Abstract
The study was aimed at comparing the effects of concentric (CONC) and eccentric (ECC) exercises of equivalent (in terms of relative work load expressed as a percentage of VO2max) moderate intensity on selected blood cytokine levels and blood creatine kinase (CK) activity. Twenty recreationally active healthy young male volunteers were randomized between two groups that performed a single 1 h bout of CONC (uphill running) or ECC (downhill running) exercise at 60% of the respective individual VO2max. Venous blood taken 1 h before, at the end, and 24 h after the exercise was processed for plasma and analyzed for CK activity and IL-6, IL-1β and TNFα levels. There was no between-group difference in these cytokines prior to or just after the exercise, and in pre-exercise CK activity. The cytokines elevated significantly and similarly in both groups during the exercise, with no significant change in CK activity. Twenty-four hours later, CK activity and IL-6 were at pre-exercise levels in the CONC group, but showed further major increases in the ECC group, resulting in marked between-group differences in these indices. Changes in IL-1β and TNFα levels during the recovery period showed only minor differences between the study groups and produced no significant between-group difference in these cytokines. However, IL-1β level normalized in the ECC but not in the CONC group. The study suggests that moderate intensity ECC exercise compared to CONC exercise of equivalent relative work load results in considerably greater muscle damage and its related elevation in circulating IL-6, but it does not cause a major systemic inflammatory response.
Keywords: concentric exercise, eccentric exercise, inflammation, metabolism, muscle damage, treadmill
INTRODUCTION
Skeletal muscles are capable of performing two types of contractions distinguished by opposing changes in muscle fibre length: lengthening (in eccentric exercise, ECC) and shortening (in concentric exercise, CONC) [26]. The two types of exercise exert different effects on the metabolism of the working muscles and induce distinct systemic circulatory and endocrine responses [13]. At equivalent absolute work load (WL), ECC involves a smaller demand for oxygen [30], while inflicting a larger damage on the muscle fibres involved [6]. Leukocytes and macrophages that invade the exercise-injured muscles for several hours post-injury [19, 24] and reside there for at least 24 h [23] and/or resident macrophages [11] can produce a number of proinflammatory cytokines, including TNFα and IL-1β that promote the breakdown of the damaged fibres [5].
Working muscle fibres being the main source of circulating IL-6 during exercise [4, 29] may significantly contribute to the resulting systemic endocrine responses. IL-6 has been considered an inflammatory cytokine, but it can also exert a strong anti-inflammatory response by enhancing production of IL-1 receptor antagonist and anti-inflammatory cytokines and by inhibiting production of proinflammatory cytokines [29, 31] and may act as a mediator of muscle healing [39]. Its response to exercise is not preceded by an increase in TNFα and is not obligatorily related to muscle damage. Hence, it was postulated that acute exercise-induced elevations in IL-6 may be more important for metabolic rather than immunological responses, and its major role in working muscles is that of an energy sensor (reviewed in [40]). This study was aimed at verifying the hypothesis that the effects of CONC and ECC exercises of moderate intensities and identical relative work load (expressed as a percentage of VO2max) on circulating levels of selected cytokines of muscle (IL-6) and systemic origin (IL-1β and TNFα) differ.
MATERIALS AND METHODS
Participants and experimental protocol
Twenty recreationally active male students of the Jerzy Kukuczka Academy of Physical Education in Katowice, all healthy and taking no medications, volunteered for the study. They were fully informed of the study protocol and the possible associated discomforts and risks, and have signed the respective informed consent. The participants were not involved in any strenuous ECC or CONC exercise for at least 4 weeks prior to the study, and – to reduce the variability related to macronutrient influence on inflammatory responses – consumed a normal mixed diet for 3 days before each test. They were also told to avoid exercise and heavy food intake for at least 2 hours before the treadmill test. All tests were performed between 8 a.m. and 12 p.m. Maximal oxygen uptake (VO2max) was determined using a model Oxycon Champion metabolic analyzer (Jaeger, Germany), two weeks before the experiment, during an incremental ‘to-exhaustion’ exercise test on a model HP Cosmos (Pulsar, Germany) treadmill as described elsewhere [20], with two minor modifications; these modifications consisted in maintaining treadmill inclination at +1% throughout the procedure, and at the beginning running speed being only 4 km · h−1. Heart rate (HR), oxygen consumption (VO2), carbon dioxide production, pulmonary ventilation and respiratory quotient were recorded breath-by-breath every 30 s during all tests; HR was measured using a model Sport Tester (Polar, Finland). After assessment of their VO2max, the subjects were randomized between the study groups (N = 10 each). One group was subject to a single 1 h bout of ECC exercise (treadmill downhill running at -10% gradient), and the other group to a single 1 h bout of CONC exercise (treadmill uphill running at +10% gradient). Each participant exercised at absolute WL set to keep VO2 at 60% of his individual VO2max. All tests were performed in an air-conditioned environment, under thermoneutral conditions. The physiological strain index was calculated as proposed by Moran et al. [25] except for using auditory canal (instead of rectal) temperature measurements; the measurements were taken using a model E Val-Flex thermometer (Ellab A/S, Denmark). The study protocol was designed in accordance with the tenets of the Helsinki Declaration and has been approved by the Research Ethics Committee of the Jerzy Kukuczka Academy of Physical Education.
Blood sampling and analysis
Blood samples were obtained from the antecubital vein about 1 h before exercise (resting samples), immediately post-exercise, and after 24 h of recovery, using sodium heparin (18 IU · mL−1) as an anticoagulant, and were immediately processed for plasma that was instantly aliquoted, frozen and stored at -70° until analyzed for IL-6, IL-1b and TNFα concentrations, and for creatine kinase (E.C.2.3.7.2; CK) activity; all the analyses were run in duplicate and the results were averaged for data analysis.
Plasma CK activity was assayed using the Boehringer Mannheim/ Hitachi 747 analysis system and the CK-NAC kit (Randox Laboratories, Crumlin, UK). IL-6 and TNFα were assessed with R&D BioSource ELISA kits (Invitrogen) according to the manufacturer's instructions; the limit of detection for each of the assays was 5 pg · ml−1. IL-1β was determined using a commercial ELISA kit (Bender MedSystems GmbH, Vienna, Austria) according to the manufacturer's instructions; the limit of detection for this assay was 0.3 pg · ml−1. The results of all these analyses were corrected for exercise-related changes in plasma volume.
Statistical analysis
All data were first tested for variance heterogeneity (by the Brown- Forsythe test) and for distribution normality (by the Shapiro-Wilk test). CK activity data showed major variance heterogeneity and deviations from distribution normality that could not be corrected by log transformation. Hence, they were analyzed for each study group separately by the Friedman ANOVA; between-group differences and within-group changes in CK activity were then tested for significance by the Mann-Whitney U test and the Wilcoxon test, respectively. Cytokine level data showed no sizeable variance heterogeneity or deviations from normal distribution, and were analyzed by 2-way ANOVA with group as the main factor and time as the repeated measures factor, followed by Student's t tests for independent and dependent variables when appropriate. The Bonferroni-Holm correction was applied to the results of all post-hoc tests to keep the family-wise probability of type I error below 0.05. Between-group differences in anthropometric and running-related characteristics were tested with Student's t-test for independent variables. Correlations between the various studied variables were tested by Spearman's rank correlation test. In all cases, P < 0.05 was considered significant. All statistical analyses (excepting the Bonferroni-Holm correction that was done ‘manually’) were performed using the Statistica 7.1 (StatSoft Inc., Tulsa, OK, USA) software.
RESULTS
There was no significant difference between the study groups in mean age, body mass index and VO2max. During exercise, O2 uptake stabilized at similar levels in both groups after 30 min of running. The two groups showed no significant difference in HR, VO2/VO2max ratio, and physiological strain index during the steady state phase of the exercise (31-60 min), while average absolute WL and running speed were markedly lower in the ECC group (Table 1).
TABLE 1.
BASIC ANTHROPOMETRIC DATA AND RUNNING PERFORMANCE CHARACTERISTICS OF THE STUDY GROUPS DURING THE STEADY-STATE PHASE OF THE ECCENTRIC (ECC) AND CONCENTRIC (CONC) EXERCISE
| Variable | ECC group (N = 10) | CONC group (N = 10) | P | ||
|---|---|---|---|---|---|
| Mean (S.D.) | Min-Max | Mean (S.D.) | Min-Max | ||
| Age, years | 21.2 ± 1.3 | 20 – 24 | 21.4 ± 1.3 | 20 –24 | 0.47 |
| Body mass index [kg · m−2] | 22.5 ± 1.9 | 19.2 –25.7 | 21.9 ± 1.6 | 20.2 –25.7 | 0.63 |
| VO2max [l · min−1] | 3.66 ± 0.20 | 3.38 –4.09 | 3.66 ± 0.23 | 3.29 –3.96 | 0.99 |
| Absolute work load [W] | 306 ± 35 | 241 –364 | 122 ± 17 | 106 –151 | < 10–6 |
| Speed at 60% VO2max [km · h−1] | 15.8 ± 1.5 | 14.0 –18.0 | 6.4 ± 0.7 | 5.9 –7.9 | < 10–6 |
| Steady-state VO2/VO2max [%] | 60.3 ± 2.8 | 55.3 –64.2 | 60.5 ± 4.9 | 53.5 –67.2 | 0.89 |
| VO2 at 60% VO2max [l · min−1] | 2.20 ± 0.05 | 2.10 –2.26 | 2.21 ± 0.06 | 2.10 –2.28 | 0.87 |
| HR at 60% VO2max [bpm] | 156 ± 6 | 149 –165 | 151 ± 8 | 139 –160 | 0.18 |
| Physiological strain index | 6.03 ± 0.84 | 4.39 –7.43 | 6.32 ± 0.57 | 5.68 –7.47 | 0.38 |
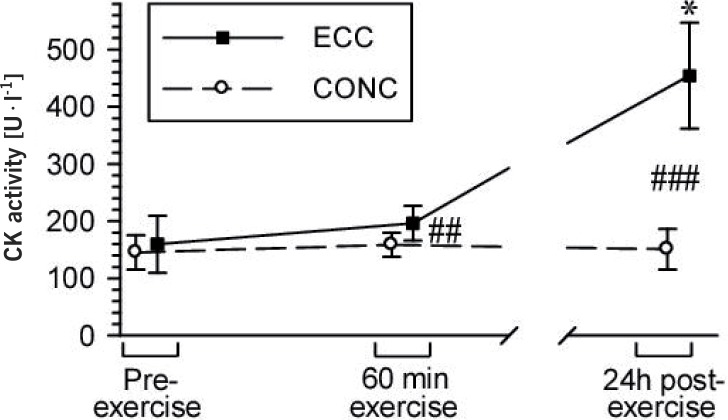
There was no significant difference in resting plasma CK activity and IL-6, TNFα and IL-1β levels between the study groups. Friedman's ANOVA yielded no significant change in CK activity in the CONC group during the observation period, while a significant change was found in the ECC group. In the latter, post-hoc analysis showed a tendency for elevation in CK activity at the end of the exercise (+31%, P = 0.037 by uncorrected Wilcoxon test) and a major increase (to 307% of the respective pre-exercise value) 24 h later; this effect translated into significant differences between the study groups at both these time points (Fig. 1).
FIG. 1.
EFFECT OF A SINGLE BOUT OF ECC EXERCISE OR CONC EXERCISE ON BLOOD CK ACTIVITY IN HEALTHY ADULT RECREATIONALLY ACTIVE MALES
Note: Friedman's ANOVA results: ECC group: χ2df = 2, N = 10 = 16.80, P = 0.0002; CONC group: χ2df = 2, N = 10 = 2.60, P = 0.27. * P < 0.05 vs. the respective pre-exercise value, by the Wilcoxon test, ## P < 0.01 vs. the other group, by the Mann-Whitney U test (with the Bonferroni-Holm correction). Data are presented as the mean ± S.D.
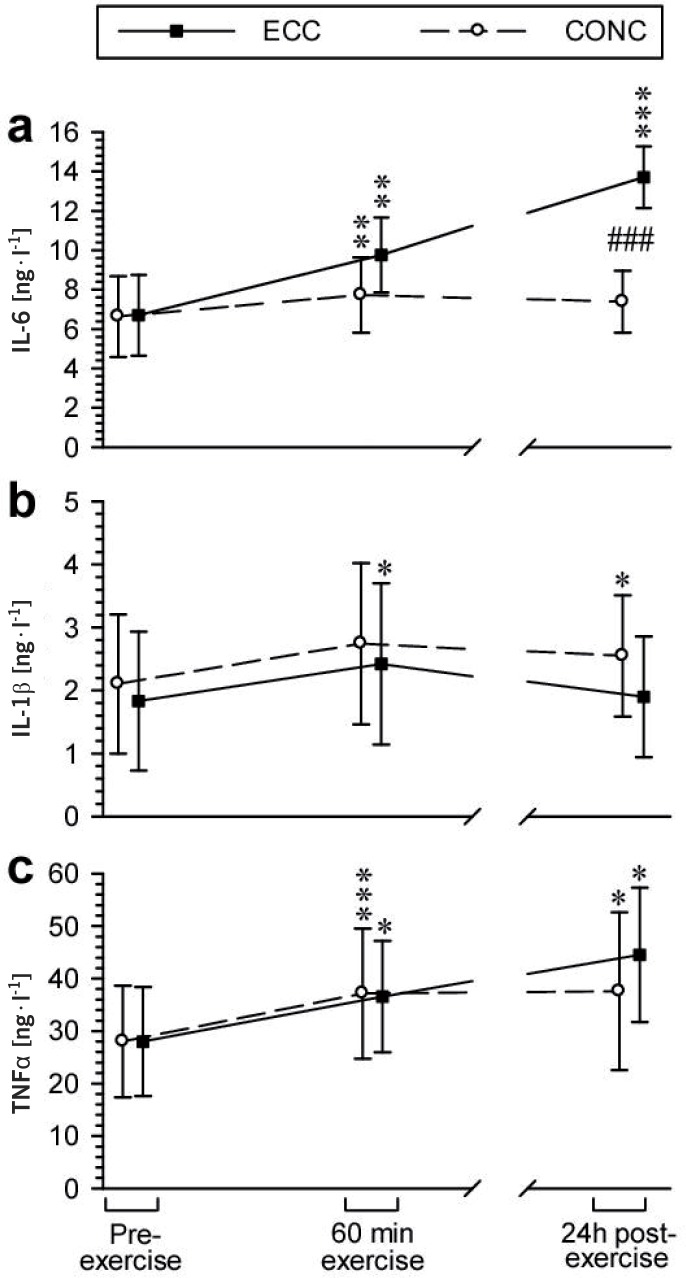
Two-way ANOVA yielded significant effects of time, exercise type and exercise type × time interaction on plasma IL-6 level. In the CONC group, IL-6 level showed a minor but significant increase (+17%) at the end of exercise, while 24 h later it was only insignificantly higher (+11%) than the respective resting value. In the ECC group, the relative increase in IL-6 at the end of the exercise was larger (+46%), but there was no significant difference between the IL-6 levels in the study groups at this time point. Twenty-four hours later plasma IL-6 level in the ECC group was markedly higher than immediately after the exercise (205% of the respective resting value) and significantly higher than that in the CONC group (Fig. 2a).
FIG. 2.
EFFECTS OF A SINGLE BOUT OF ECC EXERCISE OR CONC EXERCISE ON BLOOD IL-6 (A), IL-1β (B) AND TNFα (C) LEVELS IN HEALTHY ADULT RECREATIONALLY ACTIVE MALES
Note: Two-way ANOVA results: (a): exercise type effect: F1,18 = 12.2, P = 0.0026; time effect: F2,36 = 41.5, P < 10 6; exercise type × time interaction effect: F2,36 = 28.2, P = < 10 6; (b): exercise type effect: F1,18 = 1.30, P = 0.27; time effect: F2,36 = 5.31, P = 0.0096; exercise type × time interaction effect: F2,36 = 0.59, P = 0.56; (c) exercise type effect: F1,18 = 0.26, P = 0.61; time effect: F2,36 = 9.94, P = 0.0004; exercise type × time interaction effect: F2,36 = 0.98, P = 0.38. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. the respective pre-exercise value; ### P < 0.001 vs. the other group. Data are presented as the mean ± S.D.
Two-way ANOVA showed a significant effect solely of time on plasma IL-1β and TNFα. This effect translated into a significant but moderate and transient elevation of plasma IL-1β in the ECC group at the end of the exercise (+32%). In the CONC group, the level of IL-1β showed only a non-significant increase (+30%) immediately after the exercise, whereas 24 h later this effect reached significance at +21% above the respective resting value. There was no difference in absolute IL-1β levels between the study groups at any time point studied (Fig. 2b). Post-hoc tests showed that plasma TNFα was significantly elevated in both groups both at the end of the exercise and 24 h later. However, while plasma TNFα level continued to rise between the end of exercise and the end of the observation period in the ECC group, no such effect was found in the CONC group (Fig. 2c).
CK activity did not correlate significantly with absolute WL at the end of the exercise in either the ECC or CONC group (R = 0.02, P = 0.97, and R = 0.31, P = 0.38, respectively), whereas CK activity 24 h post-exercise correlated positively with absolute WL only in the former (R = 0.70, P = 0.025, vs. R = -0.15, P = 0.68). Excepting the positive correlation of IL-6 level in the ECC group 24 h post-exercise (R = 0.64, P = 0.048), none of the studied circulating cytokine levels correlated significantly with absolute WL at the end of the exercise or 24 h later.
DISCUSSION
This study used ECC exercise and CONC exercise to evaluate the contribution of metabolic and mechanical stress on muscle damage and systemic inflammatory responses to a single moderate intensity bout of exercise. As evidenced by VO2 data, the two exercises were performed at identical energy expenditure/metabolic stress. Hence, the observed differences in exercise-induced changes in CK activity and cytokine levels resulted from differences in absolute WL and the related mechanical stress/muscle damage. Other contributing factors might be the relative inefficiency of the muscle pump during ECC exercise as compared to that during CONC exercise [7], and ECC exercise-associated temporary impairment of local microvascular [18] and macrovascular function, in particular arterial stiffness [2], as well as reduced vasodilator response [15]. Hence, it is possible that, despite identical relative VO2 values, oxygen delivery to the working muscles differed between the two types of exercise, which might have affected the extent of muscle damage and responses to the latter.
The primary and most common determinant of muscle damage is mechanical stress [9, 19], which may cause a transient increase in myocyte membrane permeability and permanent cell damage. Few studies have examined the relationship between the extent of exercise-induced injury and the duration and intensity of the exercise. Delayed onset post-exercise muscle soreness and serum levels of intramuscular enzymes were shown to increase with both intensity and duration of exercise [36]. The present study showed a substantial tendency for increased CK activity at the end of exercise in the ECC but not in the CONC group (p = 0.04 and p = 0.28, respectively, by uncorrected Wilcoxon test), while further CK increase occurred only in the former. This suggests that only the ECC exercise increased myocyte membrane permeability and caused sizeable muscle damage. We found a positive correlation between absolute WL and plasma CK activities 24 h post-exercise only in the ECC group. This indicates that post-exercise increase in CK activity was the result of mechanical stress/muscle damage.
Physical exercise is associated with a systemic cytokine response cascade that differs considerably between damaging and nondamaging exercises [3]. Eccentric-biased cycling or downhill running results in a greater elevation in blood IL-6 than concentric contractions [16], but this difference is not fully explained by differences in muscle damage. Since IL-6 is produced upon muscle contraction [29], dynamic eccentric-biased aerobic exercise will elicit greater hemodynamic changes that possibly contribute to increased IL-6 ‘spillover’ to the blood [2, 28]. Our results show that cytokine response to exercise depends on exercise type; particularly discernible were the differences in IL-6 and TNFα levels that significantly increased only after ECC exercise. Symptoms of inflammation have been commonly reported following mechanical stress protocols. Damage-related cytokine responses were evidenced by increased concentrations of IL-1β, IL-6 and IL-10 following high intensity ECC exercise [33].
It is well known that the profile of cytokine response to exercise is related to exercise type, intensity and duration, the mass of muscle recruited [31], as well as diet macronutrient composition [8] and blood redistribution [16]. So far, the changes in circulating IL-6 levels have only been studied during and after physical exercise of high intensity or long duration (‘to exhaustion’). We compared the effects of two types of exercise of moderate intensity (60% of VO2max) and duration (estimated at ≤ 50% of that ‘to exhaustion’). The primary finding of this study is that both the CONC and ECC exercise significantly elevated blood IL-6 at the end of the exercise whereas only the ECC exercise resulted in elevated blood IL-6 24 h later. ECC exercise elevated blood IL-6 during the exercise only non significantly more than CONC exercise. This indicates that the major factor at play with regard to this cytokine during the physical effort was exercise intensity, but not the type of muscle work. Notably, no difference was found in IL-6 mRNA levels 30 min after CONC or ECC exercise using electrical muscle stimulation in anaesthetized rats to produce maximum force development [17]. There was no difference in core body temperature or physiological strain index between our study groups. This fact is of considerable importance as well, because previous studies have shown that IL-6 release from working muscles depends on body temperature [32].
Normalization of blood IL-6 level in the CONC group and the marked between-group difference in circulating IL-6 levels 24 h post-exercise suggest that the major determinant of IL-6 level at this time point was muscle damage. These observations are in general agreement with earlier studies [4] that have attributed this difference to greater ECC exercise-induced muscle damage. This suggestion is also supported by the significant positive correlation between absolute WL and the IL-6 level in the ECC group 24 h post-exercise and the short (∼7 min) half-life of IL-6 in humans [37].
ECC exercise-related muscle damage is biphasic. The primary insult appears to be mechanical in nature and to result directly from fibre contractions [1, 28], while the secondary damage is an aftermath of increased leukocyte influx into the injured fibres and enhanced production of proinflammatory cytokines [12, 25, 28]. Notably, there was no significant correlation between peak IL-6 or absolute WL and CK activity at the end of either exercise, while a significant correlation was found between blood CK activity and IL-6 level 24 h post-exercise in the ECC group. These findings indicate no considerable muscle damage and/or leukocyte reaction at the completion of either exercise type, and sizeable ECC exerciserelated damage and leukocyte reaction developed 24 h later.
The kinetics of IL-6 production show a progressive increase after muscle-damaging ECC exercise, but a rapid decline after non-damaging CONC exercise [38]. The elevated blood IL-6 after 24 h of recovery from ECC exercise was assumed to originate from inflammatory cells infiltrating exercise-damaged muscles [38]. Some authors have suggested that it plays a key role in the repair of damaged muscle fibres [39].
Both tested exercises showed similarly elevated blood TNFα at the end of exercise, and the increases persisted until the end of the observation period with no considerable differences between the study groups. This suggests no sizeable difference between these exercises with regard to muscle catabolism and loss of muscle function [35]. Some but not all reports on the effects of single bouts of ECC exercise have demonstrated elevations in circulating TNFα both at the end of exercise and at later time points (reviewed in [27]), and the reports on the effects of single bouts of CONC exercise were conflicting as well [14, 21]. All said, the existing body of evidence indicates that contracting human skeletal muscles can be the source of elevated blood TNFα. However, it has been shown that the early exercise-related increase in circulating TNFα can take place with no increase in muscle TNFα mRNA [22], which finding indicates that at least part of the increase is due to leukocyte TNFα production. Since muscle damage attracts neutrophils and macrophages, which both contribute to the degradation of the damaged tissue by releasing pro-inflammatory cytokines [28], elevated TNFα 24 h post-exercise in our study might be attributed in part to this phenomenon. However, the difference in this cytokine level between the two study groups was far from significant, indicating that this was unlikely.
Plasma IL-1β levels did not differ between the study groups over the study period and showed only minor increases at the end of either exercise type, which disappeared partly or entirely over the next 24 h. These findings are in line with some though not all earlier studies, which found only minor, if any, changes in blood IL-1(β) levels after exercise, mostly in untrained men [4, 10, 34]. However, results from the various studies are rather hard to compare because of differences in experimental designs and timing of blood sampling, and in cytokine assay specificity/sensitivity. Moreover, IL-1β is rapidly removed from the circulation [34].
CONCLUSIONS
The magnitude and time course of the post-exercise inflammatory response to ECC and CONC exercises of the same moderate relative intensity (%VO2max) depend on the type of contraction and absolute work load (mechanical stress). However, the acute-phase response does not lead to a major systemic inflammatory response even after ECC exercise that causes considerably more muscle damage.
Acknowledgments
This study was supported by statutory funds from the Jerzy Kukuczka Academy of Physical Education, Katowice, Poland.
Conflict of interest
There is no conflict of interest regarding this paper.
REFERENCES
- 1.Armstrong R.B, Warren G.L, Warren J. Mechanisms of exercise-induced muscle fibre injury. Sports Med. 1991;12:184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- 2.Barnes J.N, Trombold J.R, Dhindsa M, Lin H.F, Tanaka H. Arterial stiffening following eccentric exercise-induced muscle damage. J. Appl. Physiol. 2010;109:1102–1108. doi: 10.1152/japplphysiol.00548.2010. [DOI] [PubMed] [Google Scholar]
- 3.Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J. Leukoc. Biol. 2005;78:819–835. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- 4.Bruunsgaard H, Galbo H, Halkjaer- Kristensen J, Johansen T.L, MacLean D.A, Pedersen B.K. Exerciseinduced increase in serum interleukin-6 in humans is related to muscle damage. J. Physiol. 1997;499:833–841. doi: 10.1113/jphysiol.1997.sp021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon J.G, St. Pierre B.A. Cytokines in exertion-induced skeletal muscle injury. Mol. Cell. Biochem. 1998;179:159–167. doi: 10.1023/a:1006828425418. [DOI] [PubMed] [Google Scholar]
- 6.Clarkson P.M, Byrnes W.C, McCormick K.M, Turcotte L.P, White J.S. Muscle soreness and serum creatine kinase activity following isometric, eccentric, and concentric exercise. Int. J. Sports Med. 1986;7:152–155. doi: 10.1055/s-2008-1025753. [DOI] [PubMed] [Google Scholar]
- 7.Dean E. Physiology and therapeutic implications of negative work. Phys. Ther. 1988;68:233–237. doi: 10.1093/ptj/68.2.233. [DOI] [PubMed] [Google Scholar]
- 8.Depner C.M, Kirwan R.D, Frederickson S.J, Miles M.P. Enhanced inflammation with high carbohydrate intake during recovery from eccentric exercise. Eur. J. Appl. Physiol. 2010;109:1067–1076. doi: 10.1007/s00421-010-1448-0. [DOI] [PubMed] [Google Scholar]
- 9.Dufour S.P, Doutreleau S, Lonsdorfer-Wolf E, Lamper E, Hirth C, Piquard F, Lonsdorfer J, Geny B, Mettauer B, Richard R. Deciphering the metabolic and mechanical contributions to the exercise-induced circulatory response: insights from eccentric cycling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1641–R1648. doi: 10.1152/ajpregu.00567.2006. [DOI] [PubMed] [Google Scholar]
- 10.Evans W.J, Meredith C.N, Cannon J.G, Dinarello C.A, Frontera W.R, Hughes V.A, Jones B.H, Knuttgen H.G. Metabolic changes following eccentric exercise in trained and untrained men. J. Appl. Physiol. 1986;61:1864–1868. doi: 10.1152/jappl.1986.61.5.1864. [DOI] [PubMed] [Google Scholar]
- 11.Fielding R.A, Manfredi T.J, Ding W, Fiatarone M.A, Evans W.J, Cannon J.G. Acute phase response in exercise. III. Neutrophil and IL-1α accumulation in skeletal muscle. Am. J. Physiol. 1993;265:R166–R172. doi: 10.1152/ajpregu.1993.265.1.R166. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Lopez D, Cuevas M.J, Almar M, Lima E, De Paz J.A, Gonzalez-Gallego J. Effects of eccentric exercise on NF-κB activation in blood mononuclear cells. Med. Sci. Sports Exerc. 2007;39:653–664. doi: 10.1249/mss.0b013e31802f04f6. [DOI] [PubMed] [Google Scholar]
- 13.Gleeson M, Blannin A.K, Zhu B, Brooks S, Cave R. Cardiorespiratory, hormonal and haematological responses to submaximal cycling performed 2 days after eccentric or concentric exercise bouts. J. Sports Sci. 1995;13:471–479. doi: 10.1080/02640419508732264. [DOI] [PubMed] [Google Scholar]
- 14.Gökbel H, Okudan N, Gül I, Belviranli M, Gergerlioğlu H.S, Başaral M.K. Effects of repeated bouts of supramaximal exercise on plasma adiponectin, interleukin-6, and tumor necrosis factor-α levels in sedentary men. J. Strength Cond. Res. 2012;26:1675–1679. doi: 10.1519/JSC.0b013e318231ac1c. [DOI] [PubMed] [Google Scholar]
- 15.Heap S.J, Fulgenzi G.L, Hudlicka O. Microcirculation in rat soleus muscle after eccentric exercise: the effect of nifedipine. Eur. J. Appl. Physiol. 2006;97:687–694. doi: 10.1007/s00421-006-0239-0. [DOI] [PubMed] [Google Scholar]
- 16.Hirose L, Nosaka K, Newton M, Laveder A, Kano M, Peake J, Suzuki K. Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc. Immunol. Rev. 2004;10:75–90. [PubMed] [Google Scholar]
- 17.Jonsdottir I.H, Schjerling P, Ostrowski K, Asp S, Richter E.A, Pedersen B.K. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J. Physiol. 2000;528:157–163. doi: 10.1111/j.1469-7793.2000.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kano Y, Padilla D.J, Behnke B.J, Hageman K.S, Musch T.I, Poole D.C. Effects of eccentric exercise on microcirculation and microvascular oxygen pressures in rat spinotrapezius muscle. J. Appl. Physiol. 2005;99:1516–1522. doi: 10.1152/japplphysiol.00069.2005. [DOI] [PubMed] [Google Scholar]
- 19.Khoshkhahesh F, Siahkuhain M, Fisher G, Nakhostin-Rooh B. Influence of a low-dose cox-2 inhibitor drug on exercise-induced inflammation, muscle damage and lipid peroxidation. Biol. Sport. 2013;30:61–65. doi: 10.5604/20831862.1029824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kłapcińska B, Waśkiewicz Z, Chrapusta S.J, Sadowska-Krępa E, Czuba M, Langfort J. Metabolic responses to a 48 h ultra-marathon run in middle-aged male amateur runners. Eur. J. Appl. Physiol. 2013;113:2781–2793. doi: 10.1007/s00421-013-2714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leelarungrayub D, Khansuwan R, Pothongsunun P, Klaphajone J. N-acetylcysteine supplementation controls total antioxidant capacity, creatine kinase, lactate, and tumor necrotic factor-alpha against oxidative stress induced by graded exercise in sedentary men. Oxid. Med. Cell. Longev. 2011;2011:329643. doi: 10.1155/2011/329643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao P, Zhou J, Ji L, Zhang Y. Eccentric contraction induces inflammatory responses in rat skeletal muscle: role of tumor necrosis factor-α. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R599–R607. doi: 10.1152/ajpregu.00480.2009. [DOI] [PubMed] [Google Scholar]
- 23.Malm C, Nyberg P, Engstrom M, Sjodin B, Lenkei R, Ekblom B, Lundberg I. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J. Physiol. 2000;529:243–262. doi: 10.1111/j.1469-7793.2000.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKune A.J, Semple S.J, Peters-Futre E.M. Acute exercise-induced muscle injury. Biol. Sport. 2012;29:3–10. [Google Scholar]
- 25.Moran D.S, Shitzer A, Pandolf K.B. A physiological strain index to evaluate heat stress. Am. J. Physiol. 1998;275:R129–R134. doi: 10.1152/ajpregu.1998.275.1.R129. [DOI] [PubMed] [Google Scholar]
- 26.Padulo J, Laffaye G, Ardigò LP, Chamari K. Concentric and eccentric: muscle contraction or exercise? J. Hum. Kinet. 2013;37:5–6. doi: 10.2478/hukin-2013-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulsen G, Mikkelsen UR, Raastad T, Peake J.M. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012;18:42–97. [PubMed] [Google Scholar]
- 28.Peake J.M, Suzuki K, Wilson G, Hordern M, Nosaka K, Mackinnon L, Coombes J.S. Exercise-induced muscle damage, plasma cytokines, and markers of neutrophil activation. Med. Sci. Sports Exerc. 2005;37:737–745. doi: 10.1249/01.mss.0000161804.05399.3b. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen B.K, Febbraio M.A. Muscle as an endocrine organ: focus on musclederived interleukin-6. Physiol. Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 30.Perrey S, Betik A, Candau R, Rouillon J.D, Hughson R.I. Comparison of oxygen uptake kinetics during concentric and eccentric cycle exercise. J. Appl. Physiol. 2001;91:2135–2142. doi: 10.1152/jappl.2001.91.5.2135. [DOI] [PubMed] [Google Scholar]
- 31.Petersen A.M, Pedersen B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 32.Rhind S.G, Gannon G.A, Shephard R.J, Buguet A, Shek P.N, Radomski M.W. Cytokine induction during exertional hyperthermia is abolished by core temperature clamping: neuroendocrine regulatory mechanisms. Int. J. Hyperthermia. 2004;20:503–516. doi: 10.1080/02656730410001670651. [DOI] [PubMed] [Google Scholar]
- 33.Smith L.L, Anwar A, Fragen M, Rananto C, Johnson R, Holbert D. Cytokines and cell adhesion molecules associated with high-intensity eccentric exercise. Eur. J. Appl. Physiol. 2000;82:61–67. doi: 10.1007/s004210050652. [DOI] [PubMed] [Google Scholar]
- 34.Sprenger H, Jacobs C, Nain M, Gressner A.M, Prinz H, Wessemann W, Gemsa D. Enhanced release of cytokines, interleukin-2 receptors, and neopterin after long-distance running. Clin. Immunol. Immunopathol. 1992;63:188–195. doi: 10.1016/0090-1229(92)90012-d. [DOI] [PubMed] [Google Scholar]
- 35.Steensberg A, Keller C, Starkie R.L, Osada T, Febbraio M, Pedersen B.K. IL-6 and TNF-α expression in, and release from, contracting human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2002;283:E1272–E1278. doi: 10.1152/ajpendo.00255.2002. [DOI] [PubMed] [Google Scholar]
- 36.Tiidus P.M, Ianuzzo C.D. Effects of intensity and duration of muscular exercise on delayed soreness and serum enzyme activities. Med. Sci. Sports Exerc. 1983;15:461–465. [PubMed] [Google Scholar]
- 37.Toft A.D, Falahati A, Steensberg A. Source and kinetics of interleukin-6 in humans during exercise demonstrated by a minimally invasive model. Eur. J. Appl. Physiol. 2011;111:1351–1359. doi: 10.1007/s00421-010-1755-5. [DOI] [PubMed] [Google Scholar]
- 38.Toft A.D, Jensen L.B, Bruunsgaard H, Ibfelt T, Halkjaer-Kristensen J, Febbraio M, Pedersen B.K. Cytokine response to eccentric exercise in young and elderly humans. Am. J. Physiol. Cell Physiol. 2002;283:C289–C295. doi: 10.1152/ajpcell.00583.2001. [DOI] [PubMed] [Google Scholar]
- 39.Toumi H, F'guyer S, Best T.M. The role of neutrophils in injury and repair following muscle stretch. J. Anat. 2006;208:459–470. doi: 10.1111/j.1469-7580.2006.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh N.P, Gleeson M, Shephard R.J, Gleeson M, Woods J.A, Bishop N.C, Fleshner M, Green C, Pedersen B.K, Hoffman-Goetz L, Rogers C.J, Northoff H, Abbasi A, Simon P. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011;17:6–63. [PubMed] [Google Scholar]