Abstract
Verteporfin (VP), a benzoporphyrin derivative, is clinically used in photodynamic therapy for neovascular macular degeneration. Recent studies indicate that VP may inhibit growth of hepatoma cells without photoactivation hrough inhibition of YAP-TEAD complex. In this study, we examined the effects of VP without light activation on human retinoblastoma cell lines. Verteporfin but not vehicle control inhibited the growth, proliferation and viability of human retinoblastoma cell lines (Y79 and WERI) in a dose-dependent manner and was associated with downregulation of YAP-TEAD associated downstream proto-oncogenes such as c-myc, axl, and surviving. In addition VP affected signals involved in cell migration and angiogenesis such as CTGF, cyr61, and VEGF-A but was not associated with significant effect on the mTOR/autophagy pathway. Of interest the pluripotency marker Oct4 were downregulated by Verteporfin treatment. Our results indicate that the clinically used photosensitizer VP is a potent inhibitor of cell growth in retinoblastoma cells, disrupting YAPTEAD signaling and pluripotential marker OCT4. This study highlights for the first time the role of the YAP-TEAD pathway in Retinoblastoma and suggests that VP may be a useful adjuvant therapeutic tool in treating Rb patients.
Keywords: Cancer, Hippo, eye, intraocular, YAP, Oct4
INTRODUCTION
Retinoblastoma is the most common primary malignant intraocular tumor in infants and children. In the United States, it affects 12 per million children aged 0-4 years, representing 6.1% of all childhood cancers under the age of 5 years (Broaddus et al., 2009). Slightly more than half of the patients have the sporadic or non-inherited form of the disease, which results from the spontaneous inactivation of the retinoblastoma gene (RB1). Despite progress in the treatment of retinoblastoma, significant problems remain unsolved and metastatic disease is all too often fatal (Rodriguez-Galindo et al., 2003). Although several treatment modalities are available for retinoblastoma, including local control of small to intermediate size tumors with laser and/or cryotherapy sometimes in combination with radiation and/or chemotherapy, or enucleation with or without systemic chemotherapy to control metastatic disease, each of them has major drawbacks, especially in pediatric patients. For example, conventional external beam radiation, which is used to control large tumors, has many complications, including an increased incidence of secondary malignancies, such as osteosarcoma. This complication occurs more frequently in patients with the hereditary-form of retinoblastoma. The 30-year cumulative incidence of second malignancies is >35% for patients who received external beam therapy vs 6% for those patients without radiation (Roarty et al., 1988). Intra-atrerial chemotherapy is a more recent treatment option for retinoblastoma, however variables that affect blood flow can greatly affect drug delivery and therapy success (Marr et al., 2012) and can be complicated by retinal and choroidal vasculopathy in up to 10% to 20% of patients (Bianciotto et al., 2012; Muen et al., 2012). Direct intravitreal injection of melphalan has also been tested as an effective modality in controlling active vitreous seeds, however there is concern for tumor dissemination (Ghassemi and Shields, 2012; Munier et al., 2012). Systemic chemotherapy used for treatment for intraocular retinoblastoma with subsequent consolidation with photocoagulation, cryotherapy, or radiotherapy has a recurrence rate of 24% by 5 years (Shields et al., 2002). This increases to 50% for patients with vitreous seeds (Shields et al., 2003). The recent reports (Sussman et al., 2003; Schefler et al., 2007; Shields, 2009; Shields et al., 2009) revealed success for local control approaching 90–100% for group A–C, but in less than 50% for group D (new international classification). In addition, significant morbidity with the chemotherapy has been described previously (Benz et al., 2000). One of the drugs used for chemotherapy (etoposide) is thought to be associated with increased incidence of acute myeloblastic leukemia although the actual cases implicated so far have been low with ~20 cases reported (Nishimura et al., 2001). For these reasons, there is a pressing need for alternative treatment modalities for retinoblastoma with better safety and efficacy profiles.
Verteporfin (VP) belongs to the porphyrin family, which contains aromatic heterocyclic cyclic molecules composed of four modified pyrrole units which are interconnected at their carbon atoms via methine bridges (Liu-Chittenden et al., 2012). Photodynamic therapy (PDT) using verteporfin is a clinically approved, minimally invasive therapeutic procedure that involves administration of a photosensitizing agent followed by irradiation at a wavelength of 693nm corresponding to an absorbance band of the sensitizer (Agostinis et al., 2011). In wet age related macular degeneration (AMD), liposomal VP (trade name Visudyne) accumulates in abnormal blood vessels, where it is activated by nonthermal laser at 693nm generating reactive oxygen radicals. This results in local damage to the endothelium as well as blockage, and potential elimination of the vessels. It has been clinically used worldwide resulting in vision preservation in many patients (Miller et al., 1999). VP has also been tested but not yet approved as a light-based therapeutic modality for several human cancers (Pogue et al., 2003; Harbour, 2004; Isola et al., 2006; Celli et al., 2011; MS et al., 2011). In human cancers PDT with visudyne may act not only at the cancer vasculature but also directly on the cancer cells and act as a inducer of apoptosis or autophagy (Kessel et al., 2006).
Yes Associated Protein (YAP) is or has been suggested to be a major candidate oncogene in the human chromosome 11q22 amplicon, and mutations or abnormal expression of Hippo-pathway components are associated with human tumorigenesis (Fernandez-L et al., 2012; Liu et al., 2012; Mo et al., 2012). As a transcriptional coactivator, YAP has been reported to bind to several DNA-binding transcription factors, and among the reported YAP binding partners, the TEAD transcription factors are the best characterized (Vassilev, 2001). Recent studies indicate that VP may disrupt YAP-TEAD complex and inhibit growth of hepatocellular carcinoma without light activation (Liu-Chittenden et al., 2012).
Since YAP-TEAD pathway is usually not active in normal tissues, drugs disrupting this interaction have the potential for increased cancer specificity with minimal healthy tissue toxicity making them valuable novel potential therapeutic option. For this reason we investigated the effects of non-light activated -VP on human retinoblastoma cells.
MATERIALS AND METHODS
Reagents
Verteporfin (Visudyne) was obtained from Novartis (Novartis, Basel, Switzerland).
Metformin, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and ribonuclease-A were purchased from Sigma Aldrich (St.Louis, MO, USA). Propidium iodide was purchased from Invitrogen (Carlsbad, CA, USA). The following primary antibodies were purchased from Cell Signaling technology (Danvers, MA, USA) and used diluted 1:1000 unless stated otherwise: c-myc, axl, phospho-S6 ribosomal protein (Ser235/236), phospho-4EBP1 (Thr37/46), p21 Waf1/Cip1, p27Kip1, LC3B, phospho-p38 MAPK (Thr180/Tyr182) and p44/42 MAPK (Erk1/2), PCNA (1:2000), 4-Oct, survivin, pAkt (S473) (1:2000), pAkt (T308). The following primary antibodies were purchased from Epitomics (Burlingame, CA, USA): cyclin D1, D3, E1, E2, A2 and the following from Santa Cruz (Dallas, Texas, USA): cyr61 (1:500), VEGFA (1:500), CTGF (1:500).
Cell culture
The human retinoblastoma cells Y79 and WERI (ATCC, Manassas, VA, USA) were grown in RPMI 1640 medium (Invitrogen, Grand Island, NY, USA) supplemented with 15% fetal bovine serum (FBS) (ATCC), penicillin (100μg/ml), streptomycin (100μg/ml), 2mM L-glutamine, 10mM HEPES (all from Invitrogen). Cells were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2 and split when the cells reached approximately 90% confluence. Cells were protected from light at any time (aluminium foil) and all experiments were performed in darkness.
Assessment of growth curves and doubling time
The cells were seeded in 6-well plates at amount 4.5×105 cells per well. Verteporfin was added (final concentrations 2μg/ml or 10μg/ml) and cells were incubated for 5 days. At days 3 and 5 cell number and viability were determined by the trypan blue (0.4%) dye exclusion method. Growth curve was drawn. Experiment was performed 3 times with 2 wells per condition each time.
MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) assay: measurement of cell growth and viability
Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MTT assay is used to measure the reduction of a tetrazolium compound by the cellular mitochondria, producing an optically active soluble formazan.
Cells were cultured in 48-well plates at density 60,000 cells per well in 300ul growth medium. After 1 and 3 days of treatment with VP, MTT (5 mg/mL in PBS) was added to each well at a 1/10 volume. Cells were incubated for 1hour at 37°C and resuspended in DMSO. The absorbance at 595 nm was measured using a microplate reader. Data are displayed as percentage of control.
Flow cytometry: assessment of cell cycle
Cellular DNA content was assessed by flow cytometry. Cells were seeded in 6-well plates at density 500,000 cells per 2 ml growth medium and were treated with verteporfin (final concentrations 2μg/ml or 10μg/ml) for 48h. After overnight fixation in 75% ethanol, cells were resuspended in PBS with DNase-free RNase A at final concentration 0.3mg/ml and propidium iodide at final concentration 1mg/ml. DNA content assessed on Becton Dickinson LSRII flow cytometer. The sub-G1 peak that represented the nonviable cell population was quantified . Results were analyzed with Diva Software.
Protein extraction and western blot analysis
Cells were incubated for 48 hours in the presence or absence of VP at concentrations 2μg/ml and 10μg/ml. Control cells were treated with PBS. The samples were lysed in M-PER Mammalian Protein Extraction Reagent (Thermo-Scientific, Rockford, IL USA) supplemented with protease (according to manufacturer suggestions; Roche Applied Science) and phosphatase inhibitor cocktails (dilution 1:50; Thermo-Scientific, Pierce Protein Research Products). All cells and samples were protected from light at any time. Ten micrograms of total amount of proteins was loaded onto a 4 – 12% Bis-Tris Gel (NuPAGE; Invitrogen). The electrophoresis was performed using NuPAGE MOPS Running Buffer (Invitrogen) and then samples were transferred onto a PVDF membrane (Millipore, Billerica, MA, USA). After transfer, the membranes were stained with Comassie blue to ensure equal loading, and then blocked 45min at room temperature in 5% wt/vol BSA followed by incubation over night at 4C° with the listed above rabbit anti human primary antibodies. Then the membranes were washed three times 1xTBS 0.1% Tween 20 and incubated for 45min at room temperature with the horseradish peroxidase-labeled secondary anti-rabbit antibody at 1:50000 (Jackson ImmunoResearch, West Grove, PA, USA). The immunoreactive bands were visualized with ECL exposured onto Fuji RX film (Fujifilm,Tokyo, Japan). The results were quantified using ImageJ software.
Statistical analysis
Data are expressed as mean and standard error of the mean (SEM). Statistical significance was evaluated using the one-way ANOVA test with Dunnett's modification for multiple means comparison or t-test for two means. A p value of < 0.05 was considered to be significant. Two-tailed tests were used for all comparisons.
RESULTS
Verteporfin inhibits cell growth and viability, and increases doubling time of human retinoblastoma cell lines Y79 and WERI
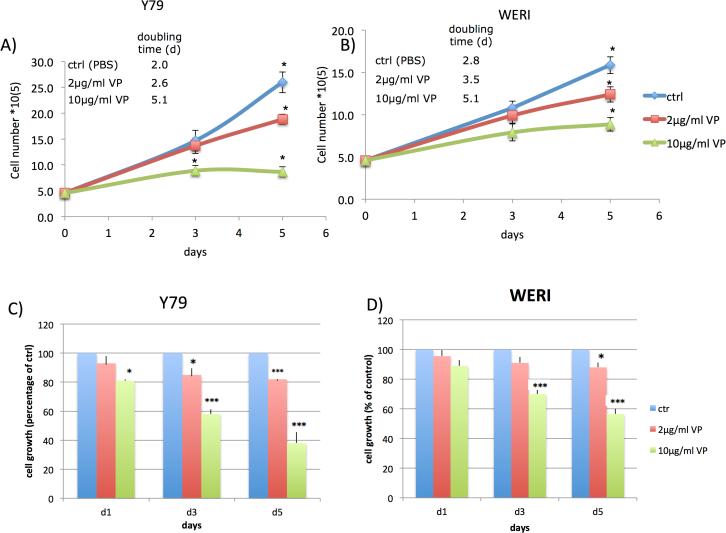
In order to determine whether VP without light activation affects human retinoblastoma cell growth and proliferation, we analyzed its effect on the human retinoblastoma cell line Y79 and WERI. Cell lines were treated with two different concentrations of VP (2 μg/ml and 10 μg/ml) or vehicle control (PBS). All cells were protected from light at any time. Five days of VP treatment resulted in a decrease in cell colony sizes and a remarkable increase of single dysmorphic cells in suspension. Cell growth and doubling time assessed by trypan blue exclusion tests showed increase in doubling time in a dose-dependent manner in both cell lines (Fig.1A,B). Similarly VP caused a statistically significant inhibition of cell growth and cell viability as assessed by MTT assay in a time- and dose-dependent manner in both cell lines (Fig.1C,D) .
Figure 1. Verteporfin (VP) inhibits growth of retinoblastoma cells Y79 and WERI without light activation.
Y79 and WERI retinoblastoma cells were left untreated (PBS control) or treated with VP for five days; control (blue), VP concentration 2μg/ml (red) and 10μg/ml (green).
(A,B) VP treatment resulted in an inhibition of cell growth and a decrease of the cell number of Y79 and WERI cells in a dose-dependent manner. The doubling time was increased.
(C,D) VP treatment resulted in a significant, time- and dose-dependent inhibition of Y79 and WERI cell growth and viability as determined by MTT assays. The results are expressed as percentage of growth (%) relative to control values. Results are average of three independent experiments. Data are presented as mean +/− SEM (n=9, *p<0.05, *** p<0.001).
VP effects on cell cycle phases
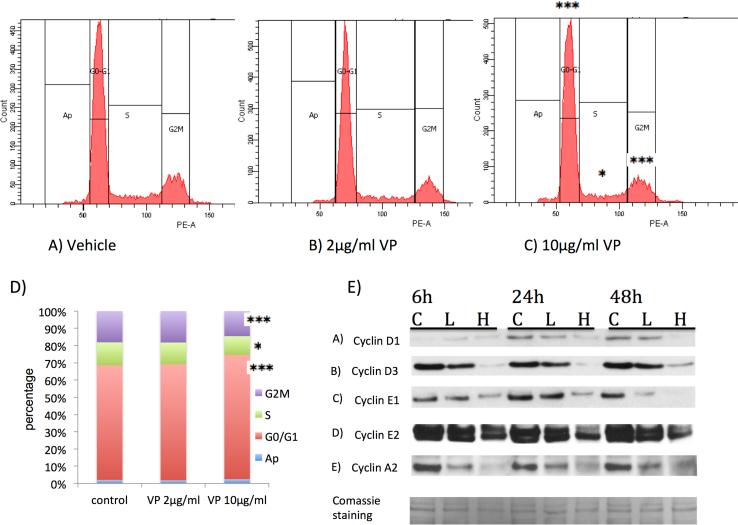
To examine the effects of VP on retinoblastoma cell cycle distribution and progression, we treated Y79 cells with 2μg/ml and 10μg/ml of VP for 48h, and analyzed cell cycle for nuclear DNA content by propidiumiodide staining and flow cytometry (Fig.2). VP treatment at 2μg/ml had minimal effects on cell cycle phases (Fig.2B,D), whereas at 10μg/ml (Fig.2C,D) led to a small but statistically significant increase inY79 cells in G0/G1 phase from 66% to 70.9% (p<0.001), and a resultant decrease in S-phase and G2/M phase (13.3% to 10.7% and 18% to 14.3% respectively, p<0.05). The cell cycle effects were associated with a time and dose dependent decrease mostly in cyclins D3, E1, E2 and A2 (Fig.2 E) as measured by western blot.
Figure 2. Verteporfin (VP) blocks cell cycle progression in retinoblastoma cells.
(A) Y79 retinoblastoma cells left untreated (PBS control) (B) treated with 2μg/ml or (C) 10μg/ml VP for 48h, were analyzed regarding their cell cycle phases for nuclear DNA content by propidium-iodide staining and flow cytometry. (D) Quantification of results shows, that VP treatment at a high concentration of 10μg/ml resulted in a significant increase of Y79 cells in G0/G1 phase, a significant decrease of cells in S-phase and a significant decrease of cells in G2/M phase. Representative data from three independent experiments are shown (n=6 per condition, *p<0.05, *** p<0.001). Data are presented as mean +/− SEM (n=6). (E) Verteporfin (VP) affects the levels of cyclins in retinoblastoma cells. Y79 retinoblastoma cells were treated with vehicle control (letter C above first blot) or with VP 2μg/ml (L) or 10μg/ml (H), for 6, 24 and 48 hours as indicated, while being protected from light any time. Western blots of cyclins D1, D3, E1, E2, A2ares shown. Data are representative out of at least two independent experiments.
Mitogen-activated protein kinases (MAPKs) play a major role in the regulation of cell cycle and apoptosis (Brancho et al., 2003), and activation of p38 MAPK is associated with an inhibition of tumor growth. VP treatment induced expression of p38 MAPK in a dose-dependent manner (Suppl Fig1) suggesting that activation of p38 MAPK may be important for the inhibition of cell proliferation of retinoblastoma cell line Y79 by VP.
VP affects YAP-TEAD proto-oncogene pathway in retinoblastoma cells
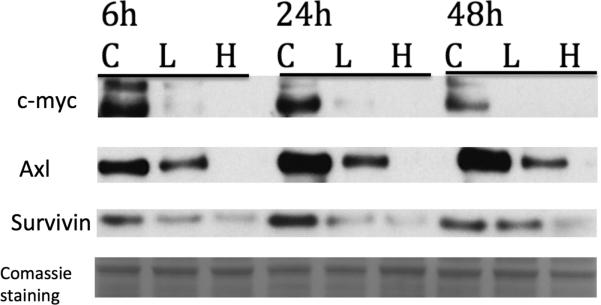
We next examined whether VP treatment results in alterations in genes regulated by TEAD. The protein c-Myc is a major proto-oncogene is activated by the HIPPO pathway and is controlled by YAP/TEAD interactions. It is found in many cancers and is highly amplified in retinoblastoma. Axl is another protooncogene regulated by the Yap/TEAD complex. It is a tyrosine receptor kinase and is another oncogene known to be associated with many cancers including CLL, colon, breast and ocular melanoma and regulated by the YAP/TEAD complex. Survivin, also called baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5 is a member of the inhibitor of apoptosis (IAP) family. The survivin protein functions to inhibit caspase activation, thereby leading to negative regulation of apoptosis or programmed cell death and is seen to be upregualted in many cancers including Retinoblastoma. Indeed cells treated with VP (2 μg/ml and 10μg/ml) for 6, 24 and 48h showed a dose-dependent downregulation of all these proto-oncogenes in retinoblastoma (Fig 3 A).
Figure 3. VP affects YAP-TEAD proto-oncogene pathway in retinoblastoma cells.
Y79 retinoblastoma cells were treated with vehicle (letter C) or with VP 2μg/ml (L) or 10μg/ml (H) for 6, 24 and 48hours. Protein expression of c-Myc, Axl, and surviving was assessed by Western Blot. Data are representative out of at least two independent experiments.
VP affects YAP-TEAD signaling pathway □in retinoblastoma cells involved in angiogenesis and migration (CYR 61, CTGF and VEGF )
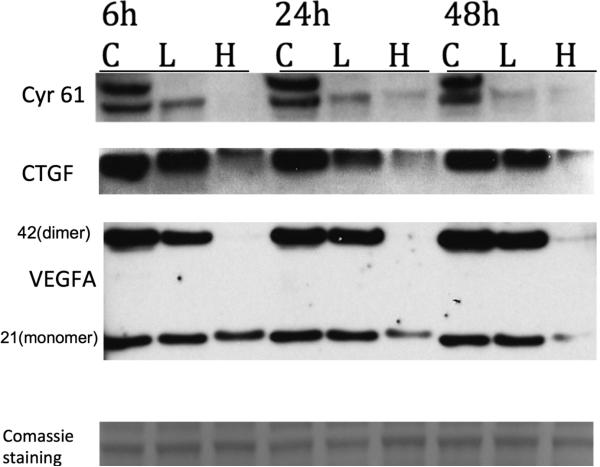
Besides oncogenes, YAP/TEAD controls the expression of molecules involved in angiogenesis and cell migration such as CYR61(CCN1) and CTGF(CCN2). Cysteine-rich angiogenic inducer 61(CYR61) or CCN family member 1 (CCN1) is critical for blood vessel formation and vascular integrity, has potent angiogenic activity upon endothelial cells and induces neovascularization. Connective tissue growth factor (CTGF), also known as CCN2 has important roles in cell adhesion, migration, proliferation and angiogenesis. In addition VEGF expression has been shown in the past that may be also controlled by TEAD via interaction with the Vestigial-like (Vgll) transcription coactivators (Teng et al., 2010). Retinoblastoma cells treated with VP (both 2 and 10 μg / ml ) showed a time and dose dependent decrease in the expression of all these proteins as examined by western blot analysis (Fig. 4).
Figure 4. Verteporfin (VP) affects YAP-TEAD signaling pathway in retinoblastoma cells involved in angiogenesis and migration (CYR 61, CTGF and VEGF ).
Y79 retinoblastoma cells were treated with vehicle (letter C) or with VP 2μg/ml (L) or 10μg/ml (H) for 6, 24 and 48hours. Protein expression of Cyr61,CTGF and VEGF-A was assessed by Western Blot. Data are representative out of at least two independent experiments.
VP down-regulates pluripotency marker OCT-4 in retinoblastoma cells
In contrast to normal cells, pluripotency cell marker OCT-4 is expressed in cancer cells and in particular in cancer stem cell subpopulation. Given the fact that YAP-TEAD is highly active and required during early development we wanted to examine the effects of VP on the pluripotency marker OCT-4. Rb cells treated with VP In our experiments VP led to a reduction of OCT4 in a dose and time dependent manner (Fig 5) suggesting that VP can act as a tool in suppressing cancer stem cells.
Figure 5. Verteporfin down-regulates pluripotency marker OCT-4 in retinoblastoma cells.
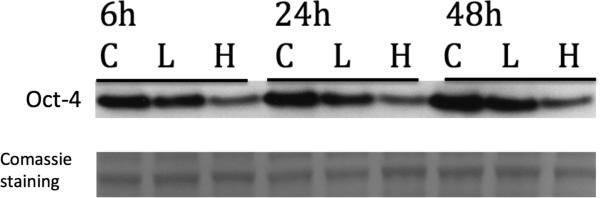
Y79 retinoblastoma cells were treated with vehicle (letter C) or with VP 2μg/ml (L) or 10μg/ml (H) for 6, 24 and 48hours. OCT-4 expression was assessed by Western Blot. Data are representative out of at least two independent experiments.
VP effects on the mTOR and autophagy pathway in retinoblastoma cells
Recently it has been determined that YAP may mediate partial crosstalk between the Hippo and PI(3)K– TOR pathways and activates the mammalian target of rapamycin (mTOR), a major regulator of cell growth (Tumaneng et al., 2012). In addition mTOR is a critical regulator of autophagy induction, with activated mTOR suppressing autophagy, and negative regulation of mTOR promoting it. To determine whether VP treatment of Y79 cells was associated with inhibition of the mTOR pathway and activation of autophagy, we examined the phosphorylation status of direct downstream targets of mTOR, ribosomal protein S6, 4EBP1 and Akt and the autophagic marker LC3 by western blot. Analysis of cells treated with VP showed minimal effects on the mTor pathway suggesting (Suppl Fig 2)
DISCUSSION
Verteporfin has been widely and safely used as a photosensitizer in PDT for neovascular macular degeneration as well as treatment of several human tumors, after it is activated by laser light to eliminate abnormal blood vessels (Miller et al 1999). In the present study, we demonstrate that verteporfin has a potential to induce growth inhibition, apoptosis, and G0/G1-phase cell cycle arrest in human retinoblastoma cells in vitro without any light activation by interfering with the YAP-TEAD growth pathway. There is a long history of clinical use of Photodynamic therapy using VP yet injury of RPE or retina damage is not seen in the area other than light is exposed. (Husain et al, 1999). Recently, evidence was acquired that VP may also have a direct inhibitory effect on the growth of cancer cells without light activation but via disruption of YAP-TEAD complex and prevention of YAP-induced oncogenic growth (Liu-Chittenden et al., 2012). Yes Associated Protein (YAP) has been implicated as the candidate oncogene in the human chromosome 11q22 amplicon, and mutations or abnormal expression of Hippo pathway components are associated with human tumorigenesis (Fernandez-L et al., 2012; Liu et al., 2012; Mo et al., 2012). As a transcriptional coactivator, YAP has been reported to bind to several DNA-binding transcription factors and among the reported YAP binding partners, the TEAD transcription factors are the best characterized (Vassilev, 2001). TEAD proteins are transcription factors that are crucial for cell development, but also play a role in cancers. Several TEAD-interacting coactivators are known including YAP and Vgll proteins. Vgll proteins upregulate the expression genes such as VEGFA (Teng et al., 2010) an important pro-angiogenic factor involved in cancer progression (Bellou et al., 2013).
Recent studies have shown that TEADs and their coactivators aid in the progression of various cancers, including glioblastoma, liver and ovarian cancers (Pobbati and Hong, 2013). They facilitate cancer progression through expression of genes promoting cancer cell proliferation, which are upregulated by YAP, such as c-myc, surviving and Axl(Xu et al., 2011; Pobbati and Hong, 2013). The c-myc oncoprotein is a transcription factor, with many of its target genes encoding proteins that initiate and maintain the transformed state and is upregulated when Rb function is lost (Prochownik, 2004). Axl (from the Greek word “anexelekto” or uncontrolled) is a receptor tyrosine kinase has recently been identified as a critical factor driving tumor cell invasion and migration in mesothelioma and to correlate with metastasis and poor prognosis in breast and ovarian cancer ( O'Bryan et al, 1991; Chen et al., 2013). Survivin is a member of the inhibitor-of-apoptosis proteins family. It is expressed during embryonic and fetal development and by many different cancer cell types (Coumar et al., 2013). Survivin has also been found in Rb pateints and its levels correlated with disease stage and response to treatment (Coumar, 2013). All these proto-oncogenes were downregulated by VP treatment in a dose and time dependent manner in retinoblastoma cells.
Another set of proteins regulated by YAP/TEAD involve factors that govern cell migration and angiogenesis such as CYR61(CCN1) and CTGF(CCN2). Cysteine-rich 61 (Cyr61), one of the tissue growth factors in the CCN family (Cyr61/CTGF/NOV), is highly expressed in various cancer tissues and cell lines, including breast cancer, endometrial cancer, MCF-7 cells, SKOV-3 cells, gastric cancer cells, benign prostatic hyperplasia, gliomas, and melanomas (Lee et al., 2012). Connective tissue growth factor (CTGF), also known as CCN2 has important roles in cell adhesion, migration, proliferation and angiogenesis and was recently shown that the PDZ binding motif of YAP is required for its co-activation of TEAD-mediated CTGF transcription and oncogenic cell transforming activity (Shimomura et al, 2014). In addition VEGF expression has been shown in the past that may be also controlled by TEAD via interaction with the Vestigial-like (Vgll) transcription coactivators (Teng et al., 2010). Indeed treatment of retinoblastoma Y79 cells with VP has demonstrated time and dose dependent down-regulation of all these proteins that are regulated by YAP-TEAD interaction.
Recently it has been determined that YAP mediates crosstalk between the Hippo and PI(3)K–TOR pathways and that YAP activates the mammalian target of rapamycin (mTOR), a major regulator of cell growth (Tumaneng et al., 2012). The phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway plays a central role in regulation of tumor cell proliferation, transcription, apoptosis, cell migration, survival and angiogenesis (Cicenas, 2008; Ewald et al., 2013; Pal and Quinn, 2013; Saini et al., 2013). mTOR activity has been shown to regulate autophagy (Sarbassov et al., 2005) a process that maintains cellular homeostasis by ensuring the lysosomal degradation of long-lived proteins and organelles. It was initially believed to promote cell survival and the generation of nutrients and energy, but later studies demonstrated that persistent stress can also promote autophagic cell death (Kondo et al., 2005). In our study, VP treatment of human retinoblastoma Y79 cells had minimal effects on the mTOR and autophagy pathway (Suppl Fig S2).
Activation of p38 MAPK has been implicated in the inhibition of tumor growth (Brancho et al., 2003; Gu et al., 2012). Consistent with these reports, the anti-proliferative action of VP on retinoblastoma Y79 cells reported in this study was associated with an activation of p38 MAPK (Suppl Fig 1). Contribution of MAPKs in cell proliferation and apoptosis has been extensively documented. As demonstrated previously, p38 MAPK activation induces apoptosis in certain cells, including acute lymphomblastic leukemia and hepatoma cells (Sengupta et al., 2007), and induces the mitochondrial apoptotic pathway (García-Fernández et al., 2002). Inhibition of mTOR also activates the MAPK pathway throughout S6K1 signaling (Carracedo et al., 2008). We therefore suggest that p38 may mediate VP's effect on retinoblastoma cells.
Although p21 is thought of as a tumor suppressor, we observed a down-regulation of p21 by VP in retinoblastoma Y79 cells (Suppl.Fig.3). This paradoxical down-regulation of p21 was also reported in our previous study of AICAR and metformin effects on retinoblastoma cells (Theodoropoulou et al., 2010; 2013)(unpublished data). Evidence suggesting an oncogenic function of p21 was also reported in some other tumors, including lymphoma and esophageal squamous cell carcinoma with p53 gene mutations; in these studies, p21 promoted tumor growth by inhibiting apoptosis and/or promoting cell proliferation (Gartel, 2009). In another study, mitogenic stimuli resulted in transient p21 induction during G1-S progression (Gartel and Radhakrishnan, 2005). Together, these data suggest that p21 may function as an oncogene in human retinoblastoma cells.
An interesting new finding of our study was the effect of VP on downregualting pluripotency marker Oct-4. Adult human differentiated cells loose the pluripotency marker Oct-4 expression and only normal adult stem cells and cancer stem cells maintain expression of Oct-4 (Trosko, 2006). A strategy to target cancer stem cells is to suppress their Oct-4 gene in order to cause the cells to differentiate (Trosko, 2006). Retinoblasotma cells are known to express stemm cell like side population and express OCT4 (Seigel , 2007; Hu, 2012). Our findings suggest that YAP/TEAD may be involved in pluripotency and suggest VP as a novel therapeutic tool in suppressing cancer stem cells.
Although advances in therapy for retinoblastoma have led to significant increases in the life expectancy of patients, significant problems still remain, such as the increased incidence of secondary malignancies after radiation and significant morbidity with current chemotherapeutic agents. This underscores the need for the development of new targets and less toxic therapies. Here, we showed for the first time, that verteporfin can inhibit growth and proliferation of human retinoblastoma cells without any light activation in vitro by interfering with the YAP/TEAD pathway. There is a long history of clinical use of Photodynamic therapy using VP yet atrophy of RPE is not seen in the area other than light is exposed (Husain et al. 1999). In addition in retina most cells (neurosensory and neuroepithelial ) are non-proliferating and as such have reduced YAPTEAD activity and thus are less affected by YAP-TEAD inhibitors. In a separate study of ours (MS in preparation) we have shown that confluent RPE cells and proliferating endothelial cells are more resistant to VP with LD50 several times higher than proliferating cancer cell lines (12-35 micro molar vs 4-5 micromolar). VP may thus have a potential as novel, non-chemotherapeutic treatment option for retinoblastoma. Future animal studies are needed to assess its potential use and effectiveness in humans.
Supplementary Material
HIGHLIGHTS.
VERTEPORFIN (VP) WITHOUT PHOTO-ACTIVATION INHIBITS RETINOBLASTOMA CELL LINES
VERTEPORFIN DOWN-REGULATES YAP-TEAD CONTROLLED PROTOONCOGES MYC,AXL AND SURVIVIN
VERTEPORFIN DOWN-REGULATES YAP-TEAD CONTROLLED CELL MIGRATION AND ANGIOGENESIS FACTORS CYR61,CTGF AND VEGF-A
VERTEPORFIN DOWN-REGULATES PLURIPOTENCY FACTOR OCT4
Non Standard Abbreviations
- VP
Verteporfin
- RB
Retinoblastoma
- MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
- PDT
Photodynamic therapy
- MAPK
Mitogen-activated protein kinases
- Vgll
Vestigial-like (Vgll)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, et al. Photodynamic therapy of cancer: An update. CA: a Cancer Journal for Clinicians. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellou S, Pentheroudakis G, Murphy C, Fotsis T. Anti-angiogenesis in cancer therapy: Hercules and hydra. Cancer Lett. 2013;338:219–228. doi: 10.1016/j.canlet.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Benz MSM, Scott IUI, Murray TGT, Kramer DD, Toledano SS. Complications of systemic chemotherapy as treatment of retinoblastoma. Arch Ophthalmol. 2000;118:577–578. [PubMed] [Google Scholar]
- Bianciotto C, Shields CL, Iturralde JC, Sarici A, Jabbour P, Shields JA. Fluorescein angiographic findings after intra-arterial chemotherapy for retinoblastoma. Ophthalmology. 2012;119:843–849. doi: 10.1016/j.ophtha.2011.09.040. [DOI] [PubMed] [Google Scholar]
- Brancho D, Tanaka N, Jaeschke A, Ventura J-J, Kelkar N, Tanaka Y, Kyuuma M, Takeshita T, Flavell RA, Davis RJ. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969–1978. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975-2004. Br J Ophthalmol. 2009;93:21–23. doi: 10.1136/bjo.2008.138750. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Baselga J, Pandolfi PP. Deconstructing feedback-signaling networks to improve anticancer therapy with mTORC1 inhibitors. Cell Cycle. 2008;7:3805–3809. doi: 10.4161/cc.7.24.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli JP, Solban N, Liang A, Pereira SP, Hasan T. Verteporfin-based photodynamic therapy overcomes gemcitabine insensitivity in a panel of pancreatic cancer cell lines. Lasers Surg Med. 2011;43:565–574. doi: 10.1002/lsm.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-X, Li Q-Y, Yang Z. Axl and prostasin are biomarkers for prognosis of ovarian adenocarcinoma. Ann Diagn Pathol. 2013 doi: 10.1016/j.anndiagpath.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Cicenas J. The potential role of Akt phosphorylation in human cancers. Int J Biol Markers. 2008 doi: 10.1177/172460080802300101. [DOI] [PubMed] [Google Scholar]
- Coumar MS, Tsai F-Y, Kanwar JR, Sarvagalla S, Cheung CHA. Treat cancers by targeting survivin: Just a dream or future reality? Cancer Treat. Rev. 2013;39:802–811. doi: 10.1016/j.ctrv.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Ewald F, Grabinski N, Grottke A, Windhorst S, Nörz D, Carstensen L, Staufer K, Hofmann BT, Diehl F, David K, et al. Combined targeting of AKT and mTOR using MK-2206 and RAD001 is synergistic in the treatment of cholangiocarcinoma. Int. J. Cancer. 2013;133:2065–2076. doi: 10.1002/ijc.28214. [DOI] [PubMed] [Google Scholar]
- Fernandez-L A, Squatrito M, Northcott P, Awan A, Holland EC, Taylor MD, Nahlé Z, Kenney AM. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31:1923–1937. doi: 10.1038/onc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Fernández LF, Losada A, Alcaide V, Alvarez AM, Cuadrado A, González L, Nakayama K, Nakayama KI, Fernández-Sousa JM, Muñoz A, et al. Aplidin induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C delta. Oncogene. 2002;21:7533–7544. doi: 10.1038/sj.onc.1205972. [DOI] [PubMed] [Google Scholar]
- Gartel AL. p21(WAF1/CIP1) and cancer: a shifting paradigm? Biofactors. 2009;35:161–164. doi: 10.1002/biof.26. [DOI] [PubMed] [Google Scholar]
- Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130:1268–1271. doi: 10.1001/archophthalmol.2012.1983. [DOI] [PubMed] [Google Scholar]
- Gu Z, Zhang F, Wang Z-Q, Ma W, Davis RE, Wang Z. The p44/wdr77-dependent cellular proliferation process during lung development is reactivated in lung cancer. Oncogene. 2012 doi: 10.1038/onc.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour JW. Photodynamic Therapy for Choroidal Metastasis From Carcinoid Tumor. Am. J. Ophthalmol. 2004:1143–1145. doi: 10.1016/j.ajo.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Hu H, Deng F, Liu Y, Chen M, Zhang X, Sun X, Dong Z, Liu X, Ge J. Characterization and retinal neuron differentiation of WERI-Rb1 cancer stem cells. Mol Vis. 2012;18:2388–97. Epub 2012 Sep 24. [PMC free article] [PubMed] [Google Scholar]
- Husain D, Kramer M, Kenny AG, Michaud N, Flotte TJ, Gragoudas ES, Miller JW. Effects of photodynamic therapy using verteporfin on experimental choroidal neovascularization and normal retina and choroid up to 7 weeks after treatment. IOVS. 1999 Sep;40(10):2322–31. PMID: 10476799. [PubMed] [Google Scholar]
- Isola V, MD, Pece A, MD, Pierro L., MD Photodynamic Therapy With Verteporfin of Choroidal Malignancy From Breast Cancer. 2006:1–3. doi: 10.1016/j.ajo.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Kessel D, Vicente MGH, Reiners JJ. Initiation of apoptosis and autophagy by photodynamic therapy. Lasers Surg Med. 2006;38:482–488. doi: 10.1002/lsm.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- Lee K-B, Byun H-J, Park SH, Park C-Y, Lee S-H, Rho SB. CYR61 controls p53 and NF-κB expression through PI3K/Akt/mTOR pathways in carboplatin-induced ovarian cancer cells. Cancer Lett. 2012;315:86–95. doi: 10.1016/j.canlet.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Liu AM, Wong K-F, Jiang X, Qiao Y, Luk JM. Regulators of mammalian Hippo pathway in cancer. Biochim. Biophys. Acta. 2012;1826:357–364. doi: 10.1016/j.bbcan.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee S-J, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr BP, Hung C, Gobin YP, Dunkel IJ, Brodie SE, Abramson DH. Success of intra-arterial chemotherapy (chemosurgery) for retinoblastoma: effect of orbitovascular anatomy. Arch Ophthalmol. 2012;130:180–185. doi: 10.1001/archophthalmol.2011.386. [DOI] [PubMed] [Google Scholar]
- Miller JW, Ursula S-E, Michael S, Constantin P, Horst L, Irene B, leonidas Z, Bertrand P, Guy D, Lane AM, et al. Photodynamic Therapy With Verteporfin for Choroidal Neovascularization Caused by Age-related Macular Degeneration. Arch Ophthalmol. 1999;117 doi: 10.1001/archopht.117.9.1161. [DOI] [PubMed] [Google Scholar]
- Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripursky M, Churgin D, Conway M, Peyman G. A Review of Photodynamic Therapy for Intraocular Tumors. J Anal Bioanal Techniques 01. 2011 [Google Scholar]
- Muen WJ, Kingston JE, Robertson F, Brew S, Sagoo MS, Reddy MA. Efficacy and complications of super-selective intra-ophthalmic artery melphalan for the treatment of refractory retinoblastoma. Ophthalmology. 2012;119:611–616. doi: 10.1016/j.ophtha.2011.08.045. [DOI] [PubMed] [Google Scholar]
- Munier FL, Gaillard M-C, Balmer A, Soliman S, Podilsky G, Moulin AP, Beck-Popovic M. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012;96:1078–1083. doi: 10.1136/bjophthalmol-2011-301450. [DOI] [PubMed] [Google Scholar]
- Nishimura SS, Sato TT, Ueda HH, Ueda KK. Acute myeloblastic leukemia as a second malignancy in a patient with hereditary retinoblastoma. J Clin Oncol. 2001;19:4182–4183. doi: 10.1200/JCO.2001.19.21.4182. [DOI] [PubMed] [Google Scholar]
- O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R, III, Le Beau MM, Earp HS, Liu ET. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Molec. Cell. Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal SK, Quinn DI. Differentiating mTOR inhibitors in renal cell carcinoma. Cancer Treat. Rev. 2013;39:709–719. doi: 10.1016/j.ctrv.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbati AV, Hong W. Emerging roles of TEAD transcription factors and its coactivators in cancers. Cancer Biol. Ther. 2013;14:390–398. doi: 10.4161/cbt.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue BW, OHara JA, Demidenko E, Wilmot CM, Goodwin IA, Chen Bin, Swartz HM, Hasan T. Photodynamic Therapy with Verteporfin in the Radiation-induced Fibrosarcoma-1 Tumor Causes Enhanced Radiation Sensitivity. Cancer Res. 2003:1–9. [PubMed] [Google Scholar]
- Prochownik EV. c-Myc as a therapeutic target in cancer. Expert Rev Anticancer Ther. 2004 doi: 10.1586/14737140.4.2.289. [DOI] [PubMed] [Google Scholar]
- Roarty JDJ, McLean IWI, Zimmerman LEL. Incidence of second neoplasms in patients with bilateral retinoblastoma. Ophthalmology. 1988;95:1583–1587. doi: 10.1016/s0161-6420(88)32971-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Galindo CC, Wilson MWM, Haik BGB, Lipson MJM, Cain AA, Merchant TET, Kaste SS, Pratt CBC. Treatment of metastatic retinoblastoma. Ophthalmology. 2003;110:1237–1240. doi: 10.1016/S0161-6420(03)00258-6. [DOI] [PubMed] [Google Scholar]
- Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE, Piccart-Gebhart MJ. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat. Rev. 2013;39:935–946. doi: 10.1016/j.ctrv.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Schefler ACA, Cicciarelli NN, Feuer WW, Toledano SS, Murray TGT. Macular retinoblastoma: evaluation of tumor control, local complications, and visual outcomes for eyes treated with chemotherapy and repetitive foveal laser ablation. CORD Conference Proceedings. 2007;114:162–169. doi: 10.1016/j.ophtha.2006.06.042. [DOI] [PubMed] [Google Scholar]
- Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F. Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis. 2007 Jun 8;13:823–32. [PMC free article] [PubMed] [Google Scholar]
- Sengupta TKT, Leclerc GMG, Hsieh-Kinser TTT, Leclerc GJG, Singh II, Barredo JCJ. Cytotoxic effect of 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) on childhood acute lymphoblastic leukemia (ALL) cells: implication for targeted therapy. Mol. Cancer. 2007;6:46–46. doi: 10.1186/1476-4598-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CL. Forget-Me-Nots in the Care of Children with Retinoblastoma. Semin Ophthalmol. 2009;23:324–334. doi: 10.1080/08820530802506029. [DOI] [PubMed] [Google Scholar]
- Shields CL, Palamar M, Sharma P, Ramasubramanian A, Leahey A, Meadows AT, Shields JA. Retinoblastoma Regression Patterns Following Chemoreduction and Adjuvant Therapy in 557 Tumors. Arch Ophthalmol. 2009;127:282–290. doi: 10.1001/archophthalmol.2008.626. [DOI] [PubMed] [Google Scholar]
- Shields CL, Shelil A, Cater J, Meadows AT, Shields JA. Development of new retinoblastomas after 6 cycles of chemoreduction for retinoblastoma in 162 eyes of 106 consecutive patients. Arch Ophthalmol. 2003;121:1571–1576. doi: 10.1001/archopht.121.11.1571. [DOI] [PubMed] [Google Scholar]
- Shields CLC, Honavar SGS, Shields JAJ, Demirci HH, Meadows ATA, Naduvilath TJT. Factors predictive of recurrence of retinal tumors, vitreous seeds, and subretinal seeds following chemoreduction for retinoblastoma. Arch Ophthalmol. 2002;120:460–464. [PubMed] [Google Scholar]
- Shimomura T, Miyamura N, Hata S, Miura R, Hirayama J, Nishina H. The PDZ-binding motif of Yes-associated protein is required for its co-activation of TEAD-mediated CTGF transcription and oncogenic cell transforming activity. Biochem Biophys Res Commun. 2014 Jan 17;443(3):917–923. doi: 10.1016/j.bbrc.2013.12.100. [DOI] [PubMed] [Google Scholar]
- Sussman DA, Escalona-Benz E, Benz MS, Hayden BC, Feuer W, Cicciarelli N, Toledano S, Markoe A, Murray TG. Comparison of retinoblastoma reduction for chemotherapy vs external beam radiotherapy. Arch Ophthalmol. 2003;121:979–984. doi: 10.1001/archopht.121.7.979. [DOI] [PubMed] [Google Scholar]
- Teng ACT, Kuraitis D, Deeke SA, Ahmadi A, Dugan SG, Cheng BLM, Crowson MG, Burgon PG, Suuronen EJ, Chen HH, et al. IRF2BP2 is a skeletal and cardiac muscle-enriched ischemia inducible activator of VEGFA expression. Faseb J. 2010;24:4825–4834. doi: 10.1096/fj.10-167049. [DOI] [PubMed] [Google Scholar]
- Theodoropoulou S, Brodowska K, Kayama M, Morizane Y, Miller JW, Gragoudas ES, Vavvas DG. Aminoimidazole Carboxamide Ribonucleotide (AICAR) Inhibits the Growth of Retinoblastoma In Vivo by Decreasing Angiogenesis and Inducing Apoptosis. PLoS ONE. 2013;8:e52852. doi: 10.1371/journal.pone.0052852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoropoulou S, Kolovou PE, Morizane Y, Kayama M, Nicolaou F, Miller JW, Evangeloss G, Ksander BR, Vavvas DG. Retinoblastoma cells are inhibited by aminoimidazole carboxamide ribonucleotide (AICAR) partially through activation of AMP-dependent kinase. Faseb J. 2010;24:2620–2630. doi: 10.1096/fj.09-152546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trosko JE. From adult stem cells to cancer stem cells: Oct-4 Gene, cell-cell communication, and hormones during tumor promotion. Ann. N. Y. Acad. Sci. 2006;1089:36–58. doi: 10.1196/annals.1386.018. [DOI] [PubMed] [Google Scholar]
- Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N, Fitamant J, Bardeesy N, Camargo FD, Guan K-L. YAP mediates crosstalk between the Hippo and PI(3)K–TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14:1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev A. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MZ, Chan SW, Liu AM, Wong KF, Fan ST, Chen J, Poon RT, Zender L, Lowe SW, Hong W, et al. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30:1229–1240. doi: 10.1038/onc.2010.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.