Abstract
We determined whether distracting the observer’s attention from an adapting stimulus could decrease the motion after-effect. Unlike previous studies we used a relatively bias-free 2AFC procedure to measure the strength of adaptation. The strength of motion adaptation was measured by the effects of a moving grating on the contrast discrimination (T vs. C) function for gratings moving in the same or opposite direction. As in previous reports, the effect of adaptation was to move the T vs. C function upwards and right-wards, consistent with an increase in the C50 (semi-saturation) response in the transduction function of the neural mechanism underlying the discrimination. On the other hand, manipulating the attentional load of a distracting task during adaptation had no consistent effect on contrast discrimination, including the absolute detection threshold. It is suggested that previous reported effects of attentional load on adaptation may have depended on response bias, rather than changes in sensitivity.
Keywords: Motion, Adaptation, Attention, Contrast detection, Contrast discrimination
1. Introduction
Several reports (Chaudhuri, 1990; Rees, Frith, & Lavie, 1997; Rezec, Krekelberg, & Dobkins, 2004; Taya et al., 2009) have indicated that the strength of the motion after effect (MAE) can be modulated by attention to an irrelevant task during the adaptation period. In what we shall refer to as the distraction method, the observer adapts to a moving stimulus either while carrying out an undemanding (low load) task or when carrying out a high-load task. Typically, the distracting task is presented at fixation while the adapting stimulus is presented in the surrounding region. For example, the distracting task might be rapid serial visual presentation (RSVP) of shapes, in which the low-load task is to respond to a change in colour, while the high-load task is to respond to a conjunction of shape and colour (Schwartz et al., 2005). The reported finding is that the strength of adaption, measured by the duration of the MAE, is reduced in the high-load task, the reason being that this distracts the observer from the adapting stimulus and thereby reduces its adapting effect.
It is important at the outset to distinguish the distraction effect from the attentional tracking effect. Experimental results (Alais & Blake, 1999; Lankheet & Verstraten, 1995; Raphael, Dillenburger, & Morgan, 2010) have convincingly shown that tracking one component of a transparent-motion display produces a motion aftereffect opposite to the direction of the attended component. This has been used to bolster the idea that distraction reduces adaptation (Rezec, Krekelberg, & Dobkins, 2004; Taya et al., 2009) but it is in fact a conceptually very different effect. Attentional tracking of one component in a motion-balanced display could work by applying a subtractive signal to an opponent mechanism (Morgan, Chubb, & Solomon, 2006), but this would not apply to a stimulus with strong directional motion energy. In fact, using an adapting stimulus with balanced expansion and contraction, Raphael, Dillenburger, and Morgan (2010) reported an effect of attentional tracking, but no effect of distraction when using only one adapting component.
Not all experiments have shown the distraction effect. In the very first experiment on this topic, carried out by Wohlgemuth (1911) a distracting RSVP task in central vision had no effect on the duration of the spiral after effect. This experiment has been neglected in subsequent reports. The evidence for the distraction effect is in fact rather weak and inconsistent. The great majority of experiments using the distraction paradigm (see Section 4 for details) have used the duration measure of the after-effect, a measure known to be highly susceptible to experimenter/subject bias (Sinha, 1952). An attempted replication using the duration measure with genuinely naive subjects failed to find an effect (Morgan, submitted for publication). For this reason, it would be desirable to measure the effect with a relatively bias-free method such as 2AFC. This was the purpose of the experiment reported here.
The cause of the distraction effect, supposing it to be real for the moment, is also somewhat mysterious. One idea is that distraction reduces the effective contrast of the adaptor, in line with the finding that it reduces to BOLD response in MT (Rees, Frith, & Lavie, 1997). However, most of the experiments so far have used a high contrast adaptor, and it has been shown that the duration of the MAE saturates rapidly with adapting contrast (Blake et al., 2006). The exception is the study by Rezec, Krekelberg, and Dobkins (2004), who measured the effect of attentional load over a wide range of adapting contrasts. They confirmed the saturation of the duration MAE at contrasts greater than ~20%, but found that the effect of load (measured in units of seconds duration difference) was the same at all adapting contrasts. Rezec et al. make the interesting suggestion that load affects not equivalent contrast but the adaptability of the neurons underling the perception of motion. Specifically, they use the standard contrast function for a mechanism:
| (1) |
where R is the response to contrast C, Rmax is the maximum response of the mechanism, C50 is the contrast at which the mechanism reaches half its maximum response (the semi-saturation contrast), n is the steepness of the function, and m is a baseline response when C = 0.
Rezec et al. suggest that adaptation moves the semi-saturation constant C50 to higher values, in line with measurements of MT neurons (Kohn & Movshon, 2003), and with psychophysical studies of the contrast discrimination function (Foley & Legge, 1981; Greenlee & Heitger, 1988; Legge & Foley, 1980; Morgan, Chubb, & Solomon, 2006; Ross, Speed, & Morgan, 1993; Stromeyer & Klein, 1974). They further suggest that distraction reduces the extent of this shift, or put this another way, that the rightwards shift of the C50 point due to adaptation is greater if the observer is fully attending to the stimulus. Using an opponent model of the MAE they show that this modulation of the C50 by attention predicts an effect on adaptation that is independent of the adapting contrast.
The contrast function described by Eq. (1) is similar to the 4-parameter function that has been used to describe the effects of adaptation on the contrast discrimination function (Foley & Legge, 1981; Legge & Foley, 1980; Morgan, Chubb, & Solomon, 2006; Ross, Speed, & Morgan, 1993; Stromeyer & Klein, 1974). The effect of adaptation is to move the contrast discrimination function, also referred to as the T vs. C function, upwards and to the right, an effect well described by an increase in the C50 parameter. This means that we can measure the T vs. C function to test the Rezec et al. model directly. To do this, we compared the T vs. C function for the adapted and unadapted directions under conditions of high and low attentional load for the distractor task. The Methods were the same as those described in a companion paper (Morgan, Chubb & Solomon, in press). We deliberately used a low-contrast adaptor (0.075) to avoid saturation of the adaptation effect.
2. Methods
2.1. Apparatus and stimuli
Stimuli were computed with MATLAB and displayed by a Cambridge Research System VSG 2/3 graphics card on a Sony monitor (resolution 640 pixels width by 479 pixels height; 100 Hz frame rate; pixel size 1.03 arcmin, mean luminance 37.5 cd/m2). Viewing distance was 2 m. Contrast was controlled by a look-up table with 15 bits resolution. To ensure a linear relation between DAC voltage and luminance, the display was calibrated with the Cambridge Research Systems OPTICAL. The three DAC’s were individually calibrated. The stimulus (both for adaptation and test) consisted of a 45° oriented, drifting 2.05 cyc/deg sinusoidal grating of temporal frequency 7.5 Hz windowed by a stationary spatial Gaussian envelope (s = 233°).
2.1.1. Psychophysics
To determine thresholds for contrast detection and discrimination, the procedure was 2AFC (temporal). On each trial there were two temporal intervals, each indicated by the fixation point turning red. In each interval a stimulus was presented for 32 frames (320 ms) with an exponential bell-shaped contrast envelope:
| (2) |
where C was the contrast in frame t; Cmax was the maximum contrast, t was the frame number (1–32) and r was the time constant (in frames), equal to 10. The first stimulus was followed by a meanluminance screen for 0.5 s and then by the second of the two stimuli. One of the two stimuli had the reference (pedestal) contrast C the other was of contrast C + ΔDC. The threshold contrast was first determined with a pedestal of zero (the detection point) and this threshold (t) was then used to determine a logarithmically-spaced range of pedestal values {0 0.25t 0.5t t 2t 4t …} up to a maximum contrast of ~0.75. The observer used a keyboard to indicate which interval was of higher contrast. The contrast increment, which the observer had to detect, (ΔC) was varied by the QUEST procedure (Watson & Pelli, 1983) using the version in the Psychtoolbox (Brainard, 1997), modified to jitter the chosen contrast on each trial in the range ±1 dB, in order to obtain fuller sampling of the psychometric function. The pedestal contrast was fixed in each block of 50 trials. There was no feedback.
The first trial in each block of 50 trials was preceded by 30 s of adaptation; later trials were preceded by 5 s of top-up adaptation. The adapting stimulus had Michelson contrast 0.075 unless otherwise stated. It was presented in a rectangularly-windowed temporal contrast envelope. Observers were instructed to keep their eyes fixed on the central fixation point during adaptation. To encourage fixation observers were given a task to perform during each adaptation period. The attentional task was based on a recent paper showing a greater BOLD response to a peripheral stimulus under low vs. high load (Schwartz et al., 2005). An area of 1.1 × 0.73° was cut-out of the centre of the adapting stimulus in its centre and replaced by a mean-luminance gray. Within this central area coloured ‘T’ like stimuli were presented at a rate of 2/s. The T could be upright or inverted and one of four colours, red, green blue and yellow. The low-load task was to press a button as quickly as possible in response to any red stimulus, independently of orientation. The high-load task was to respond to either of two conjunctions out of the eight possible combinations, for example, green-upright and blue-inverted. The actual target conjunctions were randomly resampled for each block of 50 trials, to prevent the task from becoming automatic.
2.1.2. Data analysis and modelling
Data under each combination of pedestal value, movement direction and attentional load were accumulated over sessions to obtain an overall psychometric function for that condition, which was fit by a Weibull function using the MATLAB ‘fminsearch’ fitting procedure to find the fit with maximum likelihood, defined as
where xi is the number of correct responses made at signal level i, and pi is the probability of a correct response at that level predicted by the model.
A bootstrap analysis (Efron, 1982) used the 2-parameters of the Weibull function found by the maximum likelihood method to compute 95% confidence intervals. The fitted values were used to resample the data using the exact number of trials and the Quest procedure used in the actual experiment. This was repeated 80 times and the exact range for 95% of the observations determined from the sampled distribution. It should be noted that this measure of confidence merely tells us how closely the parameters of the psychometric function are constrained by the data available. It does not tell us anything about secular variations in subject performance. A subject could produce very different results in two sessions, but when these two sessions are combined, this variability will change the slope of the psychometric function without necessarily changing the confidence limits.
The maximum likelihood fits to the individual T vs. C functions were obtained using Foley’s (1994) version of Stromeyer and Klein’s (1974) 4-parameter transducer function:
where R is the response of the detector, C is contrast, and b is a divisive inhibition factor corresponding to a semi-saturation constant. Note the similarity to Eq. (1). The parameters p and q determine the initial acceleration and later saturation of the transducer respectively. To see if two sets of data, for example, those under low load and high load, were significantly different they were first individually fit with this 4-parameter function. This gives an 8-parameter fit to the data as a whole. The data from the two conditions were then combined and fit with the 4-parameter model. A likelihood ratio test was then used to compare the fits of the two models, one with 8 parameters and the other with 4. Let Lc and Lu be the likelihoods of the best-fitting constrained and unconstrained models. We used the established theory (Hoel, Port, & Stone, 1971), that under the null hypothesis that the constrained model captures the true state of the world,
is asymptotically distributed as chi-square with 4° of freedom (for the difference in the number of parameters).
2.1.3. Subjects
The subjects in Experiment 1 were the author (initials MJM; Age 68) and a male (initials MT; age < 30), psychophysically-experienced colleague who was aware of the purpose of the experiment. Additional observations were taken with the zero pedestal condition by a male graduate student (initials AT; age < 30), also aware of the purpose of the experiment. The subjects in Experiment 2 were a female colleague (Initials ‘ABC’ age < 30), aware of the purpose of the experiment, and a female language student (initials ‘DEF’ age < 30) who was not told the purpose of the experiment. The latter two subjects did not wish to be identified by their real initials.
3. Results and discussion
4.1. Results for contrast detection
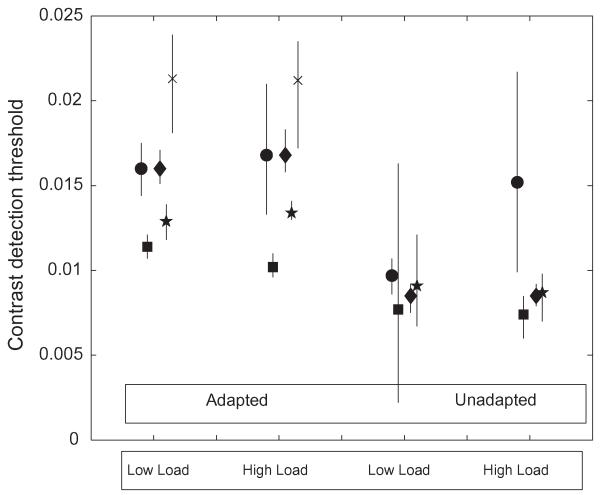
Contrast detection refers to the condition where the pedestal has zero contrast; in other words one of the 2AFC intervals is empty. This is a simple detection task. Results for this condition were collected for five subjects in all, and they provide a strong test of the prediction that adaptation will be reduced under high load. The data for detection thresholds alone for all five subjects are summarized in Fig. 2. They show no consistent or significant effect of load on detection threshold. They do, on the other hand, show the threshold elevation due to adaptation. An anomalous result is the high threshold in the unadapted/high load condition for subject ‘DEF’. This result is unexplained, but goes in the opposite direction from the prediction of the load hypothesis. The 95% confidence interval for this threshold is high.
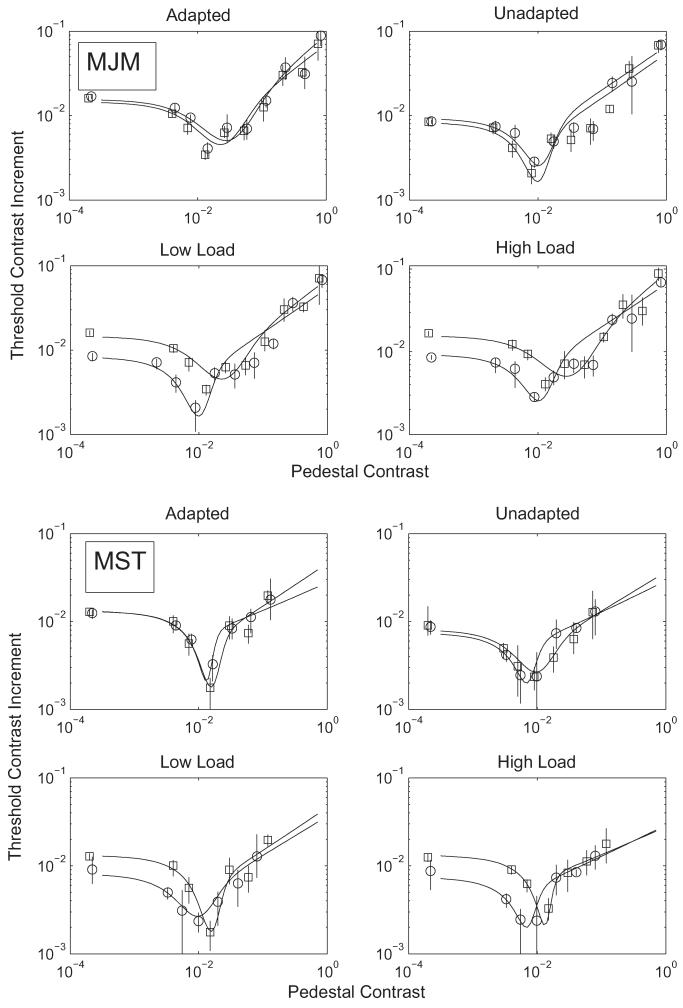
Fig. 2. The figure shows the 82% correct Threshold Contrast Increment (vertical axis) as a function of the pedestal contrast (horizontal axis).
The leftmost most point in each function was determined with a zero pedestal, and is the detection threshold for the grating. In the panels labelled ADAPTED and UNADAPTED, square symbols and dashed lines show results for the Low Attentional Load condition and circles/solid lines show the High Load condition. In the panels labelled LOW LOAD and HIGH LOAD, square symbols show results for the adapted condition and circles show the unadapted condition. The top four panels show the results for observer MJM and the bottom four show MST. The curves are the best fitting 4 parameter fits to the data. Note that the fits are not to the threshold points directly, but to all the stimulus values and their associated response probabilities used to determine those thresholds. The error bars show 95% confidence intervals.
4.2. Results for contrast discrimination: Experiment 1
Contrast discrimination differs from the detection task just described in that there is a stimulus present in both intervals of the 2AFC. The lower contrast stimulus is the pedestal, the higher contains the pedestal plus the test. The subject has to report which stimulus is of higher contrast. Adaptation is expected to have complex effects on discrimination thresholds, elevating them at some pedestal contrasts and reducing them at others (see Section 1). However, the effect of adaptation on the detection point (zero pedestal) is expected to be a simple elevation. As is conventional, we include the results of detection along with discrimination, assigning a notional contrast of 10−4 to the pedestal in this condition. Thresholds for detection are based on the same data as those in Fig. 1. The complete discrimination functions under High and Low Attentional Load and under Adapted-Direction and Unadapted-Direction for the test are shown in Fig. 2.
Fig. 1. The figure plots contrast detection thresholds (vertical axis; Michelson units) in four different conditions (labels on horizontal axis.).
Error bars show 95% confidence limits, based on 80 exact replications of the QUEST procedure in the experiment with the maximum-likelihood parameters of the psychometric function. Data for subjects MJM, MT, ABC, DEF and AT are shown with diamonds, stars, squares, circles and crosses respectively. Subject AT was not tested in the Diff (oppositely moving adaptor and test) condition.
The data show the expected effect of moving the dipper function for the adapted direction upwards and rightwards relative to the unadapted direction of test. This difference was confirmed by a likelihood analysis combining the load conditions, and comparing a single fit to the combined same/different data with the summed likelihoods of the separate fits (X2 = 129.8 (MM) and 103.8 (MT); df(4); p < 0.001). However, there was no systematic effect of the perceptual load. The impression that there was no systematic effect is confirmed by the Likelihood analysis in Table 1. None of the comparisons between combined and separate Low/High fits are significant, with the exception of MM in the different condition, where the increase in the b value in the High load condition was contrary to prediction. The Table also shows a highly significant effect of adaptation, which disappears if the b parameter is allowed different values in the same vs different adaptation conditions.
Table 1. The table shows maximum likelihoods fits of the 4 parameter {a p b q} model to the data, and the −Log Likelihood values (−L) associated with each fit.
Each rows shows the fit to a different set of data. Key: ‘Same’ refers to the condition where test and adaptor move in the same direction; ‘Different’ to the case where they are opposite. ‘Lo’ refers to the Low load case during adaptation, ‘Hi’ to the High load case. ‘Both’ means that either the two load conditions, or the two direction conditions, have been combined into a single fit. The final column shows the chi-square statistic associated with the comparison between the likelihood of the ‘both’ fits to the likelihoods of the separate fits. A single asterisk against a Chi-square value indicates a significant difference at the p < 0.05 level, and three asterisks indicate p < .001. No asterisk means that the value is not significant (p > 0.05). The last two lines for each subject show the results of constrained fits to the Same/Diff conditions where only one of the 4 parameters (a,p,b or q) was allowed to vary.
| MJM | a | p | b | q | L | χ 2 |
|---|---|---|---|---|---|---|
| 1. Same Lo | 40.611 | 2.5437 | 0.0341 | 0.3657 | 1424.1 | |
| 2. Same Hi | 39.144 | 2.4518 | 0.0379 | 0.35 | 1267.40 | |
| 3. Diff Lo | 48.7218 | 4.9983 | 0.0109 | 0.46 | 592.77 | |
| 4. Diff Hi | 39.4174 | 4.1639 | 0.012 | 0.47 | 654.44 | |
| 5. Same Both | 39.456 | 2.587 | 0.034 | 0.36 | 2693.70 | 7.0 |
| 6. Diff Both | 43.7411 | 2.4729 | 0.0162 | 0.41 | 1256.00 | 17.58** |
| 7. Lo Both | 42.5408 | 2.2945 | 0.0324 | 0.37 | 2076.30 | 118.06*** |
| 8. Hi Both | 38.2863 | 2.1692 | 0.0382 | 0.34 | 1983.30 | 122.92*** |
| Vary | a | p | b | q | ||
| 5+6 | 4014.6 | 3975.8 | 3950.9 | 3997.9 | ||
| χ 2 | 129.8*** | 52.2*** | 2.4 | 96.4*** | ||
| MST | ||||||
| 1. Same Lo | 52.637 | 6.3858 | 0.02 | 0.502 | 397.76 | |
| 2. Same Hi | 64.325 | 8.9893 | 0.0141 | 0.68 | 398.81 | |
| 3. Diff Lo | 60.2913 | 3.458 | 0.0111 | 0.55 | 310.43 | |
| 4. Diff Hi | 65.3214 | 6.2559 | 0.01 | 0.6376 | 286.38 | |
| 5. Same Both | 57.18 | 6.26 | 0.0155 | 0.5913 | 799.67 | 6.2 |
| 6. Diff Both | 66.54 | 4.7 | 0.0082 | 0.64 | 600.89 | 8.152 |
| 7. Lo Both | 53.7303 | 3.9664 | 0.0158 | 0.54 | 744.91 | 73.4276*** |
| 8. Hi Both | 58.7882 | 3.1679 | 0.0131 | 0.64 | 714.07 | 57.755*** |
| Vary | a | p | b | q | ||
| 5+6 | 1452.2 | 1425.6 | 1402.5 | 1437.1 | ||
| χ 2 | 103.28*** | 50.08*** | 3.88 | 73.08*** | ||
To confirm that the high-load task was more difficult than the low-load, the error rates and reaction times were analyzed. For MM the error rate (missed targets) was 0 for the Low load task (detecting red crosses) and 35.96% for the high-load task (detecting two different conjunctions of colour and shape). The mean RT’s were 0.99 s and 1.37 s respectively (t-test: p < 0.0001). For MST the data were somewhat different. His error rate was also zero for the low-load task but a surprisingly low 4.49% in the High-load task. This was despite the fact that the conjunctions of colour and shape were randomly chosen in each session and the subject had to infer the nature of the conjunctions from their rarity. MST seems to be unusually gifted at this task. Nevertheless, the difference in his RT’s between the two tasks was highly significant (0.954 vs. 1.206 s, p < 0.0001).
We tried two further conditions in an unsuccessful attempt to find an effect of attentional load. In the first, the low load condition was replaced by a ‘no load’ condition in which there were no red targets in the RSVP stream and the subject had no task to perform. Instead, he (MST) attended to the adapting grating. The pedestal value was zero (the detection task). Two Quest sessions under this condition (100 trials) were compared to the last two collected in the high load condition, zero pedestal. The thresholds were virtually identical in the two conditions (0.125) and the difference in likelihood between separate and combined fits was not significant (X2 = 2 − (91.3747 − 42.9814 − 48.2918) = 0.203 NS). In the second unsuccessful modification we examined the effects of spatially-directed attention. The single adapting stimulus was replaced by two smaller patches to either side of the fixation point, drifting in the same direction, and the subject (MM) attended to the one on the right. To ensure attention, the subject had to detect and respond to brief, small contrast increments in the right-hand stimulus. After each adapting period, an arrow appeared to indicate to the side on which the test would appear, but the subject maintained fixation. Half the tests were on the right and half on the left, in random order. We expected thresholds to be lower on the unattended side but this was not the case. The mean thresholds over sessions on left and right were .0257 and .0265 respectively. The hit-rate for 770 targets distributed over six Quest sessions (300 trials) was 48.9%.
4.3. Results for contrast discrimination: Experiment 2
In a further attempt to find an effect of attentional load the experiment was repeated with two further subjects, one of whom (‘DEF’) knew nothing (as far as we know) about the purpose of the experiment. The other (‘ABC’) was a psychophysically experienced colleague. The high load task was made even more difficult than before by changing the stimuli from T’s to L’s and including all four mirror images (horizontal and vertical). Half the stimuli were white and half were gray. Four of the eight colour-shape combinations were targets and four were distractors. The eight stimuli were presented with equal frequency, making the target frequency higher than in Experiment 1. The low-load task was the same as before (respond to red). Because of limited availability of the subjects, fewer pedestal levels were used and for most pedestals only two QUEST sessions of 50 trials each were run. A further difference from Experiment 1 is that subject ‘DEF’ was tested with a zero-contrast adaptor rather than an oppositely moving adaptor to the test. This was because she did not show significant directionally-specific adaption, an unusual individual difference that is worth noting. ‘DEF’ did show a significant adaptation effect relative to a zero-contrast adaptor. Subject ‘ABC’ was tested with oppositely moving tests and adaptors, as in Experiment 1.
The error rates in the High-load condition confirmed that it was indeed a difficult task for the subjects, as they asserted. The overall rate of targets missed was 21% for subject ABC and 35% for DEF. The error rate in the low-load condition was virtually zero. There were also significantly more false positives in the low load condition. As in Experiment 1, Reaction times were significantly faster in the low load condition.
The contrast discrimination results shown in Fig. 3 are similar to those in the previous experiment. Adaptation had the usual effect of shifting the T vs. C function upwards and rightwards, but there was no obvious and systematic effect of load. A likelihood analysis confirmed the highly significant effect of adaptation (p < .001) but none of load.
Fig. 3. Results of Experiment 2 for subjects ABC (top) and DEF (bottom).
The top row compares low load and high load conditions. The bottom row compares adapted and unadapted conditions. Note the rightwards and upwards shift due to adaptation. Conventions as in Fig. 2.
4. General discussion
We failed to find a systematic and significant effect of distraction on the movement after-effect, and the mechanism for the effects previously reported in the literature remain a mystery. Our results support Wohlegemuth’s conclusion that the mechanisms of motion adaptation are pre-attentional. It is worth pointing out that in other cases where negative results have been reported (Georgiades & Harris, 2002; Rees, Frith, & Lavie, 2001), they have been ascribed to a difference in conditions, rather than entertaining the possibility that the positive results are Type I errors. There is also likely to be a positive reporting bias in the literature (Rosenthal, 1979)1. We have been informed of at least one case (N. Wade, personal communication) where a negative result with 20 subjects was unreported, and of another (P. Thompson, personal communication) where a positive result has been so far unreported. The author would like to carry out a meta-analysis of experiments on attentional modulation of adaptation, and would be grateful for any hitherto unpublished material.
The great majority of experiments on the effects of attention on adaptation, including Wohlgemuth (1911) have used the duration measure of the after-effect, a measure known to be highly susceptible to experimenter/subject bias (Sinha, 1952)2. It is hard to know when a stimulus has stopped moving, particularly when it is stationary. The importance of individual criterion setting is attested to by the report (Spitz, 1966) that no less than 19 subjects had to be discarded because they pressed the ‘stop’ button as soon as the adaptor finished moving. The unstable nature of the duration measure was also note by Granit (1928) and Grindely (1930). The small, constant increase in MAE duration, independent of adapting contrast, reported by Rezec et al. could as easily be explained by a small unconscious response bias as by a change in sensitivity.
The problem of bias is not necessarily overcome by using forced-choice to determine whether a test stimulus is seen as moving left or right. This is still a measure of bias, measured from a shift in the psychometric function. Taya et al., 2009 measured the bias in left-right perceived movement after adaptation with high and low load and found an effect. The real movement required to null the perceived movement was remarkably small: 0.5 pixels/frame, corresponding to a velocity of 1.9 arcmin/s. This is very close, if not under, the reported movement velocity threshold of 2 arcmin for a 500 ms exposure (Boulton, 1987). In other words, the stimuli must have appeared almost stationary. In these circumstances, a small bias towards reporting movement against the adapting stimulus, could have shifted the psychometric function by a small amount. The effect was similar in magnitude to that reported by Winaware, Huk, and Boroditsky (2010) for the effect of purely imaginary adapting motion.
The adaptation effect of attentional tracking (Alais & Blake, 1999; Lankheet & Verstraten, 1995) have been used to support the idea that distraction reduces adaptation (Rezec, Krekelberg, & Dobkins, 2004; Taya et al., 2009). But these experiments did not use a distraction paradigm or manipulate attentional load. Instead, they showed that there is an aftereffect of attentional tracking one component in an ambiguous display, an effect that may be different from the conventional MAE, for example in not being retinotopic (Culham et al., 2000; Freeman & Sumnall, 2005; Freeman, Sumnall, & Snowden, 2003). These tracking studies do not directly show that attention to a single-component stimulus increases its adapting effect. A possible synthesis is that some motion aftereffects combine a low-level adaptation with attentional tracking, and that the latter, but not the former, is susceptible to distraction. Overt rather than attentional tracking may also be a factor, since eye movements have not been measured in the attentional load studies. However, both Rezec, Krekelberg, and Dobkins (2004) and the present study used one-dimensional sinusoidal adapting stimuli, so it is unlikely to be the nature of the adapting stimulus that explain the difference between positive and negative results.
Likewise, it is important to distinguish the effects of distraction during adaptation from more general claims about the effects of attention on motion processing, for example, that motion thresholds can be lower for an unattended than an attended stimulus (e.g. Allen & Ledgeway, 2003; Huang & Dobkins, 2005). The critical aspect of the distraction/adaptation task is that it separates the attentional manipulation from the detection task in time, and does not, therefore, suffer from the response competition and memory problems inherent in the dual-task paradigm. This is why the distraction/adaptation paradigm is especially interesting, and why it should be assessed in its own right, rather than bolstered by evidence that speaks to different issues.
Footnotes
‘For a given research are one cannot tell how many studies have been conducted but never reported. The extreme view of the ‘file drawer’ problem is that Journals are filled with the 5% of the studies that show Type 1 errors, while the file drawers are filled with [the other] 95%’.
Sinha remarks: ‘By carefully arranging the experimental set-up and by clever instructions, social factors can be generated in the laboratory. The observers may not be aware of any such influence. It, however, acts on the individual imperceptibly.’ Sinha’s own experiment cleverly measured the mean and variance of the MAE in a group of observers, and then tested them a second time after casually misinforming them of the mean of the previous MAE durations. The second set shifted towards the arbitrary group standard and showed less variance than before. The study was conducted in the Cambridge University Psychological Laboratory and I thank Donald Laming for bringing it to my attention.
References
- Alais D, Blake R. Neural strength of visual attention gauged by motion adaptation. Nature Neuroscience. 1999;2(11):1015–1018. doi: 10.1038/14814. [DOI] [PubMed] [Google Scholar]
- Allen HA, Ledgeway T. Attentional modulation of threshold sensitivity to first-order motion and second-order motion patterns. Vision Research. 2003;43(27):2927–2936. doi: 10.1016/j.visres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Blake R, Tadin D, Sobel KV, Raissian TA, Chong SC. Strength of early visual adaptation depends on visual awareness. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(12):4783–4788. doi: 10.1073/pnas.0509634103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton JC. Two mechanisms for the detection of slow motion. Journal of the Optical Society of America. A, Optics and Image Science. 1987;4(8):1634–1642. doi: 10.1364/josaa.4.001634. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Chaudhuri A. Modulation of the motion aftereffect by selective attention. Nature. 1990;344:60–62. doi: 10.1038/344060a0. [DOI] [PubMed] [Google Scholar]
- Culham JC, Verstraten FA, Ashida H, Cavanagh P. Independent aftereffects of attention and motion. Neuron. 2000;28(2):607–615. doi: 10.1016/s0896-6273(00)00137-9. [DOI] [PubMed] [Google Scholar]
- Efron B. The Jackknife, the bootstrap and other resampling plans. Society for Industrial and Applied Mathematics; Philadelphia: 1982. [Google Scholar]
- Foley JM. Human luminance-pattern vision mechanisms: Masking experiments require a new model. Journal of the Optical Society of America. A, Optics and Image Science. 1994;11:1710–1719. doi: 10.1364/josaa.11.001710. [DOI] [PubMed] [Google Scholar]
- Foley JM, Legge GE. Contrast detection and near-threshold discrimination in human vision. Vision Research. 1981;21:1041–1053. doi: 10.1016/0042-6989(81)90009-2. [DOI] [PubMed] [Google Scholar]
- Freeman TC, Sumnall JH. Extra-retinal adaptation of cortical motion-processing areas during pursuit eye movements. Proceedings of the Royal Society. Series B, Biological Sciences. 2005;272(1577):2127–2132. doi: 10.1098/rspb.2005.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman TC, Sumnall JH, Snowden RJ. The extra-retinal motion aftereffect. Journal of Vision. 2003;3(11):771–779. doi: 10.1167/3.11.11. [DOI] [PubMed] [Google Scholar]
- Georgiades M, Harris J. Evidence for spatio-temporal selectivity in attentional modulation of the motion aftereffect. Spatial Vision. 2002;16(1):21–31. doi: 10.1163/15685680260433887. [DOI] [PubMed] [Google Scholar]
- Granit R. On inhibition in after-effect of seen movement. British Journal of Psychology. 1928:XIX. [Google Scholar]
- Greenlee MW, Heitger F. The functional role of contrast adaptation. Vision Research. 1988;28(7):791–797. doi: 10.1016/0042-6989(88)90026-0. [DOI] [PubMed] [Google Scholar]
- Grindely G. Rod and cone aftereffects. Journal of Physiology. 1930;LXIX:53–59. [Google Scholar]
- Hoel PG, Port SC, Stone CJ. Introduction to statistical theory. Houghton Mifflin; Boston: 1971. [Google Scholar]
- Huang L, Dobkins KR. Attentional effects on contrast discrimination in humans: Evidence for both contrast gain and response gain. Vision Research. 2005;45(9):1201–1212. doi: 10.1016/j.visres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Kohn A, Movshon J. Neuronal adaptation to visual motion in area MT of the Macaque. Neuron. 2003;39:681–691. doi: 10.1016/s0896-6273(03)00438-0. [DOI] [PubMed] [Google Scholar]
- Lankheet MJ, Verstraten FA. Attentional modulation of adaptation to two-component transparent motion. Vision Research. 1995;35(10):1401–1412. doi: 10.1016/0042-6989(95)98720-t. [DOI] [PubMed] [Google Scholar]
- Legge GE, Foley JM. Contrast making in human vision. Journal of the Optical Society of America. 1980;70(12):1458–1471. doi: 10.1364/josa.70.001458. [DOI] [PubMed] [Google Scholar]
- Morgan M, Chubb C, Solomon J. Predicting the motion after-effect from sensitivity loss. Vision Research. 2006;46:2412–2420. doi: 10.1016/j.visres.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Chubb C, Solomon JA. Evidence for a subtractive component in motion adaptation. Vision Research. 2011;51:2312–2316. doi: 10.1016/j.visres.2011.09.002. doi:10.1016/j.visres.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Morgan M. Motion adaptation does not depend on attention to the adaptor. Vision Research. 2012;15:47–51. doi: 10.1016/j.visres.2011.12.009. Ms. No.: VR-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael S, Dillenburger B, Morgan MJ. The effect of attentional modulation on adaptation to transparent expanding/contracting motion. Society for Neuroscience; San Diego, CA: 2010. (Program No. 172.12/II17 2010 Neuroscience Meeting Planner). [Google Scholar]
- Rees G, Frith CD, Lavie N. Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science. 1997;278:1619. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- Rees G, Frith C, Lavie N. Processing of irrelevant visual motion during performance of an auditory attention task. Neuropsychologia. 2001;39(9):937–949. doi: 10.1016/s0028-3932(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Rezec A, Krekelberg B, Dobkins KR. Attention enhances adaptability: Evidence from motion adaptation experiments. Vision Research. 2004;44(26):3035–3044. doi: 10.1016/j.visres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The File Draw problem and tolerance for null results. Psychological Bulletin. 1979;86:638–641. [Google Scholar]
- Ross J, Speed H, Morgan M. The effects of adaption and masking on incremental thresholds for contrast. Vision Research. 1993;33:2050–2056. doi: 10.1016/0042-6989(93)90003-f. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J. Attentional load and sensory competition in human vision: Modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cerebral Cortex. 2005;15(6):770–786. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- Sinha D. An experimental study of a social factor in perception: The influence of an arbitrary group standard. Patna University Journal. 1952;6(1):7–16. [Google Scholar]
- Spitz H. Differential effects of central and lateral fixation on after-effects of expansion and contraction. The America Journal of Psychology. 1966;79(4):618–622. [Google Scholar]
- Stromeyer CF, III, Klein S. Spatial frequency channels in human vision as asymmetric (edge) mechanisms. Vision Research. 1974;14(12):1409–1420. doi: 10.1016/0042-6989(74)90016-9. [DOI] [PubMed] [Google Scholar]
- Taya S, Adams W, Graf E, Lavie N. The fate of task-irrelevant visual motion: Perceptual load versus feature-based attention. Journal of Vision. 2009;9(12):1–10. doi: 10.1167/9.12.12. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: A Bayesian adaptive psychometric method. Perception and Psychophysics. 1983;33(2):113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Winaware J, Huk AC, Boroditsky L. A motion aftereffect from visual imagery of motion. Cognition. 2010;114:276–284. doi: 10.1016/j.cognition.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth A. On the aftereffect of seen movement. British Journal of Psychology, Monograph, Supplement. 1911;1:1–117. [Google Scholar]