Abstract
[Purpose] Auditory hypersensitivity has been widely reported in patients with autism spectrum disorders. However, the neurological background of auditory hypersensitivity is currently not clear. The present study examined the relationship between sympathetic nervous system responses and auditory hypersensitivity induced by different types of auditory stimuli. [Methods] We exposed 20 healthy young adults to six different types of auditory stimuli. The amounts of palmar sweating resulting from the auditory stimuli were compared between groups with (hypersensitive) and without (non-hypersensitive) auditory hypersensitivity. [Results] Although no group × type of stimulus × first stimulus interaction was observed for the extent of reaction, significant type of stimulus × first stimulus interaction was noted for the extent of reaction. For an 80 dB-6,000 Hz stimulus, the trends for palmar sweating differed between the groups. For the first stimulus, the variance became larger in the hypersensitive group than in the non-hypersensitive group. [Conclusion] Subjects who regularly felt excessive reactions to auditory stimuli tended to have excessive sympathetic responses to repeated loud noises compared with subjects who did not feel excessive reactions. People with auditory hypersensitivity may be classified into several subtypes depending on their reaction patterns to auditory stimuli.
Key words: Auditory hypersensitivity, Sympathetic nervous system, Auditory stimuli
INTRODUCTION
Sensory processing disorders have been widely reported in patients with Autism Spectrum Disorder (ASD)1,2,3,4). In particular, one sensory processing problem, auditory hypersensitivity, has been observed in 23.9% to 70% of subjects with ASD5, 6), and acuity of hearing (hyperacusis) has been noted in 18% to 63%7, 8). Because auditory hypersensitivity has been reported to prevent adaptive behavior9, 10), elucidation of its neurological background and creation of an objective index of auditory hypersensitivity would be helpful for treatment interventions and for devising support methods for patients with auditory hypersensitivity. Although verification of the auditory processing mechanisms in ASD has been performed at various stages of the auditory system with electrophysiological indices, the neurological background has remained unclear11, 12). Here, we identified problems in auditory processing in a clinical setting and confirmed the presence of hypersensitivity based on questionnaires that were completed by patients, guardians, therapists, or teachers. However, we did not use an objective index.
In order to quantitatively evaluate the response of an individual to auditory stimuli, autonomic nerve reactions can be evaluated by measuring palmar sweating. Because eccrine sweat glands are induced by mental stress and are controlled by the cholinergic fibers of sympathetic nerves, measuring the amount of sweating allows for evaluation of sympathetic nerve activity during psychological stress13,14,15). In this study, we evaluated sympathetic nerve activity by using palmar sweating as an index.
In a previous study, Miller et al.16) performed experiments on patients with Fragile X syndrome who had a strong hypersensitivity towards sensation by using target and electrodermal activity as an index. In that study, the sympathetic nerve response for a given stimulus was reported to be much larger and habituation was reported to be difficult in patients with Fragile X syndrome compared with those in normally developed children. Based on the questionnaire administered by Chang et al.17) to parents of patients with targeted ASD, patients with auditory hypersensitive ASD continuously have a much stronger sympathetic nerve response to auditory stimuli compared with those in normally developed controls. Furthermore, Brown et al.18) used a self-report questionnaire in normal adults and examined the relationship between sympathetic nerve response and a sensation evaluation questionnaire. These authors classified subjects as those who had or who did not have a hypersensitive trend and measured their skin conductance. They reported that the responses were larger and habituation was difficult in subjects with auditory hypersensitivity. The findings of that report suggest the possibility of a relationship between the auditory-hypersensitive trend derived from the questionnaire and the abnormal responses of sympathetic nerves. However, the relationship between the characteristics of sound (frequency, dB) and the sympathetic nerve response to sound stimulation is not clear. In addition, the sympathetic nerve response to high- and low-frequency sound stimuli may vary because of differences in frequencies. In order to clarify sympathetic nerve responses to various types and various frequencies of auditory stimuli, we evaluated healthy adults, classified them into groups with (hypersensitive) and without (non-hypersensitive) auditory hypersensitivity using a self-report questionnaire, and measured the variations in palmar sweating elicited by sound stimuli with different characteristics.
SUBJECTS AND METHODS
In this study, 20 healthy volunteer students (4 males, 16 females) ranging in age from 19 to 25 years (mean ± standard deviation [SD], 21 ± 3 years) were recruited at Nagasaki University. None of the subjects had any diseases, medical history, or ear problems. All experimental methods were explained to the subjects. After obtaining consent for participation, we evaluated the characteristics of auditory hypersensitivity with the Adolescent/Adult Sensory Profile (AASP)19). The AASP is a standardized and self-completed questionnaire used in individuals whose ages range from 11 to 65 years. It consists of 60 items based on a 5-point Likert scale, with the answers ranging from almost never (1 point) to almost always (5 points). Higher scores indicate atypical sensory processing. AASP scores are comprised of four areas: low registration, sensation seeking, sensory sensitivity, and sensation avoiding. Neurologically, both hypersensitivity (51, I startle easily from an unexpected or loud noise; 54, I am distracted if there is a lot of noise around; or 60, I find it difficult to work with a noisy background) and sensation avoiding (53, When others are watching TV, I leave the room or ask them to turn it off; 56, I use strategies to drown out sound; or 57, I stay away from noisy settings) represent a low threshold state for auditory stimuli. In this study, we calculated the total score for hypersensitivity in hearing and sensation avoidance (30 full points) and used it as an index for auditory hypersensitivity. Additionally, we calculated the median scores and classified the subjects into the hypersensitive group if their scores were greater than the median or the non-hypersensitive group if their scores were less than the median.
Palmar sweating was recorded continuously from the thenar eminence using a probe attached to a 1-cm2 area and a sweating rate meter (SS-100, KANDS Co., Ltd., Aichi Prefecture, Japan). Auditory stimuli from the stimulator (MEB-5504, Neuro Pack∑, Nihon Kohden Corporation, Tokyo, Japan) were applied to both ears of the subject through headphones (DR-531, Nihon Kohden Corporation). Palmar sweating (mg/cm2/min) and auditory stimuli signals were simultaneously recorded with a computer and an A/D converter (PowerLab 16/23, ADInstruments Ltd., Dunedin, New Zealand) with a sampling frequency of 400 Hz. The data were analyzed with a software program (LabChart 6, ADInstruments Ltd.). The entire experiment was performed in a sound-shielded room, and the room temperature was 23 °C. Once all of the preparations were complete, each subject was requested to sit in an easy chair. The experiment was performed as described below. First, we informed the subjects that they would be hearing several series of sounds with different frequencies and magnitudes and instructed them not to move until the end of the trial, of which they would be informed by the experimenter in charge. Subsequently, each subject rested for 3 min. We ensured that huge variations in thenar sweating were not observed, and we then began the trial. For each trial, we took a baseline measure for 30-s in the beginning. One trial was comprised of repeating the same tone burst five times with a 30-s interval between bursts. We performed six such trials in total. These six trials were performed with the following six types of tone burst stimulations: 500 Hz-20 dB, 500 Hz-50 dB, 500 Hz-80 dB, 6,000 Hz-20 dB, 6,000 Hz-50 dB, and 6,000 Hz-80 dB. Each auditory stimulus was a tone burst of 1,000-ms duration, with a rise and fall duration of 10 ms. In order to counterbalance the effect of the order of stimulus presentation, the presentation order was randomized between subjects. To measure the degree of discomfort for each type of stimuli, the subjects were asked to select one of the following descriptors for each respective stimuli type: extreme discomfort, uncomfortable, a little discomfort, slight discomfort, or no discomfort at all.
To analyze the palmar sweating, the data from each subject were averaged every second. For each trial, the 0- to 30-s data before every first round of stimulation were considered the baseline values. Moreover, for each trial, after deriving the peak value of the palmar sweating from each baseline value, the variation was calculated for each stimulus, and this value was compared between the groups. To compare the reaction magnitude for each type of stimulus between the groups, we derived the amounts of palmar sweating for the baseline value and the first stimulus by analyzing the effect of the amount of reaction for group × type of stimulus × time (before vs. after the first stimulus) with a three-way analysis of variance (ANOVA).
To derive the amount of palmar sweating for the frequency of each type of stimulus for both groups, we observed the mutual interaction, the main effects of group, and the number of times by performing a two-way repeated ANOVA with a post hoc Bonferroni t-test. To analyze the differences in the degrees of discomfort between the groups in each type of stimulus, we used Mann-Whitney U tests for each stimulus type.
This study adhered to the Declaration of Helsinki and was approved by the Ethics Committee of the Department of Health Sciences, Nagasaki University Graduate School of Biomedical Sciences (12062824).
RESULTS
For the 20 healthy adult subjects, we calculated the total score of the items that reflected hypersensitivity and avoidance in the AASP auditory items. Because the median of the scores was 13 points (average ± SD score, 13.6 ± 4.73 points), subjects with scores higher than 13 points were grouped into the hypersensitive group (8 females, 2 males) and those that scored below 13 points were included in the non-hypersensitive group (8 females, 2 males). Table 1 describes participant characteristics. The groups did not significantly differ in age, gender, and sweating rate. The index for auditory hypersensitivity was significantly different between the groups (t = 7.397, p < 0.001).
Table 1. Characteristics of the subjects.
| Hyper | Non-hyper | p | |
|---|---|---|---|
| (n=10) | (n=10) | ||
| Age (yrs) | 20.9±1.7 | 20.4±1.5 | |
| Gender (m/f) | 2/8 | 2/8 | |
| Index for auditory hypersensitivity | 17.7±3.1 | 9.5±1.7 | ** |
| SR (mg/cm2/min) | 1.737±0.974 | 1.452±0.687 |
Values are mean ± SD. *p < 0.05; **p < 0.01. Hyper: hypersensitive group, Non-hyper: non-hypersensitive group, Index for auditory hypersensitivity: the total score of the items that reflected hypersensitivity and avoidance in the Adolescent/Adult Sensory Profile auditory items, SR: sweating rate
Findings regarding the discomfort with each stimulus are shown in Table 2. Based on the Mann-Whitney U tests, the degree of discomfort was significantly higher in the hypersensitive group compared with the non-hypersensitive group for the stimulations of 80 dB-6,000 Hz (p = 0.005). There were no significant differences for the other types of stimulations.
Table 2. Degree of discomfort score in both groups.
| Hyper | Non-hyper | p | |
|---|---|---|---|
| (n=10) | (n=10) | ||
| 80dB-6,000 Hz | 4.80 ± 0.4 | 4.4 ± 0.5 | |
| 80dB-500 Hz | 4.80 ± 0.4 | 3.1 ± 1.7 | ** |
| 50dB-6,000 Hz | 2.20 ± 1.0 | 2.1 ± 0.9 | |
| 50dB-500 Hz | 1.30 ± 0.5 | 1.2 ± 0.4 | |
| 20dB-6,000 Hz | 1.78 ± 1.0 | 1.7 ± 1.0 | |
| 20dB-500 Hz | 1.20 ± 0.6 | 1 |
Values are mean ± SD. *p < 0.05; **p < 0.01. Hyper: hypersensitive group, Non-hyper: non-hypersensitive group
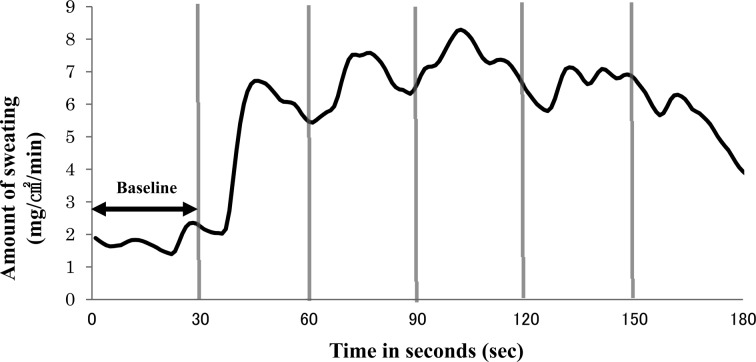
Figure 1 shows an example of a skin sweat response. Based on the three-way ANOVA, the group × type of stimulus × time (before vs. after the first stimulus) interaction was not significant for the amount of reaction, but a significant type of stimulus × time (before vs. after the first stimulus) interaction was observed for the amount of reaction (p < 0.001). Moreover, based on a simple main effect test of the amount of reaction before and after the stimulation at each level of stimulation type, a main effect was observed for 80 dB-500 Hz (p < 0.001) and 80 dB-6,000 Hz (p = 0.003). Group main effects were not observed for any type of stimulation.
Fig. 1.
Responses to 80 dB-6,000 Hz auditory stimuli of a representative individual from the hypersensitive group. Each vertical line represents an auditory stimulus.
When the change in palmar sweating was analyzed with a two-way repeated ANOVA, we found that the main effect for a group was observed only in the hypersensitive group at 80 dB-6,000 Hz (F = 6.654, p = 0.002), while the main effect for stimulation count was observed in the non- hypersensitive group at 80 dB-500 Hz (F = 5.780, p < 0.001), 50 dB-500 Hz (F = 2.933, p = 0.026), and 20 dB-6,000 Hz (F = 3.777, p = 0.008). For 80 dB-6,000 Hz, an interaction (p = 0.0348) was observed for the groups and stimulation counts. However, interaction was not observed in the other trials. Moreover, from a simple main effect test of the stimulation frequency at each level for every group, a main effect for stimulation count was observed in the hypersensitive group at 80 dB-6,000 Hz (p = 0.008), 80 dB-500 Hz (p < 0.001), 50 dB-6,000 Hz (p = 0.033), and 50 dB-500 Hz (p = 0.001). However, it was not observed in the non-hypersensitive group.
In the palmar sweating comparison tests between the groups for every stimulation count, significant differences were observed between the hypersensitive and non-hypersensitive groups at 80 dB-6,000 Hz (first time, p = 0.0003; second time, p < 0.001; third time, p < 0.001; fourth time, p < 0.001; and fifth time, p < 0.001), 80 dB-500 Hz (first time, p < 0.001; second time, p < 0.001; third time, p < 0.001; fourth time, p = 0.049; and fifth time, p = 0.008), 50 dB-6,000 Hz (first time, p = 0.0315; third time, p = 0.018; and fifth time, p = 0.046), 50 dB-500 Hz (first time, p = 0.001), 20 dB-6,000 Hz (first time, p = 0.014; second time, p = 0.007; and fifth time, p = 0.026), and 20 dB-500 Hz (fourth time, p = 0.008). The significant differences in palmar sweating variations between the stimulation frequencies in each group are shown in Table 3.
Table 3. Mean value of palmar sweating variations and standard deviation in each condition.
| 1st | 2nd | 3rd | 4th | 5th | ||
|---|---|---|---|---|---|---|
| 80 dB-6,000 Hz | Hyper | 0.139±0.217 | 0.242±0.299 | 0.257±0.302 | 0.169±0.212 | 0.141±0.182 |
| Non-hyper | 0.026±0.048 | 0.005±0.022 | 0.001±0.011 | −0.001±0.015 | −0.009±0.019 | |
| 80 dB-500 Hz | Hyper | 0.215±0.260 | 0.294±0.273 | 0.23±0.272 | 0.156±0.213 | 0.154±0.225 |
| Non-hyper | 0.073±0.137 | 0.096±0.183 | 0.084±0.168 | 0.071±0.133 | 0.036±0.074 | |
| 50 dB-6,000 Hz | Hyper | 0.035±0.060 | 0.029±0.070 | 0.037±0.093 | −0.001±0.166 | −0.030±0.130 |
| Non-hyper | −0.002±0.029 | −0.003±0.033 | −0.005±0.040 | −0.000±0.048 | 0.005±0.055 | |
| 50 dB-500 Hz | Hyper | 0.070±0.142 | 0.036±0.144 | 0.014±0.118 | 0.001±0.107 | −0.009±0.091 |
| Non-hyper | 0.006±0.016 | 0.005±0.025 | 0.005±0.033 | 0.001±0.026 | 0.000±0.027 | |
| 20 dB-6,000 Hz | Hyper | 0.059±0.087 | 0.022±0.098 | −0.011±0.120 | −0.022±0.138 | −0.017±0.157 |
| Non-hyper | 0.001±0.023 | −0.043±0.083 | −0.055±0.111 | −0.062±0.122 | −0.069±0.123 | |
| 20 dB-500 Hz | Hyper | −0.005±0.021 | 0.011±0.130 | 0.012±0.150 | 0.023±0.107 | 0.000±0.096 |
| Non-hyper | 0.005±0.026 | −0.008±0.018 | −0.020±0.034 | −0.024±0.042 | −0.019±0.055 |
Hyper: hypersensitive group, Non-hyper: non-hypersensitive group. Unit: mg/cm2/min
The differences in the variations in palmar sweating between the groups with respect to the baseline value and after the first stimulation were analyzed with one-way ANOVA. Significant differences in the average values were not found for all stimulation types. Stimulations in which the variances were significantly higher in the hypersensitive group compared with the non-hypersensitive group were 20 dB-6,000 Hz (p < 0.001), 50 dB-500 Hz (p = 0.003), and 80 dB-6,000 Hz (p = 0.002).
DISCUSSION
Although group × type of stimulus × time (before vs. after the first stimulus) interactions were not observed for the amount of reaction, a significant stimulus × time (before vs. after the first stimulus) interaction was observed for the amount of reaction. Therefore, these findings suggested that auditory stimulation influenced the palmar sweating in all of the subjects. Because the variations in palmar sweating before and after stimulation were larger for the 80-dB stimulation than for the 20-dB and 50-dB stimulations, it was likely that the influence on the sympathetic nerves was as large as the sound pressure.
We examined the habituation to stimulation and the differences in palmar sweating after stimulation of the hypersensitive and non-hypersensitive groups. Because differences occurred in the extent of palmar sweating between the hypersensitive and non-hypersensitive groups with the 80 dB-6,000 Hz stimulation, there were possible differences in habituation to the auditory stimuli between the groups. Additionally, in this regard, significant variations were observed in the palmar sweating of the hypersensitive group from the first to fifth times with the sound pressures of 80 dB and 50 dB. However, significant variations were not observed in the non-hypersensitive group. Therefore, these findings suggested that, for the non-hypersensitive group, the sympathetic nerve response was small, even for large sound pressure stimulations. For the hypersensitive group, however, the sympathetic nerve response was greater. Thus, it can be inferred that a person has a tendency for excessive autonomic nerve reactions in response to repeated loud noises if she/he routinely experiences excessive reactions to auditory stimulations. Because variations were observed in the transition of palmar sweating between the hypersensitive and non-hypersensitive groups for the stimulation types of 80 dB-6,000 Hz and 80 dB-500 Hz, it was believed that there were differences between the groups in their sympathetic nerve responses to repeated auditory stimuli. In this regard, from the first to fifth times, significant variations were observed in the palmar sweating of the hypersensitive group with the sound pressures of 80 dB and 50 dB, whereas no significant variations were observed in the non-hypersensitive group. Moreover, for the stimulations with 80 dB-500 Hz and 80 dB-6,000 Hz in the hypersensitive group, the amount of sweating clearly increased after the second stimulation, unlike in the non-hypersensitive group in which significant variations were not observed from the first to fifth times. In the hypersensitive group, the main effect of frequency was observed for 50 dB-6,000 Hz, and variations were observed between the groups in the first, third, and fifth times. Therefore, it was inferred that the sympathetic nerve responses of the subjects who regularly had excessive reactions to auditory stimuli differed from those of the persons who did not.
In a study that used healthy adults as subjects, Brown et al.18) reported that two groups with high scores for hypersensitivity and avoidance on the AASP had difficulty habituating to an auditory stimulation compared with two other groups with high scores for low registration and sensation seeking. Thus, it can be concluded that habituation to stimulation is difficult if a person’s scores for hypersensitivity and avoidance are high. Moreover, Chang et al.17) reported that children with ASD with auditory hypersensitivity exhibit high sympathetic activation and strong sympathetic reactivity to auditory stimuli and suggested that children with ASDs have difficulty habituating to auditory stimuli due to persistently strong galvanic skin responses. Furthermore, the results of this study suggested that the hypersensitive group felt significantly more discomfort with large sound pressures than the non-hypersensitive group did. The palmar sweating measured in this study could reflect mental sweating that is due to emotional changes, such as anger, fear, or anxiety. With repeated unpleasant stimuli, subjects in the hypersensitive group might have had a persistent predominant state of sympathetic nerves because they had more psychological burden and were anxiously anticipating the next stimulus.
Before and after the first stimulation, there was a difference in the variances for palmar sweating in both groups, and the variance of the hypersensitive group was larger than that of the non-hypersensitive group. Among the subjects who were classified into the hypersensitive group, subtypes may have existed depending on the auditory stimuli reaction pattern. The hypersensitive group may have included people who were prone to sympathetic nerve responses due to stimulation, people who were prone to excessive sympathetic nerve responses due to psychological factors, such as anxiety caused by repeated stimulation, and people who were subjectively hypersensitive but did not have a large reaction of sympathetic nerve response due to stimulation.
This study found that people with auditory hypersensitivity traits had different sympathetic nerve responses to repetitive and large sound pressure stimuli. There was no differentiation of the sympathetic nerve responses between 500 Hz and 6,000 Hz. However, sound pressure had an impact on the sympathetic nerve responses. People with auditory hypersensitivity traits may have a tendency for excessive sympathetic nerve reactions to sound in daily life. These findings suggested that therapists might play a role in helping people with auditory hypersensitivity traits to evaluate their needs in detail and discuss with their families their options for interventions, such as excluding unpleasant sounds.
REFERENCES
- 1.Kientz MA, Dunn W: A comparison of the performance of children with and without autism on the Sensory Profile. Am J Occup Ther, 1997, 51: 530–537 [DOI] [PubMed] [Google Scholar]
- 2.Baranek GT, David FJ, Poe MD, et al. : Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. J Child Psychol Psychiatry, 2006, 47: 591–601 [DOI] [PubMed] [Google Scholar]
- 3.Leekam SR, Nieto C, Libby SJ, et al. : Describing the sensory abnormalities of children and adults with autism. J Autism Dev Disord, 2007, 37: 894–910 [DOI] [PubMed] [Google Scholar]
- 4.Tomchek SD, Dunn W: Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am J Occup Ther, 2007, 61: 190–200 [DOI] [PubMed] [Google Scholar]
- 5.Gomes E, Rotta NT, Pedroso FS, et al. : Auditory hypersensitivity in children and teenagers with autistic spectrum disorder. Arq Neuropsiquiatr, 2004, 62: 797–801 [DOI] [PubMed] [Google Scholar]
- 6.Bromley J, Hare DJ, Davison K, et al. : Mothers supporting children with autistic spectrum disorders: social support, mental health status and satisfaction with services. Autism, 2004, 8: 409–423 [DOI] [PubMed] [Google Scholar]
- 7.Rosenhall U, Nordin V, Sandström M, et al. : Autism and hearing loss. J Autism Dev Disord, 1999, 29: 349–357 [DOI] [PubMed] [Google Scholar]
- 8.Khalfa S, Bruneau N, Rogé B, et al. : Increased perception of loudness in autism. Hear Res, 2004, 198: 87–92 [DOI] [PubMed] [Google Scholar]
- 9.Ashburner J, Ziviani J, Rodger S: Sensory processing and classroom emotional, behavioral, and educational outcomes in children with autism spectrum disorder. Am J Occup Ther, 2008, 62: 564–573 [DOI] [PubMed] [Google Scholar]
- 10.Lane AE, Young RL, Baker AE, et al. : Sensory processing subtypes in autism: association with adaptive behavior. J Autism Dev Disord, 2010, 40: 112–122 [DOI] [PubMed] [Google Scholar]
- 11.Gravel JS, Dunn M, Lee WW, et al. : Peripheral audition of children on the autistic spectrum. Ear Hear, 2006, 27: 299–312 [DOI] [PubMed] [Google Scholar]
- 12.Hitoglou M, Ververi A, Antoniadis A, et al. : Childhood autism and auditory system abnormalities. Pediatr Neurol, 2010, 42: 309–314 [DOI] [PubMed] [Google Scholar]
- 13.Homma S, Nakajima Y, Toma S, et al. : Intracerebral source localization of mental process-related potentials elicited prior to mental sweating response in humans. Neurosci Lett, 1998, 247: 25–28 [DOI] [PubMed] [Google Scholar]
- 14.Homma S, Matsunami K, Han XY, et al. : Hippocampus in relation to mental sweating response evoked by memory recall and mental calculation: a human electroencephalography study with dipole tracing. Neurosci Lett, 2001, 305: 1–4 [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Tomioka N, Ushiyama Y, et al. : Arithmetic calculation, deep inspiration or handgrip exercise-mediated pre-operational active palmar sweating responses in humans. Auton Neurosci, 2003, 104: 58–65 [DOI] [PubMed] [Google Scholar]
- 16.Miller LJ, McIntosh DN, McGrath J, et al. : Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am J Med Genet, 1999, 83: 268–279 [PubMed] [Google Scholar]
- 17.Chang MC, Parham LD, Blanche EI, et al. : Autonomic and behavioral responses of children with autism to auditory stimuli. Am J Occup Ther, 2012, 66: 567–576 [DOI] [PubMed] [Google Scholar]
- 18.Brown C, Tollefson N, Dunn W, et al. : The Adult Sensory Profile: measuring patterns of sensory processing. Am J Occup Ther, 2001, 55: 75–82 [DOI] [PubMed] [Google Scholar]
- 19.Brown CE, Dunn W: Adolescent/adult sensory profile: User’s manual. San Antonio: The Psychological Corporation,2002 [Google Scholar]