Summary
The collapse of neural networks important for memory and cognition, including death of neurons and degeneration of synapses, causes the debilitating dementia associated with Alzheimer’s disease (AD). We suggest that synaptic changes are central to the disease process. Amyloid beta and tau form fibrillar lesions that are the classical hallmarks of AD. Recent data indicate that both molecules may have normal roles at the synapse, and that the accumulation of soluble toxic forms of the proteins at the synapse may be on the critical path to neurodegeneration. Further, the march of neurofibrillary tangles through brain circuits appears to take advantage of recently described mechanisms of trans-synaptic spread of pathological forms of tau. These two key phenomena, synapse loss and the spread of pathology through the brain via synapses, make it critical to understand the physiological and pathological roles of amyloid beta and tau at the synapse.
Brains of AD patients are characterized by accumulation of amyloid beta (Aβ) into senile plaques and hyperphosphorylated tau into neurofibrillary tangles (Figure 1). Although these defining lesions were first described over a century ago by Alois Alzheimer (Alzheimer, 1907), their link to brain degeneration has remained elusive. Genetic evidence from rare familial forms of AD strongly support accumulation of Aβ as causative to the disease process. Mutations in the amyloid precursor protein (APP) and in presenilins 1 and 2, which are essential in generating Aβ, cause familial, early onset AD (Tanzi, 2012). However, there are challenges to the amyloid hypothesis suggesting that Aβ may not play a central role in the degenerative process after disease initiation. The accumulation of plaques in the brain does not correlate with cognitive impairments in patients (Giannakopoulos et al., 2003; Ingelsson et al., 2004), a large number of people without any cognitive impairment have substantial accumulations of plaques in their brains (Perez-Nievas et al., 2013), and the reduction of plaque load in the brain by immunotherapy does not result in cognitive improvement in AD patients (Holmes et al., 2008). Tangles, on the other hand, do correlate strongly with cognitive decline and with neuronal and synapse loss (Arriagada et al., 1992; Duyckaerts et al., 1998; Giannakopoulos et al., 2003; Ingelsson et al., 2004); however mutations in tau cause frontotemporal dementia, not AD (Goedert and Jakes, 2005). Of the neuropathological features of the disease, synapse loss correlates most strongly with dementia, implicating it as important to the disease process (Koffie et al., 2011). As well as frank synapse loss, it is becoming clear from animal models that dysfunction of synapses and impaired synaptic plasticity are also key components of the neurodegenerative process in AD, and that both Aβ and tau contribute to this degeneration (Crimins et al., 2013). Here we will discuss recent hypotheses about how synaptic structure and function are disrupted by Aβ and tau in the AD brain, contributing to cognitive impairment. Further, we will discuss the important role of synapses in the spread of pathology through the brain.
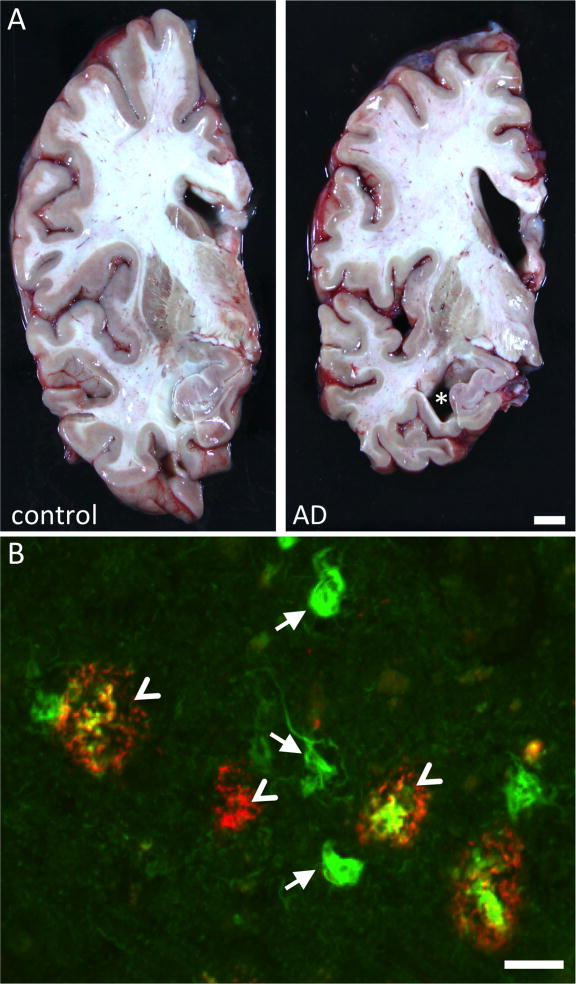
Figure 1. Neuropathology of AD.
AD brains are characterized by striking atrophy compared to control brains (A). Particularly evident is shrinkage of the cortical mantle and the hippocampus (asterisk shows hippocampal atrophy). Microscopically, AD is defined by deposition of Aβ in senile plaques (arrowheads) and tau in neurofibrillary tangles (arrows). In this micrograph, the fibrillar deposits (both plaques and tangles) are stained green with thioflavine S. Aβ is also immunostained with antibody AW7 (courtesy Dominic Walsh), illustrating the halo of soluble Aβ around fibrillar plaque cores and the heterogeneous nature of plaques. Scale bars represent 1 cm (A) and 20 μm (B).
Function of healthy synapses
In the healthy adult brain, synaptic plasticity is thought to be what allows learning and the formation of memories. The most striking symptom of AD is memory loss, so it is not surprising that the areas of the brain essential for memory, and the synaptic plasticity that forms the neurochemical and structural basis of memory degenerate. In particular, the hippocampus and neocortex are important for learning and memory (Dudai and Morris, 2013), and the circuitry connecting them is particularly impacted by AD pathology (Figure 2). During the course of AD, synaptic plasticity is altered, and many of the mechanisms involved in normal plasticity become dysregulated, leading to synapse dysfunction and collapse.
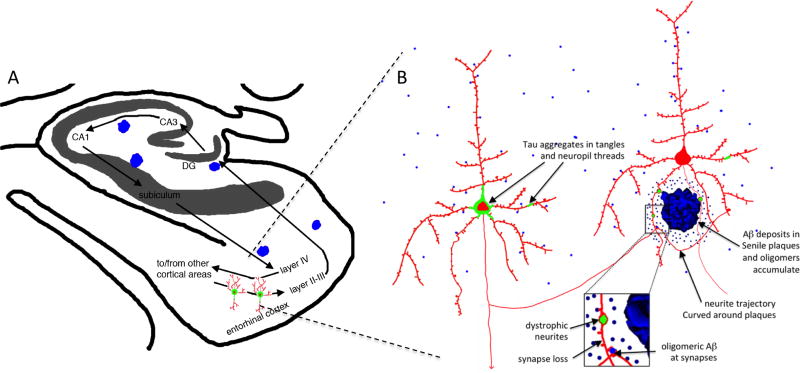
Figure 2. Structural changes in AD brain.
The neural circuitry involved in memory including the entorhinal cortex-hippocampal circuitry (A) are severely affected by AD pathology including the deposition of plaques (blue) and tangles (green) and dramatic neuronal and synapse loss. Along with the dramatic neuronal loss, there are structural changes to remaining neurons in the AD brain that are thought to contribute to neural circuit disruption and cognitive impairments (B), including damage to neurites in the halo of soluble amyloid beta surrounding plaques, tau aggregation in cell bodies and neurites, and synapse loss associated with oligomeric Aβ around plaques. Panel A modified from (Gomez-Isla et al., 2008).
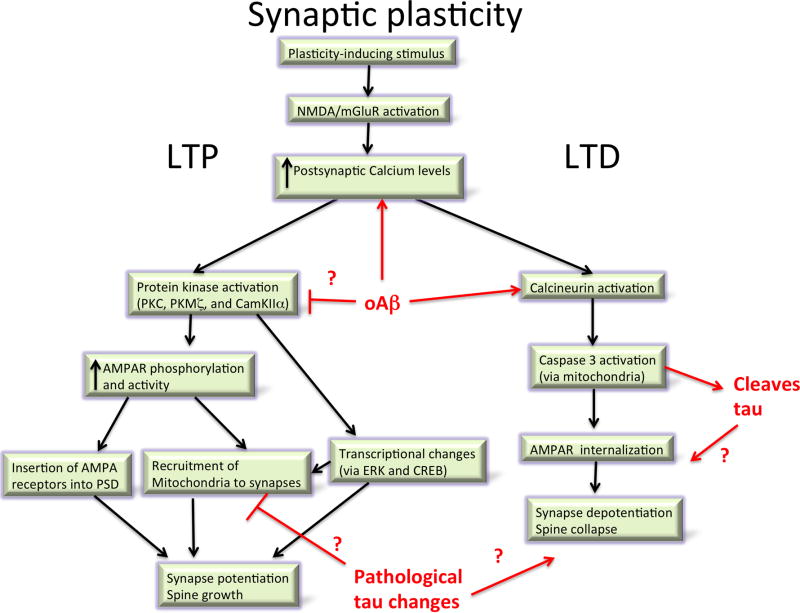
The concept of synaptic plasticity and its role in learning was put forward by Ramon y Cajal, who noted that the number of neurons in the brain did not appear to change significantly over our lifespan, making it unlikely that new memories were the result of new neurons being born and integrated into the brain. Instead, he proposed that changes in the strength of connections between existing neurons could be the mechanism for memory formation (Cajal, 1894; Jones, 1994). In 1949, Hebb expanded upon this idea when he postulated that the connection between two neurons would be strengthened if they activate simultaneously and weakened if they activate separately (Hebb, 1949). The description of long term potentiation (LTP) and its counterpart, long-term depression (LTD) from studies of animal brain slices, provide molecular understanding of the phenomenon of synapse strengthening or weakening. LTP is a specific, long-lasting increase in the strength of synaptic transmission when the pre and postsynaptic neurons are activated simultaneously, which was first described in rabbit hippocampus (Bliss and Gardner-Medwin, 1973). The mechanisms of LTP can be pre or post-synaptic postsynaptic, but postsynaptic mechanisms seem most affected in AD models. There are early and late phases of LTP, with the early phase dependent upon protein kinase activation causing several changes to synaptic AMPA receptors including phosphorylation, enhanced activity, and insertion of new receptors into the postsynaptic density. During late-phase LTP, increased levels of calcium at the postsynaptic site and persistent activation of kinases (importantly PKC, PKMζ, and CamKIIα which converge on ERK), lead to activation of transcription factors including CREB. This in turn causes production of proteins (including locally translated proteins in “tagged” synapses) which are involved in new dendritic spine formation (Bliss et al., 2003; Frey and Morris, 1997; Redondo and Morris, 2011; Sanhueza and Lisman, 2013).
LTD is a weakening of synaptic strength following a stimulus. LTD can occur via several mechanisms, which unsurprisingly have effects opposite to those seen in LTP, including internalization of AMPA receptors (Collingridge et al., 2010; Dudek and Bear, 1992; Massey and Bashir, 2007). NMDAR dependent LTD, which appears to be most affected in AD, depends on calcium influx, calcineurin activation, and non-apoptotic caspase activation (Li et al., 2010; Mulkey et al., 1993). LTD is thought to be important for clearing old memory traces and in situations requiring behavioural flexibility (Collingridge et al., 2010). Interestingly, this forgetting aspect of LTD may be hijacked during AD as very similar molecular mechanisms are involved in LTD and synapse degeneration during AD, in particular the central role of calcineurin activation.
Along with potentiation and depotentiation of synaptic strength, structural changes occur in response to brain plasticity. LTP has been associated with the formation of new dendritic spines, increases in perforated postsynaptic densities (receiving more than one presynaptic input) and with the enlargement of spine heads (Bosch and Hayashi, 2012; Maletic-Savatic et al., 1999; Nägerl et al., 2004; Van Harreveld and Fifkova, 1975). Conversely, LTD has been associated with spine shrinkage and loss (Bastrikova et al., 2008; Matsuzaki et al., 2004; Nägerl et al., 2004; Zhou et al., 2004) with recent fascinating data indicating that this process may involve non-apoptotic caspase 3 activation (D’Amelio et al., 2012; Li et al., 2010). Under conditions of environmental enrichment, substantial numbers of new dendritic spines (and corresponding excitatory synapses) and new dendritic branches form on pyramidal neurons (Mora et al., 2007; Nithianantharajah and Hannan, 2006).
During the course of Alzheimer’s disease, the normal function of synapses is impaired, synapses are eliminated, and pathological proteins are transported through synapses. Before exploring these phenomena, we will present background on the neuropathology of AD then follow with how pathological lesions affect synapses.
Alzheimer’s disease pathology – plaques and Aβ
Structural changes in the AD brain have been classified as “positive” lesions, i.e. the accumulation of plaques, tangles, neuropil threads, dystrophic neurites, cerebral amyloid angiopathy (CAA) and other lesions that are deposited in AD patients’ brains, and “negative” lesions, comprising the massive atrophy due to neuron loss and the degeneration of neurites and synapses (Serrano-Pozo et al., 2011a). Each of these lesions is present in a characteristic pattern in AD, which provides some clues about the relationship between the lesions and disease progression and symptoms. There are also structural changes in the neuropil associated with plaques and tangles, which are thought to contribute to cognitive impairments (Figure 2).
Senile plaques, first described by Alzheimer using Bielchowsky silver staining on brain sections from a patient with dementia, were determined in the early 1980s to be largely composed of the amyloid beta peptide (Glenner and Wong, 1984; Masters and Selkoe, 2012). Neuritic, or dense-cored, plaques have a dense center of amyloid surrounded by a halo of silver positive neurites. After the sequencing of the peptide and development of antibodies to Aβ, it was found that Aβ also aggregates in “diffuse” plaques of several different morphologies (Dickson and Vickers, 2001; Gomez-Isla et al., 2008; Serrano-Pozo et al., 2011a).
From cross-sectional studies of postmortem human brain, it appears that senile plaque deposition occurs early in the disease process and proceeds slowly, beginning in the neocortex and progressing through the allocortex, then to the diencephalon, striatum, and basal forebrain cholinergic nuclei, followed by progression to brainstem nuclei and finally to the cerebellum (Thal et al., 2002). Watching plaques appear in real time in the brains of mice that overexpress AD associated APP and PS1 mutations with in vivo multiphoton imaging surprisingly reveals that individual plaques coalesce from soluble Aβ remarkably rapidly. Plaques form within 24 hours the effects on surrounding neurites occur within days after plaque formation (Meyer-Luehmann et al., 2008).
Dense plaques are toxic to the surrounding brain parenchyma, causing a number of phenomena that may contribute to synapse dysfunction and loss. Many neurites surrounding plaques exhibit swollen, dystrophic morphologies and often contain aggregates of phospho-tau and multiple cellular components that likely accumulate due to disrupted cellular transport (Serrano-Pozo et al., 2011a; Woodhouse et al., 2005). The trajectories of axons and dendrites, which are usually fairly straight, are disrupted in the vicinity of amyloid plaques in mouse models of AD, which may impact synaptic integration of signal (Le et al., 2001; Spires et al., 2005; Stern et al., 2004; Urbanc et al., 2002). There is also substantial gliosis and related oxidative stress around plaques, which are likely to contribute to synaptic changes (Ingelsson et al., 2004; McLellan et al., 2003; Serrano-Pozo et al., 2011b).
Alzheimer’s disease pathology – tau
While plaques are associated with disrupted neurite morphology, gliosis, and oxidative stress, less is known about the impact of NFT on the surrounding neuropil. Neurofibrillary tangles and neuropil threads are formed of aggregated tau protein. Tau is a microtubule binding protein found largely in axons where it serves to stabilize microtubules (Goedert and Spillantini, 2006). During the course of Alzheimer’s disease, tau is hyperphosphorylated, becomes detached from the microtubules, and accumulates in the somatodendritic compartment in paired helical filaments and straight filaments (Kidd, 1963; Spillantini and Goedert, 2013; Stoothoff and Johnson, 2005). The deposition of tangles occurs in a hierarchical fashion beginning in the entorhinal cortex and progressing through the hippocampal formation, association cortices, and only affecting primary sensory areas in late stages of the disease (Arnold et al., 1991; Braak and Braak, 1991).
NFT deposition in human AD correlates with cognitive decline and neuronal loss (Arriagada et al., 1992; Duyckaerts et al., 1998; Giannakopoulos et al., 2003; Gomez-Isla et al., 1997). The association of NFT with neuronal loss and the presence of ghost tangles – NFT that remain in the brain after the neuron has died – strongly suggest that at least some neurons with tangles die during the course of the disease; however the amount of neuronal loss vastly exceeds the number of neurofibrillary tangles and ghost tangles within given brain regions, supporting the idea that a tangle is not necessary for neuron death in AD (Gomez-Isla et al., 1997). As intracellular lesions, NFT could be expected to have less impact on the surrounding environment than the extracellular accumulation of plaques, however, we recently observed gliosis in the vicinity of NFTs that correlates with disease progression (Serrano-Pozo et al., 2011b).
Synaptic dysfunction, synapse loss and relationships to pathology
Synapse loss in AD was described in the early 1990s by DeKosky and Scheff using electron microscopy and by Terry and Masliah using densitometry of immunostained synaptic proteins. These groups observed synapse loss in frontal cortex, temporal cortex, and dentate gyrus of the hippocampus, and found that synapse loss is the strongest pathological correlate of dementia (DeKosky and Scheff, 1990; DeKosky et al., 1996; Masliah et al., 1994; Terry et al., 1991). Interestingly, the entorhinal cortex, one of the earliest and most severely affected areas of the brain in terms of neuronal loss and tangle formation, does not appear to undergo loss of synapse density in the remaining neuropil (Scheff et al., 1993), despite a significant loss of synapses in the target zone of the EC in the dentate gyrus.
The association of amyloid pathology with local synapse loss was largely pioneered in animal and cell culture models. Due to the obvious neuropil disruption surrounding dense core plaques, fibrillar Aβ was long assumed to be toxic, however studies over the past decade strongly implicate soluble forms of Aβ which accumulate around dense plaques as more toxic than fibrils. Elegant experiments by several groups over the late 1990s and 2000s demonstrated that soluble forms of Aβ cause loss of dendritic spines in cultured neurons, while fibrils and monomers are comparatively inert (Klein, 2006; Lambert et al., 1998). A series of studies demonstrated that oligomeric forms of Aβ produced by cultured cells or extracted from human AD brain are toxic to synaptic function, including disrupting LTP in brain slices and impairing cognition when injected into healthy rodents in vivo (Cleary et al., 2005; Shankar et al., 2007; Shankar et al., 2008; Walsh et al., 2002; Walsh et al., 2005). There is also an association of dimers of Aβ with dementia in human brain (Mc Donald et al., 2010). In vivo imaging studies in plaque-bearing mice revealed a loss of dendritic spines around plaques due to altered structural plasticity (Figure 3)(Rozkalne et al., 2011; Spires et al., 2005; Spires-Jones et al., 2007). Removing soluble Aβ with topical application of an antibody results in increased formation of dendritic spines in vivo and long-lasting increases in synaptic markers (Rozkalne et al., 2009; Spires-Jones et al., 2009), supporting the idea that soluble forms of Aβ are toxic to synapses.
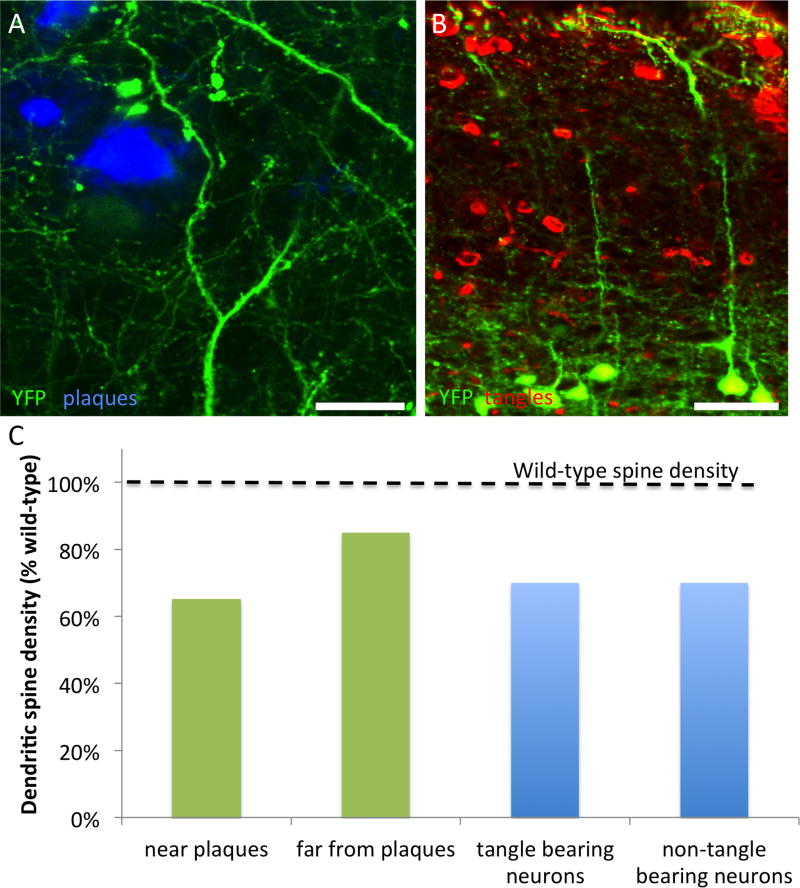
Figure 3. Dendritic spine loss in AD mouse models.
Mouse models that exhibit plaque formation or tangle formation exhibit dendritic spine loss. Crossing APP/PS1 mice (A) and rTg4510 mice (B) with YFP overexpressing lines allowed quantification of dendritic spine density on cortical pyramidal neurons (layer II/III). Dense plaques are stained with thioflavine S in (A) and neurofibrillary tangles are stained with PHF1 antibody in B, while neurons in both panels are filled with YFP due to transgenic overexpression. Similar results are found when fluorescent markers are introduced via viral infection of neurons or direct injection of fluorophores. In plaque bearing mice, dendritic spine loss is most pronounced within 50 μm of plaques, whereas in tangle bearing mice, the presence of a tangle does not affect dendritic spine density (C). Data in C adapted from (Kopeikina et al., 2012b; Kopeikina et al., 2013; Rocher et al., 2010; Rozkalne et al., 2011; Spires et al., 2005). Scale bars represent 20 μm (A) and 50 μm (B).
Despite all of the indirect evidence that oligomeric Aβ contributes to synapse dysfunction and loss, technical limitations had prevented the determination of whether oligomers of Aβ are actually physically present at synapses in the brain. This is due to the limit of the axial (z-direction) resolution of light microscopy being larger than the size of an individual synapse, which precludes accurate co-localization studies using immunofluorescence, and the difficulty of finding antibodies that work on glutaraldehyde fixed tissue for electron microscopy. Micheva and Smith developed an imaging technique that combined ultrathin sectioning of tissue into ribbons of 50–100nm serial sections with immunofluorescence techniques to allow reconstruction of three dimensional volumes of protein localization at sub-synaptic resolution (Micheva et al., 2010; Micheva and Smith, 2007). We applied this array tomography technique to plaque-bearing AD mouse brains and confirmed the presence of oligomericAβ in a subset of postsynaptic densities, particularly near plaques (Koffie et al., 2009), using Lee’s antibody that preferentially recognizes oligomeric (not monomeric) forms of Aβ (Lee et al., 2006). In these mice, the accumulation of oligomeric Aβ around plaques negatively correlated with the linear synapse loss approaching the plaque edge, and synapses containing Aβ were significantly smaller than neighboring postsynaptic densities, supporting the idea that oligomeric Aβ contributes to synapse shrinkage and collapse (Koffie et al., 2009). We then extended this technique to human autopsy tissue (Kay et al., 2013), and examined whether Aβ was present at synapses around plaques in postmortem AD brain tissue (Figure 4). We confirmed using array tomography that oligomeric Aβ is present in both pre and postsynaptic puncta, and furthermore we found an association of increased Aβ at synapses that also contain apolipoprotein Eε4 (apoE4) (Koffie et al., 2012). This is important because the APOE4 gene increases the risk for developing sporadic AD, but the mechanisms leading from APOE4 to AD are not fully understood (Corder et al., 1993; Strittmatter and Roses, 1996). Our data suggest that apoE4 contributes to AD risk at least in part by increasing the localization of toxic oligomeric Aβ to synapses.
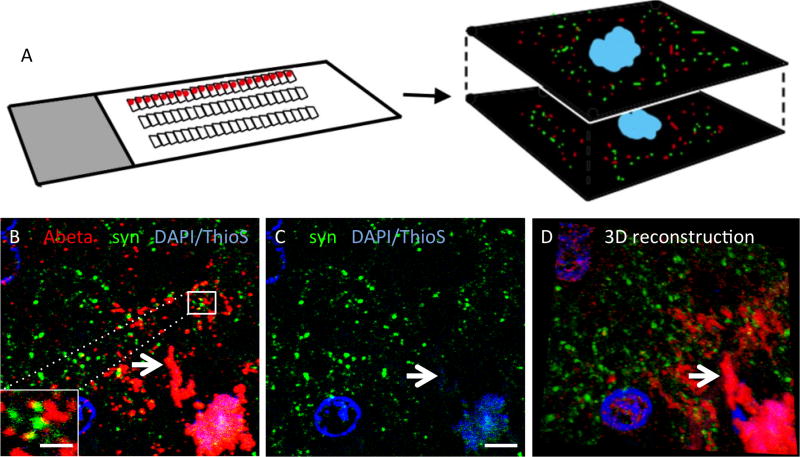
Figure 4.
Array tomography reveals colocalization of oligomeric Aβ with synapses in human brain. The array tomography technique overcomes the axial resolution of light microscopy by physically sectioning resin embedded brain tissue into ribbons of ultrathin (70nm) serial sections which are stained with immunofluorescence, imaged with a fluorescent microscope at the same place along the ribbon (red dots) and a three dimensional dataset acquired of multiple markers at synapses (A, D). Using human AD brain tissue (B–C), we observed oligomeric Aβ stained with NAB61 (red) present at a subset of synapses as can be seen in the inset in panel B (presynaptic terminals stained here with synapsin I, green). We also observe a reduction in synapse density in the halo of oligomeric Aβ surrounding the Thioflavin S (ThioS) positive dense cores of plaques (arrows). Scale bars represent 5 μm (B, C) and 1 μm (inset in B). Panel D is a reconstruction of a 36 μm × 33 μm × 1.2 μm volume (images from 17 serial sections). Panel A adapted from Micheva and Smith 2007.
Another possibility that could explain the lack of correlation of amyloid pathology with cognitive decline is that the Aβ induced synaptic changes could be important contributors early in the disease process but at later stages, tau pathology contributes more to synapse degeneration, and resultant dementia (Hyman, 2011). Tau was historically thought to reside only in axons, but recent data from several groups suggests an important role for tau in maintaining the protein composition of the postsynaptic density (PSD). Tau was observed to be important for targeting fyn kinase to the PSD (Ittner et al., 2010) and P301L mutant tau accumulation in dendritic spines in cultured neurons was observed in conjunction with disrupted synaptic transmission and altered neurotransmitter receptor composition of the PSD (Hoover et al., 2010). Overexpression of P301L tau in rTg4510 mice leads to alterations in synaptic function and loss of synapses (Figure 3)(Crimins et al., 2013; Crimins et al., 2012; Crimins et al., 2011; Kopeikina et al., 2012b; Kopeikina et al., 2013; Rocher et al., 2010). We also observed tau present in dendritic spines in the brains of rTg4510 mice using array tomography (Kopeikina et al., 2012b). While many of these studies finding tau in the PSD have been in model systems with artificially high levels of tau overexpression, other studies have found endogenous tau present in dendrites undergoing Aβ induced spine loss in cultured neurons (Zempel et al., 2010). We also observed tau in postsynaptic terminals of non-demented human control as well as AD cases strengthening the case that tau is present in the postsynaptic compartment (Tai et al., 2012).
In mouse models of tauopathy and human AD brain, tangle-bearing neurons are observed to receive fewer synapses onto their somata and to express less synaptic proteins than non tangle-bearing neurons (Callahan et al., 1999; Ginsberg et al., 2000; Katsuse et al., 2006). We also recently observed the accumulation of oligomeric forms of tau in synapses in AD brain (Tai et al., 2012), and in a recent study of AD cases versus high-pathology controls, Perez-Nievas and colleagues observed that while the total number of NFT was not associated with dementia compared to high-pathology controls, increased levels of phospho-tau specifically in the synaptic compartment were associated with dementia (Perez-Nievas et al., 2013).
Role of tau and Aβ in normal synaptic biology
Interestingly, it increasingly appears that tau, Aβ, and proteins involved in Aβ generation may play a role in healthy synaptic physiology.
Aβ has been implicated in developmental synaptic plasticity both in visual deprivation paradigms and in the development of the olfactory bulb (Cao et al., 2012; Kim et al., 2013). Moreover, in non-demented human subjects, oligomeric Aβ at a subset of synapses is associated with smaller synapse volume (Koffie et al., 2012), indicating that Aβ may play a role in synaptic plasticity. The machinery for generating Aβ is also present in the synaptic compartment (APP, beta and gamma secretases), providing support for the notion that Aβ (or APP) may have a normal role at the synapse. Generation of Aβ is enhanced by neuronal activity in vitro and in vivo (Cirrito et al., 2005; Kamenetz et al., 2003; Li et al., 2013; Sheng et al., 2012) and although the functional relevance of this activity dependent regulation remains unclear, it is possible that it plays a role in normal synaptic function.
Tau likely plays an important role in synapse function due to its regulation of microtubule stability and thus axonal transport. Interestingly, phosphorylation of tau increases during hibernation of ground squirrels and is associated with a transient, reversible loss of synaptic protein markers, indicating a physiological role for tau phosphorylation in synapse biology in these animals (Arendt et al., 2003). Beyond this indirect regulation of synaptic function, tau may also play a more direct role at the PSD in regulating NMDAR function via an interaction with fyn kinase (Ittner et al., 2010; Mondragón-Rodríguez et al., 2012). It is not yet clear whether this is a physiologic role of tau or a toxic role.
Mechanisms of synaptic dysfunction and loss
Although it is now well established that oligomeric Aβ is toxic to synapses, the exact species and the identity of the receptor(s) at synapses that are responsible for the toxic effects of Aβ are hotly debated. Oligomeric Aβ is a “sticky” molecule and multiple binding partners have been elucidated at the synapse, but which of these interactions is most important in toxicity remains to be determined (Benilova and De Strooper, 2013). Direct binding of Aβ to NMDA receptor subunits has been reported in many studies (De Felice et al., 2007; Lacor et al., 2004; Lacor et al., 2007; Rönicke et al., 2011). mGluR5 has also been proposed as an important binding partner of Aβ. In cultured neurons, Renner et al observed that quantum dot-tagged Aβ oligomers clustered at active excitatory synapses and sequestered mGluR5 into the clusters, preventing mGluR5 diffusion causing local hyperexcitability and increased calcium concentrations (Renner et al., 2010). Aβ can bind to α7-nicotinic acetylcholine receptors (Wang et al., 2000), and Greengard’s group found that these receptors are necessary for Aβ induced NMDA receptor internalization in cultured neurons (Snyder et al., 2005).
Receptor tyrosine kinases including the EphB2 receptor have also been implicated as Aβ receptors. EphB2, which regulates NMDA receptors, is depleted in the brains of plaque-bearing transgenic mice (Tg2576 line) and in human AD brain (Simón et al., 2009). Cissé et al found that oligomeric Aβ binds EphB2 leading to its degradation by the proteasome. Further, they observed that increasing EphB2 expression in a mouse model of plaque deposition (hAPP line) reversed memory deficits and reversed impairments of LTP (Cissé et al., 2011), arguing that this could be an important synaptic binding partner of oAβ.
The cellular form of the prion protein (PrPC) has also been proposed as the critical binding partner of Aβ that initiates synapse dysfunction and loss. In 2009, Laurén et al reported that binding of oligomeric Aβ with PrPC is required for the impairment of LTP (Laurén et al., 2009), a finding that became controversial when several groups failed to replicate it (Balducci et al., 2010; Benilova and De Strooper, 2010; Calella et al., 2010). More recent studies have however confirmed that antibodies targeting a specific domain of PrPC (amino acids 94–104) can prevent the oligomeric Aβ–induced inhibition of LTP (Barry et al., 2011; Freir et al., 2011), and Larson et al observed that soluble Aβ binding to PrPC in dendritic spines forms a complex with Fyn causing Fyn activation and tau phosphorylation (Larson et al., 2012). The interaction of Aβ and PrPC appears to depend on mGluR5 (Um et al., 2013). These last two studies are intriguing as they link Aβ to tau pathology via Fyn, which has been seen to be an important interacting partner of tau at the PSD; however the molecules that link PrP to Fyn remain unknown (Chen et al., 2013).
Another binding partner of Aβ surprisingly appears to be a major histocompatibility complex class 1 (MHC1) receptor. MHC1 normally functions in the immune system, but the receptors are also expressed in the brain where they contribute to synaptic plasticity (Shatz, 2009). The mouse MHC1 receptor paired immunoglobulin-like receptor B (PirB) and its human ortholog, leukocyte immunoglobulin-like receptor LilrB2 have been implicated by Shatz and colleagues as binding partners for oligomeric Aβ, (Kim et al., 2013). Further, slices from PirB knockout mice do not show impairment of LTP with application of oAβ, and crossing APP/PS1 mice with PirB knockout mice prevents cognitive impairment and deficits in ocular dominance plasticity (Kim et al., 2013).
The molecular mechanisms leading to synapse dysfunction and loss downstream of Aβ and tau have not been fully elucidated but some candidate pathways have become very clear from multiple studies (reviewed in (Dinamarca et al., 2012). In the case of Aβ, increased Ca2+ levels in dendrites and dendritic spines appear central to synapse dysfunction and loss. Application of oligomeric Aβ to cultured neurons causes increased Ca2+ concentrations associated with dendritic spine loss (Demuro et al., 2005; Hudry et al., 2012; Mattson et al., 1992; Wu et al., 2010; Zempel et al., 2010). Similar increases in calcium concentration have been observed in dendrites around plaques in vivo in AD transgenic models, which is associated with a loss of spinodendritic calcium compartmentalization (Kuchibhotla et al., 2008). This is the same region in which our postmortem observations reveal high levels of oligomeric Aβ (Koffie et al., 2009). Downstream of calcium activation, it appears that calcineurin is an important mediator of synaptic degeneration. Expressing constitutively active calcineurin both in vitro and in vivo recapitulates the morphological phenotypes associates with Aβ: dendritic spine loss, neurite curvature, and neurite dystrophies; and inhibiting calcineurin prevents these phenotypes in vitro and in vivo, providing a strong argument that calcineurin activation is both necessary and sufficient to cause synapse loss and neurite degeneration (Cavallucci et al., 2013; Rozkalne et al., 2011; Wu et al., 2010). This Aβ induced calcineurin activation begins in dendritic spines and propagates into dendrites and soma over time (Wu et al., 2012).
The changes in calcium concentrations and activation of calcineurin induced by Aβ interfere with normal synaptic plasticity (Figure 5). LTP and LTD depend on calcium influx through NMDA receptors or mGluRs, with rapid, high levels of calcium influx causing LTP and lower levels of calcium influx associated with LTD (Kullmann and Lamsa, 2007). Oligomeric Aβ robustly impairs LTP in slices and in vivo (Lambert et al., 1998; Shankar et al., 2008; Walsh et al., 2002). LTD is associated with shrinkage and loss of dendritic spines, and recent data suggest that the mechanisms of normal LTD are induced by oligomeric Aβ, indicating that this normal synaptic LTD “forgetting” machinery may be hijacked during AD causing synapse loss and memory problems. Application of oligomeric Aβ enhances LTD (Christie et al., 2001; Li et al., 2009; Shankar et al., 2008). In addition, Aβ causes the internalization of AMPA and NMDA neurotransmitter receptors through the same calcineurin-mediated pathways involved in LTD (Hsieh et al., 2006; Koffie et al., 2011; Snyder et al., 2005; Wang et al., 2004). Non-apoptotic caspase activation also plays a role in the AMPA and NMDAR internalization observed during LTD (Chen et al., 2013; Li et al., 2010), and Aβ induces caspase 3 activation, which appears to be involved in the observed enhancement of LTD and internalization of synaptic receptors (Chen et al., 2013; D’Amelio et al., 2011; Liu et al., 2010). As well as influencing internalization of synaptic receptors, altered calcium dynamics act to destabilize the cytoskeleton in dendritic spines allowing spine collapse. Some evidence of this has been found in cultured neurons where the scaffolding protein ranBP9 potentiates Aβ–induced mitochondrial dysfunction and calcium dysregulation (Roh et al., 2013).
Figure 5. Pathways involved in normal synaptic plasticity and how they may be affected in AD.
Under normal conditions, LTP promotes recruitment of neurotransmitter receptors to active synapses and causes synapse potentiation, stabilization, and growth. LTD conversely results in synapse depotentiation and spine collapse. Both of these processes are affected in animal models of AD with oligomeric Aβ clearly affecting the calcium and calcineurin pathways involved in these phenomena. Tau overexpression has been observed to affect synaptic function in transgenic models and to be necessary for oligomeric Aβ mediated synapse dysfunction, but the mechanisms by which pathological forms of tau affect synaptic plasticity are less well understood. It is possible that hyperphosphorylation affects microtubule stability and the transport of mitochondria to synapses which could affect synaptic function. The cleavage of tau by caspase 3 has also been observed which could be tied to the non-apoptotic role of caspase 3 in LTD and spine collapse.
Developmental synaptic pruning mechanisms, which allow for synapse elimination during postnatal refinement of neural circuits, may also be re-activated and contribute to synapse loss in Alzheimer’s disease. Microglia are involved in synaptic refinement where they appear to engulf and remove dendritic spines “marked” for removal by members of the classical complement cascade complement receptor 3 and C1q (Chu et al., 2010; Clarke and Barres, 2013; Harry, 2013; Paolicelli et al., 2011; Stephan et al., 2013; Tremblay et al., 2010). In AD, gliosis is a prominent feature around plaques including recruitment of activated microglia, which may contribute to local synapse loss around plaques.
The mechanisms of synapse degeneration associated with pathological changes in tau are less well established. The predominating view is that pathological changes in tau cause disruptions in microtubule-based cellular transport, since tau is a microtubule stabilizing protein (Kopeikina et al., 2012a). Disrupting cellular transport prevents the trafficking of essential cargoes to synapses including mitochondria and synaptic receptors. Tau overexpression in cultured neurons inhibits anterograde axonal transport, particularly of mitochondria, by interfering with kinesin molecular motors (Ebneth et al., 1998; Kanaan et al., 2011; LaPointe et al., 2009; Stoothoff et al., 2009a). Impairment of mitochondrial transport to pre and postsynaptic terminals is thought to cause synapse loss and eventual dying-back of axons due to the essential roles of mitochondria in ATP production and calcium buffering (Kopeikina et al., 2012a; Sheng and Cai, 2012). Accumulation of pathologically phosphorylated and misfolded tau may impair transport by directly competing with cargo or by impairing signaling cascades involved in kinesin based transport including JNK3 and GSK3 regulation (Dubey et al., 2008; Ittner et al., 2009; Morfini et al., 2009).
Impaired microtubule-based transport may also contribute to the hyperexcitability of neurons in transgenic mice expressing FTD-associated P301L mutant tau (rTg4510 line), which could be caused by impairments in trafficking of dendritically targeted ion channels (Crimins et al., 2013; Crimins et al., 2012; Rocher et al., 2010). Recent work by Hoover et al also demonstrated that abnormally phosphorylated tau impairs glutamate receptor subunit GluA1, GluA2/3 and NR1 trafficking to the postsynaptic density (Hoover et al., 2010), in support of the idea that pathological tau confers synaptic toxicity by impairing cellular transport. Presynaptically, pathological tau may also impair synaptic function. In squid axons that received microinjections of human tau, synaptic transmission was blocked (Moreno et al., 2011).
In addition to the impaired transport of mitochondria due to pathological changes in tau, mitochondria appear to be key players in the molecular cascades leading to synapse loss in AD on several fronts (Eckert et al., 2010). Mitochondrial dynamics, that is the fission and fusion of mitochondria to regulate the mitochondrial network in different subcellular compartments, are altered in AD by both and Aβ and tau (Quintanilla et al., 2012; Wang et al., 2009). Similarly, Aβ and tau both cause mitochondrial dysfuction. Tau specifically impairs mitochondrial complex I function and Aβ impairs complex IV function (Eckert et al., 2013), and mitochondrial function, particularly at the synapse is crucial for synaptic function. It further appears that mitochondria are central to the non-apoptotic caspase activation that is associated with synapse loss in AD models. The caspase activation associated with LTD is via the intrinsic mitochondrial apoptotic cascade pathway (Li et al., 2010), and caspase upregulation is associated with synapse loss in AD models (Hoover et al., 2010). Mitochondria may also play a role in spine collapse through cofilin, a filamentous-actin cleaving protein implicated in spine remodeling. Translocation of cofilin to mitochondria is an early step in apoptosis, which causes cytochrome c release and mitochondrial swelling (Chua et al., 2003; Klamt et al., 2009). Translocation of cofilin to mitochondria is also associated with Aβ mediated mitochondrial dysfunction and calcium dysregulation (Roh et al., 2013), likely due to cofilin activation through NMDA-induced activation of calcineurin (Pontrello et al., 2012). Finally, mitochondria are essential for calcium homeostasis in synapses and preventing transport of mitochondria to synapses undoubtedly impairs synaptic calcium buffering (Eckert et al., 2013).
The intersection of Aβ and tau at the synapse
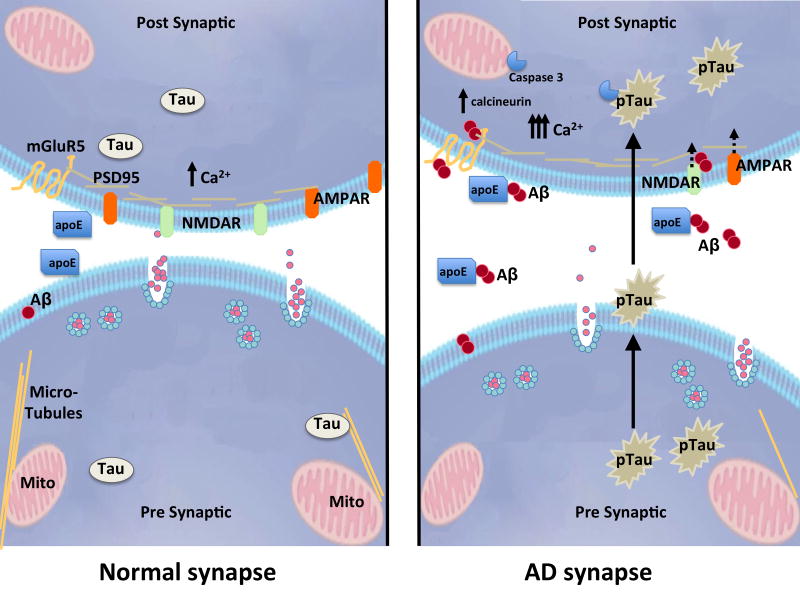
Several recent studies suggest that these two pathological proteins act in concert in synapse degeneration (Figure 6, reviewed recently by(Crimins et al., 2013; Ittner and Gotz, 2011)). An early indication that synapse dysfunction depended on the actions of both Aβ and tau came from the observation that removing endogenous tau in mutant APP overexpressing mice by crossing them with a tau knockout line prevented Aβ associated cognitive deficits and reduced the susceptibility to seizures induced by a GABA antagonist (Roberson et al., 2007). Later studies suggested that this protective effect of tau reduction is due to the normal role of tau in recruiting fyn kinase to the postsynaptic density in response to Aβ at the synapse (Ittner et al., 2010; Roberson et al., 2011). In cultured neurons, soluble Aβ oligomers isolated from human AD brain induce neuritic degeneration in concert with tau hyperphosphorylation (Jin et al., 2011). Mechanistically, a neuronal culture study by the Mandelkow group provided tantalizing evidence for the importance of both calcium and mitochondria when they observed that dendritic spine loss induced by exogenous Aβ occurs specifically in regions of the dendrite to which tau has been mis-sorted, calcium levels are elevated, microtubules are disrupted, and mitochondrial distribution is impaired (Zempel et al., 2013; Zempel et al., 2010).
Figure 6. Synaptic effects of Aβ and tau.
Many studies implicate oligomeric Aβ in synapse dysfunction and loss in models of AD. Aβ may be specifically trafficked to the synapse by apoE4, where it binds to postsynaptic receptors, causes an increase in calcium concentration, calcineurin activation, caspase 3 activation, and downstream internalization of synaptic receptors. Tau has also been implicated in synapse dysfunction downstream of Aβ, and pathological forms of tau (pTau) are transferred through synaptic circuits, although which forms of tau are transported and how they are transported remains to be determined. Figure courtesy A Hermann.
In both neuronal cultures and in vivo, calcium increases in response to Aβ have been seen to be important in synapse dysfunction and loss, and the recent data suggesting that tau is necessary for Aβ mediated synapse degeneration beg the question of whether tau is “upstream” or “downstream” of increases in calcium. Using in vivo multiphoton imaging, we observed that tau-associated dendritic spine loss in the rTg4510 mouse model is not associated with chronically increased resting calcium levels, nor does tau overexpression impair calcium responses to visual stimulation, indicating that pathological forms of tau can cause synapse collapse in a pathway independent of calcium (Kopeikina et al., 2013; Kuchibhotla et al., 2013). This would support a model in which alterations in tau were downstream of increased calcium levels induced by Aβ, but further studies are needed to confirm.
Soluble versus fibrillar species at the synapse – which are toxic?
As the defining lesions for AD, fibrillar Aβ and tau in plaques and tangles were long believed to be toxic to the brain. However, it is becoming quite clear that while fibrils themselves are not likely toxic, soluble forms of these proteins strongly contribute to toxicity. Many studies have clearly demonstrated in cell cultures and animal models that oligomers but not fibrils of Aβ are toxic to synapses and can impair cognition in wild-type animals (reviewed by (Klein, 2013; Mucke and Selkoe, 2012)). Recent data from models of tauopathy similarly indicate that soluble forms of tau, but not NFT, are toxic to synapses. In rTg4510 mice, accumulation of soluble oligomers of tau correlates with memory loss (Berger et al., 2007), neurons show electrophysiological impairments and structural degeneration that does not depend on the presence of a tangle (Crimins et al., 2012; Rocher et al., 2010), and cultured neurons from these mice have accumulation of phosphorylated tau (not aggregates) at dendritic spines which impairs synaptic function (Hoover et al., 2010). Memory deficits in rTg4510 mice (SantaCruz et al., 2005) and proaggregant TauRD mice (Sydow et al., 2010) are reversible when tau transgene expression is suppressed even in the continued presence of neurofibrillary tangle pathology, strongly arguing in favor of soluble forms of tau as toxic to synaptic function.
Deficits in axonal trafficking thought to contribute to synaptic dysfunction downstream of tau pathology also appear to be due to soluble forms of tau. Impairments to cellular transport could be due to a loss of function of tau stabilizing microtubules and/or a toxic gain-of-function of aggregated tau either “blocking” the microtubule tracks or directly interfering with cellular transport motors (Dixit et al., 2008; Mandelkow et al., 2003; Stoothoff et al., 2009b). In an assay that measures the distribution of mitochondria through the cell soma and neurites, we observed that misfolded tau in cell bodies and neuropil threads is associated with impairments in mitochondrial distribution in both rTg4510 mice and human AD. Interestingly, in rTg4510 mice, these deficits in mitochondrial distribution recovered after lowering soluble tau levels, even in the continued presence of aggregated misfolded tau (Kopeikina et al., 2011). This argues against the idea of tau aggregates as “roadblocks” and rather for soluble tau impairing trafficking either through destabilizing microtubules or disrupting the function of molecular motors.
Another strong argument against the overt toxicity of aggregates comes from observations that there is a substantial proportion of the elderly population (approximately 1/3) who have plaques and tangles in their brains without exhibiting any signs of dementia. This has been observed in many large-scale postmortem cohort studies of aging including the religious orders study (Arnold et al., 2013; Bennett, 2006; Schneider et al., 2009), the Nun Study (Tyas et al., 2007), the Baltimore Longitudinal study of Aging (O’Brien et al., 2009), and the Medical Research Council Cognitive Function and Ageing Study (Savva et al., 2009). Further, in human imaging studies using Pittsburgh Compound B positron emission tomography (PET) to assess amyloid deposition, amyloid is found in the brains of approximately 30% of cognitively normal aged individuals (Aizenstein et al., 2008; Andrews et al., 2013). This cognitive resilience in the face of pathological lesions could be due to a “reserve” of connectivity with many extra synapses perhaps resulting from a highly enriched lifestyle. Another possibility is that the amount of tau pathology and the regions it occupies may be different in individuals who convert from cognitively normal to dementia. This possibility has not been thoroughly explored in the published literature but the advent of new tau imaging markers may clarify this point (Maruyama et al., 2013).
Alternatively, the aggregates themselves may genuinely be non-toxic and people with pathology who do not have cognitive problems may have less of the toxic soluble forms of Aβ and tau that are normally associated with pathology. In support of this latter idea, a recent study in Gomez-Isla’s group found that the burden of dense-core plaques and the burden of plaques positive for oligomeric Aβ in plaques measured with Lee’s NAB61 antibody were increased in patients with dementia (Perez-Nievas et al., 2013). They also observed significantly less synapse and neuronal loss and gliosis in people who had plaques and tangles but intact cognition. A similar study comparing AD patients and high-pathology control cases used a new detection method to detect Aβ oligomers in brain lysates and found that soluble oligomer levels in demented patients were more tightly correlated with plaque burden, supporting the possibility that plaque-associated oligomers are particularly toxic (Esparza et al., 2013), similar to our previous work showing plaque-associated synaptotoxicity in mouse models and human brain. These data are also interesting in light of recent biophysical work exploring the mechanisms of Aβ aggregation. Cohen and colleagues demonstrated that fibrils catalyze the formation of oligomers (Cohen et al., 2013), in accord with the high concentration of oligomers around dense plaques. Similarly, accumulation of prefibrillar tau oligomers correlates with cognitive decline in postmortem studies of brains from people with mild cognitive impairment (Mufson et al., 2013; Vana et al., 2011).
A role for synapses in disease progression
Synapse loss correlates strongly with cognitive decline and both Aβ and tau appear to contribute to this loss, perhaps synergistically, but that is not the end of the story. Synapses may also be key to both the initiation and the spread of disease processes throughout the brain.
The accumulation of Aβ pathology appears to be related to synaptic activity. In hippocampal slices (Kamenetz et al., 2003) and in vivo in mouse models, neuronal activity increases generation of Aβ (Bero et al.; Cirrito et al., 2008), due to the increase in endocytosis associated with synaptic activity. Conversely, decreasing activity levels by denervation of somatosensory cortex causes a reduction in Aβ levels (Bero et al., 2011). In human brain, Aβ generation is higher in patients with higher neurological status, supporting the animal data that neuronal activity increases Aβ levels (Brody et al., 2008). Interestingly, the immediate early gene Arc/Arg3.1, which is involved in synaptic plasticity and AMPAR internalization, regulates the activity dependent generation of Aβ via an endosomal pathway (Wu et al., 2011). There is also a link between areas with high baseline levels of neuronal activity and Aβ generation in AD. In vivo PIB imaging of humans reveals that the earliest senile plaques appear in the neocortex, particularly in areas of the brain which are active in the “default” state (Buckner et al., 2005). These data indicate that synaptic activity plays a role in disease onset since accumulation of Aβ in the brain is widely regarded as the initial stage of Alzheimer’s disease.
Recent work also shows that tau, which is predominantly an intracellular, axonal protein, is also released from cells (Chai et al., 2012; Karch et al., 2013; Pooler et al., 2014; Saman et al., 2012). Similar to the studies of Aβ, tau release from neurons also appears to be increased with synaptic activity both in vitro and in vivo (Pooler et al., 2013a; Yamada et al., 2014).
Synapses also appear to play a role in the spread of disease through the brain. When Aβ-rich brain extracts (derived from human AD brain or transgenic mouse models) are injected into human APP transgenic models before any amyloid deposits are present, they seed plaque formation (Jucker and Walker, 2013). The induction of Aβ aggregation initially occurs near the injection site, however, there is spreading to axonally connected regions in the neural circuits suggesting that the seeds of aggregation are taken up by local neurons, travel along the axon, and propagate across the synapse to connected neurons (Eisele et al., 2009; Jucker and Walker, 2011). Restricted expression of mutant human APP to the entorhinal cortex (EC) in a mouse model also showed deposition of Aβ in plaques both locally in the EC and in the synaptically connected dentate gyrus of the hippocampus (Harris et al., 2010).
The role of synaptic connections in the spread of tau pathology is even more strongly supported. The progression of neurofibrillary tangle pathology through the brain correlates well with cognitive decline (Nelson et al., 2012), and is a very systematic process beginning in the entorhinal cortex then spreading through the hippocampal formation, limbic and association cortices, and finally affecting most brain areas in late stages of the disease (Braak and Braak, 1991; Hyman et al., 1984). This systematic march of tau pathology through the brain appears to follow neural circuits, and recent data from mouse models confirms that pathological forms of tau do progress through synaptically connected circuits (Pooler et al., 2013b). Injection of brain extracts from P301S tau mice which had tangles into mice expressing human wild-type four repeat tau (which do not usually exhibit pathology), induced tau aggregation at the site of injection which spread to neighboring brain regions (Clavaguera et al., 2009; Goedert et al., 2010).
Transgenic models to examine tau propagation have also been developed. Three independent groups generated models with P301L mutant human tau expression restricted to the entorhinal cortex (de Calignon et al., 2012; Harris et al., 2012; Liu et al., 2012). These mice express tau in the medial EC and closely associated pre and parasubiculum, which results in accumulation of misfolded, phosphorylated tau first in the EC. Over time, we observe that the misfolded human tau spreads to dentate gyrus granule cells, which do not express the human tau transgene, then further propagates to downstream regions in the neural circuit including CA3, CA1, and anterior cingulate cortex (de Calignon et al., 2012). Even before overt neurofibrillary pathology occurs in the dentate gyrus, there is evidence of hippocampal dysfunction. At an age prior to synaptic loss or the appearance of NFT in the dentate gyrus, sensitive electrophysological and molecular markers demonstrate abnormalities in the presynaptic function of the perforant pathway projection to the hippocampal formation, implicating misfolded, but soluble tau in neural system failure (Polydoro et al., 2013b). Importantly, work on the rTgTauEC model indicates that lowering soluble tau levels by transgene suppression prevents synapse loss and trans-synaptic spread of mutant tau, and surprisingly allows reversal of existing neurofibrillary tangle pathology both in the dentate gyrus and entorhinal cortex (Polydoro et al., 2013a). This has implications for therapeutics as it implies reducing tau levels will prevent both the spread of the disease through the brain and the synapse loss that appears to contribute significantly to cognitive decline.
Moving forward: outstanding questions
Although great strides have been made in understanding the role of synapses in Alzheimer’s disease, many outstanding questions remain, which need to be addressed in order to develop therapeutics to target synaptic degeneration and the trans-synaptic spread of pathology. We highlight a few of these below.
Which forms are toxic? Despite the strong evidence that soluble forms of Aβ and tau are toxic at synapses, the exact forms of the toxic species remain to be determined. There is evidence for toxicity of both low molecular weight dimers and trimers of Aβ (Jin et al., 2011; Masters and Selkoe, 2012; Mc Donald et al., 2010; Shankar et al., 2008) and larger dodecamers (Lesne et al., 2006; Reed et al., 2011); however the complete story likely includes a mix of species in complexes with other molecules including apolipoprotein E. Synaptotoxic tau is likely to be phosphorylated or misfolded oligomers (Hoover et al., 2010; Lasagna-Reeves et al., 2010; Lasagna-Reeves et al., 2011). In order to appropriately target synaptotoxic species, these will need to be accurately identified. Further, we need to better understand the relative role of soluble versus aggregated species of both Aβ and tau. Are they in equilibria with each other? If the toxic species are the soluble (oligomeric) forms, then are therapies aiming to disrupt the fibrillar aggregates potentially harmful?
What are the molecular mechanisms leading to synapse loss? An abundance of data from model systems are beginning to converge on a solid pathway from Aβ to calcium increases to synapse degeneration, but there are still holes, particularly the link between Aβ and tau in synapse loss, the receptor that Aβ binds and whether we can prevent either this binding or downstream consequences, thus saving synapses. However, to the extent that the synaptic alterations are subsequent to enhanced physiological processes, and to the extent that normal plasticity might be linked to some extent to Aβ at the synapse, a deeper understanding of what it means to manipulate this system is critical for the development of appropriately targeted therapeutics.
Should we target extracellular tau? To the extent that we now recognize extracellular tau as a normal species, increased after neuronal activity, rather than simply a marker of neuronal damage, we need to understand what its normal role is and, in particular, if it has a role at the synapse. There is exciting recent evidence that immunotherapy directed at tau is beneficial in mouse models, which could be due to the removal of the extracellular tau which is being transferred between neurons (Boutajangout et al., 2011; Chai et al., 2011; d’Abramo et al., 2013; Yanamandra et al., 2013).
In summary, synapse dysfunction and loss and the propagation of pathological proteins through synaptic connections appear to be important contributors to dementia in AD, and therapeutic approaches to prevent these deficits have the potential to prevent or reverse cognitive decline in the future.
Highlights.
Synapse loss strongly correlates with dementia in Alzheimer’s disease
Both amyloid beta and tau may have normal roles at the synapse
Pathological species of amyloid and tau contribute to synapse degeneration
Propagation through the brain likely occurs via synaptic transfer of pathology
Acknowledgments
Funding provided by NIH P50AG05134 (BTH); Alzheimer’s Research UK (TS-J), University of Edinburgh Wellcome Trust ISSF (TS-J). We would like to thank Dominic Walsh for providing AW7 antibody, Virginia Lee for NAB61 antibody, Matthew Frosch for human brain images, Abigail Hermann for aid in figure preparation, and Iris Oren and Shaun Croft for discussions about the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstein H, Nebes R, Saxton J, Price J, Mathis C, Tsopelas N, Ziolko S, James J, Snitz B, Houck P, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of neurology. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer A. Uber eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeitschrife Psychiatrie. 1907;64:146–148. [Google Scholar]
- Andrews K, Modat M, Macdonald K, Yeatman T, Cardoso M, Leung K, Barnes J, Villemagne V, Rowe C, Fox N, et al. Atrophy rates in asymptomatic amyloidosis: implications for Alzheimer prevention trials. PLoS One. 2013:8. doi: 10.1371/journal.pone.0058816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt T, Stieler J, Strijkstra AM, Hut RA, Rudiger J, Van der Zee EA, Harkany T, Holzer M, Hartig W. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci. 2003;23:6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S, Louneva N, Cao K, Wang LS, Han LY, Wolk D, Negash S, Leurgans S, Schneider J, Buchman A, et al. Cellular, synaptic, and biochemical features of resilient cognition in Alzheimer’s disease. Neurobiology of Aging. 2013;34:157–168. doi: 10.1016/j.neurobiolaging.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology. 1992;42:1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T, et al. Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2295–2300. doi: 10.1073/pnas.0911829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A, Klyubin I, Mc Donald J, Mably A, Farrell M, Scott M, Walsh D, Rowan M. Alzheimer’s disease brain-derived amyloid-β-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastrikova N, Gardner G, Reece J, Jeromin A, Dudek S. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:3123–3127. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, De Strooper B. Prion protein in Alzheimer’s pathogenesis: a hot and controversial issue. EMBO molecular medicine. 2010;2:289–290. doi: 10.1002/emmm.201000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, De Strooper B. Promiscuous Alzheimer’s Amyloid: Yet Another Partner. Science. 2013;341:1354–1355. doi: 10.1126/science.1244166. [DOI] [PubMed] [Google Scholar]
- Bennett D. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer disease and associated disorders. 2006;20:8. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]
- Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, et al. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27:3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee J-M, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-[beta] deposition. Nat Neurosci. 14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-[beta] deposition. Nature neuroscience. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T, Collingridge G, Morris R. Introduction. Long-term potentiation and structure of the issue. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2003;358:607–611. doi: 10.1098/rstb.2003.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T, Gardner-Medwin A. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. The Journal of physiology. 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Current opinion in neurobiology. 2012;22:383–388. doi: 10.1016/j.conb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutajangout A, Ingadottir J, Davies P, Sigurdsson E. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. Journal of neurochemistry. 2011;118:658–667. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brody D, Magnoni S, Schwetye K, Spinner M, Esparza T, Stocchetti N, Zipfel G, Holtzman D. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science (New York, NY) 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R, Snyder A, Shannon B, LaRossa G, Sachs R, Fotenos A, Sheline Y, Klunk W, Mathis C, Morris J, Mintun M. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SRY. The Croonian Lecture: La fine structure des centres nerveux. Proceedings of the Royal Society of London. 1894;55:444–468. [Google Scholar]
- Calella A, Farinelli M, Nuvolone M, Mirante O, Moos R, Falsig J, Mansuy I, Aguzzi A. Prion protein and Abeta-related synaptic toxicity impairment. EMBO molecular medicine. 2010;2:306–314. doi: 10.1002/emmm.201000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan L, Vaules W, Coleman P. Quantitative decrease in synaptophysin message expression and increase in cathepsin D message expression in Alzheimer disease neurons containing neurofibrillary tangles. Journal of neuropathology and experimental neurology. 1999;58:275–287. doi: 10.1097/00005072-199903000-00007. [DOI] [PubMed] [Google Scholar]
- Cao L, Schrank B, Rodriguez S, Benz E, Moulia T, Rickenbacher G, Gomez A, Levites Y, Edwards S, Golde T, et al. Aβ alters the connectivity of olfactory neurons in the absence of amyloid plaques in vivo. Nature communications. 2012;3:1009. doi: 10.1038/ncomms2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallucci V, Berretta N, Nobili A, Nisticò R, Mercuri N, D’Amelio M. Calcineurin inhibition rescues early synaptic plasticity deficits in a mouse model of Alzheimer’s disease. Neuromolecular medicine. 2013;15:541–548. doi: 10.1007/s12017-013-8241-2. [DOI] [PubMed] [Google Scholar]
- Chai X, Dage J, Citron M. Constitutive secretion of tau protein by an unconventional mechanism. Neurobiology of Disease. 2012;48:356–366. doi: 10.1016/j.nbd.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Chai X, Wu S, Murray T, Kinley R, Cella C, Sims H, Buckner N, Hanmer J, Davies P, O’Neill M, et al. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. The Journal of biological chemistry. 2011;286:34457–34467. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lin R, Xu S, Wei X, Zhang J, Wang C, Anwyl R, Wang Q. Enhancement of long-term depression by soluble amyloid β protein in rat hippocampus is mediated by metabotropic glutamate receptor and involves activation of p 38 MAPK, STEP and caspase-3. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.08.054. [DOI] [PubMed] [Google Scholar]
- Christie R, Kimchi E, Kajdasz S, Bacskai B, Hyman BT. Multiphoton microscopy and amyloid angiopathy. Amyloid. 2001;8(Suppl 1):48–50. [PubMed] [Google Scholar]
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, Prince D. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua B, Volbracht C, Tan K, Li R, Yu V, Li P. Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nature cell biology. 2003;5:1083–1089. doi: 10.1038/ncb1070. [DOI] [PubMed] [Google Scholar]
- Cirrito J, Kang JE, Lee J, Stewart F, Verges D, Silverio L, Bu G, Mennerick S, Holtzman D. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito J, Yamada K, Finn M, Sloviter R, Bales K, May P, Schoepp D, Paul S, Mennerick S, Holtzman D. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Cissé M, Halabisky B, Harris J, Devidze N, Dubal D, Sun B, Orr A, Lotz G, Kim D, Hamto P, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469:47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L, Barres B. Emerging roles of astrocytes in neural circuit development. Nature reviews Neuroscience. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nature neuroscience. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Cohen S, Linse S, Luheshi L, Hellstrand E, White D, Rajah L, Otzen D, Vendruscolo M, Dobson C, Knowles T. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9758–9763. doi: 10.1073/pnas.1218402110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G, Peineau S, Howland J, Wang Y. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Crimins J, Pooler A, Polydoro M, Luebke J, Spires-Jones T. The intersection of amyloid beta and tau in glutamatergic synaptic dysfunction and collapse in Alzheimer’s disease. Ageing Res Rev. 2013;12:757–763. doi: 10.1016/j.arr.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimins JL, Rocher AB, Luebke JI. Electrophysiological changes precede morphological changes to frontal cortical pyramidal neurons in the rTg 4510 mouse model of progressive tauopathy. Acta Neuropathol. 2012;124:777–795. doi: 10.1007/s00401-012-1038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimins JL, Rocher AB, Peters A, Shultz P, Lewis J, Luebke JI. Homeostatic responses by surviving cortical pyramidal cells in neurodegenerative tauopathy. Acta Neuropathol. 2011;122:551–564. doi: 10.1007/s00401-011-0877-0. [DOI] [PubMed] [Google Scholar]
- d’Abramo C, Acker C, Jimenez H, Davies P. Tau passive immunotherapy in mutant P 301L mice: antibody affinity versus specificity. PloS one. 2013:8. doi: 10.1371/journal.pone.0062402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, Diamantini A, De Zio D, Carrara P, Battistini L, et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nature neuroscience. 2011;14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- D’Amelio M, Sheng M, Cecconi F. Caspase-3 in the central nervous system: beyond apoptosis. Trends in neurosciences. 2012;35:700–709. doi: 10.1016/j.tins.2012.06.004. [DOI] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz D, Kopeikina K, Pitstick R, Sahara N, Ashe K, Carlson G, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice F, Velasco P, Lambert M, Viola K, Fernandez S, Ferreira S, Klein W. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. The Journal of biological chemistry. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- DeKosky S, Scheff S. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Annals of neurology. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- DeKosky S, Scheff S, Styren S. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration: a journal for neurodegenerative disorders, neuroprotection, and neuroregeneration. 1996;5:417–421. doi: 10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- Demuro A, Mina E, Kayed R, Milton S, Parker I, Glabe C. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. The Journal of biological chemistry. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- Dickson TC, Vickers JC. The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer’s disease. Neuroscience. 2001;105:99–107. doi: 10.1016/s0306-4522(01)00169-5. [DOI] [PubMed] [Google Scholar]
- Dinamarca M, Ríos J, Inestrosa N. Postsynaptic Receptors for Amyloid-β Oligomers as Mediators of Neuronal Damage in Alzheimer’s Disease. Frontiers in physiology. 2012;3:464. doi: 10.3389/fphys.2012.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey M, Chaudhury P, Kabiru H, Shea T. Tau inhibits anterograde axonal transport and perturbs stability in growing axonal neurites in part by displacing kinesin cargo: neurofilaments attenuate tau-mediated neurite instability. Cell motility and the cytoskeleton. 2008;65:89–99. doi: 10.1002/cm.20243. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Morris R. Memorable trends. Neuron. 2013;80:742–750. doi: 10.1016/j.neuron.2013.09.039. [DOI] [PubMed] [Google Scholar]
- Dudek S, Bear M. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts C, Colle MA, Dessi F, Piette F, Hauw JJ. Progression of Alzheimer histopathological changes. Acta neurologica Belgica. 1998;98:180–185. [PubMed] [Google Scholar]
- Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. The Journal of cell biology. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert A, Nisbet R, Grimm A, Götz J. March separate, strike together –Role of phosphorylated TAU in mitochondrial dysfunction in Alzheimer’s disease. Biochimica et biophysica acta. 2013 doi: 10.1016/j.bbadis.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Eckert A, Schulz K, Rhein V, Götz J. Convergence of amyloid-beta and tau pathologies on mitochondria in vivo. Molecular neurobiology. 2010;41:107–114. doi: 10.1007/s12035-010-8109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele Y, Bolmont T, Heikenwalder M, Langer F, Jacobson L, Yan ZX, Roth K, Aguzzi A, Staufenbiel M, Walker L, Jucker M. Induction of cerebral beta-amyloidosis: intracerebral versus systemic Abeta inoculation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12926–12931. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza T, Zhao H, Cirrito J, Cairns N, Bateman R, Holtzman D, Brody D. Amyloid-β oligomerization in Alzheimer dementia versus high-pathology controls. Annals of neurology. 2013;73:104–119. doi: 10.1002/ana.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freir D, Nicoll A, Klyubin I, Panico S, Mc Donald J, Risse E, Asante E, Farrow M, Sessions R, Saibil H, et al. Interaction between prion protein and toxic amyloid β assemblies can be therapeutically targeted at multiple sites. Nature communications. 2011;2:336. doi: 10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris R. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann F, Bussière T, Bouras C, Kövari E, Perl D, Morrison J, Gold G, Hof P. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Ginsberg S, Hemby S, Lee V, Eberwine J, Trojanowski J. Expression profile of transcripts in Alzheimer’s disease tangle-bearing CA1 neurons. Annals of neurology. 2000;48:77–87. [PubMed] [Google Scholar]
- Glenner G, Wong C. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends in Neurosciences. 2010;33:317–325. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochimica et biophysica acta. 2005;1739:240–250. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Spires T, De Calignon A, Hyman B. Neuropathology of Alzheimer’s disease. Handb Clin Neurol. 2008;89:233–243. doi: 10.1016/S0072-9752(07)01222-5. [DOI] [PubMed] [Google Scholar]
- Harris J, Devidze N, Verret L, Ho K, Halabisky B, Thwin M, Kim D, Hamto P, Lo I, Yu GQ, et al. Transsynaptic progression of amyloid-β-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron. 2010;68:428–441. doi: 10.1016/j.neuron.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Koyama A, Maeda S, Ho K, Devidze N, Dubal DB, Yu GQ, Masliah E, Mucke L. Human P301L-mutant tau expression in mouse entorhinal-hippocampal network causes tau aggregation and presynaptic pathology but no cognitive deficits. PLoS One. 2012;7:e45881. doi: 10.1371/journal.pone.0045881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry G. Microglia during development and aging. Pharmacology & therapeutics. 2013;139:313–326. doi: 10.1016/j.pharmthera.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior: A neuropsychological theory. 1949. [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, et al. Tau Mislocalization to Dendritic Spines Mediates Synaptic Dysfunction Independently of Neurodegeneration. Neuron. 2010;68:1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry E, Wu HY, Arbel-Ornath M, Hashimoto T, Matsouaka R, Fan Z, Spires-Jones TL, Betensky RA, Bacskai BJ, Hyman BT. Inhibition of the NFAT pathway alleviates amyloid beta neurotoxicity in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32:3176–3192. doi: 10.1523/JNEUROSCI.6439-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol. 2011;68:1062–1064. doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Horsen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Ingelsson M, Fukumoto H, Newell K, Growdon J, Hedley-Whyte E, Frosch M, Albert M, Hyman B, Irizarry M. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62:925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- Ittner L, Ke Y, Götz J. Phosphorylated Tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP 1) in Alzheimer disease. The Journal of biological chemistry. 2009;284:20909–20916. doi: 10.1074/jbc.M109.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner LM, Gotz J. Amyloid-beta and tau--a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe D. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci U S A. 2011;108:5819–5824. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Santiago Ramon y Cajal and the croonian lecture, March 1894. Trends in neurosciences. 1994;17:190–192. doi: 10.1016/0166-2236(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Jucker M, Walker L. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Annals of neurology. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M, Walker L. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kanaan N, Morfini G, LaPointe N, Pigino G, Patterson K, Song Y, Andreadis A, Fu Y, Brady S, Binder L. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:9858–9868. doi: 10.1523/JNEUROSCI.0560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch C, Jeng A, Goate A. Calcium phosphatase calcineurin influences tau metabolism. Neurobiology of Aging. 2013;34:374–386. doi: 10.1016/j.neurobiolaging.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuse O, Lin WL, Lewis J, Hutton M, Dickson D. Neurofibrillary tangle-related synaptic alterations of spinal motor neurons of P301L tau transgenic mice. Neuroscience Letters. 2006;409:95–99. doi: 10.1016/j.neulet.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Kay KR, Smith C, Wright AK, Serrano-Pozo A, Pooler AM, Koffie RM, Bastin ME, Bak TH, Abrahams S, Kopeikina KJ, et al. Studying synapses in human brain with array tomography and electron microscopy. Nature Protocols. 2013 doi: 10.1038/nprot.2013.078. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd M. Paired helical filaments in electron microscopy in Alzheimer’s disease. Nature. 1963;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, Hyman BT, Shatz CJ. Human LilrB2 Is a -Amyloid Receptor and Its Murine Homolog PirB Regulates Synaptic Plasticity in an Alzheimer’s Model. Science. 2013:341. doi: 10.1126/science.1242077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamt F, Zdanov S, Levine R, Pariser A, Zhang Y, Zhang B, Yu LR, Veenstra T, Shacter E. Oxidant-induced apoptosis is mediated by oxidation of the actin-regulatory protein cofilin. Nature cell biology. 2009;11:1241–1246. doi: 10.1038/ncb1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. Synaptic targeting by A beta oligomers (ADDLS) as a basis for memory loss in early Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2006;2:43–55. doi: 10.1016/j.jalz.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Klein W. Synaptotoxic amyloid-β oligomers: a molecular basis for the cause, diagnosis, and treatment of Alzheimer’s disease? Journal of Alzheimer’s disease: JAD. 2013;33(Suppl 1):65. doi: 10.3233/JAD-2012-129039. [DOI] [PubMed] [Google Scholar]
- Koffie RM, Hashimoto T, Tai HC, Kay KR, Serrano-Pozo A, Joyner D, Hou S, Kopeikina KJ, Frosch MP, Lee VM, et al. Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-beta. Brain. 2012;135:2155–2168. doi: 10.1093/brain/aws127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer’s disease: synapses gone cold. Molecular neurodegeneration. 2011;6:63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeikina K, Hyman B, Spires-Jones T. Soluble forms of tau are toxic in Alzheimer’s disease. Transl Neurosci. 2012a;3:223–233. doi: 10.2478/s13380-012-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeikina KJ, Carlson GA, Pitstick R, Ludvigson AE, Peters A, Luebke JI, Koffie RM, Frosch MP, Hyman BT, Spires-Jones TL. Tau accumulation causes mitochondrial distribution deficits in neurons in a mouse model of tauopathy and in human Alzheimer’s disease brain. Am J Pathol. 2011;179:2071–2082. doi: 10.1016/j.ajpath.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeikina KJ, Polydoro M, Tai HC, Yaeger E, Carlson GA, Pitstick R, Hyman BT, Spires-Jones TL. Synaptic alterations in the rTg 4510 mouse model of tauopathy. J Comp Neurol. 2012b doi: 10.1002/cne.23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeikina KJ, Wegmann SK, Pitstick R, Carlson GA, Bacskai B, Betensky RA, Hyman BT, Spires-Jones TL. Tau causes synapse loss without disrupting calcium homeostasis in the rTg 4510 model of tauopathy. PloS one. 2013 doi: 10.1371/journal.pone.0080834. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla KV, Wegmann S, Kopeikina KJ, Hawkes J, Rudinskiy N, Andermann ML, Spires-Jones TL, Bacskai BJ, Hyman BT. Neurofibrillary tangle-bearing neurons are functionally integrated in cortical circuits in vivo. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1318807111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann D, Lamsa K. Long-term synaptic plasticity in hippocampal interneurons. Nature reviews Neuroscience. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- Lacor P, Buniel M, Chang L, Fernandez S, Gong Y, Viola K, Lambert M, Velasco P, Bigio E, Finch C, et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor P, Buniel M, Furlow P, Clemente A, Velasco P, Wood M, Viola K, Klein W. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M, Barlow A, Chromy B, Edwards C, Freed R, Liosatos M, Morgan T, Rozovsky I, Trommer B, Viola K, et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe N, Morfini G, Pigino G, Gaisina I, Kozikowski A, Binder L, Brady S. The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. Journal of neuroscience research. 2009;87:440–451. doi: 10.1002/jnr.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson M, Sherman M, Amar F, Nuvolone M, Schneider J, Bennett D, Aguzzi A, Lesné S. The complex PrP(c)-Fyn couples human oligomeric Aβ with pathological tau changes in Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:16857. doi: 10.1523/JNEUROSCI.1858-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lasagna-Reeves CA, Castillo-Carranza DL, Guerrero-Muoz MJ, Jackson GR, Kayed R. Preparation and characterization of neurotoxic tau oligomers. Biochemistry. 2010;49:10039–10041. doi: 10.1021/bi1016233. [DOI] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Clos AL, Jackson GR, Kayed R. Tau Oligomers Impair Memory and Induce Synaptic and Mitochondrial Dysfunction in Wild-type Mice. Mol Neurodegener. 2011;6:39. doi: 10.1186/1750-1326-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurén J, Gimbel D, Nygaard H, Gilbert J, Strittmatter S. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le R, Cruz L, Urbanc B, Knowles R, Hsiao-Ashe K, Duff K, Irizarry M, Stanley H, Hyman B. Plaque-induced abnormalities in neurite geometry in transgenic models of Alzheimer disease: implications for neural system disruption. J Neuropathol Exp Neurol. 2001;60:753–758. doi: 10.1093/jnen/60.8.753. [DOI] [PubMed] [Google Scholar]
- Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, Lee VM. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem. 2006;281:4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson N, Walsh D, Shankar G, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Uemura K, Hashimoto T, Nasser-Ghodsi N, Arimon M, Lill C, Palazzolo I, Krainc D, Hyman B, Berezovska O. Neuronal activity and secreted amyloid β lead to altered amyloid β precursor protein and presenilin 1 interactions. Neurobiology of disease. 2013;50:127–134. doi: 10.1016/j.nbd.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jo J, Jia JM, Lo SC, Whitcomb D, Jiao S, Cho K, Sheng M. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chang L, Roselli F, Almeida O, Gao X, Wang X, Yew D, Wu Y. Amyloid-β induces caspase-dependent loss of PSD-95 and synaptophysin through NMDA receptors. Journal of Alzheimer’s disease: JAD. 2010;22:541–556. doi: 10.3233/JAD-2010-100948. [DOI] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA 1 hippocampal dendrites induced by synaptic activity. Science (New York, NY) 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Stamer K, Vogel R, Thies E, Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24:1079–1085. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, Zhang MR, Trojanowski J, Lee V, Ono M, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–1108. doi: 10.1016/j.neuron.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, DeTeresa R, Alford M, Terry R. Synaptic and neuritic alterations during the progression of Alzheimer’s disease. Neurosci Lett. 1994;174:67–72. doi: 10.1016/0304-3940(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Massey P, Bashir Z. Long-term depression: multiple forms and implications for brain function. Trends in neurosciences. 2007 doi: 10.1016/j.tins.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Masters C, Selkoe D. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2012:2. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies G, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel R. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Donald J, Savva G, Brayne C, Welzel A, Forster G, Shankar G, Selkoe D, Ince P, Walsh D Medical Research Council Cognitive F and Ageing S. The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain: a journal of neurology. 2010;133:1328–1341. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan ME, Kajdasz ST, Hyman BT, Bacskai BJ. In vivo imaging of reactive oxygen species specifically associated with thioflavine S-positive amyloid plaques by multiphoton microscopy. J Neurosci. 2003;23:2212–2217. doi: 10.1523/JNEUROSCI.23-06-02212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Busse B, Weiler NC, O’Rourke N, Smith SJ. Single-Synapse Analysis of a Diverse Synapse Population: Proteomic Imaging Methods and Markers. Neuron. 2010;68:639–653. doi: 10.1016/j.neuron.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Smith SJ. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron. 2007;55:25–36. doi: 10.1016/j.neuron.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragón-Rodríguez S, Trillaud-Doppia E, Dudilot A, Bourgeois C, Lauzon M, Leclerc N, Boehm J. Interaction of endogenous tau protein with synaptic proteins is regulated by N-methyl-D-aspartate receptor-dependent tau phosphorylation. The Journal of biological chemistry. 2012;287:32040–32053. doi: 10.1074/jbc.M112.401240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora F, Segovia G, del Arco A. Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain research reviews. 2007;55:78–88. doi: 10.1016/j.brainresrev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Moreno H, Choi S, Yu E, Brusco J, Avila J, Moreira J, Sugimori M, Llinás R. Blocking Effects of Human Tau on Squid Giant Synapse Transmission and Its Prevention by T-817 MA. Frontiers in synaptic neuroscience. 2011;3:3. doi: 10.3389/fnsyn.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Burns M, Binder L, Kanaan N, LaPointe N, Bosco D, Brown R, Brown H, Tiwari A, Hayward L, et al. Axonal transport defects in neurodegenerative diseases. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Selkoe D. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harbor perspectives in medicine. 2012:2. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson E, Ward S, Binder L. Prefibrillar Tau Oligomers in Mild Cognitive Impairment and Alzheimer’s Disease. Neuro-degenerative diseases. 2013 doi: 10.1159/000353687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey R, Herron C, Malenka R. An essential role for protein phosphatases in hippocampal long-term depression. Science (New York, NY) 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Nägerl U, Eberhorn N, Cambridge S, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan A. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- O’Brien R, Resnick S, Zonderman A, Ferrucci L, Crain B, Pletnikova O, Rudow G, Iacono D, Riudavets M, Driscoll I, et al. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA) Journal of Alzheimer’s disease: JAD. 2009;18:665–675. doi: 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli R, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira T, Guiducci E, Dumas L, et al. Synaptic pruning by microglia is necessary for normal brain development. Science (New York, NY) 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Perez-Nievas B, Stein T, Tai HC, Dols-Icardo O, Scotton T, Barroeta-Espar I, Fernandez-Carballo L, de Munain E, Perez J, Marquie M, et al. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain. 2013;136:2510–2526. doi: 10.1093/brain/awt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polydoro M, de Calignon A, Suarez-Calvet M, Sanchez L, Kay KR, Nicholls SB, Roe AD, Pitstick R, Carlson GA, Gomez-Isla T, et al. Reversal of Neurofibrillary Tangles and Tau-Associated Phenotype in the rTgTauEC Model of Early Alzheimer’s Disease. J Neurosci. 2013a;33:13300–13311. doi: 10.1523/JNEUROSCI.0881-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polydoro M, Dzhala VI, Pooler AM, Nicholls SB, McKinney AP, Sanchez L, Pitstick R, Carlson GA, Staley KJ, Spires-Jones TL, Hyman BT. Soluble pathological tau in the entorhinal cortex leads to presynaptic deficits in an early Alzheimer’s disease model. Acta Neuropathol. 2013b doi: 10.1007/s00401-013-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontrello C, Sun MY, Lin A, Fiacco T, DeFea K, Ethell I. Cofilin under control of β-arrestin-2 in NMDA-dependent dendritic spine plasticity, long-term depression (LTD), and learning. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:51. doi: 10.1073/pnas.1118803109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler A, Noble W, Hanger D. A role for tau at the synapse in Alzheimer’s disease pathogenesis. Neuropharmacology. 2014;76(Pt A):1–8. doi: 10.1016/j.neuropharm.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Pooler A, Phillips E, Lau D, Noble W, Hanger D. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO reports. 2013a;14:389–394. doi: 10.1038/embor.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler AM, Polydoro M, Wegmann S, Nicholls SB, Spires-Jones TL, Hyman BT. Propagation of tau pathology in Alzheimer’s disease: identification of novel therapeutic targets. Alzheimer’s research & therapy. 2013b;5:49. doi: 10.1186/alzrt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla R, Dolan P, Jin Y, Johnson G. Truncated tau and Aβ cooperatively impair mitochondria in primary neurons. Neurobiology of aging. 2012;33:35. doi: 10.1016/j.neurobiolaging.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo R, Morris R. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- Reed MN, Hofmeister JJ, Jungbauer L, Welzel AT, Yu C, Sherman MA, Lesne S, LaDu MJ, Walsh DM, Ashe KH, Cleary JP. Cognitive effects of cell-derived and synthetically derived Abeta oligomers. Neurobiol Aging. 2011;32:1784–1794. doi: 10.1016/j.neurobiolaging.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner M, Lacor P, Velasco P, Xu J, Contractor A, Klein W, Triller A. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR 5. Neuron. 2010;66:739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu GQ, et al. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Rocher AB, Crimins JL, Amatrudo JM, Kinson MS, Todd-Brown MA, Lewis J, Luebke JI. Structural and functional changes in tau mutant mice neurons are not linked to the presence of NFTs. Exp Neurol. 2010;223:385–393. doi: 10.1016/j.expneurol.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh S-E, Woo J, Lakshmana M, Uhlar C, Ankala V, Boggess T, Liu T, Hong Y-H, Mook-Jung I, Kim S, Kang D. Mitochondrial dysfunction and calcium deregulation by the RanBP9-cofilin pathway. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2013 doi: 10.1096/fj.13-234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönicke R, Mikhaylova M, Rönicke S, Meinhardt J, Schröder U, Fändrich M, Reiser G, Kreutz M, Reymann K. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiology of aging. 2011;32:2219–2228. doi: 10.1016/j.neurobiolaging.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Rozkalne A, Hyman B, Spires-Jones T. Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiology of disease. 2011;41:650–654. doi: 10.1016/j.nbd.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozkalne A, Spires-Jones TL, Stern EA, Hyman BT. A single dose of passive immunotherapy has extended benefits on synapses and neurites in an Alzheimer’s disease mouse model. Brain Res. 2009;1280:178–185. doi: 10.1016/j.brainres.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee A, Alvarez V, Lee N, Hall G. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. The Journal of biological chemistry. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, Lisman J. The CaMKII/NMDAR complex as a molecular memory. Molecular brain. 2013;6:10. doi: 10.1186/1756-6606-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SantaCruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva G, Wharton S, Ince P, Forster G, Matthews F, Brayne C Medical Research Council Cognitive F and Ageing S. Age, neuropathology, and dementia. The New England journal of medicine. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Sparks L, Price DA. Quantitative assessment of synaptic density in the entorhinal cortex in Alzheimer’s disease. Ann Neurol. 1993;34:356–361. doi: 10.1002/ana.410340309. [DOI] [PubMed] [Google Scholar]
- Schneider J, Aggarwal N, Barnes L, Boyle P, Bennett D. The neuropathology of older persons with and without dementia from community versus clinic cohorts. Journal of Alzheimer’s disease: JAD. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch M, Masliah E, Hyman B. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011a:1. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, Mielke M, Gómez-Isla T, Betensky R, Growdon J, Frosch M, Hyman B. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am J Pathol. 2011b;179:1373–1384. doi: 10.1016/j.ajpath.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Sabatini B, Südhof T. Synapses and Alzheimer’s disease. Cold Spring Harbor perspectives in biology. 2012:4. doi: 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng ZH, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nature reviews Neuroscience. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón A, de Maturana R, Ricobaraza A, Escribano L, Schiapparelli L, Cuadrado-Tejedor M, Pérez-Mediavilla A, Avila J, Del Río J, Frechilla D. Early changes in hippocampal Eph receptors precede the onset of memory decline in mouse models of Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2009;17:773–786. doi: 10.3233/JAD-2009-1096. [DOI] [PubMed] [Google Scholar]
- Snyder E, Nong Y, Almeida C, Paul S, Moran T, Choi E, Nairn A, Salter M, Lombroso P, Gouras G, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nature neuroscience. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Spillantini M, Goedert M. Tau pathology and neurodegeneration. Lancet neurology. 2013;12:609–622. doi: 10.1016/S1474-4422(13)70090-5. [DOI] [PubMed] [Google Scholar]
- Spires T, Meyer-Luehmann M, Stern E, McLean P, Skoch J, Nguyen P, Bacskai B, Hyman B. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Meyer-Luehmann M, Osetek JD, Jones PB, Stern EA, Bacskai BJ, Hyman BT. Impaired spine stability underlies plaque-related spine loss in an Alzheimer’s disease mouse model. Am J Pathol. 2007;171:1304–1311. doi: 10.2353/ajpath.2007.070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Mielke ML, Rozkalne A, Meyer-Luehmann M, de Calignon A, Bacskai BJ, Schenk D, Hyman BT. Passive immunotherapy rapidly increases structural plasticity in a mouse model of Alzheimer disease. Neurobiol Dis. 2009;33:213–220. doi: 10.1016/j.nbd.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan A, Madison D, Mateos J, Fraser D, Lovelett E, Coutellier L, Kim L, Tsai HH, Huang E, Rowitch D, et al. A Dramatic Increase of C1q Protein in the CNS during Normal Aging. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:13460–13474. doi: 10.1523/JNEUROSCI.1333-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EA, Bacskai BJ, Hickey GA, Attenello FJ, Lombardo JA, Hyman BT. Cortical synaptic integration in vivo is disrupted by amyloid-beta plaques. J Neurosci. 2004;24:4535–4540. doi: 10.1523/JNEUROSCI.0462-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoothoff W, Jones P, Spires-Jones T, Joyner D, Chhabra E, Bercury K, Fan Z, Xie H, Bacskai B, Edd J, et al. Differential effect of three-repeat and four-repeat tau on mitochondrial axonal transport. J Neurochem. 2009a;111:417–427. doi: 10.1111/j.1471-4159.2009.06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoothoff W, Jones PB, Spires-Jones TL, Joyner D, Chhabra E, Bercury K, Fan Z, Xie H, Bacskai B, Edd J, et al. Differential effect of three-repeat and four-repeat tau on mitochondrial axonal transport. J Neurochem. 2009b;111:417–427. doi: 10.1111/j.1471-4159.2009.06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoothoff WH, Johnson GV. Tau phosphorylation: physiological and pathological consequences. Biochimica et biophysica acta. 2005;1739:280–297. doi: 10.1016/j.bbadis.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- Sydow A, Van der Jeugd A, Zheng F, Ahmed T, Balschun D, Petrova O, Drexler D, Zhou L, Rune G, Mandelkow E, et al. Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic Tau mutant. J Neurosci. 2010;31:2511–2525. doi: 10.1523/JNEUROSCI.5245-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai HC, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol. 2012;181:1426–1435. doi: 10.1016/j.ajpath.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry R, Masliah E, Salmon D, Butters N, DeTeresa R, Hill R, Hansen L, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Annals of neurology. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Thal D, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Tremblay M-È, Lowery R, Majewska A. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010:8. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyas S, Salazar J, Snowdon D, Desrosiers M, Riley K, Mendiondo M, Kryscio R. Transitions to mild cognitive impairments, dementia, and death: findings from the Nun Study. American journal of epidemiology. 2007;165:1231–1238. doi: 10.1093/aje/kwm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um J, Kaufman A, Kostylev M, Heiss J, Stagi M, Takahashi H, Kerrisk M, Vortmeyer A, Wisniewski T, Koleske A, et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer aβ oligomer bound to cellular prion protein. Neuron. 2013;79:887–902. doi: 10.1016/j.neuron.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanc B, Cruz L, Le R, Sanders J, Ashe K, Duff K, Stanley H, Irizarry M, Hyman B. Neurotoxic effects of thioflavin S-positive amyloid deposits in transgenic mice and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2002;99:13990–13995. doi: 10.1073/pnas.222433299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harreveld A, Fifkova E. Swelling of dendritic spines in the fascia dentata after stimulation of the perforant fibers as a mechanism of post-tetanic potentiation. Exp Neurol. 1975;49:736–749. doi: 10.1016/0014-4886(75)90055-2. [DOI] [PubMed] [Google Scholar]
- Vana L, Kanaan N, Ugwu I, Wuu J, Mufson E, Binder L. Progression of tau pathology in cholinergic Basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. The American journal of pathology. 2011;179:2533–2550. doi: 10.1016/j.ajpath.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Shankar GM, Townsend M, Fadeeva JV, Betts V, Podlisny MB, Cleary JP, Ashe KH, Rowan MJ, Selkoe DJ. The role of cell-derived oligomers of Abeta in Alzheimer’s disease and avenues for therapeutic intervention. Biochemical Society transactions. 2005;33:1087–1090. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- Wang H, Lee D, Davis C, Shank R. Amyloid peptide Abeta(1–42) binds selectively and with picomolar affinity to alpha 7 nicotinic acetylcholine receptors. J Neurochem. 2000;75:1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Walsh D, Rowan M, Selkoe D, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:3370–3378. doi: 10.1523/JNEUROSCI.1633-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee H-g, Li X, Perry G, Smith M, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse A, West AK, Chuckowree JA, Vickers JC, Dickson TC. Does beta-amyloid plaque formation cause structural injury to neuronal processes? Neurotoxicity research. 2005;7:5–15. doi: 10.1007/BF03033772. [DOI] [PubMed] [Google Scholar]
- Wu HY, Hudry E, Hashimoto T, Uemura K, Fan ZY, Berezovska O, Grosskreutz C, Bacskai B, Hyman B. Distinct dendritic spine and nuclear phases of calcineurin activation after exposure to amyloid-β revealed by a novel fluorescence resonance energy transfer assay. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:5298–5309. doi: 10.1523/JNEUROSCI.0227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, et al. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Petralia R, Kurushima H, Patel H, Jung M-y, Volk L, Chowdhury S, Shepherd J, Dehoff M, Li Y, et al. Arc/Arg 3.1 regulates an endosomal pathway essential for activity-dependent β-amyloid generation. Cell. 2011;147:615–628. doi: 10.1016/j.cell.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Holth J, Liao F, Stewart F, Mahan T, Jiang H, Cirrito J, Patel T, Hochgräfe K, Mandelkow EM, Holtzman D. Neuronal activity regulates extracellular tau in vivo. The Journal of experimental medicine. 2014;211:387–393. doi: 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra K, Kfoury N, Jiang H, Mahan T, Ma S, Maloney S, Wozniak D, Diamond M, Holtzman D. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80:402–414. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempel H, Luedtke J, Kumar Y, Biernat J, Dawson H, Mandelkow E, Mandelkow EM. Amyloid-β oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL 6 and spastin. The EMBO journal. 2013;32:2920–2937. doi: 10.1038/emboj.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempel H, Thies E, Mandelkow E, Mandelkow EM. A{beta} Oligomers Cause Localized Ca2+ Elevation, Missorting of Endogenous Tau into Dendrites, Tau Phosphorylation, and Destruction of Microtubules and Spines. J Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma K, Poo M-m. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]