Abstract
Prolonged adaptation to a stimulus, such as a drifting grating, lowers sensitivity for detecting similar stimuli, and changes their appearance, for example, making gratings of the same orientation appear of lower contrast and move more slowly. It has been suggested that adaptation is increased by sustained attention to the adapting stimulus and is decreased by distracting attention with a competing task. This paper describes a novel 2AFC (spatial) measure of adaptation in which adaptation and bias are carefully distinguished by the random interleaving of different test conditions. The experiment revealed significant adaptation of perceived velocity, but no effect of attentional distraction.
Keywords: adaptation, aftereffects, attention
Introduction
The “attentional load” theory (Lavie, 2005) states that if attention is drawn by one stimulus, there is less attention available for another. If sustained attention increases the magnitude of sensory adaptation (Beck, Rees, Frith, & Lavie, 2001; Chaudhuri, 1990; Rezec, Krekelberg, & Dobkins, 2004; Taya, Adams, Graf, & Lavie, 2009), load theory predicts that distracting attention from an adapting stimulus will decrease its adapting effect. A key experiment for load theory (Rees, Frith, & Lavie, 1997) showed this to be true for the movement after-effect caused by large-field moving stimuli with a distracting stimulus placed foveally in its center. Furthermore, the same study showed that directing attention toward or away from a spatially-located visual target has large and robust effects on the blood oxygenation level–dependent (BOLD) effects in the human primary visual cortex (Rees et al., 1997; Schwartz et al., 2005; Watanabe et al., 2011), suggesting that sustained attention to a stimulus is equivalent to increasing its contrast (Ling & Carrasco, 2006).
However, there are problems with this reasoning. The first is the absence of a clear psychophysical linking hypothesis (Brindley, 1960) relating the level of the BOLD signal to the strength of adaptation. Presumably, the linking hypothesis is that the positive BOLD signal is correlated with increased neural activity and that increases in neural activity are positively correlated with adaptation. If this is so, attending to an adaptor should have an effect on adaptation equivalent to an increase in its contrast, but this was not demonstrated in the experiment (Rees et al., 1997), which used only the same high-contrast (100%) adaptor in both attentional conditions. The strength of the motion after-effect saturates very rapidly as contrast is raised (Blake, Tadin, Sobel, Raissian, & Chong, 2006; Morgan, Chubb, & Solomon, 2011; Rezec et al., 2004), so it is implausible that the effect of the high-contrast adaptor would be significantly modulated by attention. The linking hypothesis thus justifies skepticism, particularly since it involves the counterintuitive notion that attending to a stimulus over a period of time reduces its effective contrast.
The second problem is that the behavioral effect of attention in increasing adaptation is by no means clearly established. Some studies have found an effect (Beck et al., 2001; Chaudhuri, 1990; Rezec et al., 2004; Taya et al., 2009), but others have found no effect (Morgan, 2011, 2012; Rees, Frith, & Lavie, 2001; Wohlgemuth, 1911), while yet others have found both positive and negative results in conceptual replications (Georgiades & Harris, 2002; Nishida & Ashida, 2000). A problem with most of the experiments is that they have used behavioral measures known to be susceptible to observer cognitive bias and expectation (see Discussion).
Morgan, Dillenburger, Raphael, and Solomon (2012) have shown that the standard method of single stimuli (MSS) for measuring the effects of adaptation cannot distinguish between genuine perceptual biases and a change in decisional criterion. Subjects can voluntarily shift their psychometric functions without changing their slope. The strategy here is to design a test procedure such that it is difficult for a subject to influence the effect of adaptation with a response or decisional bias, even if they know the expected direction of adaptation. As pointed out elsewhere (Garcia-Perez & Alcala-Quintana, 2012) a good start can be made in removing response bias by using 2AFC rather than MSS. The test stimulus can be added either to an adapted or an unadapted location, or even better to two differently-adapted locations. The 2AFC design can be refined by randomly interleaving different conditions of test such a change in adaptation state does not correspond to a simple change in decisional criterion.
This paper presents a proof-of-concept of the multicondition 2AFC design for measuring adaptation. We use the reduction in perceived velocity of a test stimulus after adapting to a stimulus moving in the same direction as the test (Thompson, 1981). The subject is adapted to two vertically-aligned moving grating patches moving in opposite directions, and tested in a two-alternative forced procedure (2AFC spatial) procedure with patches in which the physical speed difference is varied between the top and bottom patches (Figure 1). The subject has to decide which is moving more quickly.
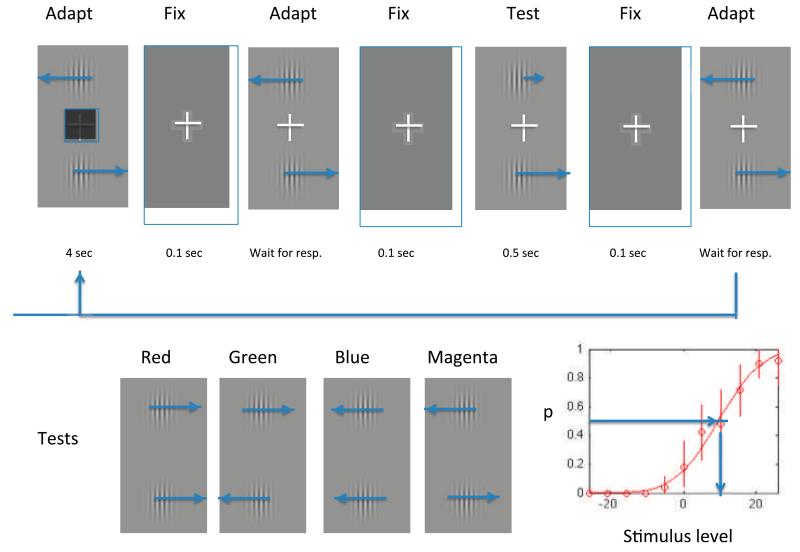
Figure 1.
The timeline at the top of the Figure shows the sequence of events in a single trial, which begins with a 4-s presentation of the adapting gratings during which the size of the black square in the center varies pseudorandomly over time, and subjects have to decide whether the average size on that trial is greater or smaller than a standard size. They indicate their decision by pressing either the up (greater) or down (smaller) arrow key on the keyboard, as soon as possible when the adapting gratings re-appear without the central square. Their key press triggers another 0.1-s blank interval followed by the test stimulus. The subject then makes a key press as quickly as possible to indicate which of the two stimuli (top or bottom) is moving more quickly, and their key press initiates the next trial. The lower half of the Figure shows the configurations of the test stimuli, which have all four possible combinations of direction in the top and bottom patches. In two of the tests (1 and 3) the patches move in the same direction and adaptation is expected to affect their perceived relative speed. In the other two tests (2 and 4) the stimuli move in opposite directions and adaptation is not expected to affect their relative speed. The Legend above each test shows the color in which the condition is coded in later Figures. The inset at the bottom right shows an illustrative psychometric function, from which the value of the mean (μ) is derived from the 0.5 point as shown, and the just-noticeable-difference is derived from the standard deviation (σ) of the best-fitting cumulative Gaussian function.
Methods
The trial sequence and the four test stimuli are illustrated in Figure 1.
The four test conditions were randomly interleaved over a series of 256 trials, and contained all four possible combinations of movement of the top and bottom patches. In two of these (Tests 1 and 3) the two patches move in the same direction. In these cases, if adaptation affects perceived velocity we expect the patch moving in the same direction as the adaptor to appear to move more slowly. In Test 1 this will be the lower patch, in Test 3 it will be the upper patch; thus any response bias towards upper or lower patch will cancel out between the two kinds of tests. In the other two cases (Test 2 and 4) the test patches move in opposite directions, as do the adapting patches; therefore no effect of adaptation on relative velocity of the upper and lower patches would be expected. The effect of this complex design is that subjects would have to adopt a similarly complex branching strategy to bias their performance in the direction expected from a genuine sensory adaptation. They would have to adopt no bias in the case of Tests 2 and 4, and opposite biases in the case of Tests 1 and 3. The perceptual task, on the other hand, was simple: to decide whether the top or bottom patch moved more quickly.
In the distracting task to manipulate perceptual load the subject had to decide whether the mean size of a set of rapidly presented black squares in the center of the fixation cross during adaptation was larger or smaller than the sampled mean of all squares previously seen in the experiment. The set of sizes on a particular trial was drawn from a uniform distribution with an expected value that varied randomly between trials. In the control task the squares were absent and the subjects attended to the adapting stimuli. For details see the section on Stimuli.
Apparatus
Stimuli were presented on the LCD display of a MacBookPro laptop computer (Apple Inc., Cupertino, CA) (refresh rate 60 Hz) with screen dimensions 33 cm × 20.7 cm (1440 × 900 pixels) viewed at 0.57 m so that 1 pixel subtended 1.25 arcmin visual angle. The background screen luminance was 50 cd/m2. Stimulus presentation was controlled by MATLAB and the PTB3 version of the Psychtoolbox (Brainard, 1997). Stimuli were viewed binocularly through natural pupils with appropriate corrective lenses for each subject if normally worn. Testing took place in a dimly lit room.
Subjects
The subjects in Experiment 1 were the (male) author (S1), a male colleague (S2), a male postdoctoral vision researcher (S3), a female PhD student (S4), and a male paid student volunteer (S5). Two additional female postdoctoral researchers (S6 and S7) took part in Experiment 2. An added näive female undergraduate volunteer (S8) was unable to perform in Experiment 1 due to visual discomfort caused by the moving adaptor and flashing squares, but served in Experiment 2. All of the subjects other than the author were younger than 60 years. None of the subjects other than the author knew that there were four interleaved tests, or what their significance was.
Stimuli
The adapting and test stimuli consisted of moving vertical sinusoidal gratings (spatial frequency 2.4 c/°) windowed with a stationary Gaussian envelope (standard deviation 0.625°) and drifting at a rate of 45 deg of phase angle (π/4) every frame, equivalent to a temporal frequency of 7.48 Hz. Mean luminance of the grating was 50 cd/m2 and the Michelson contrasts of adaptor and test were 52% and 26% respectively. A large white fixation cross was present in the centre of the screen with the two stimuli placed symmetrically above and below at distances of +/− 3.125° (see Figure 1).
The squares in the distracting task were presented at and overlapped transparently with the center of the fixation cross. The squares were black (minimum luminance). Presentation rate was 2 Hz with a 50% duty cycle. Thus each square was presented for 0.25 s followed by a 0.25-s blank interval before the next square. The average (expected value) square size throughout the experiment was 1.098. On any given trial a value m was sampled from a uniform distribution U with limits 0.81° and 1.37°. Then on each presentation the actual square size was sampled from a uniform distribution with limits m and m+0.575°. In effect this meant that the squares size varied by approximately 50% of their mean size throughout the experiment.
The orientation change that the subjects had to detect in the secondary task was a ramped increase in orientation of the adaptor over eight frames followed by a decrease back to zero over the next eight frames. The incremental change in orientation per frame was initially set at 0.5° but was altered over trials by a staircase, such that two successive “hits” decreased the increment by 0.25 (with a floor of 0) and either a false positive or a “miss” increased it by 0.25. The probability of the change occurring in a trial was 0.25 (without replacement) and the change could start randomly at any point in the trial between 0 and 16 frames from the end.
Procedure
Each trial began with a 4-s presentation of the adaptor with fixation cross and flashing squares (in the high load distraction condition). After 4 s the screen went blank (mean luminance) for 0.1 s and then reappeared with only the fixation cross and moving adaptors. This was the cue to the subject to press a key on the computer keyboard (up arrow or down arrow) to indicate whether the squares had been larger or smaller than the overall average. (In the low load condition, subjects pressed either key). Immediately the key was pressed, which subjects were instructed to do as quickly as possible, the two test stimuli appeared for 0.5 s. At the end of the test presentation the screen went blank once more for 0.1 s and then reappeared with only the fixation cross and moving adaptors. This was the cue to tell the subject to press a key to indicate whether the upper or lower test was faster. As soon as the key had been pressed (as quickly as possible) a square was presented at fixation for 0.5 s, and then the next trial began with reappearance of the adaptor and fixation cross. In the baseline training condition the square was white or black to indicate a correct and incorrect response respectively. In adaptation sessions it was a neutral gray.
In summary, the sequence was: Adapt-Respond-Test (0.5 s)-Respond-Adapt (4 s)—and so on (Figure 1). Every 64 trials after the second response the sequence was interrupted with a screen instructing the observer to take a rest. After 30 s a message appeared on the screen instructing the observer to “press any key to resume the experiment.” They could wait for as long as they liked and stretch their legs before doing so.
Further sessions were run after completion of the main experiment in which subjects attempted to detect brief orientation changes in the adapting stimulus while also carrying out the square averaging and control tasks. The purpose of this was to see whether the high-load task was successful in distracting attention from the adapting gratings. For details see the section on Stimuli.
Psychophysics and data analysis
The procedure was 2AFC (spatial) with a test that was always slower than the standard by an amount abs (DV) and which was presented randomly in the upper or lower position according to the sign of ΔV. [cf. 2AFC methodology for contrast discrimination (Morgan et al., 2011)]. In each block of trials the four test conditions (see Figure 1) were randomly interleaved until each had been presented 64 times. Each test was controlled by an independent adaptive procedure designed to select a velocity difference ΔV with a value placed at +/− 1 standard deviation around the mean of the subject“s psychometric function (Watt & Andrews, 1981). The standard moved with velocity V and the test with a velocity V-abs (ΔV). In other words the test always moved more slowly than the standard, this being a 2AFC procedure. If the value of ΔV was positive the test was in the top position, if it was negative the test was in the bottom position. The observer pressed a key on the keyboard to indicate whether the top of bottom stimulus appeared to move more quickly, irrespective of its direction. In other words, speed was reported, not velocity.
The resulting psychometric functions, plotting the probability of an “upper” response against ΔV were fit with a cumulative normal distribution to extract the maximum-likelihood values for the parameters μ and σ, representing the subject’s bias and sensitivity respectively. The value of the mean (μ) is derived from the 0.5 point as shown, and the just-noticeable-difference is derived from the standard deviation (σ) of the best-fitting cumulative Gaussian function.
An illustrative example is shown in the inset to Figure 1. Confidence limits for these parameters were obtained by a bootstrapping procedure in which the experiment was rerun with a simulated observer having the previously-determined maximum-likelihood values for μ and σ. The experiment was simulated 160 times and the exact 95% confidence limits for the resampled values of μ and σ were calculated.
Results
Experiment 1: High contrast adaptation
In this first experiment the adapting stimulus had 52% contrast in order to produce a robust adaptation effect. For further details see Methods.
Baseline results
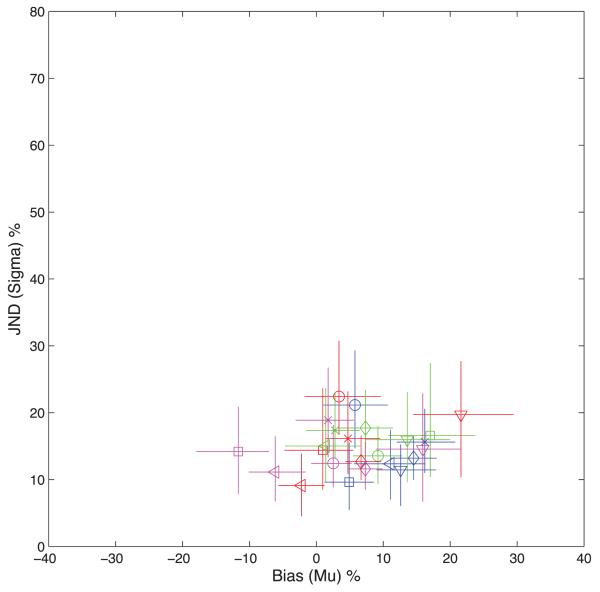
Baseline measurements were taken with a stationary adapting stimulus and showed that subjects could discriminate the test-standard velocity difference ΔV with a just-noticeable-difference (JND) of 14.9% (average over conditions and subjects). The JND is defined as σ of the psychometric function. There was an overall bias in the direction of reporting the stimulus below fixation, and thus in the lower visual field, as being slower than the upper (μ = 6.7 % averaged over all subjects and all four test conditions). All six subjects showed a bias in the same direction, averaged over test conditions. There were no systematic differences between test conditions over subjects. The complete dataset for individual subjects and test conditions with 95% confidence limits is shown graphically in Figure 2 in the form of a plot of σ against μ.
Figure 2.
The Figure shows the results for the baseline measurements with a stationary adaptor. Each point shows the Bias (abscissa) against the JND of the psychometric function for a particular subject, indicated by symbol shape, and test condition indicated by symbol color. Results for Subjects 1–5 are indicated by circles, squares, diamonds, crosses, and triangles respectively. The significance of the colors is as explained in Figure 1. If the movement of the top and bottom test patches is denoted RR for Condition 1 (Red), then the remaining conditions are RL (Green), LL (Blue), and LR (Magenta). A positive bias indicates that the subject saw the upper visual field stimulus as moving slower than an equivalent lower visual field stimulus. The bars indicate 95% confidence limits based on 120 bootstrapping simulations of the experiment.
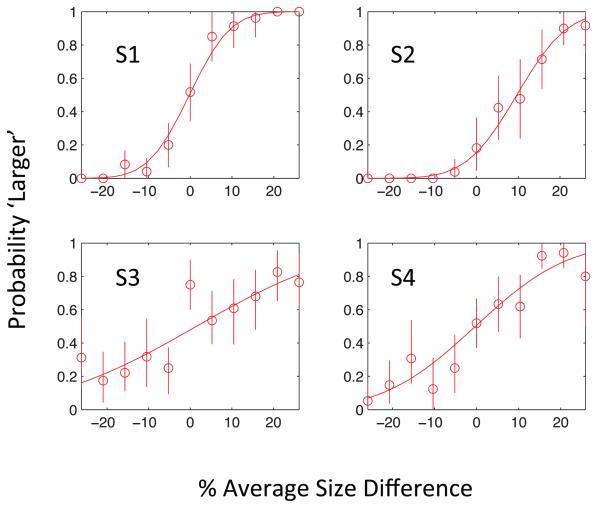
Performance in the square-averaging task
All subjects were able to perform the task, as show by the orderly psychometric functions relating mean size on a trial to the probability of a “greater” response. Illustrative examples are shown in Figure 3. There were large differences in accuracy between different subjects as shown by the σ values of the psychometric functions: (1) 7.7%; (2) 28%; (3) 9.48%; (4) 17.2%; (5) 12.3%.
Figure 3.
The Figure shows psychometric functions for the average square task used during adaptation to produce a high perceptual load. The data points show the probability (ordinate) of responding “larger” as a function of the difference in the mean value of the squares from the grand mean square size over the whole session. Vertical bars show 95% confidence limits for the binomial distribution based on the number of observations in each data point.
Was the high load task successful in distracting attention from the adaptor?
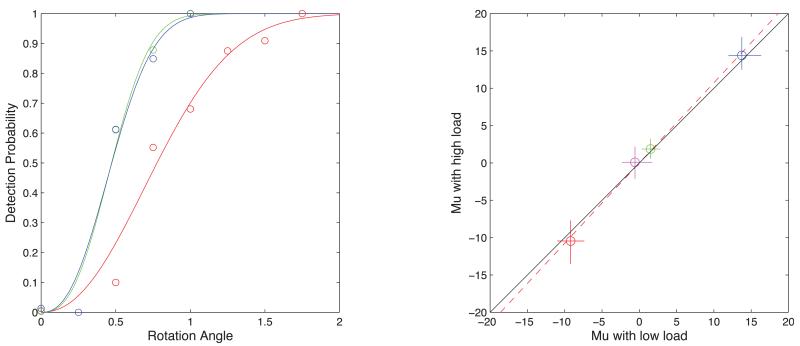
To demonstrate that the high-load task uses greater attentional resources it is conventional to show that reaction times and errors are greater than in the low-load task (Rees et al., 1997). However, the RT/Errors test does not prove anything directly about the allocation of attentional resources. Subjects could devote equal resources to the two tasks, and for that very reason respond more quickly and accurately in the low-load condition. What is required is a direct demonstration that subjects are attending less to the adaptor in the high load condition. We tested this by giving observers a secondary task of responding to a brief (16-frame) orientation change in the adaptor occurring randomly on 25% of trials at a random chosen time. Subjects were instructed to make the central square-averaging task their priority and only to respond to the orientation change if they happened to notice it. In one control condition there were no central squares and subjects gave their full attention to the adaptor; in another control the squares were present but subjects ignored them. The response to both the primary and secondary task was made at the end of the adapting period, by selecting one of four response keys. The size of the orientation cue was varied by a one-up-two-down staircase (see Methods). Results showed significantly higher error rates in the high-load task versus the control condition. A representative psychometric function in Figure 4 compares the high-load and control condition error rates. There was no significant effect of load on μ values in any of the conditions.
Figure 4.
Left panel: data for one subject (S1) detecting brief changes in the orientation of the adapting grating under three conditions. The conditions are: Red—the task was secondary to carrying out the high-load foveal task of square averaging; Green—the foveal squares were not presented and the orientation task was primary; and Blue—the foveal squares were presented but the subject was instructed to ignore them and make the orientation task primary. Right Panel: Biases (Mu) in the “Blue” low load condition plotted against those in the “Red” High load condition. (See Figure 2 caption for details).
Adaptation results
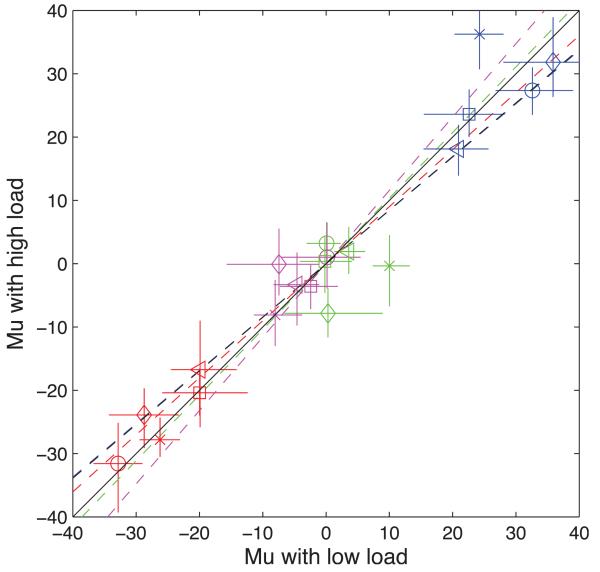
Subjects could discriminate the test-standard velocity difference ΔV with a JND of 14.7% (average over conditions and subjects). This was not significantly different from the value of 14.9% in the baseline condition. Thus, adaptation had no effect on sensitivity. It did, however, produce strong biases (μ) in the expected direction. Values of μ derived from the psychometric functions are shown in Figure 5, with different symbol shapes for different subjects and different symbol colors for test conditions. Recall from Figure 1 that there were four test conditions, consisting of all possible combinations of motion direction between top and bottom grating patches. The conditions numbered 1–4 in Figure 1 are colored Red, Green, Blue, and Magenta respectively in Figure 5. The data points show clear segregation by colour and thus by Test condition. Conditions 2 and 4 (Green and Magenta) show relatively small biases, as would be expected from the fact that the test patches move in opposite directions so that the effects of adaptation cancel out. Condition 1 (Red) shows a strong negative bias, indicating that the upper patch appeared to move more slowly. This would be expected from the fact that the upper patch in this test condition moved in the same direction as its adapting patch. Conversely, with Test 3 (blue) subjects had positive biases, meaning that the lower patch was slower. This would be expected from the fact that the lower patch moved in the same direction as its adapting patch.
Figure 5.
The Figure shows that effect of adaptation, measured from the bias (μ) of the psychometric function) did not differ as a function of perceptual load. If it had, data points would not fall on the diagonal line. The Figure also shows that the extent of adaptation did differ between the different conditions in the experiment. Results for Subjects 1–5 are indicated by circles, squares, diamonds, crosses, and triangles respectively. The significance of the colors is as explained in Figure 1. If the movement of the top and bottom test patches is denoted RR for Condition 1 (Red), then the remaining conditions are RL (Green), LL (Blue) and LR (Magenta). Each data point represents the μ value from the psychometric function in the low load task (abscissa) plotted against the value in the high load task (ordinate). The error bars are 95% confidence intervals obtained by bootstrapping with 120 experimental simulations. The colored lines show regression lines through the four test results separately for each subject. The intercept of these lines has been removed from the data so that the lines all cross zero in the middle. The black line shows the locus of points expected if the high and low load results are identical.
There was no obvious effect of perceptual load on the data, which would have been evident in departures of the data points from the diagonal. To see if there were any statistically significant effects of load, a Chi-squared test based on likelihood ratios was used (Table 2). A fit to the combined high-load and low-load psychometric functions using different values of μ for the two conditions was compared to a fit using the same value of μ. Twice the difference in log likelihoods between the two fits was assumed to be distributed as Chi-squared with 1 degrees of freedom (Hoel, Port, & Stone, 1971). If the two-μ fit is significantly better than a one-μ fit we can conclude that load has a significant effect on adaptation. A significance criterion of p < 0.001 was used in order to account of multiple comparisons. Results (Table 1) showed only one case of a significant difference, in Subject 4, and this was in a Test Condition (2) that would not be expected to show adaptation. The same analysis for values of σ showed a significant effect only for Subject 1, Condition 1.
Table 1.
Chi-squared values for the effects of perceptual load on μ values (top five lines) and σ values (bottom 5 lines) of the psychometric function. (Bold signifies p < 0.01).
| Subject | Condition 1 | Condition 2 | Condition 3 | Condition 4 |
|---|---|---|---|---|
| 1 | 0.71 | 4.51 | 0.66 | 0.54 |
| 2 | 0.06 | 0.01 | 1.86 | 0.00 |
| 3 | 0.28 | 2.07 | 0.08 | 0.55 |
| 4 | 2.84 | 15.64 | 5.73 | 0.96 |
| 5 | 0.66 | 8.37 | 7.23 | 2.97 |
| 1 | 11.11 | 2.27 | 5.14 | 1.75 |
| 2 | 0.99 | 3.36 | 0.15 | 0.02 |
| 3 | 0.08 | 4.73 | 0.22 | 0.46 |
| 4 | 1.45 | 0.94 | 0.71 | 0.90 |
| 5 | 0.00 | 0.70 | 6.94 | 0.14 |
Table 2.
Chi-squared values for the effects of test condition on μ values of the Psychometric function (Red color indicates p > 0.01). Each cell shows the results of a pairwise combination of two test conditions.
| Low Load | High Load | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Cond 1 | Cond 2 | Cond 3 | Cond 4 | Cond 1 | Cond 2 | Cond 3 | Cond 4 | |
| 1 | Cond 1 | 61.39 | 76.57 | 19.72 | 53.42 | 119.11 | 41.88 | ||
| Cond 2 | 61.39 | 60.46 | 0.00 | 53.42 | 43.59 | 0.48 | |||
| Cond 3 | 76.57 | 60.46 | 28.16 | 119.11 | 43.59 | 28.27 | |||
| Cond 4 | 19.72 | 0.00 | 28.16 | 41.88 | 0.48 | 28.27 | |||
| 2 | Cond 1 | 20.60 | 33.92 | 12.68 | 10.43 | 38.01 | 15.90 | ||
| Cond 2 | 20.60 | 29.74 | 0.43 | 10.43 | 12.55 | 0.17 | |||
| Cond 3 | 33.92 | 29.74 | 24.79 | 38.01 | 12.55 | 18.44 | |||
| Cond 4 | 12.68 | 0.43 | 24.79 | 15.90 | 0.17 | 18.44 | |||
| 3 | Cond 1 | 13.91 | 41.86 | 7.80 | 23.46 | 33.40 | 18.26 | ||
| Cond 2 | 13.91 | 11.12 | 2.34 | 23.46 | 33.38 | 0.24 | |||
| Cond 3 | 41.86 | 11.12 | 36.55 | 33.40 | 33.38 | 33.25 | |||
| Cond 4 | 7.80 | 2.34 | 36.55 | 18.26 | 0.24 | 33.25 | |||
| 4 | Cond 1 | 69.30 | 86.36 | 22.38 | 20.21 | 64.53 | 26.49 | ||
| Cond 2 | 69.30 | 20.53 | 31.82 | 20.21 | 38.18 | 3.91 | |||
| Cond 3 | 86.36 | 20.53 | 41.28 | 64.53 | 38.18 | 34.96 | |||
| Cond 4 | 22.38 | 31.82 | 41.28 | 26.49 | 3.91 | 34.96 | |||
| 5 | Cond 1 | 52.27 | 86.64 | 22.11 | 21.10 | 42.97 | 5.49 | ||
| Cond 2 | 52.27 | 32.69 | 13.00 | 21.10 | 18.50 | 2.33 | |||
| Cond 3 | 86.64 | 32.69 | 39.54 | 42.97 | 18.50 | 40.50 | |||
| Cond 4 | 22.11 | 13.00 | 39.54 | 5.49 | 2.33 | 40.50 | |||
The same type of analysis for the effects of adaptation on μ showed highly significant differences between Test Conditions 1 and 3 (Red and Blue) but not between 2 and 4 (Green and Magenta). The results of all possible pairwise comparisons of Test Conditions for the High-Load condition are shown in Table 2. The color code for significance reveals only one case where a 1 versus 3 comparison is not significant and very few cases where 2 versus 4 is significant.
Experiment 2: Low adapting contrast
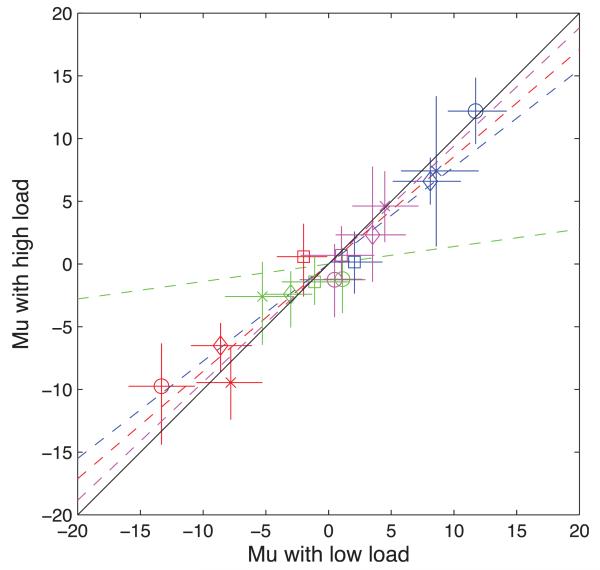
One observer (S8) found the high contrast (52%) moving adapting stimulus visually disturbing, as were the rapidly changing squares in the perceptual load task. There was no indication of adaptation in her data. Her partial data were not included in the analysis. To see if she could tolerate lower contrast stimuli the adapting stimulus was reduced to 26% (the same as the test) and the luminance of the squares was reduced by 50%. These stimuli were no longer disturbing to her so a full data set was collected, along with one subject from Experiment 1 (S1) and two new subjects (S6 and S7). Another good reason for using a low contrast adaptor is that the effects of attentional load may be too weak to be found with strong adaptors. The results of this experiment are shown in Figure 6, using the same conventions as Figure 5.
Figure 6.
Results for the low-contrast adaptation. Results for Subject are indicated by circles (S1), squares (S8), diamonds (S6), and crosses (S7). If the movement of the top and bottom test patches is denoted RR for Condition 1 (Red), then the remaining conditions are RL (Green), LL (Blue), and LR (Magenta). Other conventions as in Figure 3 but note the change in y-axis scale.
Comparing Figures 5 and 6 we see that adaptation was reduced by the lower contrast adaptor in Experiment 2. (Note the difference in y-axis scale between the two figures.) Indeed, S8 showed no evidence for adaptation at all (squares). Because all but one (S1) of the subjects were different between the two experiments it is not possible to conclude that this was an effect of the adaptor, but in S1 the difference in μ values between the two experiments, with σ allowed to vary, was highly significant for both conditions that were expected to show adaptation. (χ2 = 16.1, p < 0.01 and 7.70, p, 0.01). Interpretation is complicated by a smaller but still significant difference in the case of one of the two control conditions [χ2 = 5, 56* and 1.21 (p > 0.05)]. However, the balance of evidence suggests that adaptation was reduced by the lower contrast adaptor.
Discussion
The consistent bias found in Baseline data was in the direction of seeing “lower as slower.” Hemifield biases in velocity comparisons (Johnston & Wright, 1986; Smith & Hammond, 1986) have been reported previously but were subject-varying in direction. With data from only six subjects it would not be profitable to speculate about mechanisms for the consistent bias that we have observed.
Adaptation reduced the apparent speed of a stimulus moving in the same place and direction of the adapting stimulus, in agreement with previous work (Thompson, 1981). Because of the design we have used this was not equivalent to a response bias, or a perceptual bias in favor of one location rather than another.
There was no consistent effect of perceptual load on the extent of adaptation, consistent with the first experiment to be performed on this topic (Wohlgemuth, 1911). As argued elsewhere (Morgan, 2011) previous reports to the contrary may have reflected the use of inappropriate statistics in conceptual replications, and methods that are susceptible to cognitive and response biases. One of these is the perceived duration of the motion aftereffect, in which the observer looks at a stationary stimulus and decides when it stops appearing to move (Rees et al., 1997). Obviously this must involve some criterion, since the subject knows (from the fact that there is no displacement) that the stimulus is not really moving. One experiment (Sinha, 1952) showed that subjects could be manipulated into changing their duration estimates merely by informing them of the results of other observers. Another study (Grindley & Wilkinson, 1953) showed that it is not uncommon for observers to report zero duration for the aftereffect.
Superficially more free of bias are determinations of the 50% point (μ) on the psychometric function, in which the amount of real movement in the test stimulus is varied, in order to find the velocity at which the subject is equally likely to report that the stimulus moved in any direction (Taya et al., 2009). However, this measure also requires the subject to adopt a response criterion, and it can be shown that observers can easily shift their criterion in a way that mimics adaptation, without affecting the slope of the psychometric function, by choosing one direction of response when they are unsure (Morgan et al., 2012).
Experiments in social psychology have shown how easy it is for experimenters to bias the behavior of their subjects in perceptual experiments, even without intending to (Rosenthal & Rubin, 1978). Given that it is so easy to bias measures of adaptation based on duration or on the MSS, it is important that subjects should not be tested by experimenters who have an expectation of a particular result, but it is not clear that this condition has been met in any of the studies carried out so far. A degree of skepticism is therefore justified, as in the case of the linking hypothesis.
Two experiments have studied the effects of sustained attention on contrast detection, as opposed to stimulus appearance. Morgan (2011) found that adaptation increased contrast detection thresholds for tests moving in the same direction as the adaptor and moved contrast-discrimination “dipper functions” upwards and rightwards as in previous studies (Morgan, Chubb, & Solomon, 2006), but there was no effect of a distracting attentional task either in experienced or naive subjects. Ling and Carrasco (2006) adapted observers to four vertically-oriented Gabor patches and measured contrast detection thresholds by an orientation discrimination task in one of the four positions. If subjects were instructed to attend to the adapting patch in the to-be-tested location rather than to all four positions, the extent of adaptation was reduced for adaptation periods of up to about 2 s, and thereafter increased up to a maximum at about 4 s, before decreasing again to zero (in two subjects). It is noteworthy that the adaptation period at which attention had its maximum effect on the extent of adaptation effect (4 s) is the same as that used in the present study and similar to that (5 s top up) used in a previous experiment (Morgan, 2011). It therefore seems unlikely that our failure to find an effect of sustained attention on adaptation is due to an unfortunate choice of adaptation period, but there are many other differences between our studies and that of Ling and Carrasco (2006) and the interaction between attention, adaptation, and adaptation strength might repay closer investigation.
Even if it turns out that there are special conditions under which sustained attention can be equivalent to a slight increase in effective contrast, this would still not explain the reported effects of distraction on the motion aftereffect, given that motion adaptation saturates rapidly with adapting contrast, and the fact that most previous studies have used high contrast adaptors (an exception being Morgan, 2012, which found no effect of attentional load). Distracting attention from a moving grating with a central task has been shown to decrease the MT+ BOLD response to a moving grating of 20% contrast (Huk, Ress, & Heeger, 2001). It would be interesting to know if this is also true for 100% contrast stimuli.
Questioning a role of sustained attention in low-level adaptation has no implications for other claims for the role of “attention,” which are almost too numerous and various to mention (Morgan, 2011). In particular, the small effect of exogenous cueing on contrast sensitivity is well-established (Pestilli, Viera, & Carrasco, 2007), even if the lack of a capacity limit for the effect makes it very different from endogenous attention (Solomon, 2004). Likewise, it is clear that “attentional tracking” of a transparent or ambiguous motion display can enhance adaptation (Lankheet & Verstraten, 1995), but this may depend on a specialized mechanism connected with an extraretinal signal (Freeman, Sumnall, & Snowden, 2003) rather than the retinotopic motion aftereffect, which is the strict topic under consideration in the present paper. In general, the protean effects claimed for the ill-defined term “attention” suggest caution in bolstering claims for the effect of one kind of “attention” by evidence from other kinds.
If there is indeed no clear effect of attention on motion adaptation, the functional significance for the decreased BOLD signal due to attentional distraction is not obvious. It is also noteworthy that fMRI adaptation shows robust effect of stimulus repetition even in the presence of a distracting task (Larsson & Smith, 2012). What initially seemed to be strong evidence for an association of a change in BOLD with a behavioral effect on adaptation now appears to show the reverse.
Acknowledgments
Supported by grants from The Wellcome Trust to MM, the Engineering and Physical Sciences Research Council UK to JA Solomon and MM, and a Senior Fellowship to MM from the Max-Planck Society.
Footnotes
Commercial relationships: none.
References
- Beck DM, Rees G, Frith CD, Lavie N. Neural correlates of change detection and change blindness. Nature Neuroscience. 2001;4(6):645–650. doi: 10.1038/88477. [DOI] [PubMed] [Google Scholar]
- Blake R, Tadin D, Sobel KV, Raissian TA, Chong SC. Strength of early visual adaptation depends on visual awareness. Proceedings of the National Academy of Sciences, USA. 2006;103(12):4783–4788. doi: 10.1073/pnas.0509634103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Brindley GS. Physiology of the retina and visual pathway. Edward Arnold; London: 1960. [Google Scholar]
- Chaudhuri A. A motion illusion generated by afternystagmus suppression. Neuroscience Letters. 1990;118:91–95. doi: 10.1016/0304-3940(90)90256-9. [DOI] [PubMed] [Google Scholar]
- Freeman TC, Sumnall JH, Snowden RJ. The extra-retinal motion aftereffect. Journal of Vision. 2003;3(11):11, 771–779. doi: 10.1167/3.11.11. http://www.journalofvision.org/content/3/11/11. doi:10.1167/3.11.11. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez M, Alcala-Quintana R. Shifts of the psychometric function: Distinguishing bias from perceptual effects. The Quarterly Journal of Experimental Psychology, iFirst. 2012:1–19. doi: 10.1080/17470218.2012.708761. [DOI] [PubMed] [Google Scholar]
- Georgiades M, Harris J. Evidence for spatio-temporal selectivity in attentional modulation of the motion aftereffect. Spatial Vision. 2002;16(1):21–31. doi: 10.1163/15685680260433887. [DOI] [PubMed] [Google Scholar]
- Grindley GC, Wilkinson RT. The aftereffect of seen movement on a plain field. Quarterly Journal of Experimental Psychology. 1953;5:183–184. [Google Scholar]
- Hoel PG, Port SC, Stone CJ. Introduction to statistical theory. Houghton Mifflin; Boston: 1971. [Google Scholar]
- Huk AC, Ress D, Heeger DJ. Neuronal basis of the motion aftereffect reconsidered. Neuron. 2001;32(1):161–172. doi: 10.1016/s0896-6273(01)00452-4. [DOI] [PubMed] [Google Scholar]
- Johnston A, Wright MJ. Matching velocity in central and peripheral vision. Vision Research. 1986;26(7):1099–1109. doi: 10.1016/0042-6989(86)90044-1. [DOI] [PubMed] [Google Scholar]
- Lankheet MJ, Verstraten FA. Attentional modulation of adaptation to two-component transparent motion. Vision Research. 1995;35(10):1401–1412. doi: 10.1016/0042-6989(95)98720-t. [DOI] [PubMed] [Google Scholar]
- Larsson J, Smith AT. fMRI repetition suppression: Neuronal adaptation or stimulus expectation? Cerebral Cortex. 2012;22(3):567–576. doi: 10.1093/cercor/bhr119. doi: 10.1093/cercor/bhr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: Selective attention under load. [Review] Trends in Cognitive Sciences. 2005;9(2):75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Ling S, Carrasco M. When sustained attention impairs perception. Nature Neuroscience. 2006;9(10):1243–1245. doi: 10.1038/nn1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M, Chubb C, Solomon JA. Predicting the motion after-effect from sensitivity loss. Vision Research. 2006;46(15):2412–2420. doi: 10.1016/j.visres.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Morgan M, Dillenburger B, Raphael S, Solomon JA. Observers can voluntarily shift their psychometric functions without losing sensitivity. Attention, Perception & Psychophysics. 2012;74(1):185–193. doi: 10.3758/s13414-011-0222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ. Wohlgemuth was right: distracting attention from the adapting stimulus does not decrease the motion after-effect. Vision Research. 2011;51(20):2169–2175. doi: 10.1016/j.visres.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ. Motion adaptation does not depend on attention to the adaptor. Vision Research. 2012;55:47–51. doi: 10.1016/j.visres.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ, Chubb C, Solomon JA. Evidence for a subtractive component in motion adaptation. Vision Research. 2011;51(21-22):2312–2316. doi: 10.1016/j.visres.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Nishida S, Ashida H. A hierarchical structure of motion system revealed by interocular transfer of flicker motion aftereffects. Vision Research. 2000;40(3):265–278. doi: 10.1016/s0042-6989(99)00176-5. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Viera G, Carrasco M. How do attention and adaptation affect contrast sensitivity? Journal of Vision. 2007;7(7):9, 1–12. doi: 10.1167/7.7.9. http://www.journalofvision.org/content/7/7/9. doi:10.1167/7.7.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Frith C, Lavie N. Processing of irrelevant visual motion during performance of an auditory attention task. Neuropsychologia. 2001;39(9):937–949. doi: 10.1016/s0028-3932(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Rees G, Frith CD, Lavie N. Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science. 1997;278:1619–1619. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- Rezec A, Krekelberg B, Dobkins KR. Attention enhances adaptability: Evidence from motion adaptation experiments. Vision Research. 2004;44(26):3035–3044. doi: 10.1016/j.visres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rubin D. Interpersonal expectancy effects: The first 345 studies. The Behavioral and Brain Sciences. 1978;3:377–386. [Google Scholar]
- Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J. Attentional load and sensory competition in human vision: Modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cerebral Cortex. 2005;15(6):770–786. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- Sinha D. A experimental study of a social factor in perception: The influence of an arbitrary group standard. Patna University Journal. 1952:7–16. [Google Scholar]
- Smith AT, Hammond P. Hemifield differences in perceived velocity. Perception. 1986;15(2):111–117. doi: 10.1068/p150111. [DOI] [PubMed] [Google Scholar]
- Solomon JA. The effect of spatial cues on visual sensitivity. Vision Research. 2004;44(12):1209–1216. doi: 10.1016/j.visres.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Taya S, Adams W, Graf E, Lavie N. The fate of task-irrelevant visual motion: Perceptual load versus feature-based attention. Journal of Vision. 2009;9(12):12, 1–10. doi: 10.1167/9.12.12. http://www.journalofvision.org/content/9/12/12. doi:10.1167/9.12.12. [DOI] [PubMed] [Google Scholar]
- Thompson P. Velocity aftereffects: The effects of adaptation to moving stimuli on the perception of subsequently seen moving stimuli. Vision Research. 1981;21:337–345. doi: 10.1016/0042-6989(81)90161-9. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Cheng K, Murayama Y, Ueno K, Asamizuya T, Tanaka K, et al. Attention but not awareness modulates the BOLD signal in the human V1 during binocular suppression. Science. 2011;334(6057):829–831. doi: 10.1126/science.1203161. [DOI] [PubMed] [Google Scholar]
- Watt RJ, Andrews DP. APE: Adaptive probit estimation of a psychometric function. Current Psychological Reviews. 1981;1:205–214. [Google Scholar]
- Wohlgemuth A. On the aftereffect of seen movement. British Journal of Psychology, Monograph. 1911;1(Supplement):1–117. [Google Scholar]