molecular definition of renal disease. Clin Exp Nephrol. 2006;10:91–98. doi: 10.1007/s10157-006-0421-z. [DOI] [PubMed] [Google Scholar]
- 44.Kamath-Rayne BD, Smith HC, Muglia LJ, Morrow AL. Amniotic fluid: the use of high-dimensional biology to understand fetal well-being. Reprod Sci. 2014;21:6–19. doi: 10.1177/1933719113485292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hogan MC, Manganelli L, Woollard JR, et al. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol. 2009;20:278–288. doi: 10.1681/ASN.2008060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dear JW, Street JM, Bailey MA. Urinary exosomes: a reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics. 2013;13:1572–1580. doi: 10.1002/pmic.201200285. [DOI] [PubMed] [Google Scholar]
- 47.Zhou H, Kajiyama H, Tsuji T, et al. Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. Am J Physiol Renal Physiol. 2013;305:F553–F559. doi: 10.1152/ajprenal.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alkhalaf A, Zürbig P, Bakker SJ, et al. for the PREDICTIONS Group. Multicentric validation of proteomic biomarkers in urine specific for diabetic nephropathy. PLoS One. 2010;5:e13421. doi: 10.1371/journal.pone.0013421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ling XB, Sigdel TK, Lau K, et al. Integrative urinary peptidomics in renal transplantation identifies biomarkers for acute rejection. J Am Soc Nephrol. 2010;21:646–653. doi: 10.1681/ASN.2009080876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drube J, Schiffer E, Lau E, et al. Urinary proteome analysis to exclude severe vesicoureteral reflux. Pediatrics. 2012;129:e356–e363. doi: 10.1542/peds.2010-3467. [DOI] [PubMed] [Google Scholar]
- 51.Dai Y, Sui W, Lan H, Yan Q, Huang H, Huang Y. Comprehensive analysis of microRNA expression patterns in renal biopsies of lupus nephritis patients. Rheumatol Int. 2009;29:749–754. doi: 10.1007/s00296-008-0758-6. [DOI] [PubMed] [Google Scholar]
- 52.Lv LL, Cao YH, Ni HF, et al. MicroRNA-29c in urinary exosome/ microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol. 2013;305:F1220–F1227. doi: 10.1152/ajprenal.00148.2013. [DOI] [PubMed] [Google Scholar]
- 53.Zhang A, Sun H, Wu X, Wang X. Urine metabolomics. Clin Chim Acta. 2012;414:65–69. doi: 10.1016/j.cca.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Vilasi A, Cutillas PR, Maher AD, et al. Combined proteomic and metabonomic studies in three genetic forms of the renal Fanconi syndrome. Am J Physiol Renal Physiol. 2007;293:F456–F467. doi: 10.1152/ajprenal.00095.2007. [DOI] [PubMed] [Google Scholar]
- 55.Desai AN, Jere A. Next-generation sequencing: ready for the clinics? Clin Genet. 2012;81:503–510. doi: 10.1111/j.1399-0004.2012.01865.x. [DOI] [PubMed] [Google Scholar]
- 56.Koboldt DC, Steinberg KM, Larson DE, Wilson RK, Mardis ER. The next-generation sequencing revolution and its impact on genomics. Cell. 2013;155:27–38. doi: 10.1016/j.cell.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Artuso R, Fallerini C, Dosa L, et al. Advances in Alport syndrome diagnosis using next-generation sequencing. Eur J Hum Genet. 2012;20:50–57. doi: 10.1038/ejhg.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCarthy HJ, Bierzynska A, Wherlock M, et al. for the RADAR the UK SRNS Study Group. Simultaneous sequencing of 24 genes associated with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2013;8:637–648. doi: 10.2215/CJN.07200712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Otto EA, Ramaswami G, Janssen S, et al. for the GPN Study Group. Mutation analysis of 18 nephronophthisis associated ciliopathy disease genes using a DNA pooling and next generation sequencing strategy. J Med Genet. 2011;48:105–116. doi: 10.1136/jmg.2010.082552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shapiro E, Biezuner T, Linnarsson S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat Rev Genet. 2013;14:618–630. doi: 10.1038/nrg3542. [DOI] [PubMed] [Google Scholar]
- 61.Baker M. Big biology: the ‘omes puzzle. Nature. 2013;494:416–419. doi: 10.1038/494416a. [DOI] [PubMed] [Google Scholar]
- 62.Köhler S, Doelken SC, Mungall CJ, et al. The Human Phenotype Ontology project: linking molecular biology and disease through phenotype data. Nucleic Acids Res. 2014;42:D966–D974. doi: 10.1093/nar/gkt1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jouret F, Walrand S, Parreira KS, et al. Single photon emission-computed tomography (SPECT) for functional investigation of the proximal tubule in conscious mice. Am J Physiol Renal Physiol. 2010;298:F454–F460. doi: 10.1152/ajprenal.00413.2009. [DOI] [PubMed] [Google Scholar]
- 64.Schreiber A, Shulhevich Y, Geraci S, et al. Transcutaneous measurement of renal function in conscious mice. Am J Physiol Renal Physiol. 2012;303:F783–F788. doi: 10.1152/ajprenal.00279.2012. [DOI] [PubMed] [Google Scholar]
- 65.Grubb SC, Bult CJ, Bogue MA. Mouse Phenome Database. Nucleic Acids Res. 2013;42:D825–D834. doi: 10.1093/nar/gkt1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng S, Geghman K, Shenoy S, Li C. Retake the center stage-new development of rat genetics. J Genet Genomics. 2012;39:261–268. doi: 10.1016/j.jgg.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Brown AJ, Fisher DA, Kouranova E, et al. Whole-rat conditional gene knockout via genome editing. Nat Methods. 2013;10:638–640. doi: 10.1038/nmeth.2516. [DOI] [PubMed] [Google Scholar]
- 68.Drummond IA. Kidney development and disease in the zebrafish. J Am Soc Nephrol. 2005;16:299–304. doi: 10.1681/ASN.2004090754. [DOI] [PubMed] [Google Scholar]
- 69.Drummond I. Studying cilia in zebrafish. Methods Cell Biol. 2009;93:197–217. doi: 10.1016/S0091-679X(08)93011-9. [DOI] [PubMed] [Google Scholar]
- 70.Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol. 2012;23:1039–1047. doi: 10.1681/ASN.2011080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahmood F, Mozere M, Zdebik AA, et al. Generation and validation of a zebrafish model of EAST (epilepsy, ataxia, sensorineural deafness and tubulopathy) syndrome. Dis Model Mech. 2013;6:652–660. doi: 10.1242/dmm.009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weavers H, Prieto-Sánchez S, Grawe F, et al. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature. 2009;457:322–326. doi: 10.1038/nature07526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang F, Zhao Y, Chao Y, Muir K, Han Z. Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J Am Soc Nephrol. 2013;24:209–216. doi: 10.1681/ASN.2012080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Müller RU, Zank S, Fabretti F, Benzing T. Caenorhabditis elegans, a model organism for kidney research: from cilia to mechanosensation and longevity. Curr Opin Nephrol Hypertens. 2011;20:400–408. doi: 10.1097/MNH.0b013e3283471a22. [DOI] [PubMed] [Google Scholar]
- 75.Beets I, Janssen T, Meelkop E, et al. Vasopressin/oxytocin-related signaling regulates gustatory associative learning in C. elegans . Science. 2012;338:543–545. doi: 10.1126/science.1226860. [DOI] [PubMed] [Google Scholar]
- 76.Devuyst O, Antignac C, Bindels RJ, et al. The ERA-EDTA Working Group on inherited kidney disorders. Nephrol Dial Transplant. 2012;27:67–69. doi: 10.1093/ndt/gfr764. [DOI] [PubMed] [Google Scholar]
- 77.Tong A, Wong G, Hodson E, Walker RG, Tjaden L, Craig JC. Adolescent views on transition in diabetes and nephrology. Eur J Pediatr. 2013;172:293–304. doi: 10.1007/s00431-012-1725-5. [DOI] [PubMed] [Google Scholar]
- 78.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 2011;80:338–347. doi: 10.1038/ki.2011.134. [DOI] [PubMed] [Google Scholar]
- 79.Trudu M, Janas S, Lanzani C, et al. for the Swiss Kidney Project on Genes in Hypertension (SKIPOGH) team. Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat Med. 2013;19:1655–1660. doi: 10.1038/nm.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Böger CA, Chen MH, Tin A, et al. for the CKDGen Consortium. CUBN is a gene locus for albuminuria. J Am Soc Nephrol. 2011;22:555–570. doi: 10.1681/ASN.2010060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chasman DI, Fuchsberger C, Pattaro C, et al. for the CARDIoGRAM Consortium and the ICBP Consortium and the CARe Consortium and the WTCCC2. Integration of genome-wide association studies with biological knowledge identifies six novel genes related to kidney function. Hum Mol Genet. 2012;21:5329–5343. doi: 10.1093/hmg/dds369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stuiver M, Lainez S, Will C, et al. CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am J Hum Genet. 2011;88:333–343. doi: 10.1016/j.ajhg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 84.Gbadegesin RA, Winn MP, Smoyer WE. Genetic testing in nephrotic syndrome--challenges and opportunities. Nat Rev Nephrol. 2013;9:179–184. doi: 10.1038/nrneph.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cochat P, Rumsby G. Primary hyperoxaluria. N Engl J Med. 2013;369:649–658. doi: 10.1056/NEJMra1301564. [DOI] [PubMed] [Google Scholar]
- 86.Jézégou A, Llinares E, Anne C, et al. Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc Natl Acad Sci USA. 2012;109:E3434–E3443. doi: 10.1073/pnas.1211198109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8:622–633. doi: 10.1038/nrneph.2012.195. [DOI] [PubMed] [Google Scholar]
- 88.Robben JH, Kortenoeven ML, Sze M, et al. Intracellular activation of vasopressin V2 receptor mutants in nephrogenic diabetes insipidus by nonpeptide agonists. Proc Natl Acad Sci USA. 2009;106:12195–12200. doi: 10.1073/pnas.0900130106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brodin-Sartorius A, Tête MJ, Niaudet P, et al. Cysteamine therapy elays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012;81:179–189. doi: 10.1038/ki.2011.277. [DOI] [PubMed] [Google Scholar]
- 90.Cravedi P, Kopp JB, Remuzzi G. Recent progress in the pathophysiology and treatment of FSGS recurrence. Am J Transplant. 2013;13:266–274. doi: 10.1111/ajt.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu CC, Fornoni A, Weins A, et al. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369:2416–2423. doi: 10.1056/NEJMoa1304572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zuber J, Fakhouri F, Roumenina LT, Loirat C, Frémeaux-Bacchi V for the French Study Group for aHUS/C3G. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643–657. doi: 10.1038/nrneph.2012.214. [DOI] [PubMed] [Google Scholar]
- 93.Lemaire M, Frémeaux-Bacchi V, Schaefer F, et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet. 2013;45:531–536. doi: 10.1038/ng.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takahashi K, Makita N, Manaka K, et al. V2 vasopressin receptor (V2R) mutations in partial nephrogenic diabetes insipidus highlight protean agonism of V2R antagonists. J Biol Chem. 2012;287:2099–2106. doi: 10.1074/jbc.M111.268797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bernier V, Morello JP, Zarruk A, et al. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol. 2006;17:232–243. doi: 10.1681/ASN.2005080854. [DOI] [PubMed] [Google Scholar]
- 96.Bolignano D, Nagler EV, Van Biesen W, Zoccali C. Providing guidance in the dark: rare renal diseases and the challenge to improve the quality of evidence. Nephrol Dial Transplant. 2013 doi: 10.1093/ndt/gft344. published online Sept 11. [DOI] [PubMed] [Google Scholar]
- 97.Forman J, Taruscio D, Llera VA, et al. for the International Conference for Rare Diseases and Orphan Drugs (ICORD) The need for worldwide policy and action plans for rare diseases. Acta Paediatr. 2012;101:805–807. doi: 10.1111/j.1651-2227.2012.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schlingmann KP, Kaufmann M, Weber S, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. 2011;365:410–421. doi: 10.1056/NEJMoa1103864. [DOI] [PubMed] [Google Scholar]
- 99.Aymé S, Kole A, Groft S. Empowerment of patients: lessons from the rare diseases community. Lancet. 2008;371:2048–2051. doi: 10.1016/S0140-6736(08)60875-2. [DOI] [PubMed] [Google Scholar]
- 100.Ingelfinger JR, Drazen JM. Patient organizations and research on rare diseases. N Engl J Med. 2011;364:1670–1671. doi: 10.1056/NEJMe1102290. [DOI] [PubMed] [Google Scholar]