Abstract
Background
Various allergens play a role in the elicitation or exacerbation of eczematous skin lesions in atopic dermatitis (AD), and much research effort has been focused on improving diagnostic tests to identify causative allergens.
Objective
The purpose of this study was to evaluate the diagnostic effectiveness of a newly introduced microarray-based specific immunoglobulin E detection assay, ImmunoCAP ISAC, for use in AD patients.
Methods
The serum samples of 25 AD patients were tested by using ISAC and a multiple allergen simultaneous test-enzyme immunoassay (MAST-EIA). In addition, 10 of the 25 patients underwent skin prick testing (SPT). The positive reaction rates to allergens in each test and the agreements, sensitivities, and specificities of ISAC and MAST-EIA were evaluated versus the SPT results.
Results
For ISAC versus SPT, the overall results were as follows: sensitivity, 90.0%; specificity, 98.2%; positive predictive value (PPV), 90.0%; and negative predictive value (NPV), 98.2%. The total agreement and κ value for ISAC versus SPT were 96.9% and 0.882, respectively. For MAST-EIA versus SPT, the sensitivity was 80.0%, specificity 92.7%, PPV 66.7%, and NPV 96.2%. The total agreement and κ value for MAST-EIA versus SPT were 90.8% and 0.672, respectively. The overall agreement between the ISAC and MAST-EIA results was 88%.
Conclusion
The ISAC results in AD correlated well with the SPT results, and compared favorably to the MAST-EIA results. This study demonstrates the potential of ISAC as a convenient allergic diagnostic method in AD patients.
Keywords: Atopic dermatitis, Microarray-based specific IgE detection assay
INTRODUCTION
Although atopic dermatitis (AD) remains a challenging condition to treat in clinical practice because of its obscure and varied pathogenic mechanisms, convincing evidence indicates that in some patients, allergens play a role in the elicitation and exacerbation of eczematous skin lesions1. To identify causative allergens in AD patients, which is crucial for the control and treatment of symptoms, many in vivo and in vitro diagnostic techniques have been devised.
The skin prick test (SPT) is a typical in vivo test that uses the principle of immediate hypersensitivity to allergens and is being extensively used as a standard diagnostic tool. The SPT provides a cheap, quick, and reliable means of detecting specific immunoglobulin E (sIgE) among a large number of allergens. On the other hand, it is invasive, time-consuming, produces false-positive reactions, and is more difficult to perform than venous puncture in young children. Furthermore, it is a clinically important issue that the SPT cannot be conducted on patients taking antihistamine2.
To overcome these limitations of the SPT, new in vitro tools have been devised to identify sIgE and allow the identification of a wide spectrum of sensitizing allergens. Three representative methods-the radioallergosorbent test, the Pharmacia CAP test, and the multiple allergen simultaneous test-chemiluminescent assay (MAST-CLA)-can be used to test for various types of allergens. MAST-CLA has been popularly applied as a diagnostic tool owing to its simplicity, convenience, and safety3. An enzyme immunoassay (EIA), which is more straightforward and faster than MAST-CLA, was recently developed. It has been widely used in outpatient clinics in Korea4.
This EIA that is based on MAST uses an immunoblot technique involving solid-phase allergen absorption and immobilization on nitrocellulose, and can be used to measure the total IgE and a dozen sIgEs simultaneously in human serum. The recently introduced AdvanSure Allo-Screen assay (MAST-EIA; LG Life Sciences, Seoul, Korea) provides three panels: an inhalant panel, a food panel, and an atopy panel. A report on this assay showed that it has good clinical efficacy with respect to specificity and sensitivity; however, more scientific studies are needed5.
In parallel to the developments of new techniques for measuring sIgE, a revolution occurred in the field of allergen extracts. Initially, crude natural and nonstandardized extracts were used; however, gradually, standardized extracts with more precise allergenic component contents and improved diagnostic values were introduced. Recently, these standardized extracts were subdivided into specific allergen proteins and common allergen proteins. In addition, protein microarrays now allow sIgEs against multiple molecules to be detected in one assay, and thus, enable molecular or component-resolved diagnosis (CRD)6,7. The ImmunoCAP ISAC assay (ISAC; Phadia, Uppsala, Sweden) was commercialized by Phadia and is the first protein microarray developed for the detection of sIgEs. The current version (2012) contains 112 components.
In the present study, we evaluated the clinical outcomes of AD patients and the diagnostic usefulness of the ImmunoCAP ISAC assay for the detection of allergen sIgEs, and compared its performance with two other methods, namely, SPT and MAST-EIA, that are popularly used in Korea. In addition, we provide a review of the literature about the characteristics, roles, and values of CRD microarrays.
No benefits in any form have been received or will be received from any commercial party related directly or indirectly to the subject of this article.
MATERIALS AND METHODS
Patients
Twenty-five AD patients who registered at the Department of Dermatology at Gachon University Gil Medical Center were enrolled in this study. This study was approved by the ethics committee of Gachon University Gil Medical Center (GIRBA 2809-2012), and all participants provided written informed consent. Diagnoses were established according to the criteria of Hanifin and Rajka8. To evaluate trigger allergens and the clinical suitability of the CRD microarray, the serum samples of all 25 patients were tested by using a CRD microarray-based sIgE detection assay (ImmunoCAP ISAC) and MAST-EIA (AdvanSure AlloScreen). In addition, 10 of the 25 patients underwent SPT (Allergy Ergo; Allergopharma, Reinbek, Germany). All 25 patients were positive for various allergens in three multiple allergen tests. No patient had a chronic comorbidity or recent treatment history (previous 2 weeks) of antihistamine or steroid use.
Allergy diagnostic testing
1) In vitro allergen sIgE detection: ImmunoCAP ISAC and AdvanSure AlloScreen
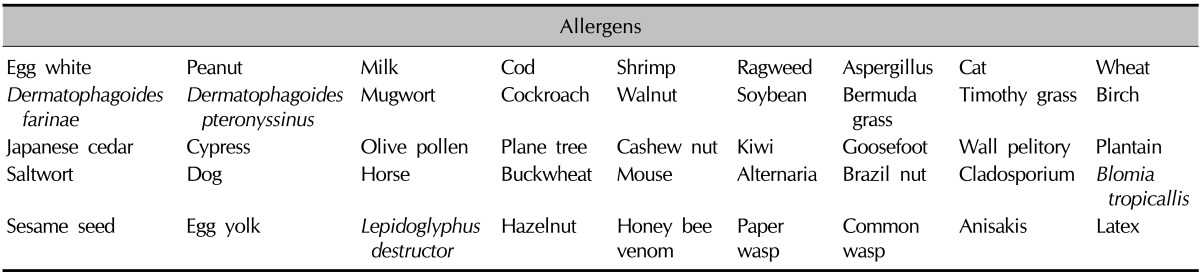
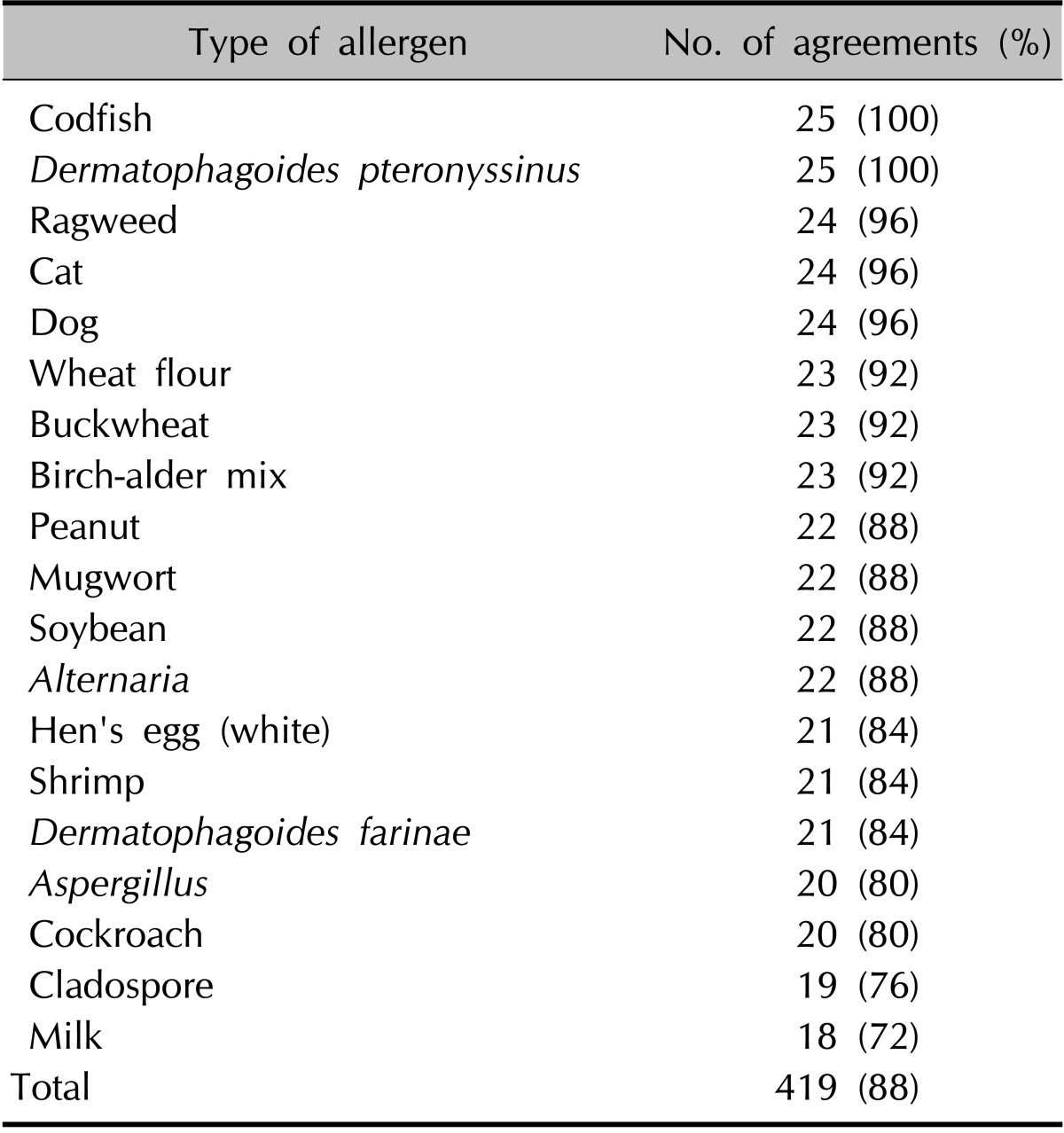
The 25 patients underwent two diagnostic tests, ImmunoCAP ISAC and AdvanSure AlloScreen, for the simultaneous detection of allergen sIgEs. ISAC is a multiple allergen component test based on a solid-phase multiple immunoassay, containing immobilized proteins (purified recombinant or natural allergens). Antibodies present in serum are captured by different allergens and then detected by using a second fluorescent-labeled anti-IgE antibody. The 45 allergens (Table 1), which were composed of 82 mainly species-specific components and 30 crossreactive components, were bound to the solid phase in triplicate, to ensure test reproducibility. Results were analyzed on a semiquantitative basis, and IgE values are presented in arbitrary units called ISAC standardized units (from 0.3 to 100 ISU). Values of >0.3 ISU were considered positive9.
Table 1.
List of the 45 allergens in the ISAC
On the other hand, MAST-EIA can simultaneously measure 41 different sIgE antibodies (Table 2) by using an atopy panel provided by the manufacturer. The allergens consist of food, mold, pollen, and inhalant allergens that most commonly induce reactions in Koreans. SIgE scales range from class 0 (<0.35 kU/L) to class 6 (>100 kU/L). A rating of class 2 or more was considered positive. All methods were performed according to the manufacturer's instructions.
Table 2.
List of the 41 allergens in MAST-EIA
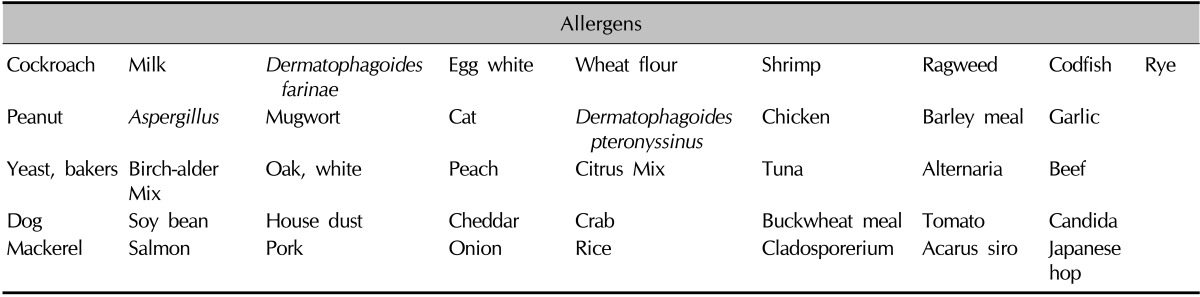
MAST-EIA: multiple allergen simultaneous test-enzyme immunoassay.
2) In vitro SPTs
Ten of the enrolled patients underwent SPT testing (Allergy Ergo) by using 50 commercial extracts in our dermatology department (Table 3). All SPTs were performed by using disposable 1-mm-tip lancets. Readings were taken at 20 min after application, and a mean wheal diameter of >3 mm greater than the negative control was considered positive. SPT was also conducted with histamine (10 mg/ml) and saline as positive and negative controls, respectively.
Table 3.
List of the 50 skin prick test allergens
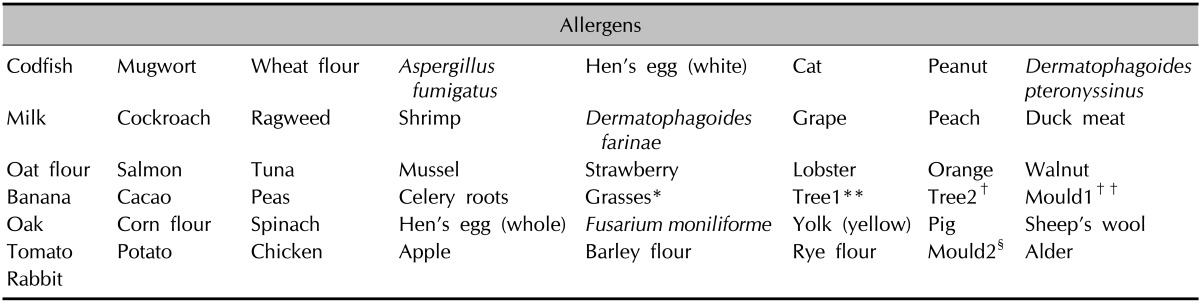
*Grasses: velvet, orchard, rye, timothy grass, Kentucky blue meadow fescue, **Tree1: alder, hazel, poplar, elm, willow, †Tree2: birch, beech, oak, plane tree, ††Mould1: alternaria, botrytis, cladosporium, curvularia, fusarium, helminthosporium, §Mould2: aspergillus, mucor, penicillium, pullularia, rhizopus, serpula.
Statistical analysis
Statistical analysis was conducted by using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Agreements between results (Cohen's κ analysis) were calculated to evaluate the consistencies of ISAC and MAST-EIA versus SPT. We assessed and categorized κ values as almost perfect (0.8~1.0), substantial (0.6~0.8), moderate (0.4~0.6), fair (0.2~0.4), or poor (<0.2)10. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each allergen in the ISAC and MAST-EIA assays were calculated with respect to SPT results.
RESULTS
Patient distribution
All three tests were done in 1 day. Patients' ages ranged from 7 months to 33 years (mean, 14 years). Of the 25 study subjects, 14 were male and 11 were female. In addition, 10 patients underwent supplementary SPT testing, and their ages ranged from 4 to 33 years (mean, 14 years).
Numbers of positive reactions in the three allergen tests
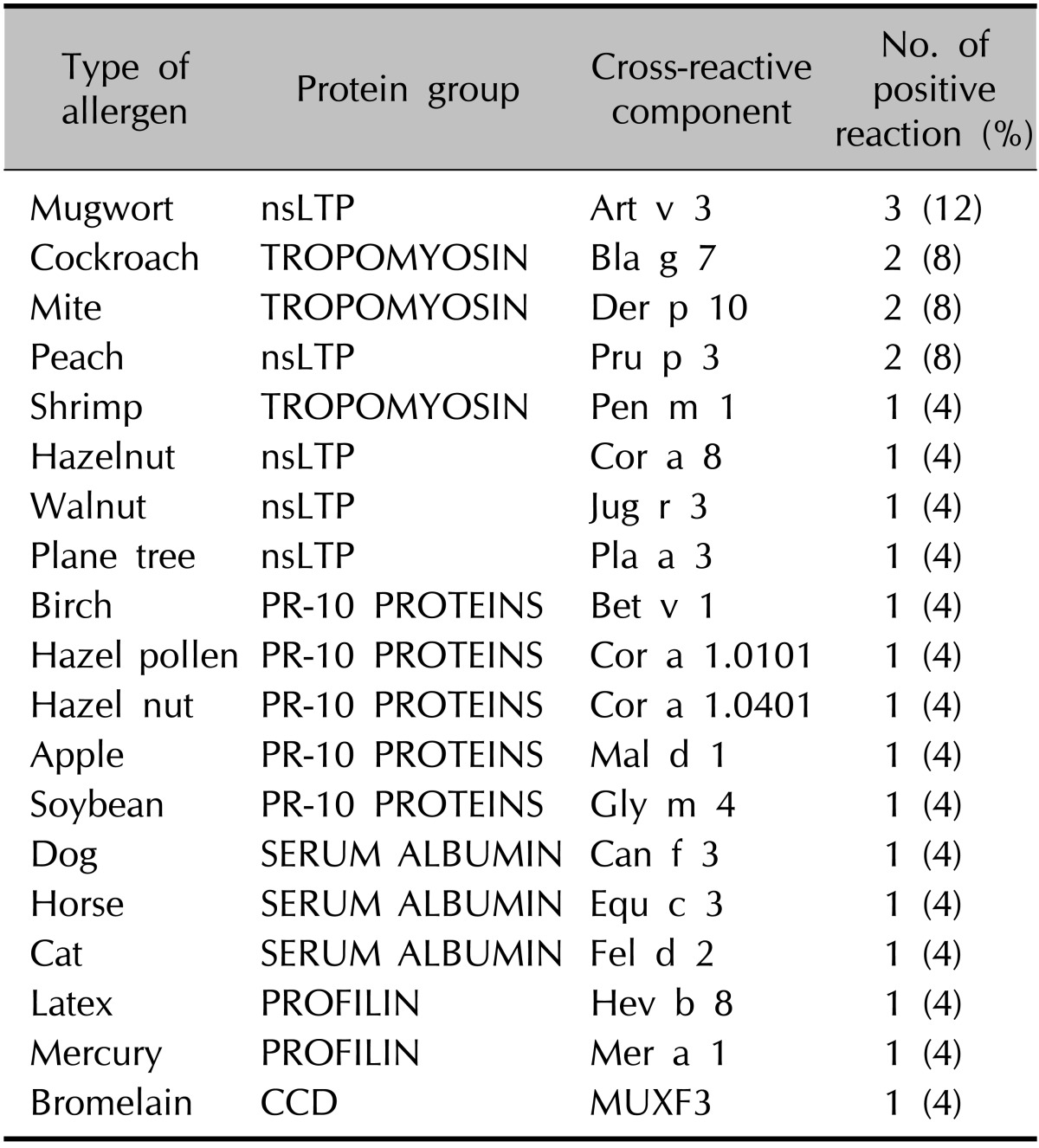
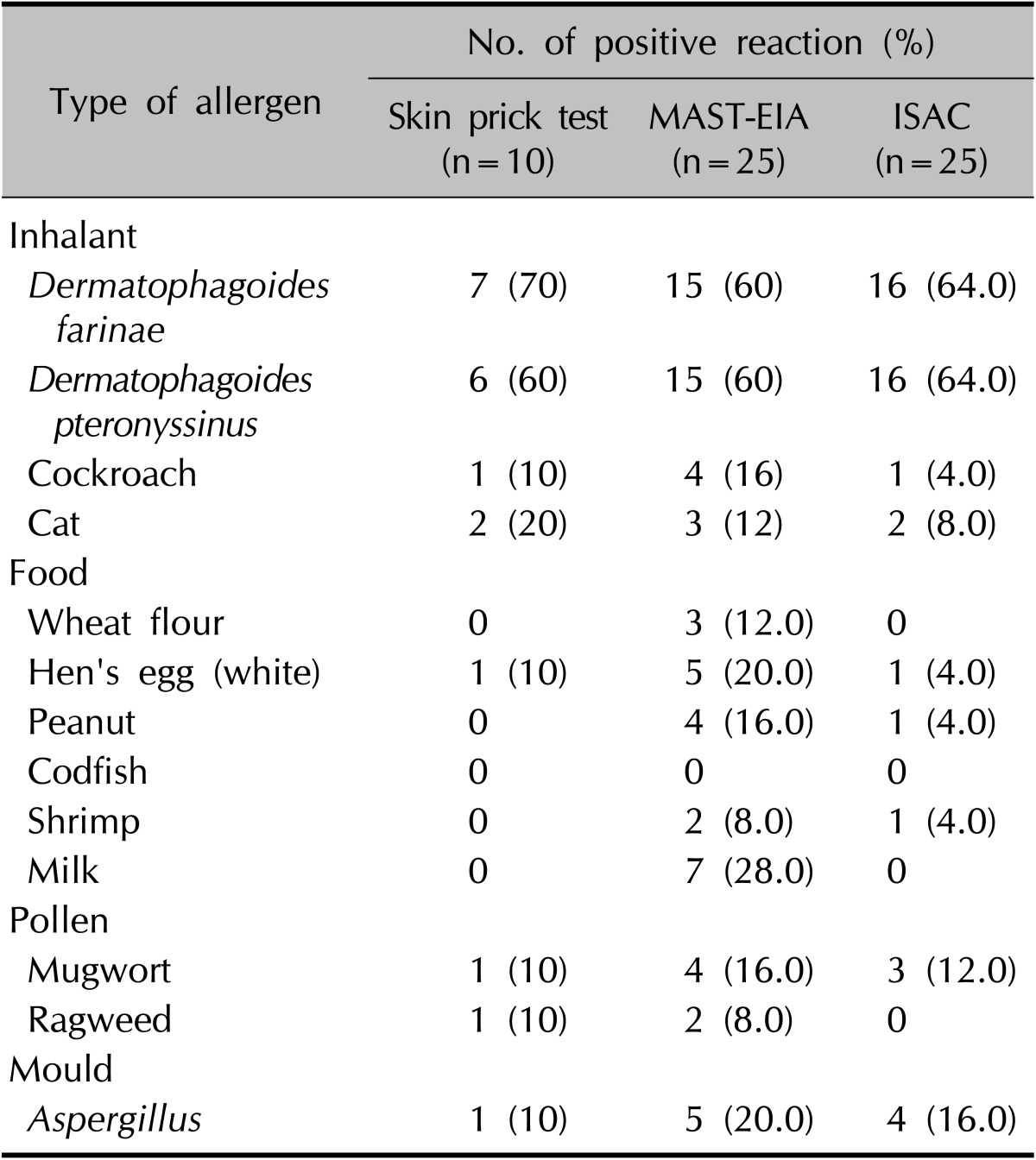
The numbers of patients with a positive reaction for one or more specific allergens in the three tests were slightly different. Eight of the 10 patients had a positive SPT reaction (Table 4). Table 5 provides the SPT positivity rates for each allergen. Dermatophagoides farinae and Dermatophagoides pteronyssinus had the highest reaction rates of 70% and 60%, respectively. Tree2, which included birch, beech, oak, and plane, was the next with 30%. Celery roots and cat hair both had rates of 20%. By MAST-EIA, all 25 patients had more than one positive reaction. Table 6 lists the allergens in order. D. farinae and D. pteronyssinus were also found to have the highest reaction rates of 60%, and Acarus siro, house dust, and Candida albicans had rates of 44%, 40%, and 36%, respectively. In ISAC, 20 of the 25 patients showed positivity. Table 7 shows that D. farinae and D. pteronyssinus had the highest reaction rates. Alternaria, Aspergillus, and Bermuda grass followed with rates of 44% each, and walnut, olive, mugwort, cypress, timothy, and blomia had rates of 40%. We were able to divide the 45 allergens into two groups: 82 mainly species-specific components and 30 cross-reactive components. Tables 8 and 9 provide the ISAC positivity rates for these two groups.
Table 4.
Number of positive allergens by the skin prick test, MAST-EIA, and ISAC
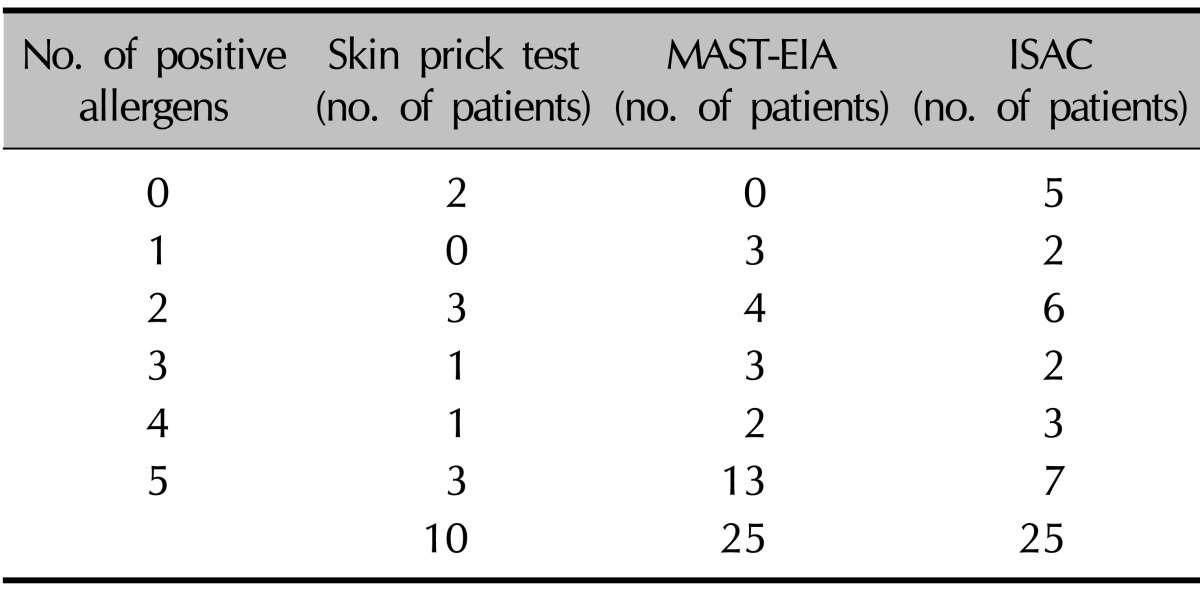
MAST-EIA: multiple allergen simultaneous test-enzyme immunoassay.
Table 5.
Positive rates of 50 allergens by the skin prick test (N=10)
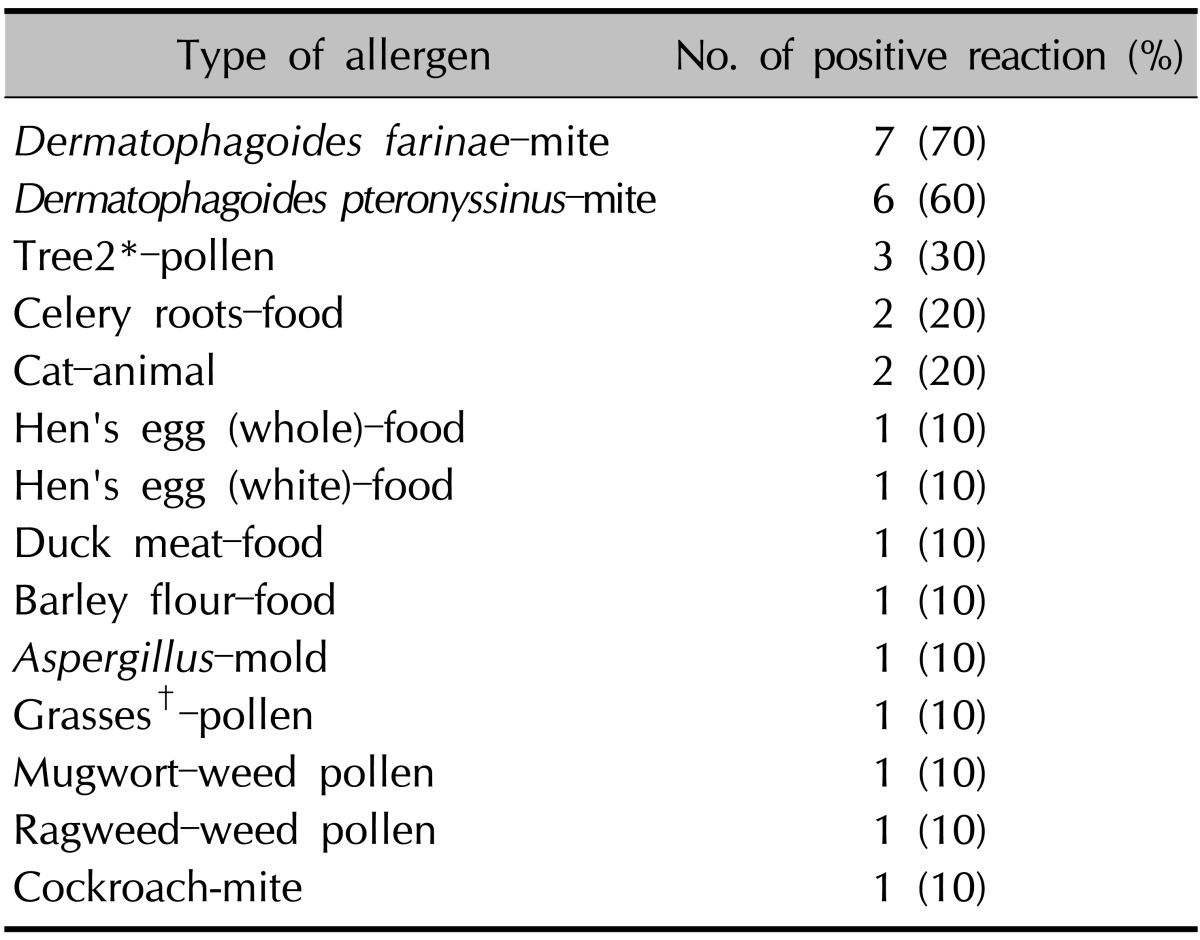
*Tree2: birch, beech, oak, plane tree, †Grasses: velvet, orchard, rye, timothy grass, Kentucky blue meadow fescue.
Table 6.
Positive rates of 41 allergens by the MAST-EIA (N=25)
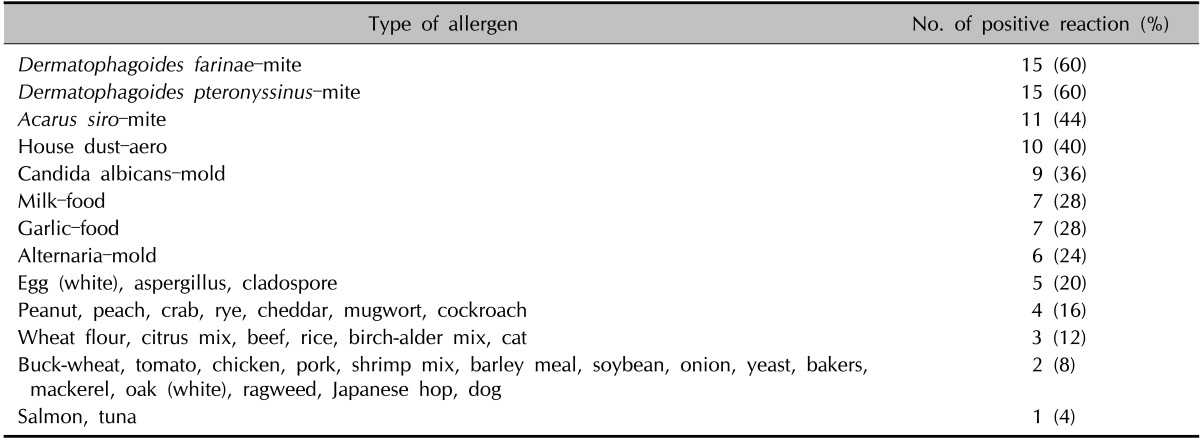
MAST-EIA: multiple allergen simultaneous test-enzyme immunoassay.
Table 7.
Positive rates of 45 allergens by the ISAC assay (N=25)
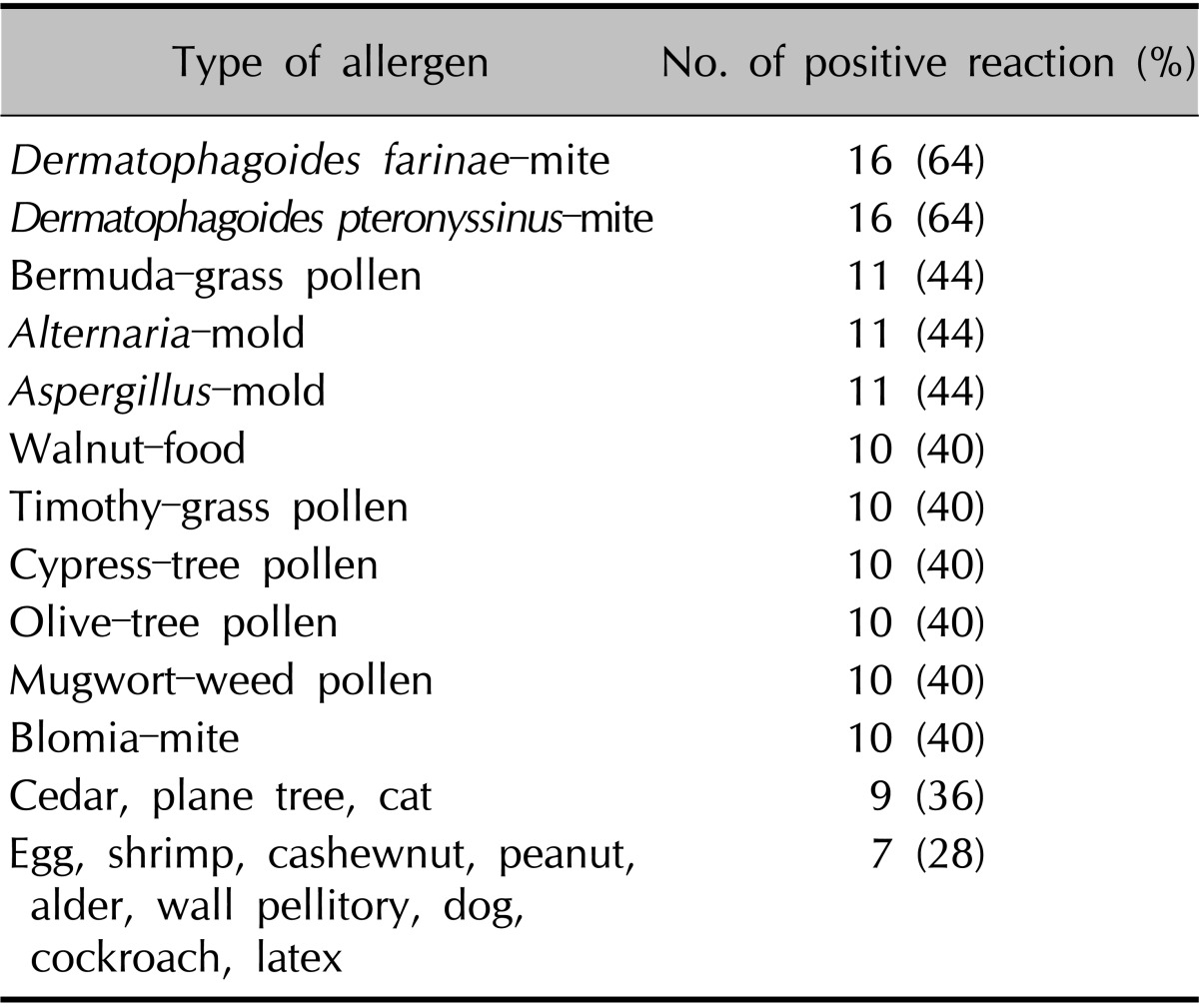
Table 8.
Positive rates of ISAC (N=25) for mainly species-specific components
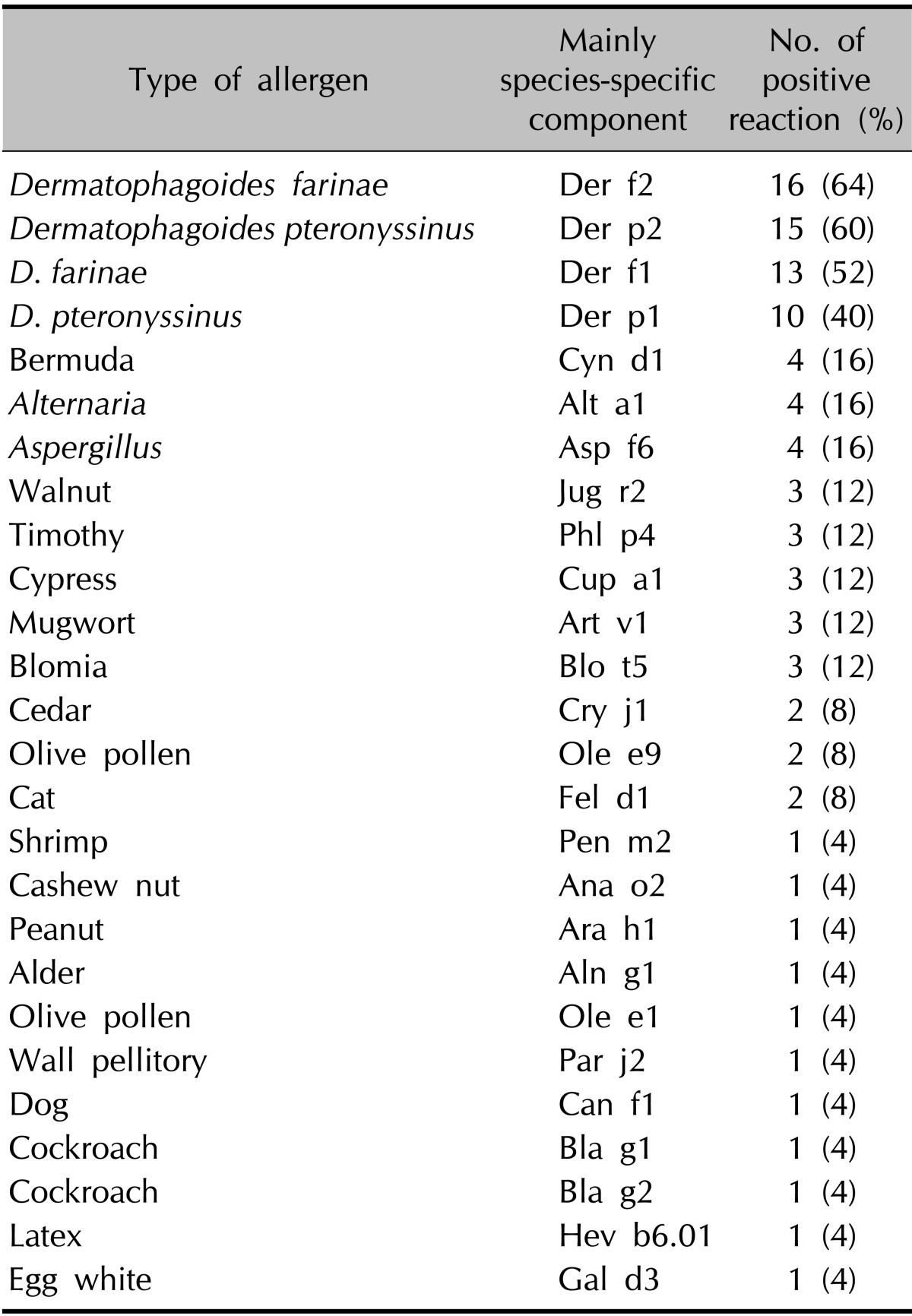
Table 9.
Positive rates of ISAC for cross-reactive components
Comparison of three tests with 13 common allergens
Because each of the three methods contains different types of allergens, we selected 13 allergens common to the three methods for comparative purposes; Table 10 shows their positivity rates.
Table 10.
Comparison of the common allergen positive rates of the three tests
MAST-EIA: multiple allergen simultaneous test-enzyme immunoassay.
Comparative analysis of ISAC and MAST-EIA versus SPT
We calculated the agreements, κ values, sensitivities, specificities, PPVs, and NPVs for MAST-EIA and ISAC versus SPT for 13 common allergens representing four categories (inhalants, food, pollens, and molds).
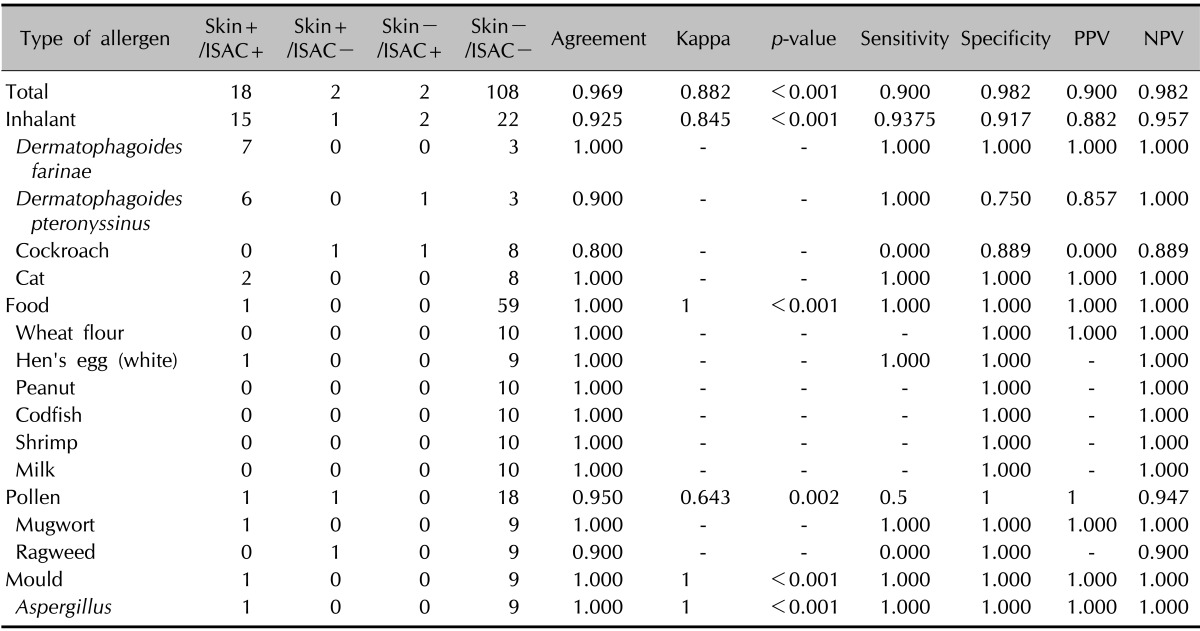
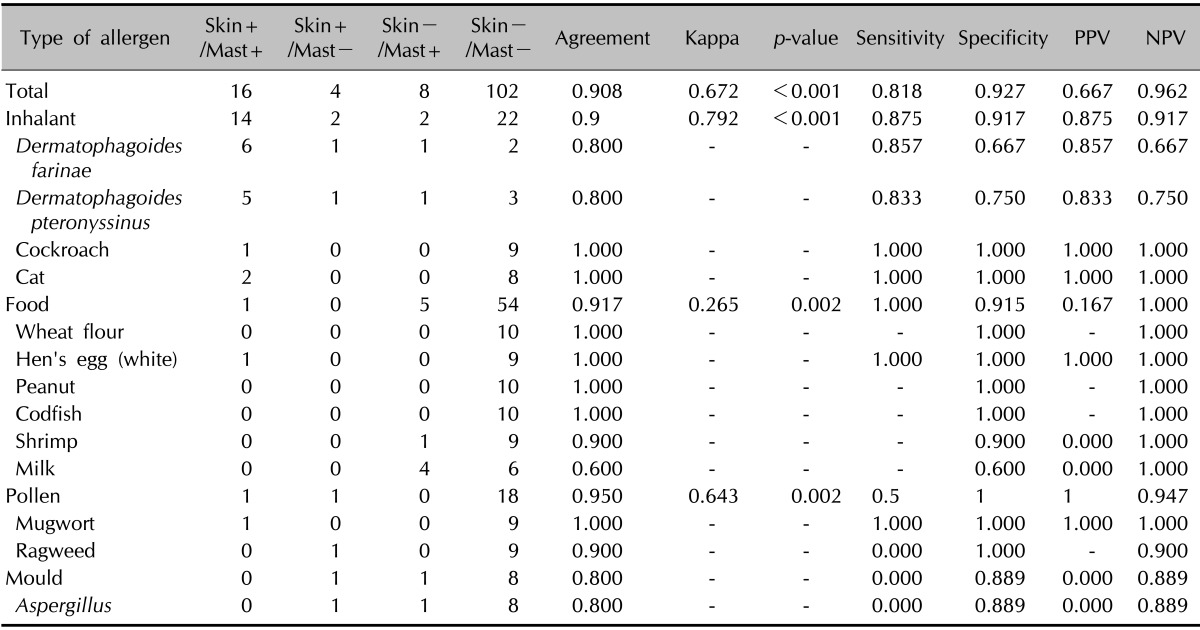
For ISAC, the sensitivity was 90.0%, specificity 98.2%, PPV 90.0%, and NPV 98.2% versus SPT. The total agreement and κ value for ISAC versus SPT were 96.9% and 0.882, respectively (Table 11). After dividing the 13 allergens into four categories, we examined the results in each category. Inhalants had an agreement of 92.5% and a κ value of 0.882, and foods had an agreement of 100% and a κ value of 1.0. For the MAST-EIA assay, the overall agreement was 90.8%, κ 0.672, sensitivity 80.0%, specificity 92.7%, PPV 66.7%, and NPV 96.2%. Inhalants had an agreement of 90.0%, a κ value of 0.792, a sensitivity of 81.8%, a specificity of 91.7%, a PPV of 87.5%, and an NPV of 91.7%, whereas foods had an agreement of 91.7%, a κ value of 0.265, a sensitivity of 100.0%, a specificity of 91.5%, a PPV of 16.7%, and an NPV of 100.0% (Table 12). In addition, we checked the agreements between ISAC and MAST-EIA for 19 common allergens in the 25 patients. The overall agreement between the two assays was 88% (Table 13).
Table 11.
Comparison between ISAC and the skin prick test (N=10)
PPV: positive predictive value, NPV: negative predictive value.
Table 12.
Comparison between MAST-EIA and the skin prick test (N=10)
MAST-EIA: multiple allergen simultaneous test-enzyme immunoassay, PPV: positive predictive value, NPV: negative predictive value.
Table 13.
Degrees of agreement between MAST-EIA and ISAC (N=25)
MAST-EIA: multiple allergen simultaneous test-enzyme immunoassay.
DISCUSSION
AD is a chronic, relapsing inflammatory skin disease that is frequently associated with abnormalities of the skin barrier function and allergen sensitization driven by heightened IgE responses against various allergens1. Because of the huge number of factors possibly involved, innovative investigative tools are required for the simultaneous analysis of many sensitizers and elicitors of IgE reactivity11.
SPT and the MAST-CLA are the most popular tools used in the Republic of Korea in diagnosing allergic skin diseases12, and recently, MAST-EIA was reported to show good clinical efficacy5. However, both tests are limited by low specificity and allergenic potency because the allergen extracts of these traditional diagnostic tools are derived from natural sources that contain mixtures of allergenic and nonallergenic molecules13,14. In addition, because all allergens in MAST-EIA are influenced by one conjugated anti-IgE antibody and the potency of one allergen may be influenced by the activities of others, results may be confusing, especially in patients with a high total IgE5.
To overcome these limitations of the existing methods, CRD with microarray technology, which is based on recombinant DNA technology, was recently introduced into clinical allergy practice for allergen characterization. The term "CRD" is used to designate diagnostic tests on the basis of pure allergen proteins, which are either produced by the recombinant expression of allergen-encoding complementary DNA or by purification from natural allergen sources15. A greater number of recombinant allergens with immunological properties comparable to those of natural allergens are now available7,11,16. All allergens can be considered species-specific allergen components, which are unique markers of the allergen source, or as other allergen components, which are classified as markers of cross-reactivity because of their similar protein structures and IgE-binding properties.
In this study, we examined ISAC (a CRD microarray assay), which is based on 112 components of 45 allergens (82 mainly species-specific components and 30 cross-reactive components) in AD patients, and compared its results with those of SPT and MAST-EIA. By comparing ISAC with these diagnostic tools, we hoped to determine whether it could provide an effective allergen screening method for AD patients. Table 4 shows that the positive rates of SPT and ISAC were both 80%, which is similar to that reported previously in AD (70%~84%)17,18,19,20. Although all patients were positive for one or more specific allergens in MAST-EIA, this may have been due to the inclusion of a few patients suspected to show sensitizations to allergens. About the positive rates of each allergen, D. farinae and D. pteronyssinus have generally been reported to show the highest positive rates in Korean AD patients3,18,19,20. This study shows similar positive rates for SPT (70% and 60%, respectively), ISAC (64% and 64%), and MAST-EIA (60% and 60%), and also shows that the house dust mite is the most common allergen. However, other allergens showed large positivity rate differences. We believe that these differences were caused by the recruitment of only 10 patients and the low allergen positive rates, which concurs with the results of previous studies3,18,19,20. In addition, because the three methods examined in this study are commercial diagnostic methods, we only compared 13 common allergens. The mite allergen (Acarus siro) had relatively high positive rates but could only be detected with MAST-EIA. On the basis of our results, we suggest that a panel of allergens optimized for Korean AD patients be selected.
With no standard reference sIgE test, in vivo SPT is widely used as a standard for the evaluation of in vitro tests, and thus, we also compared the ISAC and MAST-EIA results with the SPT results21,22. In a previous study, MAST-CLA had a concordance rate of 91.4%, a sensitivity of 34.1%, a specificity of 98.7%, and PPV and NPV of 77% and 92.2%, respectively23. In another study, it had a concordance rate of 88.9%~100%, a sensitivity of 22.2%~97.6%, and a specificity of 81.3%~98.9%24. Sensitivity values tend to vary appreciably between studies, and we attribute this to statistical errors caused by the small sample numbers. In general, AD patients present high positivity for inhalant allergens but low positivity for allergens such as food and pollen. In addition, MAST-CLA showed a lower sensitivity than SPT for food and pollen allergens19,23,24,25. Therefore, the more allergens like food and pollen are used in MAST-CLA, the lower or more variable the mean sensitivity might become. In the present study, MAST-EIA had a concordance rate of 90.8%, a sensitivity of 81.8%, a specificity of 92.7%, a PPV of 66.7%, and an NPV of 96.2%, which indicate a higher sensitivity and a lower PPV than those in previous studies. On the other hand, ISAC achieved higher values in all categories; that is, a concordance rate of 96.9%, a sensitivity of 90%, a specificity of 98.2%, a PPV of 90%, and an NPV of 98.2%. Kappa values of MAST and ISAC versus SPT were 0.672 or "substantial" and 0.882 or "perfect," and ISAC demonstrated higher reliability than MAST-EIA. In a previous study with D. farinae plus D. pteronyssinus, which had the highest positive rate, the concordance rate, sensitivity, and specificity of MAST-CLA versus SPT were 72.8%, 58.9%, and 86.1%, respectively20; those of MAST-EIA were 80.0%, 84.6%, and 71.4%; and those of ISAC were higher at 95.5%, 100%, and 85.7%, respectively. The κ value of MAST-EIA was 0.56 (moderate), whereas that of ISAC was 0.886 (perfect), which showed that ISAC was the more reliable method. In previous studies, SPT was generally found to have higher sensitivity and lower specificity than MAST-CLA. In the present study, MAST-EIA and ISAC both showed high sensitivity and specificity, but ISAC had better reliability than SPT. We believe that the allergen extracts used for ISAC could increase the test reliability, and we suggest that ISAC could be a more effective method for allergen screening.
The measurement of sIgE to individual components could be useful in polysensitized AD patients. In two previous studies, 35.5%~61.6% of AD patients were sensitive to two or more allergens3,23, and in another, the average numbers of sensitized allergens in pediatric and adult patients were found to be 3.4 and 4.1, respectively19. Thus, we sometimes confuse the sensitized allergens in AD patients as clinically important or believable information. For CRD microarrays, whereas species-specific allergen components are theoretically unique markers, cross-reactive allergen components are classified as markers of cross-reactivity owing to their similar protein structures and IgE-binding properties. These components can be present in many different allergen sources, sometimes even in unrelated sources. Thus, the identification of markers of cross-reactivity provides valuable information on the possible sensitization to several different sources7. For example, cross-reactive carbohydrate determinants of MUXF3 (carbohydrate epitope, oligosaccharide from pineapple stem bromelain), which are found in almost all plants and invertebrates, are a cause of false-positives and a potential source of interferences in in vitro tests15,26. Thus, identifying whether sensitization is genuine or due to cross-reactivity could prevent the risk of sensitization to different allergen sources. As a result, the ability of CRD microarray technology to identify allergens to which patients are sensitized, including species-specific components and cross-reactive components, could aid in the elucidation of the clinical relevance of allergic sensitization in AD.
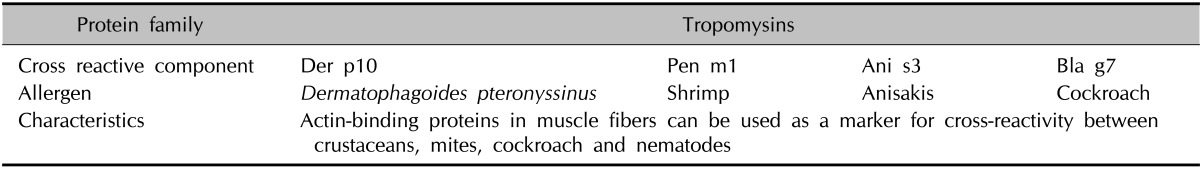
CRD microarrays can also be used to adapt treatment strategies. For example, this method can lead to successful specific immunotherapy against allergens such as D. farinae and D. pteronyssinus Tables 8 and 9 show that Der p1, Der p2 and tropomyosin Der p10 are among the species-specific and cross-reactive components with the highest positive rates. Furthermore, patients with sIgE to tropomyosin Der p10 are believed to react to other mite allergens. Table 14 shows that cross-reactions exist between antibodies to Der p10 of mites, Pen a1 from shrimps, and Ani s3 from Anisakis. Accordingly, CRD microarray technology enables the selection of p10-negative patients who primarily possess sIgE to Der p1 and/or Der p2. Thus, CRD microarray could be important for evaluating the potential of species-specific immunotherapy27,28.
Table 14.
Characteristics of cross-reactive components, tropomysins
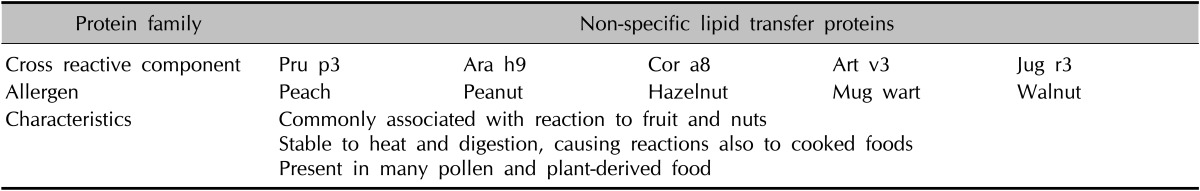
CRD microarrays could also be useful for predicting the severities of clinical reactions. Instead of measuring the sIgE response to complex allergen extracts, specific responses at the level of individual allergenic proteins make it possible to determine the IgE reactivity profiles of patients and assess clinical sensitization patterns. For example, clinical manifestations of lipid transfer protein (LTP; a plant panallergen) sensitization are of varying severity and could result in anaphylaxis or have effects restricted to the oropharyngeal area, skin, or gastrointestinal tract29. Table 15 shows that Pru p3 of peach has structural similarities to other components, which suggests that Pru p3 sensitization could lead to LTP cross-reactions. Furthermore, peach Pru p3, mugwort Art v3, and hazelnut Cor a8 could play roles in pollen-food syndromes associated with weed pollen30,31. Although this study was performed on AD patients, some patients had positive results for LTPs, which raises issues of cross-reactivity. Accordingly, with the understanding of the clinically relevant cross-reactivities between plant foods and pollens, that is, multiple plant food sensitization, CRD microarray technology could allow appropriate clinical decisions and the creation of individualized treatment plans for AD patients.
Table 15.
Characteristics of cross-reactive components, non-specific lipid transfer proteins
Much research effort is being directed toward the development of diagnostic tests with improved prognostic performances. In the present study, the CRD microarray results in AD showed a better correlation and accordance with the SPT results than did the MAST-EIA results. Nevertheless, this study was limited by the small number of patients enrolled and the inclusion of only 13 common allergens for each of the methods examined. Thus, we suggest that further investigations be performed to evaluate the detection performance for each allergen on a larger scale. The risks of overdiagnosis and misinterpretation caused by the complexities of the results produced are drawbacks of the CRD microarray test32; however, conventional diagnostic tests based on natural allergen extracts composed of mixtures of species-specific allergens, nonallergenic components, and cross-reactive allergens cannot precisely identify disease-eliciting allergens, particularly in polysensitized AD patients. The present study demonstrates that CRD microarray testing is a convenient allergic diagnostic method based on single measurements that can be obtained from minute amounts of serum.
ACKNOWLEDGMENT
The authors wish to acknowledge the financial support of the Gachon University, Graduate School of Medicine and the Gil Medical Center Research Foundation.
References
- 1.Scalabrin DM, Bavbek S, Perzanowski MS, Wilson BB, Platts-Mills TA, Wheatley LM. Use of specific IgE in assessing the relevance of fungal and dust mite allergens to atopic dermatitis: a comparison with asthmatic and nonasthmatic control subjects. J Allergy Clin Immunol. 1999;104:1273–1279. doi: 10.1016/s0091-6749(99)70024-2. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Marcos L, Sanchez-Solis M, Martinez-Torres AE, Lucas Moreno JM, Hernando Sastre V. Phadiatop compared to skin-prick test as a tool for diagnosing atopy in epidemiological studies in schoolchildren. Pediatr Allergy Immunol. 2007;18:240–244. doi: 10.1111/j.1399-3038.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 3.Shin JW, Jin SP, Lee JH, Cho S. Analysis of MAST-CLA results as a diagnostic tool in allergic skin diseases. Ann Dermatol. 2010;22:35–40. doi: 10.5021/ad.2010.22.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park DS, Cho JH, Lee KE, Ko OS, Kim HR, Choi SI, et al. Detection rate of allergen-specific IgE by multiple antigen simultaneous test-immunoblot assay. Korean J Lab Med. 2004;24:131–138. [Google Scholar]
- 5.Lee JH, Park KH, Kim HS, Kim KW, Sohn MH, Kim CH, et al. Specific IgE measurement using AdvanSure® system: comparison of detection performance with ImmunoCAP® system in Korean allergy patients. Clin Chim Acta. 2012;413:914–919. doi: 10.1016/j.cca.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Ferrer M, Sanz ML, Sastre J, Bartra J, del Cuvillo A, Montoro J, et al. Molecular diagnosis in allergology: application of the microarray technique. J Investig Allergol Clin Immunol. 2009;19(Suppl 1):19–24. [PubMed] [Google Scholar]
- 7.Sastre J. Molecular diagnosis in allergy. Clin Exp Allergy. 2010;40:1442–1460. doi: 10.1111/j.1365-2222.2010.03585.x. [DOI] [PubMed] [Google Scholar]
- 8.Hanifin JM, Rajka RG. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;60(Suppl 92):44–47. [Google Scholar]
- 9.Lucas JM. Microarrays: molecular allergology and nanotechnology for personalised medicine (I) Allergol Immunopathol (Madr) 2010;38:153–161. doi: 10.1016/j.aller.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 11.Mari A, Scala E, Alessandri C. The IgE-microarray testing in atopic dermatitis: a suitable modern tool for the immunological and clinical phenotyping of the disease. Curr Opin Allergy Clin Immunol. 2011;11:438–444. doi: 10.1097/ACI.0b013e32834a41dd. [DOI] [PubMed] [Google Scholar]
- 12.Lim HS, Kim HS, Oh H. Current status of serum allergen tests in Korea. Korean J Lab Med. 2008;28:124–129. doi: 10.3343/kjlm.2008.28.2.124. [DOI] [PubMed] [Google Scholar]
- 13.Kniker WT. Is the choice of allergy skin testing versus in vitro determination of specific IgE no longer a scientific issue? Ann Allergy. 1989;62:373–374. [PubMed] [Google Scholar]
- 14.Sampson HA. Improving in-vitro tests for the diagnosis of food hypersensitivity. Curr Opin Allergy Clin Immunol. 2002;2:257–261. doi: 10.1097/00130832-200206000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Valenta R, Vrtala S. Recombinant allergens for specific immunotherapy. Allergy. 1999;54(Suppl 56):43–44. doi: 10.1111/j.1398-9995.1999.tb04441.x. [DOI] [PubMed] [Google Scholar]
- 16.Treudler R. Update on in vitro allergy diagnostics. J Dtsch Dermatol Ges. 2012;10:89–97. doi: 10.1111/j.1610-0387.2011.07860.x. [DOI] [PubMed] [Google Scholar]
- 17.Sampson HA, McCaskill CC. Food hypersensitivity and atopic dermatitis: evaluation of 113 patients. J Pediatr. 1985;107:669–675. doi: 10.1016/s0022-3476(85)80390-5. [DOI] [PubMed] [Google Scholar]
- 18.Lee EJ, Piao YJ, Kim KH, Suhr KB, Lee JH, Park JK. The relationship among the clinical evaluation, total ige, and allergen-specific IgE of MAST-CLA in atopic dermatitis. Korean J Dermatol. 2003;41:197–206. [Google Scholar]
- 19.Kim HD, Cho MK, Lee SY, Lee JS, Park YL, Whang KU, et al. Evaluation of the MAST CLA allergy system for measuring total and allergen specific IgE in child and adult atopic dermatitis. Korean J Dermatol. 2007;45:413–421. [Google Scholar]
- 20.Chun TJ, Lee KS, Seo SJ, Kim MN, Hong CK, Ro BI. A comparison study of prick test and chemiluminescent assay. Korean J Dermatol. 1999;37:1450–1456. [Google Scholar]
- 21.Garcia-Marcos L, Sanchez-Solis M, Martinez-Torres AE, Lucas Moreno JM, Hernando Sastre V. Phadiatop compared to skin-prick test as a tool for diagnosing atopy in epidemiological studies in schoolchildren. Pediatr Allergy Immunol. 2007;18:240–244. doi: 10.1111/j.1399-3038.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 22.Contin-Bordes C, Petersen A, Chahine I, Boralevi F, Chahine H, Taïeb A, et al. Comparison of ADVIA Centaur and Pharmacia UniCAP tests in the diagnosis of food allergy in children with atopic dermatitis. Pediatr Allergy Immunol. 2007;18:614–620. doi: 10.1111/j.1399-3038.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee IW, Kim TH, Ahn SK, Choi EH. Comparison of MAST chemiluminescent assay (MAST-CLA) with skin prick, test in patients with atopic dermatitis. Korean J Dermatol. 1999;37:297–304. [Google Scholar]
- 24.Jang WR, Nahm CH, Kim JH, Lim DH, Jang TY, Moon YS, et al. Allergen specific IgE measurement with Polycheck Allergy: comparison of three multiple allergen simultaneous tests. Korean J Lab Med. 2009;29:465–472. doi: 10.3343/kjlm.2009.29.5.465. [DOI] [PubMed] [Google Scholar]
- 25.Jeon HJ, Bang HD, Kim KH, Park CW, Kim KJ, Lee CH. A study of food allergy in atopic dermatitis using CAP-RAST FEIA and open food challenge test. Korean J Dermatol. 2003;41:1034–1040. [Google Scholar]
- 26.Jin C, Hantusch B, Hemmer W, Stadlmann J, Altmann F. Affinity of IgE and IgG against cross-reactive carbohydrate determinants on plant and insect glycoproteins. J Allergy Clin Immunol. 2008;121:185–190. doi: 10.1016/j.jaci.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 27.Resch Y, Weghofer M, Seiberler S, Horak F, Scheiblhofer S, Linhart B, et al. Molecular characterization of Der p 10: a diagnostic marker for broad sensitization in house dust mite allergy. Clin Exp Allergy. 2011;41:1468–1477. doi: 10.1111/j.1365-2222.2011.03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frati F, Incorvaia C, David M, Scurati S, Seta S, Padua G, et al. Requirements for acquiring a high-quality house dust mite extract for allergen immunotherapy. Drug Des Devel Ther. 2012;6:117–123. doi: 10.2147/DDDT.S30908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuidmeer L, van Ree R. Lipid transfer protein allergy: primary food allergy or pollen/food syndrome in some cases. Curr Opin Allergy Clin Immunol. 2007;7:269–273. doi: 10.1097/ACI.0b013e32814a5401. [DOI] [PubMed] [Google Scholar]
- 30.Lombardero M, García-Sellés FJ, Polo F, Jimeno L, Chamorro MJ, García-Casado G, et al. Prevalence of sensitization to Artemisia allergens Art v 1, Art v 3 and Art v 60 kDa. Crossreactivity among Art v 3 and other relevant lipid-transfer protein allergens. Clin Exp Allergy. 2004;34:1415–1421. doi: 10.1111/j.1365-2222.2004.02053.x. [DOI] [PubMed] [Google Scholar]
- 31.Gadermaier G, Harrer A, Girbl T, Palazzo P, Himly M, Vogel L, et al. Isoform identification and characterization of Art v 3, the lipid-transfer protein of mugwort pollen. Mol Immunol. 2009;46:1919–1924. doi: 10.1016/j.molimm.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Knol EF, Knulst AC. Application of multiplexed immunoglobulin E determination on a chip in component-resolved diagnostics in allergy. Clin Exp Allergy. 2010;40:190–192. doi: 10.1111/j.1365-2222.2009.03402.x. [DOI] [PubMed] [Google Scholar]


