Abstract
Ubiquitylation is a covalent post-translational modification that regulates protein stability and is involved in many biological functions. Proteins may be modified with mono-ubiquitin or ubiquitin chains. Viruses have evolved multiple mechanisms to perturb the cell ubiquitin system and manipulate it to their own benefit. Here, we report ubiquitylation of vaccinia virus (VACV) protein N1. N1 is an inhibitor of the nuclear factor NF-κB and apoptosis that contributes to virulence, has a Bcl-2-like fold, and is highly conserved amongst orthopoxviruses. The interaction between N1 and ubiquitin occurs at endogenous protein levels during VACV infection and following ectopic expression of N1. Biochemical analysis demonstrated that N1 is covalently ubiquitylated, and heterodimers of ubiquitylated and non-ubiquitylated N1 monomers were identified, suggesting that ubiquitylation does not inhibit N1 dimerization. Studies with other VACV Bcl-2 proteins, such as C6 or B14, revealed that although these proteins also interact with ubiquitin, these interactions are non-covalent. Finally, mutagenesis of N1 showed that ubiquitylation occurs in a conventional lysine-dependent manner at multiple acceptor sites because only an N1 allele devoid of lysine residues remained unmodified. Taken together, we described a previously uncharacterized modification of the VACV protein N1 that provided a new layer of complexity to the biology of this virulence factor, and provided another example of the intricate interplay between poxviruses and the host ubiquitin system.
Introduction
Ubiquitylation is a post-translational modification consisting of the covalent attachment of an ~8 kDa ubiquitin (Ub) protein onto a recipient protein. This process involves the sequential action of at least three cellular enzymes, E1, E2 and E3, the third of which provides the specificity to target the desired protein (Komander & Rape, 2012; Pickart, 2001). Ubiquitylation is a reversible process due to the action of deubiquitinases. Conjugation of Ub occurs via the coupling of the C-terminal glycine of Ub to internal lysine residues within the substrate, although other target residues (such as cysteines, threonines, serines and terminal amino groups) can also be ubiquitylated non-canonically. Ubiquitylation can occur at a single (mono-ubiquitylation) or multiple (multi-ubiquitylation) acceptor sites within the same target protein. In addition, the lysine residues of Ub can themselves be ubiquitylated, leading to the formation of Ub chains. These chains can contain from two to >10 Ubs that can have additional complexity due to the varying linkages between these molecules. Ub chains may mark proteins for proteasomal degradation, particularly those formed via the Lys48 of ubiquitin and, to a lesser extent, Lys11 (Komander & Rape, 2012; Pickart, 2001). However, an expanding body of evidence indicates that Ub chains also have essential roles in endocytosis, trafficking or signalling, amongst others (Bhoj & Chen, 2009; Gerlach et al., 2011; Welchman et al., 2005).
Several viruses have evolved mechanisms to manipulate the Ub system and to regulate it in an advantageous manner. They may encode Ub ligases and deubiquitinases, and hijack components of the Ub machinery to target cellular components (reviewed by Gustin et al., 2011; Randow & Lehner, 2009). In addition, viruses can use ubiquitylation as a reversible post-translational modification to fulfil multiple biological functions with a limited number of proteins. For example, the human immunodeficiency virus protein Gag (Demirov et al., 2002; Garrus et al., 2001) and the Ebola virus protein VP40 (Yasuda et al., 2003) are ubiquitylated for virion budding and release, and ubiquitylation and deubiquitylation of influenza NP protein correlates with viral replication (Liao et al., 2010). Alternatively, Ub conjugation serves as a cellular mechanism to restrict pathogen propagation by degrading viral components as reported for the flavivirus NS5 protein (Taylor et al., 2011) or the human papilloma virus protein E7 (Kamio et al., 2004).
Poxviruses are large dsDNA viruses that replicate in the cytoplasm and express numerous proteins to manipulate the host cell environment (Moss, 2007). Poxviruses modulate the Ub system by multiple mechanisms (reviewed by Barry et al., 2010; Shchelkunov, 2010; Zhang et al., 2009): they code for their own Ub ligases such as p28 (Guerin et al., 2002; Nerenberg et al., 2005), express adaptors of cellular Ub complexes such as kelch and ankyrin proteins (Mohamed et al., 2009; Sonnberg et al., 2008; Sperling et al., 2008; van Buuren et al., 2008; Wilton et al., 2008), or inhibit directly the function of cellular E3 ligases such as A49 or PACR (Mansur et al., 2013; Mo et al., 2009). In addition, research with vaccinia virus (VACV), the most intensively studied poxvirus and the vaccine employed to eradicate smallpox, has demonstrated the requirement for active proteasomes to complete the viral life cycle (Mercer et al., 2012; Satheshkumar et al., 2009; Teale et al., 2009).
VACV encodes multiple proteins that promote immune evasion, called immunevasins (Smith et al., 2013). One of them, N1, is a 14 kDa intracellular protein that is expressed early during infection and contributes to virulence (Bartlett et al., 2002). Structurally, N1 is a homodimer and has a Bcl-2-like fold (Aoyagi et al., 2007; Cooray et al., 2007). Five other VACV proteins (B14, A52, F1, K7 and A46) have also been shown to have a Bcl-2 structure (Fedosyuk et al., 2014; Graham et al., 2008; Kalverda et al., 2009; Kvansakul et al., 2007) and other VACV proteins are predicted to be members of this family (González & Esteban, 2010; Graham et al., 2008). VACV Bcl-2 proteins have diverse functions including inhibition of apoptosis, inflammasome activation and signalling cascades leading to activation of the nuclear factor NF-κB and IFN regulatory factor 3 (IRF-3) (reviewed by Smith et al., 2013). Protein N1 was reported to inhibit the activation of IRF3 and NF-κB (DiPerna et al., 2004), and although the inhibition of NF-κB has been confirmed (Chen et al., 2008; Cooray et al., 2007; DiPerna et al., 2004; Maluquer de Motes et al., 2011), the mechanism by which N1 mediates this inhibition is uncertain. N1 can also protect cells against apoptosis via a surface groove that is conserved in cellular anti-apoptotic proteins, but is occluded in VACV Bcl-2-like proteins that lack anti-apoptotic activity (Cooray et al., 2007; Graham et al., 2008; Maluquer de Motes et al., 2011). The ability of N1 to prevent apoptosis has been disputed (Postigo & Way, 2012).
Here, we describe an additional feature of VACV protein N1. Whilst investigating potential cellular interacting partners for N1 using a recombinant VACV expressing an epitope-tagged N1, we noted that N1 exhibited multiple forms during infection and these represented ubiquitylated N1 proteins. N1 was ubiquitylated both during viral infection and following ectopic expression, and ubiquitylation was only abolished after mutagenesis of all N1 lysine residues. This previously uncharacterized interaction of N1 and ubiquitin added a new layer of complexity to the VACV protein N1, and provides another example of the interplay between poxviruses and the host Ub system.
Results
Generation of a recombinant tandem affinity purification (TAP)-tagged N1 virus
VACV protein N1 has multiple functions, some of which are not understood mechanistically. To gain better insight into how N1 performs these functions, a recombinant VACV strain, Western Reserve (WR), was generated by transient dominant selection (see Methods) in which the N1L gene was fused at the 3′ end to DNA encoding a TAP tag (vN1.TAP) consisting of a streptavidin-binding sequence and a FLAG epitope (Gloeckner et al., 2007) (Fig. 1a). This virus expressed N1 at a similar level to WT VACV as revealed by immunoblotting using an anti-N1 rabbit serum (Bartlett et al., 2002), indicating that the fusion did not affect the expression or stability of the protein (Fig. 1b). In addition, only protein N1 expressed from vN1.TAP infection could be detected by immunoblotting using an anti-FLAG antibody (Fig. 1b).
Fig. 1.
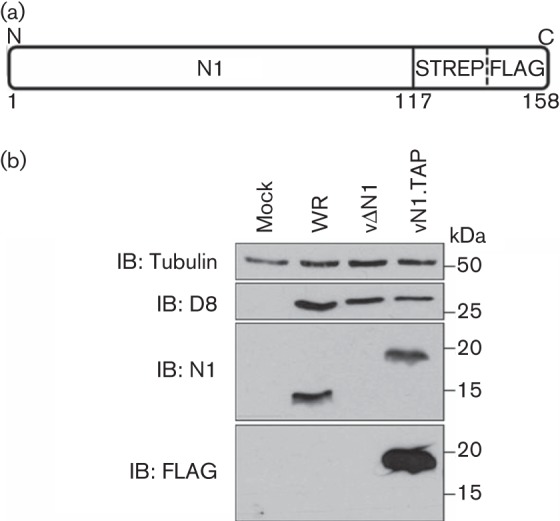
Generation of a recombinant TAP-tagged N1 virus. (a) Fusion of a TAP [streptavidin (STREP) and FLAG] tag at the C terminus of N1. (b) Infection of BS-C-1 cells with VACV strain WR, a recombinant VACV lacking the N1L gene (vΔN1) or a recombinant VACV expressing TAP-tagged N1 (vN1.TAP) at 2 p.f.u. per cell for 16 h. Whole-cell lysates were resolved by SDS-PAGE and immunoblotted (IB) with the indicated antibodies. Molecular mass markers are also included.
N1 interacts with ubiquitin during viral infection
To identify N1 binding partners, RAW247.1 cells (murine macrophages) were infected with vN1.TAP or vC6.TAP, a control virus in which the C6 protein was tagged in the same way (Methods), at 2 p.f.u. per cell for 16 h. The cell lysates were subjected to sequential affinity purification, and concentrated protein eluates were fractionated in Novex 4–12 % Bis-Tris protein gels and analysed by silver staining or subjected to SDS-PAGE and immunoblotting. In the silver-stained gels, numerous bands were observed for both N1 and C6 that were unique for each protein (Fig. 2a). For N1, intense bands were observed at ~16 and 32 kDa, which were consistent with the expected size of monomeric and dimeric N1.TAP, respectively (Bartlett et al., 2002; Maluquer de Motes et al., 2011). Accordingly, these bands were detected by an anti-FLAG antibody (Fig. 2b). MS analysis further confirmed the identity of these bands as monomeric and dimeric N1 (data not shown).
Fig. 2.
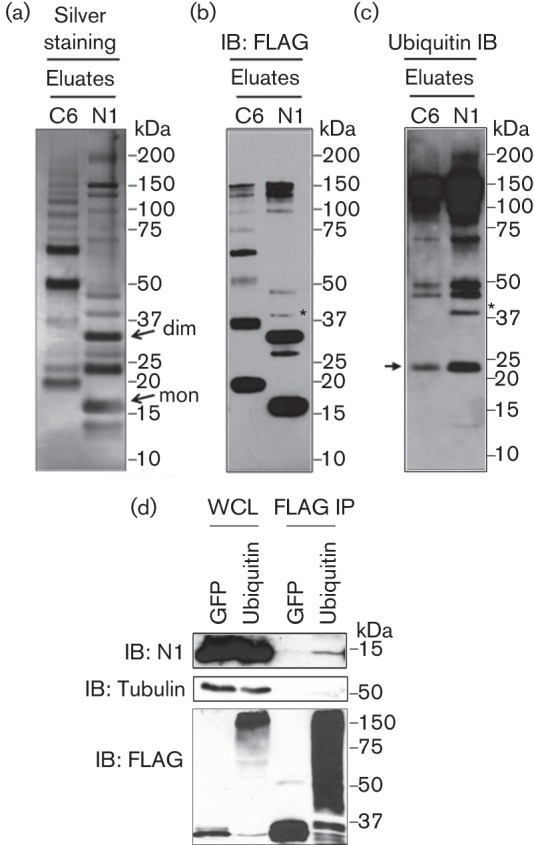
(a–c) N1 interacts with ubiquitin during infection. RAW247.1 macrophages were infected at 5 p.f.u. per cell with vC6.TAP and vN1.TAP, and subjected to TAP pull-down. Final eluates were resolved in Novex 4–12 % Bis-Tris protein gels and subjected to silver staining (a), or immunoblotting (IB) with anti-FLAG (b) or anti-ubiquitin (c) antibodies. A band of ~40 kDa that is recognized by anti-FLAG and anti-Ub antibodies is marked with an asterisk. The positions of the monomer (mon) and dimer (dim) are marked with arrows in (a). The position of the IgG light chain (LC) is indicated in (c). (d) Immunoprecipitation of FLAG-tagged Ub or GFP in cells infected with VACV WR. Whole-cell lysates (WCL) and FLAG immunoprecipitates (FLAG IP) were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. Molecular mass markers are also included. Data shown are representative of at least two experiments showing similar results.
FLAG immunoblotting also revealed other bands that did not correlate with predicted oligomers of N1. In particular, two bands were noted above the N1 dimer with sizes of ~40 and 48 kDa, and differing in ~8 kDa between each other and the N1 dimer. We speculated that these bands might correspond to ubiquitylated forms of the N1 dimer. To address this, samples were blotted with a mouse anti-Ub antibody and this showed the ~40 kDa band to be positive for Ub (Fig. 2c, asterisk). Unfortunately, we could not determine if the ~48 kDa band was ubiquitylated N1 due to non-specific signal present in both the N1 and C6 samples. These results revealed a previously unnoticed interaction between N1 and endogenous Ub in VACV-infected cells.
To confirm this interaction, a reciprocal immunoprecipitation was conducted. After FLAG immunoprecipitation, N1 co-immunoprecipitated with FLAG-Ub, but not FLAG-GFP (Fig. 2d), and this interaction was with monomeric N1 (the possible presence of ubiquitylated N1 forms could not be assessed due to non-specific signal when immunoblotting with anti-N1 serum). Therefore, N1 monomers may be immunoprecipitated with Ub due to their dimerization with ubiquitylated N1 monomers. In conclusion, N1 associated with Ub during viral infection.
Interaction between N1 and Ub does not require virus infection
To determine whether the interaction between N1 and Ub was dependent on virus infection, HEK 293T cells were co-transfected with FLAG-tagged Ub and haemagglutinin (HA)-tagged N1 plasmids, and 24 h later cell lysates were subjected to FLAG immunoprecipitation followed by anti-HA immunoblotting. Up to three HA-positive bands were observed after co-transfection of N1 and Ub (Fig. 3a). Two of these bands matched the size of ubiquitylated N1 molecules (~23 and ~30 kDa).
Fig. 3.
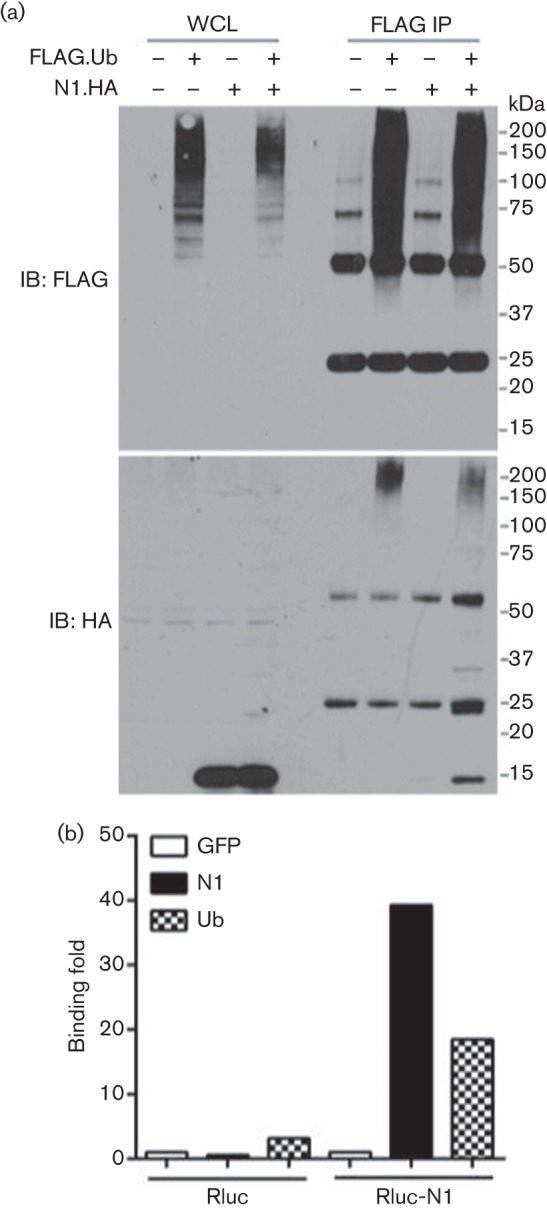
Interaction between N1 and Ub in transfected HEK 293T cells. (a) Immunoprecipitation of FLAG-tagged Ub in the presence (+) or absence (–) of HA-tagged N1. Whole-cell lysates (WCL) and FLAG immunoprecipitates (FLAG IP) were resolved in Novex 4–12 % Bis-Tris protein gels and immunoblotted (IB) with the indicated antibodies. The positions of molecular mass markers are indicated. (b) Interaction between FLAG-tagged versions of GFP, N1 or Ub and Rluc fused to N1 (Rluc-N1) or RLuc was assessed by LUMIER. Ratios between luciferase activity in lysates and eluates were calculated for each condition and plotted as a binding fold over control reactions. Data shown are from one experiment of three experiments showing indistinguishable results.
Independently, the N1–Ub interaction was analysed by LUMIER (luminescence-based mammalian interactome mapping; Barrios-Rodiles et al., 2005), taking advantage of a plasmid that codes for N1 fused with Renilla luciferase (Rluc) at its N terminus (Maluquer de Motes et al., 2011). HEK 293T cells were transfected with Rluc-N1, or Rluc only, together with FLAG-tagged versions of GFP, N1 or Ub. Luciferase activity was measured before and after FLAG immunoprecipitation, and the ratios for each condition were plotted as binding fold over a control reaction. Interaction was observed between Rluc-N1 and FLAG-N1 due to the ability of N1 to dimerize, and between Rluc-N1 and FLAG-Ub (Fig. 3b). These interactions were not present in the Rluc control reactions, and this confirmed that N1 and Ub could interact after ectopic expression in cells in the absence of viral infection.
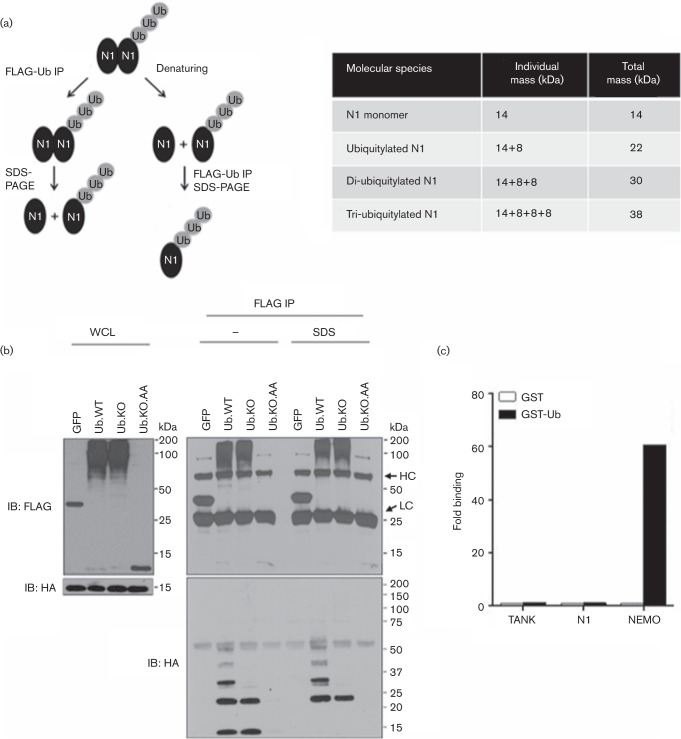
N1 is covalently ubiquitylated
Ub is an ~8 kDa protein that is covalently attached and thus remains conjugated to its target after denaturation. To determine whether N1 was ubiquitylated, we performed an experiment in which lysates from HEK 293T cells transfected with HA-tagged N1 and FLAG-tagged Ub were boiled in the presence of 1 % SDS to destroy all non-covalent interactions, and subsequently immunoprecipitated and analysed by SDS-PAGE (illustrated in Fig. 4a). Two Ub mutants were also included: a mutant in which all lysine residues were replaced by arginines, but the two C-terminal glycines were left intact (Ub.KO), and a Ub.KO mutant in which the glycine residues were replaced by alanines (Ub.KO.AA). Given this mutagenesis, mutant Ub.KO could be attached to substrates and preformed Ub chains, but could not support Ub chain formation, whereas Ub.KO.AA would be impaired for both attachment and chain formation. Accordingly, after overexpression the input samples for Ub.WT and Ub.KO mostly resolved as a smear in the upper part of the gel, whereas Ub.KO.AA resolved only as a monomer (Fig. 4b, left). After FLAG immunoprecipitation of denatured lysates, an HA-positive band of ~22 kDa was detected for Ub.WT and Ub.KO, but not for Ub.KO.AA, indicating that this band was an N1 monomer covalently attached to a Ub molecule. Some higher-molecular-mass bands, separated by ~8 kDa, were also visible for Ub.WT, but not for Ub.KO, indicating that they were subsequent attachments of Ub moieties onto the first ubiquitylated N1 monomer. When the FLAG IP was performed under normal conditions, an additional HA-positive band appeared corresponding to monomeric non-ubiquitylated N1 (~14 kDa). This demonstrated that non-covalent interactions such as N1 dimerization were disrupted by the denaturing treatment applied and that non-ubiquitylated monomeric N1 could associate with ubiquitylated N1 molecules in cells. The latter also suggested that the co-precipitation of monomeric non-ubiquitylated N1 with ubiquitin observed in previous experiments (Fig. 2d) was mediated by ubiquitylated heterodimers.
Fig. 4.
N1 is ubiquitylated. (a) Schematic of experimental design and the sizes of N1 and ubiquitylated derivatives. (b) HEK 293T cells were transfected with HA-tagged N1 and FLAG-tagged GFP or Ub.WT or Ub mutants, and lysates were subjected to immunoprecipitation under denaturing (SDS) or non-denaturing (–) conditions. Whole-cell lysates (WCL) and FLAG immunoprecipitates (FLAG IP) were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. The HA immunoblot of the FLAG IP samples was resolved in Novex 4–12 % Bis-Tris protein gels. The positions of molecular mass markers and of the IgG light chain (LC) and heavy chain (HC) are indicated. (c) Interaction between Rluc-fused TANK, N1 or NEMO with recombinant GST-tagged Ub as assessed by LUMIER. Lysates from HEK 293T cells transfected with Rluc fusions were affinity purified with recombinant GST or GST-tagged Ub, and ratios between luciferase activity in lysates and eluates were calculated for each condition. Data were plotted as fold binding over the TANK control reaction. Data shown are from one experiment of three experiments showing indistinguishable results.
To test whether N1 could also bind Ub non-covalently, we purified glutathione S-transferase (GST)-fused ubiquitin chains from bacteria and incubated them with lysates from cells expressing Rluc-N1. Cells expressing Rluc fusions for other unrelated cellular proteins (TANK and NEMO) were also included as negative and positive controls for the assay, respectively (Thurston et al., 2009). Ratios of luciferase activity were measured before and after the GST pull-down and, as expected, these revealed the Ub-binding capacity of NEMO (Fig. 4c). However, no such ability was observed for N1. Taken together, these results indicate that the interaction between N1 and Ub is mediated by ubiquitylation rather than an ability to bind Ub.
N1 does not accumulate after proteasome inhibition
Protein ubiquitylation is a crucial regulator of protein degradation in cells and most ubiquitylated proteins are targeted for degradation. Previous experiments had revealed that the levels of N1 protein were not altered by overexpression of Ub, suggesting that N1 ubiquitylation did not affect N1 stability or trigger its degradation. To confirm this, cells expressing N1 were treated with MG-132, an inhibitor of the proteasome, for different lengths of time and levels of N1 protein were determined by immunoblotting. No difference in N1 levels was observed after inhibition of the proteasome for up to 8 h (Fig. 5), suggesting that ubiquitylation of N1 did not trigger its proteasomal degradation. Conversely, p27, a well-known target of Skp2 that is constitutively degraded by the proteasome, accumulated progressively.
Fig. 5.
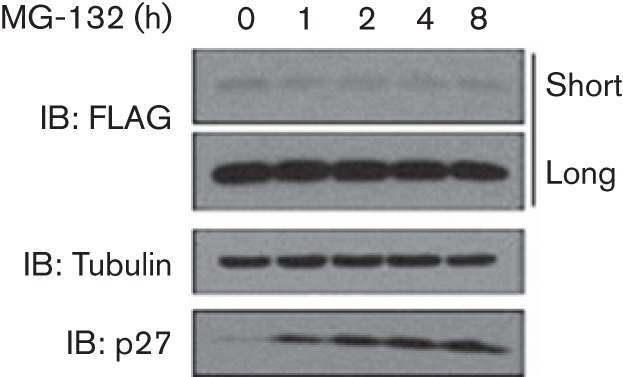
N1 is not targeted for proteasomal degradation. Lysates from HEK 293T cells transfected with TAP-tagged N1 and treated with MG-132 for the indicated lengths of time were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. Short and long exposures are shown for the FLAG (N1) panel. Data shown are from one experiment of three experiments showing indistinguishable results.
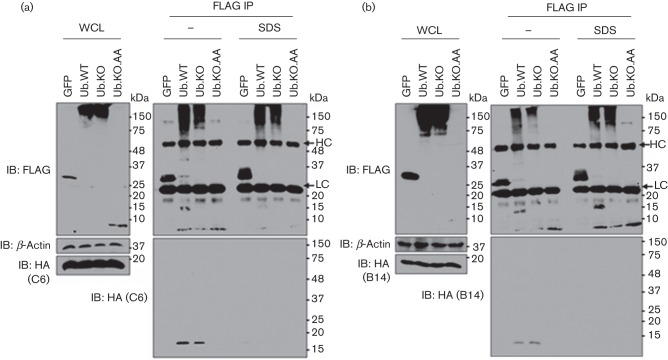
Vaccinia proteins C6 and B14 are not ubiquitylated
To determine whether ubiquitylation was unique for N1 or could also occur on other members of the VACV Bcl-2-like family, proteins C6 and B14 were studied. Similarly, in previous experiments, cells expressing different FLAG-tagged Ub alleles were infected with recombinant VACVs expressing HA-tagged C6 (Unterholzner et al., 2011) or HA-tagged B14 (Chen et al., 2006). As expected, each Ub mutant migrated differently according to their properties (Fig. 6a). Both C6 (Fig. 6a) and B14 (Fig. 6b) immunoprecipitated with Ub under normal but not denaturing conditions, indicating that their interactions with Ub are non-covalent.
Fig. 6.
VACV proteins C6 and B14 are not ubiquitylated. (a, b) Immunoprecipitation of FLAG-tagged WT or mutant Ub after infection with recombinant VACVs expressing HA-tagged C6 (a) or B14 (b) under denaturing (SDS) and non-denaturing (–) conditions. Ub mutants lacking all lysines (Ub.KO) or lacking all lysines and the C-terminal glycines (Ub.KO.AA) were used. Whole-cell lysates (WCL) and FLAG immunoprecipitates (FLAG IP) were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. The positions of the molecular mass marker and of the IgG light (LC) and heavy chain (HC) are indicated. Data shown are from one experiment of three experiments showing indistinguishable results.
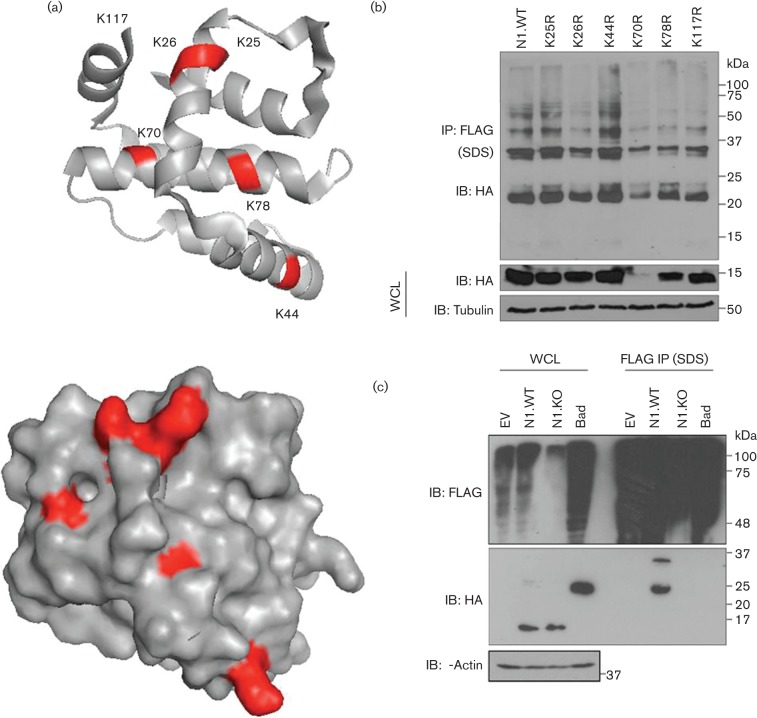
N1 is ubiquitylated in a lysine-dependent manner
To further understand the role of N1 ubiquitylation, single lysine N1 mutants were generated and their ability to be ubiquitylated was studied. N1 contains six lysine residues at positions 25, 26, 44, 70, 78 and 117. All of these are located at the surface on the molecule (although residues 70 and 78 are exposed to a lesser degree) and therefore have the potential to be accessible to the ubiquitylating machinery (Fig. 7a). We mutated each lysine residue to arginine individually and overexpressed each mutant in cells together with FLAG-Ub. All mutants expressed to comparable levels as the WT with the exception of mutant K70R. After immunoprecipitation under denaturing conditions all mutants, including K70R, co-precipitated with Ub, indicating that they were all ubiquitylated (Fig. 7b). To test whether K25 and K26 could compensate for each other due to their proximity, a double mutant K25,26R was also tested and similar ubiquitylation to N1.WT was observed (data not shown). To assess whether N1 could have multiple lysine acceptor sites or whether ubiquitylation could occur at alternative residues other than lysines, a lysine-free N1 (N1.KO) construct was purchased. N1.KO was expressed at similar levels to N1.WT, but failed to be ubiquitylated after co-expression with FLAG-tagged Ub and immunoprecipitation under denaturing conditions (Fig. 7c). In addition, protein Bad, a cellular protein with a Bcl-2-like fold, was included in the assay and failed to be ubiquitylated under the conditions tested, indicating that ubiquitylation was specific to N1 rather than the Bcl-2-like fold. Collectively, these data showed that N1 is covalently linked to Ub via one or more lysine residues.
Fig. 7.
Ubiquitylation of N1 lysine mutants. (a) Structural depiction of the N1 monomer in a ribbon-based or surface drawing obtained using PyMOL software. Lysine residues are numbered and highlighted in red. Residue K117 was not resolved in the crystal structure (Protein Data Bank ID: 2I39). (b, c) Immunoprecipitation under denaturing conditions of FLAG-tagged WT Ub in the presence of HA-tagged N1 mutants. Single lysine N1 mutants (b) or lysine-free N1 (N1.KO) (c) were used and compared with WT N1. HA-tagged Bad was also included. Whole-cell lysates (WCL) and FLAG immunoprecipitates were resolved by SDS-PAGE and immunoblotted (IB) with the indicated antibodies. The positions of molecular mass markers are indicated. Data shown are from one experiment of two experiments showing indistinguishable results.
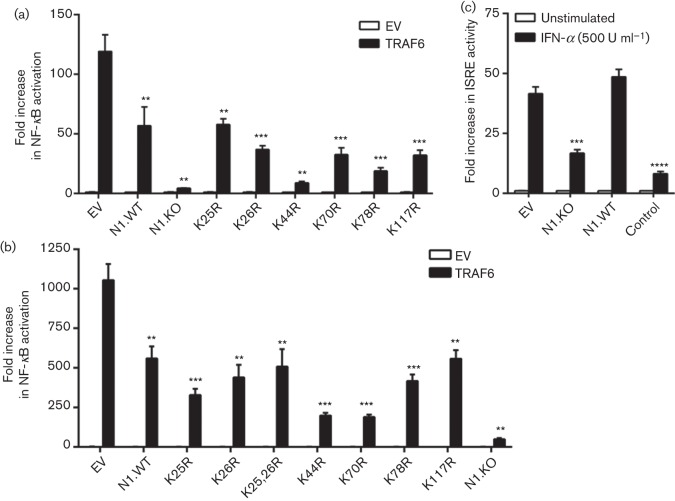
To determine whether ubiquitylation of N1 could affect its function, N1 lysine mutants were transfected in HEK 293T together with a reporter expressing luciferase under control of the NF-κB consensus promoter sequence. NF-κB activation was triggered by co-transfection with TNF-associated factor 6 (TRAF6) and luciferase activity was measured after 24 h (Fig. 8a) or 36 h (Fig. 8b). In both assays, all N1 lysine mutants retained the ability to inhibit NF-κB activation. However, N1.KO showed the greatest inhibition. To characterize this mutant further, N1.KO was tested in reporter assays where N1 was known not to have inhibitory activity. HEK 293T cells were transfected with a reporter containing IFN-stimulated reporter element (ISRE) together with N1.WT, N1.KO or a control molecule. Unexpectedly, N1.KO blocked ISRE activity upon treatment with IFN-α. These data demonstrated that N1.KO inhibited non-specifically the activation of unrelated reporter assays for reasons that remain unknown.
Fig. 8.
Inhibition of NF-κB and ISRE reporter activity by N1 lysine mutants. (a, b) Cells were transfected with the indicated plasmids together with pNF-κB-Luc and pTK-Ren. NF-κB activity was triggered by co-transfection with TRAF6. Cells were harvested 24 (a) or 36 h (b) post-transfection, and luciferase activity was plotted as fold activation over control conditions. (c) Cells were transfected with the indicated plasmids together with pISRE-Luc and pTK-Ren, and stimulated with IFN-α (500 U ml−1) for 8 h. Data are mean±sd with statistical analysis (Student’s t-test, **P<0.01, ***P<0.005, ****P<0.001) and are representative of at least two experimental repeats each. EV, Empty vector.
Discussion
In this study, a VACV expressing TAP-tagged N1 was generated to search for N1-interacting partners by TAP pull-down coupled to MS. Although no binding partners whose activity could be linked to the biological activities reported for N1 were found, Ub was specifically identified as an N1-interacting partner by immunoblotting (Fig. 2). To confirm this interaction, conventional immunoprecipitation was performed and this demonstrated that the interaction occurred not only with endogenous Ub during viral infection, but also after ectopic expression in transfected cells (Fig. 3). Furthermore, biochemical experiments revealed that N1 is directly and covalently ubiquitylated (Fig. 4b), and does not bind purified recombinant Ub (Fig. 4c), which is consistent with the lack of Ub-binding domains in the Bcl-2 structural fold (Chipuk et al., 2010). Given this, the non-covalent interactions observed between Ub and VACV proteins B14 and C6 are likely to be indirect and mediated by a third cellular or viral protein(s).
Ubiquitylation can occur in multiple forms: conjugation of a single Ub monomer (mono-ubiquitylation), conjugation of multiple monomers at different acceptor sites (multi-mono-ubiquitylation), conjugation of subsequent monomers onto each other to form Ub chains and combinations of these (Komander, 2009). In our experiments, Ub laddering was observed after denaturing. This could be caused by the conjugation of ubiquitin chains or by the simultaneous presence of multiple Ub monomers at different sites. Using a lysine-free Ub molecule that could not support chain elongation, we determined the formation of Ub chains, rather than multi-mono-ubiquitylation, on N1 (Fig. 4b). Ubiquitylation is commonly known as a cellular mechanism to target proteins for degradation. This is normally achieved by the formation of Lys48 Ub chains (Pickart, 2001). Although Ub chains were observed for N1, our attempts to determine the linkage between the different Ub monomers were inconclusive (data not shown). To investigate if N1 was targeted for proteasomal degradation, pharmacological inhibitors of the proteasome were used and such treatment did not affect the levels of N1 protein (Fig. 5).
Poxvirus protein N1 contains six lysine residues, and the crystal structure of N1 (Aoyagi et al., 2007; Cooray et al., 2007) showed that all of these are near the protein surface. Lys25, 26, 44 and 117 are very exposed, whereas Lys70 and 78 are in partially hidden positions (Fig. 7a), and the mutation of K70R reduced protein stability. Mutagenesis studies were performed to pinpoint which lysine residue was responsible for N1 ubiquitylation. These demonstrated that ubiquitylation on N1 could not be abrogated after mutagenesis of any single lysine residue, suggesting that more than one lysine can act as a lysine recipient (Fig. 7). However, replacement of all lysines prevented ubiquitylation, indicating that ubiquitylation of N1 occurs in a conventional lysine-dependent manner, with no involvement of non-canonical residues including the N-terminal group. Also, this suggests that even if a preferential acceptor site exists, alternative lysine residues can be used for Ub attachment. Identification of these residues and of their relative importance will require further studies.
Poxvirus protein N1 has been reported to contain at least two different functions: it can prevent apoptosis (Cooray et al., 2007; Graham et al., 2008; Maluquer de Motes et al., 2011) and it can inhibit intracellular signalling, particularly activation of NF-κB (Chen et al., 2008; Cooray et al., 2007; DiPerna et al., 2004; Maluquer de Motes et al., 2011). Inhibition of apoptosis by N1 has recently been mapped to a surface groove that mimics those of cellular anti-apoptotic Bcl-2 proteins (Maluquer de Motes et al., 2011). Mutations that limit the accessibility to this groove prevent N1 from binding cellular pro-apoptotic proteins Bad and Bid, and consequently from preventing cell death. In contrast, the mechanism employed by N1 to interfere with intracellular signalling remains unclear. Given this and the importance of Ub in the regulation of inflammatory signalling (Bhoj & Chen, 2009; Gerlach et al., 2011), it is tempting to speculate a correlation between the ubiquitylation of N1 and its ability to inhibit NF-κB. Unfortunately, functional analysis using lysine-free N1 revealed non-specific, off-target inhibition of different unrelated reporter activities for unknown reasons. Thus, whether there is a formal relationship between N1 ubiquitylation and the inhibition of inflammatory signalling remains unknown. Nonetheless, previous work reported that mutation I6E in N1 abrogated dimer formation and the ability to inhibit NF-κB by N1 (Maluquer de Motes et al., 2011). This indicated that either N1 needs to be a dimer to block NF-κB or that the same interface in N1 is used for both functions. No lysines are present in this interface, suggesting that ubiquitylation of N1 might not interfere with dimer formation. Given that ubiquitylation can occur after dimerization, further experiments are required to determine the ability of ubiquitylated N1 monomers to dimerize de novo.
Identification and characterization of viral proteins that undergo ubiquitylation is important to understand the complex mechanisms employed by viruses to modulate the host cell environment. Ub and Ub-like molecules are involved in many aspects of cell biology, including endocytosis, trafficking, transcription and the innate immune response. Given that viruses manipulate host cell machinery, interplay between viruses and the Ub system is to be expected. For some aspects, such as retrovirus budding and egress (Martin-Serrano, 2007) or degradation of tumour suppressor protein p53 (Beaudenon & Huibregtse, 2008; Mammas et al., 2008), it is clear that viruses have advantageously hijacked the cellular apparatus to facilitate the viral life cycle. For some others, however, it is difficult to distinguish between viral manipulation of host machinery and cellular inhibition of viral replication, or even adventitious modification of viral proteins. In this work, we demonstrate that VACV virulence factor N1 is ubiquitylated. Similar ubiquitylation has also been demonstrated for VACV protein E3 (González-Santamaria et al., 2011). Interestingly, both N1 and E3 are intracellular factors with multiple functions. Post-translational ubiquitylation may thus account for the diverse biological effects mediated by these proteins during VACV infection.
Methods
Cells, viruses and reagents.
BS-C-1, HEK 293T and RAW247.1 macrophages were grown at 37 °C in a 5 % CO2 atmosphere in Dulbecco’s modified Eagle’s medium supplemented with 10 % FBS (Invitrogen), 100 U penicillin ml−1 (Invitrogen) and 100 mg streptomycin ml−1 (Invitrogen). vC6.HA and vB14.HA were as described previously (Chen et al., 2006; Unterholzner et al., 2011). MG-132 (Sigma-Aldrich) was dissolved in DMSO and used at 20 µM. IFN-α was from Peprotech.
Expression plasmids.
HA-tagged N1 and Rluc -fused N1 were as described previously (Maluquer de Motes et al., 2011). Site-directed mutagenesis of both the HA-tagged N1 and FLAG-tagged Ub was performed using the QuikChange mutagenesis kit (Stratagene). The N1 allele where all lysine residues had been replaced by arginines (N1.KO) was obtained from Geneart (Invitrogen). FLAG-tagged GFP, TRAF6 and Rluc fusions for TANK and NEMO were gifts from F. Randow (MRC Laboratory of Molecular Biology, UK). HA-tagged Bad was from Xin Lu (Ludwig Institute for Cancer Research, UK). FLAG-tagged Ub was from Christel Brou (Institute Pasteur, France). All sequences were verified by DNA sequencing.
Generation of vN1.TAP.
N1 fused with a C-terminal TAP tag (Maluquer de Motes et al., 2011), as well as the flanking regions of the N1L gene of the VACV WR strain, were cloned into the transfer vector pUC13 containing EGFP and EcoGPT selection/marker genes as described previously (Ember et al., 2012). BS-C-1 cells infected with WR lacking N1 (vΔN1) (Bartlett et al., 2002) were transfected with pUC13-N1.TAP using Fugene (Roche) and recombinant viruses were selected as described previously (Maluquer de Motes et al., 2011; Unterholzner et al., 2011). Recombinant vN1.TAP viruses were screened by PCR using primers located in the flanking regions, and finally expanded and titrated by plaque assay in BS-C-1 cells. Protein expression was analysed after infection of BS-C-1 cells for 16 h with 2 p.f.u. per cell. A recombinant virus expressing TAP-tagged C6 was generated in a similar manner after cloning of the C6L gene and its flanking regions into the pUC13 vector.
TAP.
TAP was performed as described previously, with minor modifications (Gloeckner et al., 2007; Peters et al., 2013). RAW247.1 macrophages were infected overnight with 2 p.f.u. per cell and subsequently lysed in PBS supplemented with 0.5 % NP-40. Lysates were incubated with streptavidin beads (Thermo Scientific) for 2 h at 4 °C. Beads were washed three times and eluted in PBS supplemented with biotin (Thermo Scientific) for 1 h at 4 °C. Eluates were incubated with anti-FLAG M2 agarose beads (Sigma) and eluted in PBS supplemented with FLAG peptide (Sigma) in a similar manner. Final eluates were concentrated using 3 kDa Amicon filters (Millipore). Proteins retained on streptavidin and FLAG beads, as well as final eluates, were resolved in Novex 4–12 % Bis-Tris protein gels (Invitrogen) and analysed by silver staining (Invitrogen), or immunoblotting with anti-FLAG (Sigma-Aldrich) or anti-ubiquitin (FK2; Enzo Life Sciences) antibodies.
Immunoprecipitation and LUMIER.
HEK 293T cells were transfected using calcium chloride and lysed 24 h later with IP buffer (20 mM Tris/HCl, pH 7.4, 150 mM NaCl, 10 % glycerol, 0.1 % Triton X-100 and protease inhibitors; Roche) as described previously (Maluquer de Motes et al., 2011). Whole-cell lysates and immunoprecipitates were analysed in Novex 4–12 % Bis-Tris protein gels (Invitrogen) or by home-made SDS-PAGE and immunoblotting with the following antibodies: anti-N1 (Bartlett et al., 2002), anti-D8 (Parkinson & Smith, 1994), anti-FLAG (Sigma-Aldrich), anti-tubulin (Upstate Biotech), anti-HA (Covance), anti-p27 (Cell Signalling) and anti-β-actin (Abcam). For LUMIER with GST fusions, GST and GST-fused Ub were expressed in BL21 bacterial cells, lysed in BugBuster lysis buffer (Novagen), and purified by affinity chromatography as described previously (Thurston et al., 2009). Recombinant protein (50 ng per sample) was incubated with GST Sepharose beads (Amersham Pharmacia) for 1 h at 4 °C and mixed with cell lysates containing Rluc fusions. Samples were further incubated for 2 h at 4 °C and washed three times with lysis buffer. GST complexes were eluted in Passive Lysis Buffer (Promega) supplemented with 100 mM glutathione (Amersham Pharmacia) and luciferase activity was measured. Ratios between the whole-cell lysates and the GST pull-down eluates were calculated and compared with controls.
Ubiquitylation assay.
To examine ubiquitylation, HEK 293T cells were transfected using calcium chloride and/or infected with the indicated viruses, and 24 h later were lysed in TNE buffer (50 mM Tris/HCl, pH 7.5, 250 mM NaCl, 1 % NP-40, 1 mM EDTA and 1 mM DTT) supplemented with N-ethylmaleimide (Sigma-Aldrich) as described previously (Yamazaki et al., 2009). Cell lysates were centrifuged (10 000 g for 30 min) and cleared supernatants were mixed with 1 vol. 2 % SDS TNE. Samples were heated at 90 °C for 10 min to destroy all non-covalent interactions. Lysates were diluted 10-fold in TNE buffer and subjected to FLAG immunoprecipitation for 16 h using FLAG M2 resin (Sigma-Aldrich). Samples were washed three times in TNE buffer and finally analysed by immunoblotting.
Reporter gene assays.
HEK 293T cells were transfected with 100 ng per well of the indicated plasmids together with 70 ng per well of pNF-κB-Luc and 10 ng per well of pTK-Ren using Fugene (Roche). NF-κB was triggered by co-transfection of 10 ng per well of TRAF6. Luciferase activity was measured 24 or 36 h later and expressed as fold activation over controls. Similarly, HEK 293T cells were transfected with the indicated plasmids together with pISRE-Luc and stimulated with IFN-α for 8 h.
Acknowledgements
We thank Felix Randow and David Komander for helpful discussion. This work was supported by grants from the Medical Research Council and the Wellcome Trust. C. M. was a ‘Beatriu de Pinos’ Research Fellow from the Generalitat de Catalunya and G. L. S. is a Wellcome Trust Principal Research Fellow.
References
- Aoyagi M., Zhai D., Jin C., Aleshin A. E., Stec B., Reed J. C., Liddington R. C. (2007). Vaccinia virus N1L protein resembles a B cell lymphoma-2 (Bcl-2) family protein. Protein Sci 16, 118–124 10.1110/ps.062454707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios-Rodiles M., Brown K. R., Ozdamar B., Bose R., Liu Z., Donovan R. S., Shinjo F., Liu Y., Dembowy J. & other authors (2005). High-throughput mapping of a dynamic signaling network in mammalian cells. Science 307, 1621–1625 10.1126/science.1105776 [DOI] [PubMed] [Google Scholar]
- Barry M., van Buuren N., Burles K., Mottet K., Wang Q., Teale A. (2010). Poxvirus exploitation of the ubiquitin–proteasome system. Viruses 2, 2356–2380 10.3390/v2102356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett N., Symons J. A., Tscharke D. C., Smith G. L. (2002). The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J Gen Virol 83, 1965–1976 [DOI] [PubMed] [Google Scholar]
- Beaudenon S., Huibregtse J. M. (2008). HPV E6, E6AP and cervical cancer. BMC Biochem 9 (Suppl 1), S4. 10.1186/1471-2091-9-S1-S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoj V. G., Chen Z. J. (2009). Ubiquitylation in innate and adaptive immunity. Nature 458, 430–437 10.1038/nature07959 [DOI] [PubMed] [Google Scholar]
- Chen R. A., Jacobs N., Smith G. L. (2006). Vaccinia virus strain Western Reserve protein B14 is an intracellular virulence factor. J Gen Virol 87, 1451–1458 10.1099/vir.0.81736-0 [DOI] [PubMed] [Google Scholar]
- Chen R. A., Ryzhakov G., Cooray S., Randow F., Smith G. L. (2008). Inhibition of IkappaB kinase by vaccinia virus virulence factor B14. PLoS Pathog 4, e22. 10.1371/journal.ppat.0040022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk J. E., Moldoveanu T., Llambi F., Parsons M. J., Green D. R. (2010). The BCL-2 family reunion. Mol Cell 37, 299–310 10.1016/j.molcel.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooray S., Bahar M. W., Abrescia N. G., McVey C. E., Bartlett N. W., Chen R. A., Stuart D. I., Grimes J. M., Smith G. L. (2007). Functional and structural studies of the vaccinia virus virulence factor N1 reveal a Bcl-2-like anti-apoptotic protein. J Gen Virol 88, 1656–1666 10.1099/vir.0.82772-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirov D. G., Ono A., Orenstein J. M., Freed E. O. (2002). Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc Natl Acad Sci U S A 99, 955–960 10.1073/pnas.032511899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPerna G., Stack J., Bowie A. G., Boyd A., Kotwal G., Zhang Z., Arvikar S., Latz E., Fitzgerald K. A., Marshall W. L. (2004). Poxvirus protein N1L targets the I-kappaB kinase complex, inhibits signaling to NF-kappaB by the tumor necrosis factor superfamily of receptors, and inhibits NF-kappaB and IRF3 signaling by Toll-like receptors. J Biol Chem 279, 36570–36578 10.1074/jbc.M400567200 [DOI] [PubMed] [Google Scholar]
- Ember S. W., Ren H., Ferguson B. J., Smith G. L. (2012). Vaccinia virus protein C4 inhibits NF-κB activation and promotes virus virulence. J Gen Virol 93, 2098–2108 10.1099/vir.0.045070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedosyuk S., Grishkovskaya I., de Almeida Ribeiro E., Jr, Skern T. (2014). Characterization and structure of the vaccinia virus NF-κB antagonist A46. J Biol Chem 289, 3749–3762 10.1074/jbc.M113.512756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrus J. E., von Schwedler U. K., Pornillos O. W., Morham S. G., Zavitz K. H., Wang H. E., Wettstein D. A., Stray K. M., Côté M. & other authors (2001). Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107, 55–65 10.1016/S0092-8674(01)00506-2 [DOI] [PubMed] [Google Scholar]
- Gerlach B., Cordier S. M., Schmukle A. C., Emmerich C. H., Rieser E., Haas T. L., Webb A. I., Rickard J. A., Anderton H. & other authors (2011). Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 471, 591–596 10.1038/nature09816 [DOI] [PubMed] [Google Scholar]
- Gloeckner C. J., Boldt K., Schumacher A., Roepman R., Ueffing M. (2007). A novel tandem affinity purification strategy for the efficient isolation and characterisation of native protein complexes. Proteomics 7, 4228–4234 10.1002/pmic.200700038 [DOI] [PubMed] [Google Scholar]
- González J. M., Esteban M. (2010). A poxvirus Bcl-2-like gene family involved in regulation of host immune response: sequence similarity and evolutionary history. Virol J 7, 59. 10.1186/1743-422X-7-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Santamaría J., Campagna M., García M. A., Marcos-Villar L., González D., Gallego P., Lopitz-Otsoa F., Guerra S., Rodríguez M. S. & other authors (2011). Regulation of vaccinia virus E3 protein by small ubiquitin-like modifier proteins. J Virol 85, 12890–12900 10.1128/JVI.05628-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S. C., Bahar M. W., Cooray S., Chen R. A., Whalen D. M., Abrescia N. G., Alderton D., Owens R. J., Stuart D. I. & other authors (2008). Vaccinia virus proteins A52 and B14 Share a Bcl-2-like fold but have evolved to inhibit NF-kappaB rather than apoptosis. PLoS Pathog 4, e1000128. 10.1371/journal.ppat.1000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin J. L., Gelfi J., Boullier S., Delverdier M., Bellanger F. A., Bertagnoli S., Drexler I., Sutter G., Messud-Petit F. (2002). Myxoma virus leukemia-associated protein is responsible for major histocompatibility complex class I and Fas-CD95 down-regulation and defines scrapins, a new group of surface cellular receptor abductor proteins. J Virol 76, 2912–2923 10.1128/JVI.76.6.2912-2923.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin J. K., Moses A. V., Früh K., Douglas J. L. (2011). Viral takeover of the host ubiquitin system. Front Microbiol 2, 161. 10.3389/fmicb.2011.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalverda A. P., Thompson G. S., Vogel A., Schröder M., Bowie A. G., Khan A. R., Homans S. W. (2009). Poxvirus K7 protein adopts a Bcl-2 fold: biochemical mapping of its interactions with human DEAD box RNA helicase DDX3. J Mol Biol 385, 843–853 10.1016/j.jmb.2008.09.048 [DOI] [PubMed] [Google Scholar]
- Kamio M., Yoshida T., Ogata H., Douchi T., Nagata Y., Inoue M., Hasegawa M., Yonemitsu Y., Yoshimura A. (2004). SOCS1 [corrected] inhibits HPV-E7-mediated transformation by inducing degradation of E7 protein. Oncogene 23, 3107–3115 10.1038/sj.onc.1207453 [DOI] [PubMed] [Google Scholar]
- Komander D. (2009). The emerging complexity of protein ubiquitination. Biochem Soc Trans 37, 937–953 10.1042/BST0370937 [DOI] [PubMed] [Google Scholar]
- Komander D., Rape M. (2012). The ubiquitin code. Annu Rev Biochem 81, 203–229 10.1146/annurev-biochem-060310-170328 [DOI] [PubMed] [Google Scholar]
- Kvansakul M., van Delft M. F., Lee E. F., Gulbis J. M., Fairlie W. D., Huang D. C., Colman P. M. (2007). A structural viral mimic of prosurvival Bcl-2: a pivotal role for sequestering proapoptotic Bax and Bak. Mol Cell 25, 933–942 10.1016/j.molcel.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Liao T. L., Wu C. Y., Su W. C., Jeng K. S., Lai M. M. (2010). Ubiquitination and deubiquitination of NP protein regulates influenza A virus RNA replication. EMBO J 29, 3879–3890 10.1038/emboj.2010.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maluquer de Motes C., Cooray S., Ren H., Almeida G. M. F., McGourty K., Bahar M. W., Stuart D. I., Grimes J. M., Graham S. C., Smith G. L. (2011). Inhibition of apoptosis and NF-κB activation by vaccinia protein N1 occur via distinct binding surfaces and make different contributions to virulence. PLoS Pathog 7, e1002430. 10.1371/journal.ppat.1002430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammas I. N., Sourvinos G., Giannoudis A., Spandidos D. A. (2008). Human papilloma virus (HPV) and host cellular interactions. Pathol Oncol Res 14, 345–354 10.1007/s12253-008-9056-6 [DOI] [PubMed] [Google Scholar]
- Mansur D. S., Maluquer de Motes C., Unterholzner L., Sumner R. P., Ferguson B. J., Ren H., Strnadova P., Bowie A. G., Smith G. L. (2013). Poxvirus targeting of E3 ligase β-TrCP by molecular mimicry: a mechanism to inhibit NF-κB activation and promote immune evasion and virulence. PLoS Pathog 9, e1003183. 10.1371/journal.ppat.1003183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Serrano J. (2007). The role of ubiquitin in retroviral egress. Traffic 8, 1297–1303 10.1111/j.1600-0854.2007.00609.x [DOI] [PubMed] [Google Scholar]
- Mercer J., Snijder B., Sacher R., Burkard C., Bleck C. K., Stahlberg H., Pelkmans L., Helenius A. (2012). RNAi screening reveals proteasome- and Cullin3-dependent stages in vaccinia virus infection. Cell Rep 2, 1036–1047 10.1016/j.celrep.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Mo M., Fleming S. B., Mercer A. A. (2009). Cell cycle deregulation by a poxvirus partial mimic of anaphase-promoting complex subunit 11. Proc Natl Acad Sci U S A 106, 19527–19532 10.1073/pnas.0905893106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M. R., Rahman M. M., Lanchbury J. S., Shattuck D., Neff C., Dufford M., van Buuren N., Fagan K., Barry M. & other authors (2009). Proteomic screening of variola virus reveals a unique NF-kappaB inhibitor that is highly conserved among pathogenic orthopoxviruses. Proc Natl Acad Sci U S A 106, 9045–9050 10.1073/pnas.0900452106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. (2007). Poxviridae: the viruses and their replicaton. In Fields Virology, 5th edn, pp. 2905–2946 Edited by Knipe D. M., Howley P. M., Griffin D. E., Lamb R. A., Martin M. A., Roizman B., Straus S. E. Philadelphia, PA: Lippincott Williams & Wilkins [Google Scholar]
- Nerenberg B. T., Taylor J., Bartee E., Gouveia K., Barry M., Früh K. (2005). The poxviral RING protein p28 is a ubiquitin ligase that targets ubiquitin to viral replication factories. J Virol 79, 597–601 10.1128/JVI.79.1.597-601.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. E., Smith G. L. (1994). Vaccinia virus gene A36R encodes a Mr 43–50 K protein on the surface of extracellular enveloped virus. Virology 204, 376–390 10.1006/viro.1994.1542 [DOI] [PubMed] [Google Scholar]
- Peters N. E., Ferguson B. J., Mazzon M., Fahy A. S., Krysztofinska E., Arribas-Bosacoma R., Pearl L. H., Ren H., Smith G. L. (2013). A mechanism for the inhibition of DNA-PK-mediated DNA sensing by a virus. PLoS Pathog 9, e1003649. 10.1371/journal.ppat.1003649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart C. M. (2001). Mechanisms underlying ubiquitination. Annu Rev Biochem 70, 503–533 10.1146/annurev.biochem.70.1.503 [DOI] [PubMed] [Google Scholar]
- Postigo A., Way M. (2012). The vaccinia virus-encoded Bcl-2 homologues do not act as direct Bax inhibitors. J Virol 86, 203–213 10.1128/JVI.05817-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randow F., Lehner P. J. (2009). Viral avoidance and exploitation of the ubiquitin system. Nat Cell Biol 11, 527–534 10.1038/ncb0509-527 [DOI] [PubMed] [Google Scholar]
- Satheshkumar P. S., Anton L. C., Sanz P., Moss B. (2009). Inhibition of the ubiquitin–proteasome system prevents vaccinia virus DNA replication and expression of intermediate and late genes. J Virol 83, 2469–2479 10.1128/JVI.01986-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchelkunov S. N. (2010). Interaction of orthopoxviruses with the cellular ubiquitin–ligase system. Virus Genes 41, 309–318 10.1007/s11262-010-0519-y [DOI] [PubMed] [Google Scholar]
- Smith G. L., Benfield C. T., Maluquer de Motes C., Mazzon M., Ember S. W., Ferguson B. J., Sumner R. P. (2013). Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J Gen Virol 94, 2367–2392 10.1099/vir.0.055921-0 [DOI] [PubMed] [Google Scholar]
- Sonnberg S., Seet B. T., Pawson T., Fleming S. B., Mercer A. A. (2008). Poxvirus ankyrin repeat proteins are a unique class of F-box proteins that associate with cellular SCF1 ubiquitin ligase complexes. Proc Natl Acad Sci U S A 105, 10955–10960 10.1073/pnas.0802042105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling K. M., Schwantes A., Schnierle B. S., Sutter G. (2008). The highly conserved orthopoxvirus 68k ankyrin-like protein is part of a cellular SCF ubiquitin ligase complex. Virology 374, 234–239 10.1016/j.virol.2008.02.018 [DOI] [PubMed] [Google Scholar]
- Taylor R. T., Lubick K. J., Robertson S. J., Broughton J. P., Bloom M. E., Bresnahan W. A., Best S. M. (2011). TRIM79α, an interferon-stimulated gene product, restricts tick-borne encephalitis virus replication by degrading the viral RNA polymerase. Cell Host Microbe 10, 185–196 10.1016/j.chom.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale A., Campbell S., Van Buuren N., Magee W. C., Watmough K., Couturier B., Shipclark R., Barry M. (2009). Orthopoxviruses require a functional ubiquitin–proteasome system for productive replication. J Virol 83, 2099–2108 10.1128/JVI.01753-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston T. L., Ryzhakov G., Bloor S., von Muhlinen N., Randow F. (2009). The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol 10, 1215–1221 10.1038/ni.1800 [DOI] [PubMed] [Google Scholar]
- Unterholzner L., Sumner R. P., Baran M., Ren H., Mansur D. S., Bourke N. M., Randow F., Smith G. L., Bowie A. G. (2011). Vaccinia virus protein C6 is a virulence factor that binds TBK-1 adaptor proteins and inhibits activation of IRF3 and IRF7. PLoS Pathog 7, e1002247. 10.1371/journal.ppat.1002247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren N., Couturier B., Xiong Y., Barry M. (2008). Ectromelia virus encodes a novel family of F-box proteins that interact with the SCF complex. J Virol 82, 9917–9927 10.1128/JVI.00953-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welchman R. L., Gordon C., Mayer R. J. (2005). Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol 6, 599–609 10.1038/nrm1700 [DOI] [PubMed] [Google Scholar]
- Wilton B. A., Campbell S., Van Buuren N., Garneau R., Furukawa M., Xiong Y., Barry M. (2008). Ectromelia virus BTB/kelch proteins, EVM150 and EVM167, interact with cullin-3-based ubiquitin ligases. Virology 374, 82–99 10.1016/j.virol.2007.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K., Gohda J., Kanayama A., Miyamoto Y., Sakurai H., Yamamoto M., Akira S., Hayashi H., Su B., Inoue J. (2009). Two mechanistically and temporally distinct NF-kappaB activation pathways in IL-1 signaling. Sci Signal 2, ra66. 10.1126/scisignal.2000387 [DOI] [PubMed] [Google Scholar]
- Yasuda J., Nakao M., Kawaoka Y., Shida H. (2003). Nedd4 regulates egress of Ebola virus-like particles from host cells. J Virol 77, 9987–9992 10.1128/JVI.77.18.9987-9992.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Villa N. Y., McFadden G. (2009). Interplay between poxviruses and the cellular ubiquitin/ubiquitin-like pathways. FEBS Lett 583, 607–614 10.1016/j.febslet.2009.01.023 [DOI] [PubMed] [Google Scholar]