Abstract
Monitoring serum antibodies against natural infections or after immunizations has been a standard clinical diagnostic procedure. However, collecting blood samples requires trained personnel, and may cause discomfort and increase the risk of complications. In this study, we investigated whether tear samples could serve as a surrogate for serum samples to measure specific antibodies. A widely used preclinical cottontail rabbit papillomavirus (CRPV)/rabbit model has been a surrogate model for high-risk human papillomavirus (HPV) infections. New Zealand white rabbits, either naturally infected with CRPV or immunized with two clinically available HPV vaccines (Gardasil and Cervarix), were examined for antibody generation in both tear and serum samples. We demonstrated that antibodies were detectable in tears from both naturally infected as well as vaccinated animals. Overall, the antibody levels in tears were ~10-fold lower than those from the corresponding serum samples, but background noise was lower in tear samples. The isotypes of antibodies in tears were predominantly IgA and IgG. These findings showed clearly that tears could be a surrogate for serum samples for monitoring antibody responses. As collecting tears causes no discomfort and poses no risk to patients, it represents a novel and promising method for monitoring future HPV epidemiological studies as well as for use in clinical practice.
Introduction
Monitoring antibodies in serum samples has been used routinely either to evaluate the efficacy of vaccination or to test potential infections in patients. Although blood collection is a well-accepted procedure for patients, it may increase the risk to both patients and health care workers of infections from human immunodeficiency virus (HIV), hepatitis and other serious diseases in areas where sterilization is ineffective. Moreover, collecting blood can be a very stressful process, especially for young patients, and used syringes and needles create a major waste disposal problem. Therefore, a non-invasive method to monitor antibodies is highly desirable.
Human papillomaviruses (HPVs) are associated with anogenital warts and are the causative factor for cervical cancer in women worldwide. Currently, two prophylactic vaccines (Gardasil and Cervarix) successfully prevent infection by two of the most prevalent and cancer-associated HPV types (HPV-16 and -18). To measure the efficacy of these vaccines, serum antibody levels are monitored from vaccinated individuals. For large epidemiological studies, it is labour-intensive and challenging to collect serum samples. Previous studies have demonstrated that antibodies can be detected in body fluids such as nasal secretions, tears, saliva, etc. (Douglas et al., 1967; Friedman et al., 1989; Little et al., 1969; Madar et al., 2002). Antibodies against an array of viruses, such as measles virus, herpes simplex virus (HSV) and HIV, have been detected in tear samples (Allansmith, 1973; Friedman, 1990; Langford et al., 2003; Liotet et al., 1987; Rozanova et al., 2006). Detection of antibodies against papillomavirus in body fluids such as tears is untested. In addition, a comparative study of antibody titres from tear samples and serum samples has not been performed. If we can demonstrate antibodies in body fluids as well as in serum samples, we can apply this non-invasive method to monitor antibody levels in immunized populations. In this study, we used a cottontail rabbit papillomavirus (CRPV)/domestic rabbit model to address these questions.
For immunization studies, two clinically available HPV vaccines (Gardasil and Cervarix) together with a CRPV L1 virus-like particle (VLP) vaccine were used. For natural infection studies, CRPV viral DNA or infectious virions were used. Type-specific antibodies were detected from both serum and tear samples in both studies. Much higher titres were found in immunized animals when compared with naturally infected animals. In addition, significantly higher antibody titres were found in serum samples when compared with corresponding tear samples. However, much lower background was found in tear samples, which can help to improve the specificity and sensitivity of the assay. The major subtypes of antibodies in tears were IgA followed by IgG and IgM, whilst the major isotype in serum samples was IgG. Rabbits with higher serum titres also had higher titres in their corresponding tear samples. These findings suggest that collecting tears can be an alternative strategy to monitor antibodies in both papillomavirus-vaccinated and naturally infected animals, and may be potentially applicable in human studies and clinical diagnosis.
Results
Antibody detection in tear samples from rabbits naturally infected and immunized with CRPV
Our previous studies have demonstrated that anti-L1 antibodies could be detected from rabbit serum samples that were naturally infected with either viral DNA or infectious virions (Hu et al., 2007b). In this study, we collected tear and serum samples from three rabbits infected with virus and one rabbit infected with viral DNA. Anti-CRPV L1 antibodies were detected by standard ELISA. As shown in Table 1, all the rabbits generated detectable anti-CRPV L1 antibodies in both serum and tear samples. In addition, two rabbits immunized with CRPV L1 VLPs were tested for antibody generation in both serum and tear samples (Table 1). Regardless of the treatment, the antibody levels were ~10-fold lower in tear samples than those in the corresponding serum samples. Interestingly, much lower background was detected in normal tear samples when compared with the corresponding serum samples.
Table 1. Half-maximal ELISA titres of antibodies in tear and serum samples.
| Rabbit ID no. | Treatment | Anti-CRPV L1 antibody titre | |
| Tears | Serum | ||
| 2885 | Virus infection | 1 : 1000 | 1 : 10 000 |
| 2904 | 1 : 500 | 1 : 2000 | |
| 2908 | 1 : 2000 | 1 : 20 000 | |
| 2853 | Viral DNA | 1 : 1000 | 1 : 10 000 |
| 2937 | CRPV L1 VLP vaccination | 1 : 800 | 1 : 20 000 |
| 2938 | 1 : 600 | 1 : 10 000 | |
| 2923 | Naive | 0 | 0 |
To further confirm the findings, we collected tear and serum from nine additional animals infected with viral DNA for antibody detection. All animals showed positivity against CRPV VLPs in both tear and serum samples (Fig. 1a). These observations validated the possibility of using tear samples for monitoring natural infection.
Fig. 1.
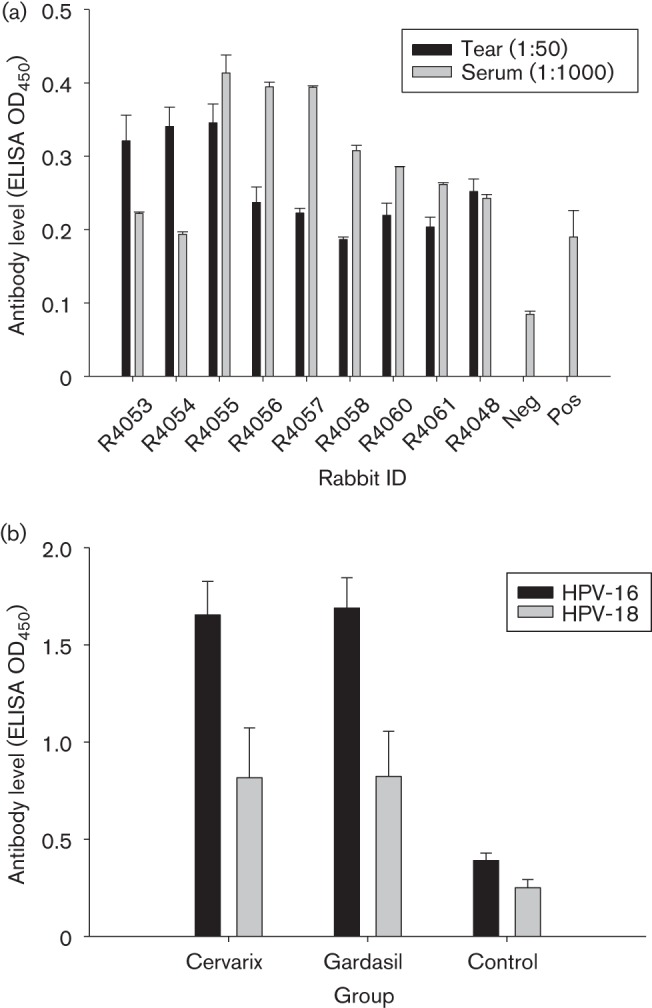
(a) Anti-CRPV antibodies detection in rabbits (N = 9) infected with CRPV DNA. (b) Anti-HPV-16 and -18 antibodies in tears of rabbits (N = 5) vaccinated with Cervarix or Gardasil. Tear (1 : 50) and serum (1 : 1000) samples from rabbits infected with 5 µg WT CRPV DNA or vaccinated with the human dose of Cervarix and Gardasil were collected ~4 weeks post-infection. Standard ELISA was conducted using a 96-well plate coated with CRPV L1 VLPs or HPV-16 L1 and -18 L1 VLPs (0.5–1 µg per well) in 1× PBS buffer at room temperature for 30 min. The plate was washed with 1× PBS buffer for at least three times and blocked with 5 % dry milk/1× PBS buffer for >1 h at room temperature. Tear and serum samples (duplicates) were incubated with VLPs at 4 °C overnight. In-house mAbs CRPV1A (1 : 100), H16.V5 (1 : 100) and H18.J4 (1 : 100) were used as positive controls (Pos). Negative control (Neg) was without primary antibody. Tears and serum from animals not infected with CRPV or vaccinated were also used as negative controls in our studies. After three washes, the secondary antibody (goat anti-rabbit IgG conjugated with alkaline phosphatase, 1 : 2000; SouthernBiotech) was added and incubated for >1 h at room temperature. The plate was then washed three times with 1× PBS, developed with 1 mg ml−1 of 4-nitrophenyl phosphate disodium salt hexahydrate tablet (Sigma) in alkaline phosphatase buffer (pH 9.5) and analysed at 450 nm with an Opsys MR microplate reader (Dynex Technologies). All rabbits showed a positive reaction after 2 h of development. Values are given as mean±se.
Antibody detection in tear samples of Gardasil- and Cervarix-immunized rabbits
Our previous studies had shown that HPV L1 VLP immunization in rabbits successfully promotes high titres of type-specific antibodies that lead to strong type-specific protection in New Zealand white (NZW) rabbits (Mejia et al., 2006). In this study, we further investigated whether antibodies could be detected in the tear samples from L1 VLP-immunized animals. In addition to our laboratory-generated CRPV L1 VLPs, we also used two commercially available HPV vaccines: Gardasil (containing HPV-16, -18, -6 and -11 L1 VLPs) and Cervarix (HPV-16 and -18 L1 VLPs). Two or three rabbits were used for each group as shown in Table 2. Doses of 50 µg CRPV L1 VLPs were used, whereas the clinical dose for humans was used for Gardasil and Cervarix immunizations. Three immunizations at 2-week intervals were administered to the animals. Tear and serum samples were harvested for serological analysis 1 week after the final booster immunization.
Table 2. Half-maximal ELISA titres of antibodies in tears and serum samples from L1 VLP-immunized animals.
| Rabbit ID no. | Vaccine | Half-maximum titre of antibodies against | |||
| HPV-16 | HPV-18 | ||||
| Tears | Serum | Tears | Serum | ||
| 2932 | Gardasil | 1 : 1000 | 1 : 51 200 | 1 : 600 | 1 : 10 000 |
| 2933 | 1 : 600 | 1 : 20 000 | 1 : 600 | 1 : 10 000 | |
| 2941 | Cervarix | 1 : 1000 | 1 : 51 200 | 1 : 600 | 1 : 20 000 |
| 2942 | 1 : 2000 | 1 : 51 200 | 1 : 600 | 1 : 20 000 | |
| 2943 | 1 : 3000 | 1 : 51 200 | 1 : 1000 | 1 : 25 600 | |
Antibodies to HPV-16, -18, -6 and -11 VLPs were examined from all the vaccinated animals. As expected, all the rabbits vaccinated with Gardasil produced antibodies against all four VLP types, whilst Cervarix-vaccinated rabbits generated antibodies against HPV-16 and -18 VLPs only. These data confirmed that type-specific antibody responses were induced in all vaccinated rabbits. We further compared the antibody levels (at half-maximum titre) in both tear and serum samples side by side from all the animals. As shown in Table 2, higher titres of type-specific antibodies were detected in serum samples when compared with those in tear samples.
Additional studies with Gardasil and Cervarix immunization in rabbits reproducibly showed anti-HPV-16, -18, -6 and -11 antibodies were generated in the tear samples of these animals (Fig. 1b and data not shown).
Anti-HPV-31 and -45 cross-reactive antibodies were detected in both serum and tear samples
In previous studies, we observed that cross-protection against closely related HPV types such as HPV-31 and -45 in HPV-16 and -18 L1 VLP-immunized animals was achieved (unpublished observations). We therefore hypothesized that the cross-protective immunity was due to cross-reacting antibodies generated in Gardasil- and Cervarix-vaccinated animals. As expected, detectable levels of tear and serum antibodies against HPV-31 and -45 were found in these vaccinated animals, indicating that cross-protection is possible by L1 VLP immunization. We did not test titres against HPV-31 and -45 in rabbit tears due to limited samples. The titres of these cross-reactive antibodies in serum samples are shown in Table 3.
Table 3. Half-maximal ELISA titres of antibodies in serum samples of L1 VLP-immunized animals.
| Rabbit ID no. | Vaccine | Half-maximum titre of antibodies against | |
| HPV-31 | HPV-45 | ||
| 2932 | Gardasil | 1 : 2000 | 1 : 600 |
| 2933 | 1 : 3000 | 1 : 600 | |
| 2941 | Cervarix | 1 : 2000 | 1 : 600 |
| 2942 | 1 : 2000 | 1 : 600 | |
| 2943 | 1 : 1800 | 1 : 1000 | |
IgA is a dominant antibody subtype in tear samples
We next tested the antibody subtypes in both serum and tear samples. In serum samples, IgG was the dominant isotype, whilst IgA was prevalent in most tear samples (Table 4, Fig. 2). IgG was also very common in tear samples of most animals.
Table 4. Typing the antibody subtypes in serum and tear samples.
| Rabbit ID no. | Challenge | Serum | Tears | ||
| HPV-16 | HPV-18 | HPV-16 | HPV-18 | ||
| 2932 | Gardasil | IgG, IgM | IgG | IgA, IgG | IgA, IgG |
| 2933 | IgG | IgG | IgM, IgG | IgG | |
| 2941 | Cervarix | IgG | IgG | IgA, IgG | IgA, IgG |
| 2942 | IgG, IgM | IgG, IgM | IgA, IgG | IgA, IgG | |
| 2943 | IgG | IgG | IgA, IgG | IgA, IgG | |
Fig. 2.
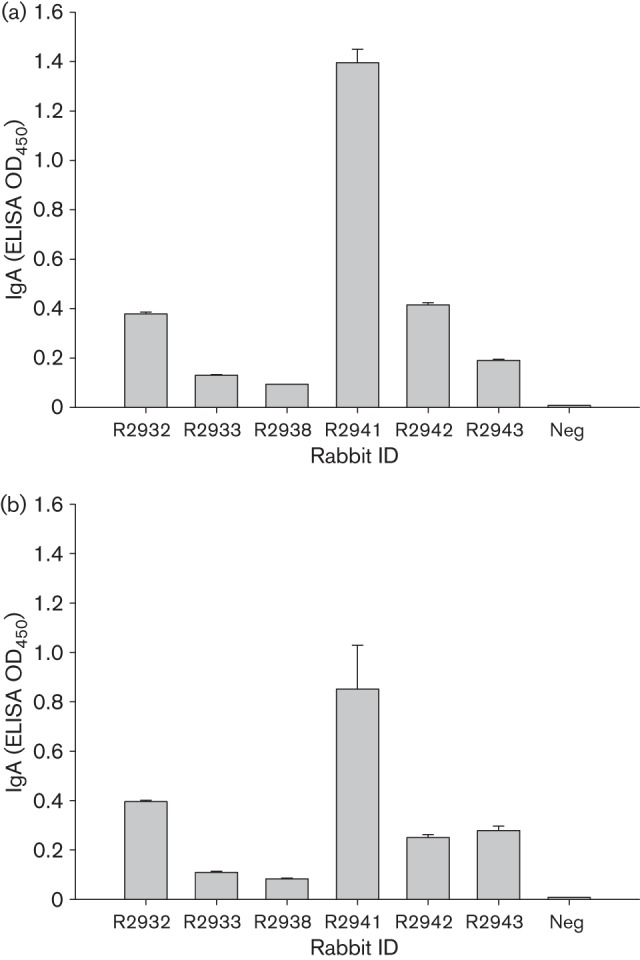
Anti-HPV-16 and -18 IgA antibodies in tears of rabbits vaccinated with Cervarix (R2941, R2942, R2943), Gardasil (R2932, R2933) or CRPV L1 (R2938). Tears (1 : 50) from rabbits vaccinated with the human dose of Cervarix and Gardasil or 50 µg CRPV L1 VLPs three times. Standard ELISA was conducted using a 96-well plate coated with CRPV L1 VLPs or HPV-16 L1 and -18 L1 VLPs (0.5–1 µg per well) in 1× PBS buffer at room temperature for 30 min. The plate was washed with 1× PBS buffer for at least three times and blocked with 5 % dry milk/1× PBS buffer for >1 h at room temperature. Tear samples (duplicates or triplicates) were incubated with VLPs at 4 °C overnight. In-house mAbs CRPV1A (1 : 100), H16.V5 (1 : 100) and H18.J4 (1 : 100) were used as positive controls. Negative control (Neg) was without primary antibody. Tears from animals not infected with CRPV or vaccinated were also used as negative controls in our studies. After three washes, the secondary antibody (goat anti-rabbit IgA conjugated with alkaline phosphatase, 1 : 2000; Bethyl) was added and incubated for >1 h at room temperature. The plate was then washed three times with 1× PBS, developed with 1 mg ml−1 of 4-nitrophenyl phosphate disodium salt hexahydrate tablet (Sigma) in alkaline phosphatase buffer (pH 9.5) and analysed at 450 nm with an Opsys MR microplate reader (Dynex Technologies). Values are given as mean±se.
Neutralizing antibody detection in serum and tear samples
We subsequently tested whether the antibodies in serum and tears were neutralizing. Rabbits vaccinated with either Cervarix (N = 5) or control vaccine (N = 5) were used for this study. One week after the final booster immunization, serum and tear samples were collected and tested for neutralization in vitro. The serum and tears samples were incubated with pseudoviruses, and then added to 293TT cells and cultured for 3 days. The infectivity was determined using a colorimetric method as reported previously (Pastrana et al., 2004). Strong neutralizing antibodies were detected in serum samples of all Cervarix-vaccinated animals (P<0.05 versus the control group, unpaired Student’s t-test; Fig. 3a, b). Anti-HPV-16 and -18 neutralizing antibodies were also detected from the tear samples, although the titres were significantly lower than those of the corresponding serum samples (P<0.01 versus the control group, unpaired Student’s t-test; Fig. 4a, b). Interestingly, tears from the control animals also showed non-specific neutralizing antibodies against both HPV-16 and -18 (P<0.01 versus the control group, unpaired Student’s t-test; Fig. 4a, b).
Fig. 3.
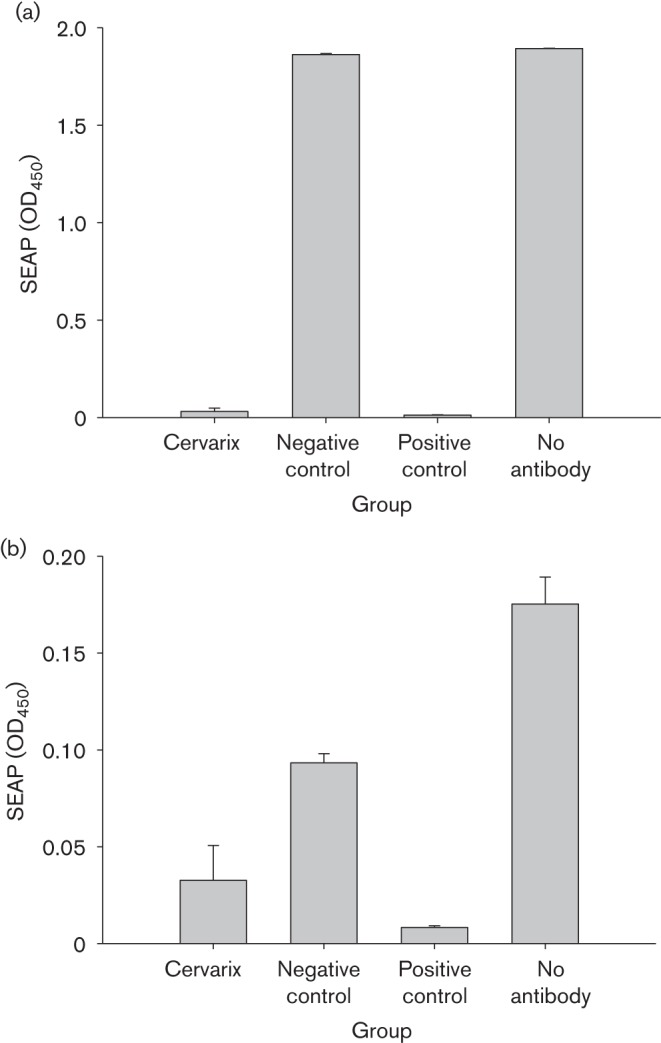
Neutralizing antibodies in rabbit serum samples. Serum samples from rabbits immunized with Cervarix (N = 5) and vector control (N = 5) were tested for neutralizing (a) HPV-16 and (b) HPV-18 pseudoviruses in 293TT cell culture as described previously. Serum samples (1 : 100) were pre-incubated with pseudovirions for 1 h at 37 °C prior to infecting triplicate wells of 293TT cells seeded the previous day in 96-well plates at 3×104 cells per well. At 3–4 days post-infection, spent media were analysed for secreted alkaline phosphatase (SEAP) levels by using a colorimetric assay. mAbs against HPV-16 L1 (H16V5) and HPV-18 L1 (H18J4) were used as positive controls. Neutralizing antibodies against HPV-16 and -18 were detected in Cervarix-immunized rabbits. Non-specific neutralizing antibodies against HPV-18, but not HPV-16 were detected in the control rabbits. Values are given as mean±se.
Fig. 4.
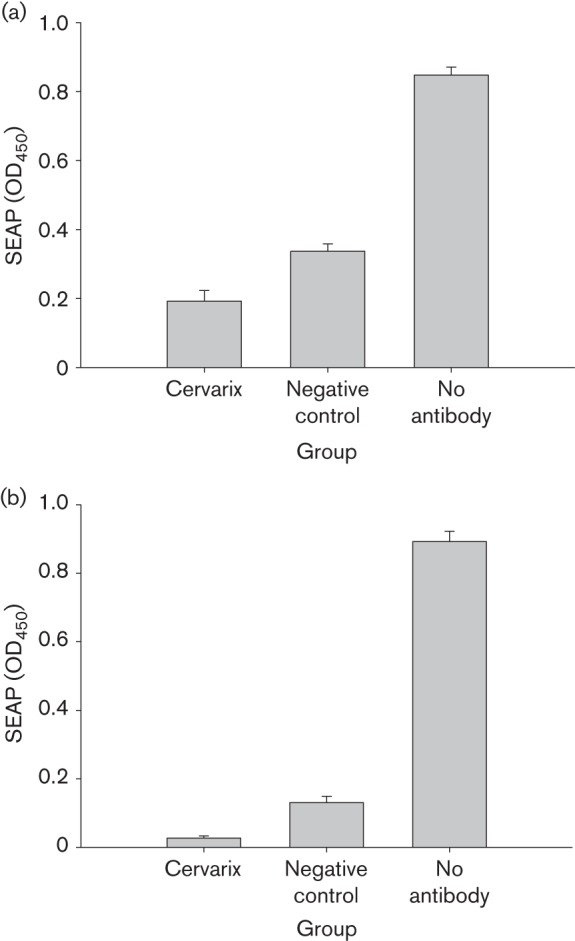
Neutralizing antibodies in rabbit tear samples. Tear samples from rabbits immunized with Cervarix (N = 5) and vector control (N = 5) were tested for neutralizing (a) HPV-16 and (b) HPV-18 pseudoviruses in 293TT cell culture as described previously. Tear samples were pre-incubated with pseudovirions (1 : 50) for 1 h at 37 °C prior to infecting triplicate wells of 293TT cells seeded the previous day in 96-well plates at 3×104 cells per well. At 3–4 days post-infection, spent media were analysed for secreted alkaline phosphatase (SEAP) levels by using a colorimetric assay. A significant difference was found between Cervarix-immunized rabbits and the control group (P<0.01) in neutralization against HPV-16 and -18. A significant difference was also found between the control group and the positive control group, indicating that tears contain non-specific neutralizing antibodies as the first defence against viral infection. Values are given as mean±se.
Discussion
Serum sample collection has been used routinely as a source for detecting antibodies against natural infections and in response to vaccination. However, collecting blood samples is stressful for most people and, in particular, impractical for large epidemiological field studies. The risk of needle contamination and the safety of needle disposal can also be problematic. Therefore, a non-invasive method to obtain samples for routine diagnosis as well as large studies is highly desirable. Previous studies have shown that body fluids such as tears, saliva and mucus have detectable antibodies (Coyle & Sibony, 1986; Langford et al., 2003). The mucosal immune system functions as a first-line barrier against a variety of extrinsic infectious agents and environmental antigens. For example, mucosal surfaces are the portal of entry for most of the neurotropic viruses that infect humans. Healthy human tears showed a high prevalence of antibodies to HSV-1 and Epstein–Barr virus (Nesburn, 1980). However, no studies have been performed to test if anti-HPV antibodies are present in tear samples.
Tear responses reflect changes within the local microenvironment. The normal tear film contains 10 % of the total protein concentration of plasma. More than 400 proteins have been identified in human tear film fluid (Zhou et al., 2012). Among these proteins, two highly cationic polypeptides, lactoferrin and lysozyme, are abundant in saliva, tear and cervicovaginal mucosal fluid, and have broad antimicrobial properties (Välimaa et al., 2009). In addition to these antimicrobial proteins, antibodies against different viruses can be detected in tear samples and the prevalent antibody isotype is IgA (Coyle & Sibony, 1988). In the current study, we detected antibodies in serum samples from rabbits naturally infected with papillomavirus as well as those vaccinated with VLPs, which agreed with previous findings (Fox et al., 1986). We also successfully detected type-specific and cross-reacting antibodies in tear samples from these rabbits, although the titres in tear samples were significantly lower than those in serum samples. These findings indicate that systemic serum antibody production for papillomavirus infection or immunization may be more effective. On the contrary, antibodies against HSV-1 were found to be much higher in tear samples because the eye is a major entry site for HSV-1 infection (Fox et al., 1986). Papillomaviruses can attack both cutaneous and mucosal tissues; the antibody titres may be higher at their primary target sites. Nevertheless, antibodies in tear samples from our rabbits were detectable and measurable. We know that both sensitivity and specificity are very important in detecting antibody levels in test samples. Although we found lower titres of antibodies in tear samples, we also found a significantly lower background in tear samples when compared with the corresponding serum samples. This property of tear samples will help to increase the specificity of detection that can be a complication of clinical serum tests.
We also detected some cross-reacting antibodies in the immunized rabbits. In previous studies performed to test the efficacy of Gardasil and Cervarix in our CRPV/rabbit model, we observed that both vaccines provided cross-protection against vaccine-related HPV types such as HPV-31 and -45 (unpublished observations). Similar observations were reported in clinical studies (Einstein et al., 2009; Giroglou et al., 2001; McCormack & Joura, 2011; Villa et al., 2005). Therefore, the detection of the antibodies in animal tears either by a natural infection or by active immunization could be a good surrogate measure in the clinical setting.
Although we detected specific neutralizing antibodies from the tear samples, we still face some challenges. First, harvesting tears from rabbit eyes turned out to be more challenging than expected. According to reports in the literature, ~1–2 µl tears min−1 can be collected (Van Haeringen, 1981). For most cases, when rabbits were under anaesthesia, we used 20–40 µl of 1× PBS to rinse the eyes briefly and recovered about half of the original solution. Second, we demonstrated that antibody titres in rabbit tears are ~10-fold lower than those in the serum samples and therefore the corresponding neutralizing antibody levels were not expected to be as high as those in the serum samples. The variation in serum neutralizing titres detected in the vaccinated rabbits was also reflected in the tear samples. Although no detectable specific anti-HPV-16 and -18 antibodies were found in the control animal tears, non-specific neutralizing activities were detected. This latter finding implied that tears may contain molecules that provide the first defence against viral infection in hosts. The vaccinated animals, however, did show additional neutralizing activity to both HPV-16 and -18, indicating that the antibodies we detected from their tears by ELISA were actually neutralizing. These results were consistent with those reported in the literature for other viral infections (Nesburn, 1980). Therefore, tear samples can be used as a surrogate for serum samples in the detection of neutralizing antibodies.
Tears are a good source of biological material for identifying many disease-related biomarkers (Zhou & Beuerman, 2012) and the use of tears is an optimal non-invasive method for diagnosing human disease in clinical practice. A recent study demonstrated a correlation between glucose levels from matching tear and serum samples in diabetic patients (Yan et al., 2011). This non-invasive sample collection strategy can bypass the currently painful process of the needle pricks these diabetic patients must undergo for daily monitoring of their glucose levels. Monitoring antibodies in tear samples has also been recommended for some viral infections in human populations (Van Haeringen, 1981). For large epidemiological studies, it is often difficult to obtain follow-up serum samples. Collecting successive tear samples instead might be more achievable. One future direction is to establish a standard protocol to analyse tear samples for either biomarker screening or disease-related changes in study populations.
The quality of tear samples is crucial for consistent and reproducible data analysis and interpretation. The different methods used currently to collect and store tears need to be standardized. For tear induction in humans, several methods, including ammonia and tear guns, have been used to induce more tears (Van Haeringen, 1981). A comprehensive study is needed to establish consistency in collection methods.
In summary, it would be very interesting to see whether antibodies from tear samples of HPV-immunized patients correlate with the titres of their serum samples. If HPV-specific antibodies are found, then a new non-invasive method to measure the efficacy of vaccination in human populations would be beneficial for future large epidemiological studies.
Methods
Animals and viral infection.
NZW rabbits were maintained in the animal facility of the Pennsylvania State University College of Medicine. The studies were approved by the Institutional Animal Care and Use Committee of Pennsylvania State University. Hershey CRPV progressive strain DNA was prepared using the Qiagen Maxi Prep system, purified by caesium chloride density ultracentrifugation and adjusted to 100 µg ml−1 in 1× TE buffer for challenge on animals as described previously (Hu et al., 2007a). Rabbits were sedated using ketamine [40 mg (kg body weight)−1]/xylazine [5 mg (kg body weight)−1] anaesthesia before scarification and infection. The back skin of the animals was scarified with a scalpel blade to create an abrasion. Three days later and under anaesthesia, the wounded sites were lightly scratched with a scalpel blade to introduce nicks into the scabs. Each site was then challenged with 5 µg DNA in 50 µl of 1× TE buffer or 50 µl 10−2 virions in 1× PBS per site from Hershey CRPV virus stock (Hu et al., 2007b). The DNA and virions were worked into the wound using a 30G1/2 needle as described previously (Cladel et al., 2008). Monitoring of papilloma outgrowth began 2 weeks after infection and continued until the time of sacrifice of the animals.
VLP immunization.
CRPV L1 VLPs were produced in our laboratory as described previously (Mejia et al., 2006). Two commercially available HPV vaccines – Gardasil (HPV-16, -18, -6 and -11 L1 VLPs) and Cervarix (HPV-16 and -18 L1 VLPs) – were purchased. Three immunizations (50 µg for each immunization for CRPV L1 and the equivalent human dose for HPV vaccines) were administered to NZW rabbits intramuscularly at 2-week intervals. Before the first immunization and after the final immunization, tear and serum samples were collected for subsequent serology analysis. For serum collection, blood was drawn from the ear aural artery. For tear collection, the tears were harvested with a micro pipette tip. In cases where tears could not be collected directly, 20 µl of 1× PBS was used to wet the eyes and the rinsed liquid was harvested. All serum and tear samples were stored at 4 °C until use.
ELISA.
Standard ELISA was used to measure plasma and tear titres of anti-CRPV L1, HPV-16 L1 and HPV-18 L1 antibodies as described previously (Hu et al., 2007a). Our in-house-produced mAbs against CRPV L1 (CRPV1A and 4B), HPV-16 (H16V5) and HPV-18 (H18J4) were used as positive controls (Christensen et al., 1990). Maxisorp 96-well ELISA plates (Nunc) were coated with 0.5–1 µg per well L1 VLPs (generated in our laboratory) at room temperature for 30 min in 1× PBS, pH 7.4. After several washes with 1× PBS, the wells were blocked with 5 % non-fat milk protein in PBS for 1 h. Rabbit serum and tears were diluted at 1 : 50 in 5 % non-fat milk protein in PBS and added to the wells. After 1 h incubation at room temperature, the plates were washed three times and then incubated with a 1 : 2000 dilution of an alkaline phosphatase-conjugated swine anti-rabbit antibody (Dako) for 1 h. Secondary antibodies against rabbit IgA (Bethyl), IgG (Biomeda) and IgM (Dako) were used to type the antibodies. The plates were then washed three times with 1× PBS, developed with 1 mg ml−1 4-nitrophenyl phosphate disodium salt hexahydrate (Sigma) in alkaline phosphate buffer (pH 9.5) and analysed at 450 nm with an Opsys MR microplate reader (Dynex Technologies). For titration of the antibody levels, serum and tear samples were diluted into serial 1 : 2 dilutions (1 : 200, 1 : 400, 1 : 800, etc.). Triplicates were set up for each dilution. The half-maximal titres of antibodies from both tear and serum samples were determined using SigmaPlot 11.0 software.
In vitro neutralization assay.
Tear and serum samples were tested for neutralization in vitro by incubating with HPV-16, HPV-18 or CRPV pseudoviruses encapsidating secreted alkaline phosphatase (SEAP)-expressing DNA as described previously (Buck et al., 2005). Specific mAbs against HPV-16 (H16V5) and HPV-18 (H18J4) L1 VLPs were used as positive controls for neutralization. Tears and sera from rabbits immunized with CRPV L1 VLPs were used as negative controls. Pseudoviruses were produced in 293TT cells using a standard protocol (Pastrana et al., 2005). Serum samples (1 : 100) and tear samples (1 : 50) were pre-incubated with pseudovirions for 1 h at 37 °C, prior to infecting triplicate wells of 293TT cells seeded the previous day in 96-well plates at 3×104 cells per well. At 3–4 days post-infection, spent media were analysed for SEAP levels using a colorimetric assay. Mean optical density readings are representative of three wells receiving pseudovirions incubated with control tear or serum samples. The percentage of neutralization was calculated as the ratio between test animals versus the negative controls. Reduction in SEAP signal (optical density value) in the test samples from the rabbits immunized with different vaccines compared with the pseudovirus control was analysed with one-way ANOVA for multiple groups or the unpaired Student’s t-test for two groups; a significant difference (P<0.05) between the two groups was interpreted as successful neutralization.
Acknowledgements
This work was supported by the National Institutes of Health (grant R01 CA47622) and the Jake Gittlen Memorial Golf Tournament. We thank Nancy Cladel for critically reviewing this manuscript.
References
- Allansmith M. (1973). Immunology of the tears. Int Ophthalmol Clin 13, 47–72 10.1097/00004397-197301310-00006 [DOI] [PubMed] [Google Scholar]
- Buck C. B., Pastrana D. V., Lowy D. R., Schiller J. T. (2005). Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med 119, 445–462 [DOI] [PubMed] [Google Scholar]
- Christensen N. D., Kreider J. W., Cladel N. M., Galloway D. A. (1990). Immunological cross-reactivity to laboratory-produced HPV-11 virions of polysera raised against bacterially derived fusion proteins and synthetic peptides of HPV-6b and HPV-16 capsid proteins. Virology 175, 1–9 10.1016/0042-6822(90)90180-Y [DOI] [PubMed] [Google Scholar]
- Cladel N. M., Hu J., Balogh K., Mejia A., Christensen N. D. (2008). Wounding prior to challenge substantially improves infectivity of cottontail rabbit papillomavirus and allows for standardization of infection. J Virol Methods 148, 34–39 10.1016/j.jviromet.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle P. K., Sibony P. A. (1986). Tear immunoglobulins measured by ELISA. Invest Ophthalmol Vis Sci 27, 622–625 [PubMed] [Google Scholar]
- Coyle P. K., Sibony P. A. (1988). Viral antibodies in normal tears. Invest Ophthalmol Vis Sci 29, 1552–1558 [PubMed] [Google Scholar]
- Douglas R. G., Jr, Rossen R. D., Butler W. T., Couch R. B. (1967). Rhinovirus neutralizing antibody in tears, parotid saliva, nasal secretions and serum. J Immunol 99, 297–303 [PubMed] [Google Scholar]
- Einstein M. H., Baron M., Levin M. J., Chatterjee A., Edwards R. P., Zepp F., Carletti I., Dessy F. J., Trofa A. F. & other authors (2009). Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin 5, 705–719 10.4161/hv.5.10.9518 [DOI] [PubMed] [Google Scholar]
- Fox P. D., Khaw P. T., McBride B. W., McGill J. I., Ward K. A. (1986). Tear and serum antibody levels in ocular herpetic infection: diagnostic precision of secretory IgA. Br J Ophthalmol 70, 584–588 10.1136/bjo.70.8.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. G. (1990). Antibodies in human tears during and after infection. Surv Ophthalmol 35, 151–157 10.1016/0039-6257(90)90070-C [DOI] [PubMed] [Google Scholar]
- Friedman M. G., Phillip M., Dagan R. (1989). Virus-specific IgA in serum, saliva, and tears of children with measles. Clin Exp Immunol 75, 58–63 [PMC free article] [PubMed] [Google Scholar]
- Giroglou T., Sapp M., Lane C., Fligge C., Christensen N. D., Streeck R. E., Rose R. C. (2001). Immunological analyses of human papillomavirus capsids. Vaccine 19, 1783–1793 10.1016/S0264-410X(00)00370-4 [DOI] [PubMed] [Google Scholar]
- Hu J., Budgeon L. R., Cladel N. M., Culp T. D., Balogh K. K., Christensen N. D. (2007a). Detection of L1, infectious virions and anti-L1 antibody in domestic rabbits infected with cottontail rabbit papillomavirus. J Gen Virol 88, 3286–3293 10.1099/vir.0.82879-0 [DOI] [PubMed] [Google Scholar]
- Hu J., Cladel N. M., Balogh K., Budgeon L., Christensen N. D. (2007b). Impact of genetic changes to the CRPV genome and their application to the study of pathogenesis in vivo. Virology 358, 384–390 10.1016/j.virol.2006.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford M. P., Orillac R., Chen D., Texada D. (2003). Systemic and ocular antibody responses to inactivated acute hemorrhagic conjunctivitis (AHC) virus; enterovirus 70 (EV70). Ocul Immunol Inflamm 11, 197–209 10.1076/ocii.11.3.197.17352 [DOI] [PubMed] [Google Scholar]
- Liotet S., Hartmann C., Batellier L., Chaumeil C., Frottier J. (1987). Anti-HIV-antibodies in tears of patients with AIDS. Fortschr Ophthalmol 84, 340–341 [PubMed] [Google Scholar]
- Little J. M., Centifanto Y. M., Kaufman H. E. (1969). Immunoglobulins in human tears. Am J Ophthalmol 68, 898–905 [DOI] [PubMed] [Google Scholar]
- Madar R., Straka S., Baska T. (2002). Detection of antibodies in saliva – an effective auxiliary method in surveillance of infectious diseases. Bratisl Lek Listy (Tlacene Vyd) 103, 38–41 [PubMed] [Google Scholar]
- McCormack P. L., Joura E. A. (2011). Spotlight on quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine(Gardasil®) in the prevention of premalignant genital lesions, genital cancer, and genital warts in women. BioDrugs 25, 339–343 10.2165/11205060-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Mejia A. F., Culp T. D., Cladel N. M., Balogh K. K., Budgeon L. R., Buck C. B., Christensen N. D. (2006). Preclinical model to test human papillomavirus virus (HPV) capsid vaccines in vivo using infectious HPV/cottontail rabbit papillomavirus chimeric papillomavirus particles. J Virol 80, 12393–12397 10.1128/JVI.01583-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesburn A. B. (1980). Common viral eye diseases and latent infections. Ophthalmology 87, 1202–1207 10.1016/S0161-6420(80)35103-8 [DOI] [PubMed] [Google Scholar]
- Pastrana D. V., Buck C. B., Pang Y. Y., Thompson C. D., Castle P. E., FitzGerald P. C., Krüger Kjaer S., Lowy D. R., Schiller J. T. (2004). Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321, 205–216 10.1016/j.virol.2003.12.027 [DOI] [PubMed] [Google Scholar]
- Pastrana D. V., Gambhira R., Buck C. B., Pang Y. Y., Thompson C. D., Culp T. D., Christensen N. D., Lowy D. R., Schiller J. T., Roden R. B. (2005). Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology 337, 365–372 10.1016/j.virol.2005.04.011 [DOI] [PubMed] [Google Scholar]
- Rozanova E. B., Teplinskaia L. E., Kaliberdina A. F., Barisani-Asenbauer T. (2006). Cytomegalovirus antibodies in tear fluid of patients with retinitis. Arch Virol 151, 2407–2417 10.1007/s00705-006-0813-0 [DOI] [PubMed] [Google Scholar]
- Välimaa H., Tenovuo J., Waris M., Hukkanen V. (2009). Human lactoferrin but not lysozyme neutralizes HSV-1 and inhibits HSV-1 replication and cell-to-cell spread. Virol J 6, 53 10.1186/1743-422X-6-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haeringen N. J. (1981). Clinical biochemistry of tears. Surv Ophthalmol 26, 84–96 10.1016/0039-6257(81)90145-4 [DOI] [PubMed] [Google Scholar]
- Villa L. L., Costa R. L., Petta C. A., Andrade R. P., Ault K. A., Giuliano A. R., Wheeler C. M., Koutsky L. A., Malm C. & other authors (2005). Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 6, 271–278 10.1016/S1470-2045(05)70101-7 [DOI] [PubMed] [Google Scholar]
- Yan Q., Peng B., Su G., Cohan B. E., Major T. C., Meyerhoff M. E. (2011). Measurement of tear glucose levels with amperometric glucose biosensor/capillary tube configuration. Anal Chem 83, 8341–8346 10.1021/ac201700c [DOI] [PubMed] [Google Scholar]
- Zhou L., Beuerman R. W. (2012). Tear analysis in ocular surface diseases. Prog Retin Eye Res 31, 527–550 10.1016/j.preteyeres.2012.06.002 [DOI] [PubMed] [Google Scholar]
- Zhou L., Zhao S. Z., Koh S. K., Chen L., Vaz C., Tanavde V., Li X. R., Beuerman R. W. (2012). In-depth analysis of the human tear proteome. J Proteomics 75, 3877–3885 10.1016/j.jprot.2012.04.053 [DOI] [PubMed] [Google Scholar]


