Abstract
Objectives
To investigate the effect of interleukin (IL)-1β on matrix metalloproteinase (MMP)-9 expression in cochlea and regulation of IL-1β-mediated MMP-9 expression by dexamethasone and the molecular and signaling mechanisms involved.
Methods
House ear institute-organ of Corti 1 (HEI-OC1) cells were used and exposed to IL-1β with/without dexamethasone. Glucocorticoid receptor antagonist, RU486, was used to see the role of dexamethasone. PD98059 (an extracellular signal-regulated kinases [ERKs] inhibitor), SB203580 (a p38 mitogen-activated protein kinases [MAPK] inhibitor), SP600125 (a c-Jun N-terminal kinase [JNK] inhibitor) were also used to see the role of MAPKs signaling pathway(s) in IL-1β-induced MMP-9 expression in HEI-OC1 cells. Reverse transcription-polymerase chain reaction and gelatin zymography were used to measure mRNA expression level of MMP-9 and activity of MMP-9, respectively.
Results
Treatment with IL-1β-induced the expression of MMP-9 in a dose- and time-dependent manner. IL-1β (1 ng/mL)-induced MMP-9 expression was inhibited by dexamethasone. Interestingly, p38 MAPK inhibitor, SB203580, significantly inhibited IL-1β-induced MMP-9 mRNA and MMP-9 activity. However, inhibition of JNKs and ERKs had no effect on the IL-1β-induced MMP-9 expression.
Conclusion
These results suggest that the pro-inflammatory cytokine IL-1β strongly induces MMP-9 expression via activation of p38 MAPK signaling pathway in HEI-OC1 cells and the induction was inhibited by dexamethasone.
Keywords: Interleukin-1beta, Matrix metalloproteinase 9, p38 Mitogen-activated protein kinases, Cochlea
INTRODUCTION
The exact pathophysiologic mechanism of the sensorineural hearing loss has remained elusive. Various inflammatory cytokines, especially interleukin (IL)-1β, are identified in the middle ear of otitis media with effusion and in the cerebrospinal fluid of bacterial meningitis patients. However, their roles in the pathogenesis of cochlear dysfunction have not been fully investigated [1]. Recently some investigations have demonstrated that matrix metalloproteinase (MMP) is important in the cochlear function [2].
MMPs are zinc-binding proteolytic enzymes, which degrade structural components of extracellular matrix (ECM) in various physiological and pathological conditions. These enzymes degrade damaged matrix under normal condition, and maintain normal tissue homeostasis. However, MMPs may be produced in excess and may also contribute to tissue damage under pathologic conditions [3]. MMPs are classified into four groups according to a substrate specificity and structural similarity; collagenase, gelatinase, stromelysins, and membrane type MMP. Among human MMPs, MMP-9 (gelatinase B, 92 kDa) is key enzyme of degrading type IV collagen, which is a major component of the basement membrane. Expression levels of MMP-9 are associated with chronic otitis media with effusion and tumor metastasis for various human cancers [4].
Glucocorticoids have long been used by physicians for the treatment of sensorineural hearing loss in inner ear disorders. Recent studies have reported that dexamethasone can protect the inner ear against cytokine induced [5]. However, the molecular and signaling mechanisms behind dexamethasone's ability to protect against this cytokines induced inner ear damage are unknown.
The purpose of this study was to investigate the effect of IL-1β on MMP-9 expression and regulation of IL-1β-mediated MMP-9 by dexamethasone, and the molecular and signaling mechanisms involved in house ear institute-organ of Corti 1 (HEI-OC1) cells [6], a murine cochlear cell line.
MATERIALS AND METHODS
Cell culture
HEI-OC1 cells, derived from long-term cultures of immortomouse cochleae were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT, USA), 20 mM Hepes buffer, and 100 µg/mL gentamicin at 37℃ and 10% CO2.
Materials
Recombinant murine IL-1β was bought from R&D Systems (Minneapolis, MN, USA). PD98059, SB203580, and SP600125 were obtained from Biomol (Plymouth Meeting, PA, USA). Bradford reagent was from Bio-Rad (Richmond, CA, USA). Dexamethasone and RU486 were obtained from Sigma (St. Louis, MO, USA). DMEM and gentamicin were purchased from Gibco-BRL (Gaithersburg, MD, USA).
Gelatin substrate gel zymograph
To determine MMP-9 activity, cells were treated with 1 ng/mL IL-1β or in the presence or absence of dexamethasone, RU486, N-acetylcysteine (NAC), PD98059, SB203580, and SP600125. Zymography was performed by the procedure described by Overall et al. [7] with minor modification. The conditioned medium was electrophoresed in a 10% polyacrylamide gel containing 1 mg/mL gelatin. The gel was then washed at room temperature for 2 hours with 2.5% Triton X-100 and then at 37℃ overnight in a buffer containing 10 mM CaCl2, 150 mM NaCl, and 50 mM Tris-HCl, pH 7.5. The gel was stained with 0.25% Coomassie blue, and then destained for 1 hour in a solution of acetic acid and methanol. The proteolytic activity was evidenced as clear bands (zones of gelatin degradation) against the blue background of stained gelatin.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
To determine whether the increased amounts of MMP-9 activity were a result of increased levels of mRNA, we compared the mRNA levels of MMP-9 in HEI-OC1 cells. Total RNA was isolated using the TriZol reagent (Life Technologies, Gaithersburg, MD, USA), and the cDNA was prepared using M-MLV RT (Gibco-BRL) according to the manufacturers' instructions. The following primers were used for the amplification of MMP-9 mRNA and actin: MMP-9 (sense) 5'-CACTGTCCACCCCTCAGAGC-3' and (anti-sense) 5'-GCCACTTGTCGGCGATAAGG-3'; actin (sense) 5'-ACAGCTTCTTTGCAGCTCCTT-3' and (antisense) 5'-CGG AGTCCATCACAATGCCT-3'. The PCR amplification was carried out using the following cycling conditions: 94℃ for 3 minutes followed by 17 (actin) or 23 cycles (MMP-9) of 94℃ for 45 seconds, 58℃ for 45 seconds, 72℃ for 1 minute, and a final extension at 72℃ for 10 minutes. The amplified products were separated by electrophoresis on a 1.5% agarose gel and detected under UV light.
Densitometry
The band intensities were scanned and quantified using the gel analysis plugin for the open source software ImageJ 1.46 (Imaging Processing and Analysis in Java, http://rsb.info.nih.gov/ij).
Statistical analysis
The data were analyzed using a one-way analysis of variance (ANOVA) and post-hoc comparisons (Student-Newman-Keuls) using the SPSS ver. 8.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
IL-1β induces MMP-9 mRNA expression and MMP-9 activity in HEI-OC1 cells
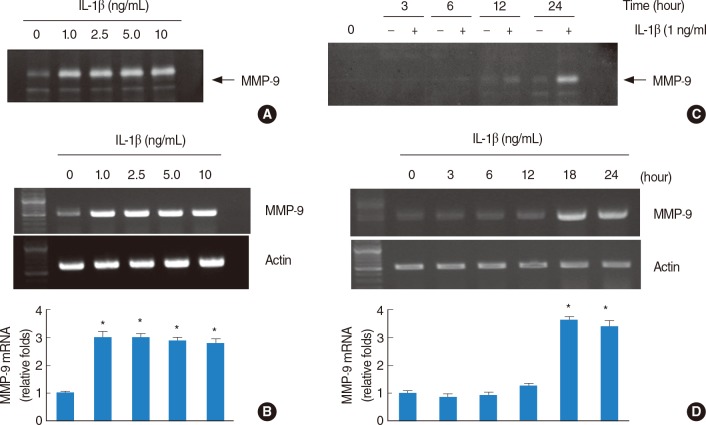
It was initially measured the effects of different concentrations of IL-1β on MMP-9 mRNA expressions and activity in HEI-OC1 cells. As shown in Fig. 1A, results of gelatin zymography demonstrated that treatment with IL-1β (1 ng/mL) for 20 hours appear to have reached a maximal MMP-9 activity in HEI-OC1 cells. Interestingly, 1 ng/mL concentration of IL-1β was sufficient to induce high activity of MMP-9 in HEI-OC1 cells. We next asked whether the IL-1β induces MMP-9 mRNA expression in HEI-OC1 cells. Similar to the pattern of MMP-9 activity, MMP-9 mRNA expression levels were reached maximal levels at 1 ng/mL IL-1β in HEI-OC1 cells (Fig. 1B). With 1 ng/mL concentration of IL-1β, we next investigated the time kinetic studies of MMP-9 activity and mRNA expression in HEI-OC1 cells. As shown in Fig. 1C, D, time-dependent increased of MMP-9 activity and mRNA expression were observed in HEI-OC1 cells. Control actin protein was not changed by treatment with IL-1β in different doses and times (Fig. 1B, D), suggesting the specificity of MMP-9 induction by IL-1β in HEI-OC1 cells.
Fig. 1.
Effect of interleukin (IL)-1β on matrix metalloproteinase (MMP)-9 activity in house ear institute-organ of Corti 1 (HEI-OC1) cells. IL-1β increases MMP-9 activity and mRNA expression. (A, B) HEI-OC1 cells were treated with various concentrations of IL-1β for 20 hours. Conditional media were collected after 20 hours and gelatin zymography was performed (A). The MMP-9 mRNA expression levels were determined by reverse transcription-polymerase chain reaction (RT-PCR). The levels of actin were used as a loading control (B). (C, D) HEI-OC1 cells were treated with the 1 ng/mL IL-1β for the indicated time periods. Conditional media were collected after 20 hours and gelatin zymography was performed (C). The MMP-9 mRNA expression levels were determined by RT-PCR. The levels of actin were used as a loading control. The values in (B, D) represent the mean±SD from three independent samples. *P<0.001 compared to the control. The data represent three independent experiments.
Dexamethasone inhibits IL-1β-induced MMP-9 expression in HEI-OC1 cells
To investigate whether dexamethasone can inhibit IL-1β-induced MMP-9 expression, HEI-OC1 cells were pretreated for 30 minutes with various concentrations of dexamethasone and subsequently treated with 1 ng/mL IL-1β. As shown in Fig. 2A, dexamethasone decreases IL-1β-induced MMP-9 activity in a dose-dependent manner, while the activity of MMP-2 is not reduced in dexamethasone-treated cells. The treatment of HEI-OC1 cells with dexamethasone also down-regulates IL-1β-stimulated MMP-9 mRNA levels in a dose-dependent manner, while the mRNA expression of actin is not altered in dexamethasone-treated cells (Fig. 2B).
Fig. 2.
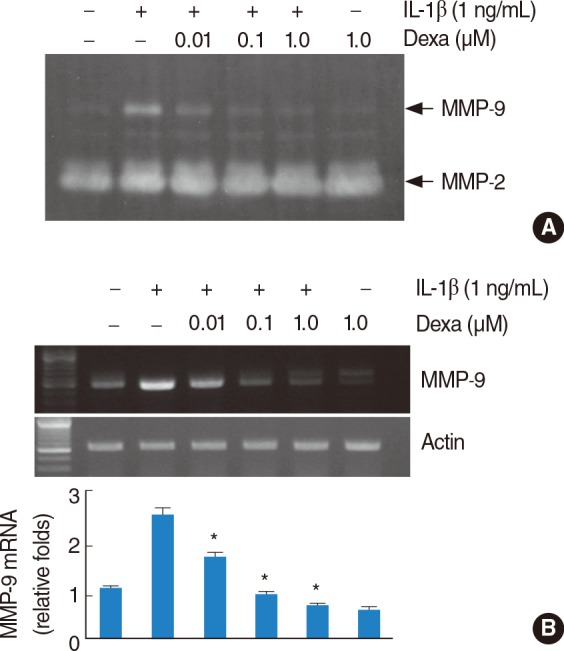
Effect of dexamethasone on interleukin (IL)-1β induced matrix metalloproteinase (MMP)-9 activity and MMP-9 mRNA expression. Dexamethasone inhibits IL-1β-induced MMP-9 activity and mRNA expression. House ear institute-organ of Corti 1 (HEI-OC1) cells were treated with the indicated concentrations of dexamethasone in the absence of presence of 1 ng/mL IL-1β for 20 hours. Conditional media were collected after 20 hours and gelatin zymography was performed (A). The MMP-9 mRNA expression levels were determined by reverse transcription-polymerase chain reaction. The levels of actin were used as a loading control. The values in (B) represent the mean±SD from three independent samples. *P<0.001 compared to IL-1β. The data represent three independent experiments.
Effect of RU486 on inhibitory effect of dexamethasone in IL-1β-induced MMP-9 activity
To investigate the mechanisms of dexamethasone inhibition of IL-1β-induced MMP-9 activity, the specific steroid receptor antagonist RU486 was used. The dexamethasone-induced inhibition of IL-1β-induced MMP-9 activity is recovered by RU486, strongly suggesting that the dexamethasone action is mediated by receptor activation (Fig. 3A). RU486 alone has no significant effect on MMP-9 activity. We also investigated whether RU486 recovered MMP-9 mRNA. As shown in Fig. 3B, similar to zymography, the expression levels of MMP-9 mRNA is recovered by RU486.
Fig. 3.
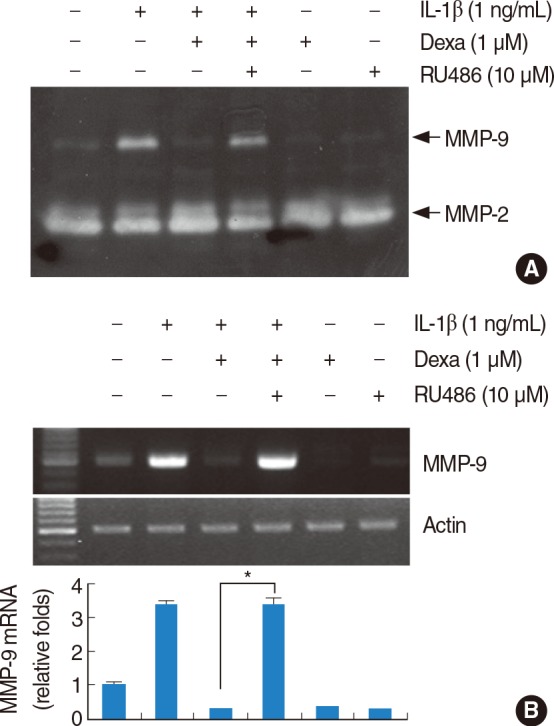
Effect of RU486 on inhibitory effect of dexamethasone in interleukin (IL)-1β-induced matrix metalloproteinase (MMP)-9 activity and mRNA expression. RU486 blocks inhibitory effect of dexamethasone in IL-1β-induced MMP-9 activity and mRNA expression. House ear institute-organ of Corti 1 (HEI-OC1) cells were pretreated with RU486 (10 µM) for 30 minutes, and then treated with the 1 ng/mL IL-1β in the absence or presence of 1 µM dexamethasone (Dex) for 20 hours. Conditional media were collected and gelatin zymography was performed (A). The MMP-9 mRNA expression levels were determined by reverse transcription-polymerase chain reaction. The levels of actin were used as a loading control. The values in (B) represent the mean±SD from three independent samples. *P<0.001 compared to IL-1β plus Dex. The data represent three independent experiments.
Effects of NAC on IL-1β-induced MMP-9 activity and mRNA expression
Generation of reactive oxygen species (ROS) during inflammation is believed to play critical role in various diseases. Therefore, we examined whether the induction of IL-1β-induced MMP-9 activity is mediated through the generation of ROS, HEI-OC1 cells were pretreated for 30 minutes with NAC, a free radical scavenger, and subsequently treated with IL-1β. As shown in Fig. 4A, B, NAC had no significant effect on MMP-9 activity and MMP-9 mRNA expression. The results suggest that IL-1β-induced MMP-9 activity and mRNA expression may be not related to ROS.
Fig. 4.
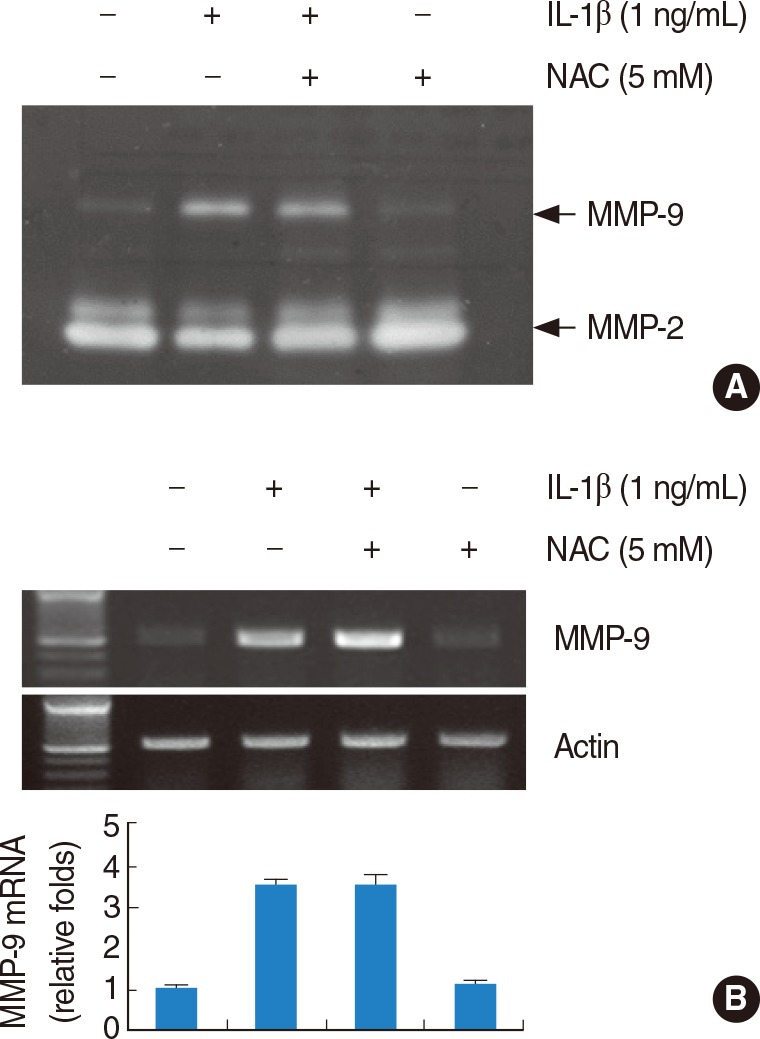
Effects of N-acetylcysteine (NAC) on interleukin (IL)-1β-induced matrix metalloproteinase (MMP)-9 activity and mRNA expression. Reactive oxygen species signaling is not associated with IL-1β-indcued MMP-9 activity and mRNA expression. House ear institute-organ of Corti 1 (HEI-OC1) cells were pretreated with NAC (5 mM) for 30 minutes, and then added 1 ng/mL IL-1β for 20 hours. Conditional media were collected and gelatin zymography was performed (A). The MMP-9 mRNA expression levels were determined by reverse transcription-polymerase chain reaction. The levels of actin were used as a loading control. The values in (B) represent the mean±SD from three independent samples. The data represent three independent experiments.
Activation of p38 MAPK signaling pathway is important for IL-1β-induced MMP-9 activity and mRNA expression in HEI-OC1 cells
To investigate whether the extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinases (MAPK) pathways are involved in IL-1β-induced MMP-9 activity, we examined whether selective MAPK inhibitors could affect IL-1β-stimulated MMP-9 expression. SB203580 (a p38 MAPK inhibitor) profoundly inhibited IL-1β-induced MMP-9 activity and MMP-9 mRNA expression. However, treatment with PD98059 (a potent inhibitor of ERK) or SP600125 (a potent inhibitor of JNK) did not significantly affect on MMP-9 activity and mRNA expression (Fig. 5). Taken together, these results indicate that the activation of p38 MAPK pathway plays an important role in regulating IL-1β-induced MMP-9 expression in HEI-OC1 cells.
Fig. 5.
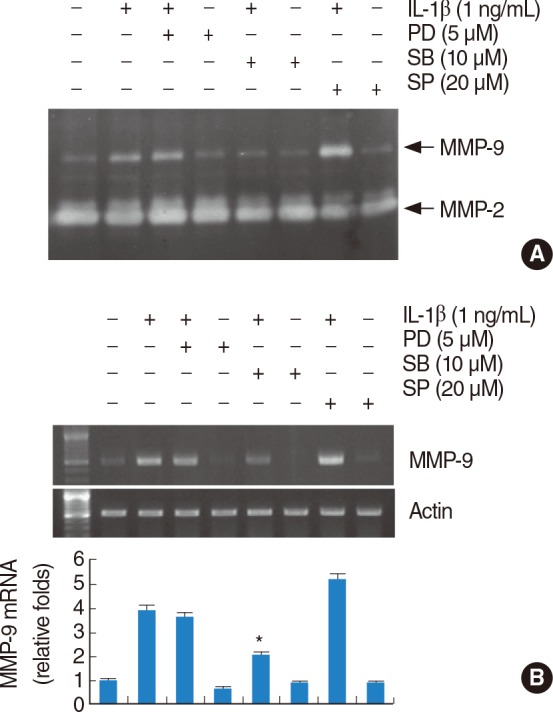
The p38 mitogen-activated protein kinases (MAPK) signaling pathways play important roles in interleukin (IL)-1β-induced matrix metalloproteinase (MMP)-9 activity and mRNA expression in house ear institute-organ of Corti 1 (HEI-OC1) cells. IL-1β induces MMP-9 activity and mRNA expression via the p38 MAPK signaling. (A) HEI-OC1 cells were pretreated with 50 µM ERK inhibitor (PD98059), 10 µM p38 MAPK inhibitor (SB203580), and 20 µM JNK inhibitor (SP600125), and then stimulated with 1 ng/mL IL-1β for 20 hours. Conditional media were collected and gelatin zymography was performed (A). The MMP-9 mRNA expression levels were determined by reverse transcription-polymerase chain reaction. The levels of actin were used as a loading control. The values in (B) represent the mean±SD from three independent samples. *P<0.001 compared to IL-1β. The data represent three independent experiments.
DISCUSSION
The stiffness and mass of basilar membrane, which are important factor for cochlear micromechanics, are affected by the structural organization of macromolecules of the ECM. Collagens are major constituents of ECMs. Laminin, entactin, type IV collagen and heparin sulfate proteoglycan were found in large amounts within cochlear basement membrane [8,9,10]. Basement epithelium supports the organ of Corti on which rests the sensory epithelium. The intercellular matrices are rich in collagen and a homeostasis is maintained by regulating the turnover of matrix composition. Most often in disease in which elevated matrix accumulation is observed, it is accompanied by elevated MMP expression [11]. Expression of proteases such as MMP-9 is regulated by diverse growth factors, and cytokines. However, the molecular mechanism involved in IL-1β-induced MMP-9 expression was poorly understood in a cochlear cell line. In this study, we observed that IL-1β induced the MMP-9 expression. These findings may be suggest that IL-1β in the inner ear under inflammatory conditions leads to production of MMP-9, and induces hearing loss.
We were further interested in the inhibitory mechanism of IL-1β-induced MMP-9 expression. Dexamethasone, a synthetic steroid analog, has been a therapeutic modality used via intratympanic injection for patients who suffer from sudden idiopathic sensorineural hearing loss with diabetes mellitus [12]. Recent studies have shown that dexamethasone protects inner ear against tumor necrosis factor (TNF)-α induced loss of cochlear hair cells, and TNF-α's ototoxicity is mediated through an up-regulation of Bax and TNF receptor-1 expression [5]. Glucocorticoid receptor (GR) expression in the inner ear has been shown by several investigators [13,14]. We investigated the inhibitory effect of dexamethasone on IL-1β-induced MMP-9 expression. GR antagonist reverses dexamethasone-mediated suppression of IL-1β induced MMP-9 activity. However, ROS may be not related to IL-1β-induced MMP-9 expression. To our knowledge, these findings are the first to report IL-1β induced MMP-9 transcriptional down-regulation by dexamethasone in HEI-OC1 cells.
Several studies reported that MAPK pathways are associated with up-regulation of IL-1β-mediated MMP-9 [15,16,17]. For examples, IL-1β increased MMP-9 expression in rat brain astrocytes, rat glomerular mesangial cells and human tracheal smooth muscle cells via ERK, p38 MAPK and JNK activation [15,16,17]. Furthermore, other factors, include the epidermal growth factor, scatter factor/hepatocyte growth factor, and TNF-α, also induced MMP-9 via MAPK activation [18,19]. However, the role of MAPKs in the regulation of MMP-9 expression in cochlear cells has not been investigated. The present study has examined what intracellular signaling proteins are activated in HEI-OC1 cells after treatment with IL-1β. Interestingly, we have found that only SB203580 down-regulated IL-1β-induced MMP-9 activity and mRNA expression in HEI-OC1 cells. These results suggest that activation of p38 MAPK signaling pathway is critical for the IL-1β-induced MMP-9 protein and mRNA expressions in HEI-OC1 cells.
In conclusion, the present study demonstrates that the inflammatory cytokine, IL-1β is able to strongly induce MMP-9 expression in HEI-OC1 cells. IL-1β-induced MMP-9 activity was inhibited by dexamethasone or inhibitor of p38 MAPK signaling pathway. Given the biological importance of MMP-9, it is certain that any inhibitor or compound to affect MMP-9 expression or other MMPs production has the potential to be clinically useful.
ACKNOWLEDGMENTS
The author thanks Federico Kalinec, PhD (House Ear Institute) for provided HEI-OC1 cells. This work was supported by the research promoting grant from the Keimyung University Dongsan Medical Center in 2007.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM. Proinflammatory cytokine expression in the endolymphatic sac during inner ear inflammation. J Assoc Res Otolaryngol. 2003 Jun;4(2):139–147. doi: 10.1007/s10162-002-3025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gratton MA, Rao VH, Meehan DT, Askew C, Cosgrove D. Matrix metalloproteinase dysregulation in the stria vascularis of mice with Alport syndrome: implications for capillary basement membrane pathology. Am J Pathol. 2005 May;166(5):1465–1474. doi: 10.1016/S0002-9440(10)62363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okada Y, Morodomi T, Enghild JJ, Suzuki K, Yasui A, Nakanishi I, et al. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts: purification and activation of the precursor and enzymic properties. Eur J Biochem. 1990 Dec;194(3):721–730. doi: 10.1111/j.1432-1033.1990.tb19462.x. [DOI] [PubMed] [Google Scholar]
- 4.Jang CH, Shin SH, Cho HH, Moon SJ, Cho YB. Expression of matrix metalloproteinase-9 and -2 in pediatric chronic otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2006 Jul;70(7):1155–1158. doi: 10.1016/j.ijporl.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Dinh CT, Haake S, Chen S, Hoang K, Nong E, Eshraghi AA, et al. Dexamethasone protects organ of corti explants against tumor necrosis factor-alpha-induced loss of auditory hair cells and alters the expression levels of apoptosis-related genes. Neuroscience. 2008 Nov;157(2):405–413. doi: 10.1016/j.neuroscience.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Kalinec GM, Webster P, Lim DJ, Kalinec F. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurootol. 2003 Jul-Aug;8(4):177–189. doi: 10.1159/000071059. [DOI] [PubMed] [Google Scholar]
- 7.Overall CM, Wrana JL, Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan;264(3):1860–1869. [PubMed] [Google Scholar]
- 8.Ishii K, Schröter-Kermani C, Xu D, Merker HJ, Jahnke V. Extracellular matrix in the rat spiral limbus. Eur Arch Otorhinolaryngol. 1992;249(4):224–230. doi: 10.1007/BF00178474. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove D, Samuelson G, Pinnt J. Immunohistochemical localization of basement membrane collagens and associated proteins in the murine cochlea. Hear Res. 1996 Aug;97(1-2):54–65. [PubMed] [Google Scholar]
- 10.Cosgrove D, Rodgers KD. Expression of the major basement membrane-associated proteins during postnatal development in the murine cochlea. Hear Res. 1997 Mar;105(1-2):159–170. doi: 10.1016/s0378-5955(96)00203-1. [DOI] [PubMed] [Google Scholar]
- 11.Rao VH, Lees GE, Kashtan CE, Nemori R, Singh RK, Meehan DT, et al. Increased expression of MMP-2, MMP-9 (type IV collagenases/gelatinases), and MT1-MMP in canine X-linked Alport syndrome (XLAS) Kidney Int. 2003 May;63(5):1736–1748. doi: 10.1046/j.1523-1755.2003.00939.x. [DOI] [PubMed] [Google Scholar]
- 12.Chandrasekhar SS. Intratympanic dexamethasone for sudden sensorineural hearing loss: clinical and laboratory evaluation. Otol Neurotol. 2001 Jan;22(1):18–23. doi: 10.1097/00129492-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Pitovski DZ, Drescher MJ, Drescher DG. Glucocorticoid receptors in the mammalian inner ear: RU 28362 binding sites. Hear Res. 1994 Jun;77(1-2):216–220. doi: 10.1016/0378-5955(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 14.Rarey KE, Curtis LM. Receptors for glucocorticoids in the human inner ear. Otolaryngol Head Neck Surg. 1996 Jul;115(1):38–41. doi: 10.1016/S0194-5998(96)70133-X. [DOI] [PubMed] [Google Scholar]
- 15.Liang KC, Lee CW, Lin WN, Lin CC, Wu CB, Luo SF, et al. Interleukin-1beta induces MMP-9 expression via p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-kappaB signaling pathways in human tracheal smooth muscle cells. J Cell Physiol. 2007 Jun;211(3):759–770. doi: 10.1002/jcp.20992. [DOI] [PubMed] [Google Scholar]
- 16.Eberhardt W, Huwiler A, Beck KF, Walpen S, Pfeilschifter J. Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J Immunol. 2000 Nov;165(10):5788–5797. doi: 10.4049/jimmunol.165.10.5788. [DOI] [PubMed] [Google Scholar]
- 17.Wu CY, Hsieh HL, Jou MJ, Yang CM. Involvement of p42/p44 MAPK, p38 MAPK, JNK and nuclear factor-kappa B in interleukin-1beta-induced matrix metalloproteinase-9 expression in rat brain astrocytes. J Neurochem. 2004 Sep;90(6):1477–1488. doi: 10.1111/j.1471-4159.2004.02682.x. [DOI] [PubMed] [Google Scholar]
- 18.McCawley LJ, Li S, Wattenberg EV, Hudson LG. Sustained activation of the mitogen-activated protein kinase pathway: a mechanism underlying receptor tyrosine kinase specificity for matrix metalloproteinase-9 induction and cell migration. J Biol Chem. 1999 Feb;274(7):4347–4353. doi: 10.1074/jbc.274.7.4347. [DOI] [PubMed] [Google Scholar]
- 19.Cho A, Graves J, Reidy MA. Mitogen-activated protein kinases mediate matrix metalloproteinase-9 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000 Dec;20(12):2527–2532. doi: 10.1161/01.atv.20.12.2527. [DOI] [PubMed] [Google Scholar]