Abstract
[Purpose] A number of different stimulation devices are used in basic and clinical research studies, and their frequencies of use vary. However, whether or not they are equally effective has not been investigated. The purpose of the present study was to investigate neural activity in the brain during the illusion of motion evoked by stimulating the tendons of the wrist extensor muscles using various vibration devices. [Subjects] Twelve right-handed university students with no history of nervous system disorder or orthopedic disease participated in the study. [Methods] The wrist extensor tendon was stimulated using 3 different devices: 1) a vibration stimulation device (SL-0105 LP; Asahi Seisakusho Co., Ltd., Saitama, Japan), frequency 80 Hz; 2) a handy massager (YCM-20; Yamazen Corporation, Osaka, Japan), frequency 70 Hz; and 3) a handy massager (Thrive MD-01; Thrive Co., Ltd., Osaka, Japan), frequency 91.7 Hz. Brain activity was recorded during stimulation by using functional near-infrared spectroscopy. [Results] Increased neural activity was observed in both the premotor cortices and the parietal region in both hemispheres in all 3 cases. The level and localization of neural activity was comparable for all 3 stimulation devices used. [Conclusion] This suggests that subjects experience the illusion of motion while the tendon is being stimulated using any vibration device.
Key words: Illusion of motion, Tendon vibration, fNIRS
INTRODUCTION
In recent years, several neuroimaging techniques have been employed to investigate the neural activity associated with motor imagery and the illusion of motion. The human brain is able to recognize changes in limb position and movement from both visual and somatosensory stimuli, and the information conveyed to the brain by limb position and musculoskeletal movement plays a vital role in forming a representational body image within the brain. Collectively, these senses are referred to as kinesthesia. In this context, it has been reported that the illusion of motion evoked by vibrating the tendon of a limb can be effective in stimulating motor control and that it can aid motor learning1).
The illusion of motion elicited by vibrating the tendon of a limb at the appropriate frequency is mediated by firing of Ia afferent fibers that occurs in response to muscle spindle activity. The muscle spindle receptors are particularly sensitive to the direction and speed of limb movements; thus, during tendon vibration, subjects experience an illusion of motion, such as stretching of the affected muscles. Previous research has shown that the tendon vibration frequency optimal for eliciting the illusion of motion lies between 70 and 100 Hz2,3,4,5). One research group used magnetoencephalography (MEG) to record brain activity while subjects experienced the illusion of motion evoked by vibrating the tendon of a limb6) and revealed that this type of stimulation activated regions within the primary sensorimotor cortex, supplementary motor area, and angular gyrus. Another research group used functional magnetic resonance imaging (fMRI) to measure neural activity during the same illusion of motion1, 3) and reported neural activation in the contralateral primary sensory motor cortex, dorsal premotor cortex, supplementary motor area, cingulate motor cortex, and ipsilateral cerebellum. These motor areas are also known to be activated during both active limb movements and motor imagery7, 8).
With regard to clinical implications, Gay et al. reported that patients with complex regional pain syndrome (CRPS) type I experienced an improvement in both their pain symptoms and range of motion following vibration of the tendon of the affected limb and the associated illusion of motion9). Goble et al. reported an improvement in standing balance after vibration of the triceps tendon10). Moreover, Roll et al. reported that the stimulation of illusory movements prevented the cortical disruption normally caused by immobilization in healthy people and that the illusion of motion evoked by vibrating the tendon of a limb could likely prove to be an effective prophylactic treatment following surgery and cast immobilization with conservative therapy11).
These basic and clinical research studies used a number of different stimulation devices, and the optimal vibration frequency used to evoke the illusion of motion varied between these studies. While the neural activation patterns occurring during the illusion of motion have been described previously, differences in brain activity associated with the use of different vibration stimulation devices, or with varying vibration frequencies, have not yet been investigated. In a clinical setting, it is important to determine the most effective method of stimulation, as well the optimal stimulation frequency to evoke a beneficial illusion of motion; if the therapy can be delivered in a simple and cost-effective manner, it can be made available to a larger number of patients. Therefore, in this study, we aimed to compare the effects of a conventional vibration stimulation device with those of 2 handy massagers, which are widely available at electronics stores. We used functional near-infrared spectroscopy (fNIRS) to compare the patterns of neural activity occurring during the illusion of motion evoked by vibrating a tendon with these 3 devices.
SUBJECTS AND METHODS
Twelve right-handed college students (7 women and 5 men; mean age, 21.6 years; range, 20–22 years) with no previous history of neurological or other diseases participated in this study. All subjects provided written informed consent after being fully informed of the protocols and purpose of the study. This study was approved by the institutional ethics review board.
For each subject, the extensor tendon of the wrist was vibrated under 3 different conditions. Condition 1 was use of a vibration stimulation device (SL-0105 LP; Asahi Seisakusho Co., Ltd, Saitama, Japan) set at 80 Hz, which was determined to be the optimal frequency in a previous study3); condition 2 was use of a handy massager (YCM-20; Yamazen Corporation, Osaka, Japan) at a frequency of 70 Hz; and condition 3 was use of a handy massager (Thrive MD-01; Thrive Co., Ltd, Osaka, Japan) set at a frequency of 91.7 Hz. The frequency of the 2 massage devices were limited by their factory settings. The order in which the devices were used on each subject was randomized.
During the trial, subjects were instructed to relax in a sitting position with their eyes closed. It has been reported that the illusion of motion is unlikely to occur until the muscle is relaxed1). Subjects were also instructed not to open their eyes until instructed to do so, as visual information can disrupt the illusion of motion12). The location selected for vibration stimulation was the common digital extensor tendon of the right wrist joint. The stimulation protocol consisted of 15 s of rest followed by 30 s of stimulation and was applied three times for each condition. The intensity of the illusion evoked was assessed by each subject using a Visual Analog Scale (VAS) immediately after each condition. The illusion of motion was confirmed to reproduce movement of the wrist, and the range of the illusion was measured using an electrical goniometer (SG150; Biometrics Ltd., Newport, United Kingdom). We defined the “range of the illusion” as the range of tendon vibration at which subjects experienced the illusion of motion. As a control, the skin surrounding the extensor tendon of the wrist was stimulated using the same protocol. This allowed us to subtract the brain activity evoked by skin irritation and excitement of Meissner and Pacinian receptors from the brain activity evoked by the illusory motion3, 4).
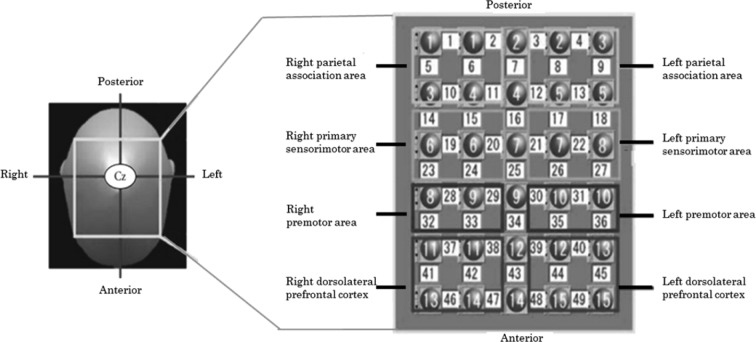
We measured neural activity in the brain by using an fNIRS system (FOIRE-3000; Shimadzu Corporation, Kyoto, Japan). The fNIRS system, which had continuous wave laser diodes with wavelengths of 780, 805 and 830 nm, was used to record cortical activity at a sampling rate of 5 Hz. In brief, we employed a 49-channel system with 30 optodes (15 light sources and 15 detectors). This system detects changes in cortical concentrations (mM · mm) of oxygenated hemoglobin (oxyHb), deoxygenated hemoglobin, and total hemoglobin, by applying the modified Beer-Lambert law. The optodes were positioned according to the International 10/20 system, with Cz located beneath the 7th light source and the other optodes located at intervals of 3.0 cm, centering around the 7th light source. The fNIRS topographic map covered the front parietal area, which was divided into 8 different regions of interest (ROIs, Fig. 1), based on the functional anatomy of the parietal and prefrontal regions. The left sensorimotor cortex was covered by channels 17, 18, 21, 22, 26, and 27; the right sensorimotor cortex was covered by channels 14, 15, 19, 20, 23, and 24; the left and right premotor cortices were covered by channels 30, 31, 35, and 36, and by channels 28, 29, 32, and 33, respectively; the left and right prefrontal cortices were covered by channels 37, 38, 41, 42, 46, and 47, and channels 39, 40, 44, 45, 48, and 49, respectively; and the left and right parietal areas were covered by channels 3, 4, 8, 9, 12, and 13 and channels 1, 2, 5, 6, 10, and 1, respectively (Fig. 1). It has been reported that a delayed reaction is inherent in fNIRS recordings; therefore, data were extracted beginning 5 s before the rest and task periods13).
Fig. 1.
Measurements using functional near-infrared spectroscopy. We employed a 49-channel system, with 30 optodes. Fifteen light sources (red numbers) and 15 detectors (blue numbers) covered the frontoparietal area. Solid white numbers denote measuring channels, which were divided into 8 regions of interest.
To compare the range of the illusion of motion and the intensity of the illusion under each of the 3 conditions, we used a one-way analysis of variance (ANOVA). We used Spearman’s rank correlation coefficient to examine the relationship between the range of illusion of motion and the reported intensity of the illusion.
In the fNIRS analysis, we selected oxyHb levels as markers of cortical activity because oxyHb is the most sensitive indicator of locomotion-related changes in regional cerebral blood flow14,15,16). Moreover, there are considerable individual differences in task-related changes in deoxyHb levels, probably due to variable neurovascular coupling in the elderly16,17,18,19). Changes in the oxyHb levels were calculated during the task phases of rest and vibration, which were defined as follows: the rest phase was the 15 s before the beginning of the 30 s task (vibration) phase. Regional changes in the oxyHb level during the control and vibration phases were obtained from each channel in each subject. Data for 3 repetitions were averaged for each channel; then the value for each region of interest was obtained by averaging data from several channels (Fig. 1) in each subject.
To evaluate the effect of cortical activation during the control and vibration periods, we calculated the effect size (ES) to adjust the influence of differential path length factors among subjects and cortical regions on oxyHb levels20). The ES for the effect of the vibration task on activities was calculated by the following formula: mean oxyHb value during vibration task-mean oxyHb value during control task/standard deviation of oxyHb value during control task.
We calculated ES for each channel16, 21) and analyzed ROIs, including the right and left primary sensory motor area, premotor area, and parietal association area. Moreover, we employed ROI analysis for the primary sensory motor area, premotor area, and parietal association area to compare the responses between the left and right hemispheres under each condition. For this analysis, we used two-way ANOVA and performed multiple comparisons using Scheffe’s method. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) ver#17.0 (SPSS, Chicago, IL, USA). The threshold for statistical significance was set at p = 0.05 for all analyses.
RESULTS
Under all 3 conditions, all 12 subjects experienced the illusion of motion. There was no significant difference in the range of illusion or in the intensity of the illusion between the 3 conditions (p > 0.05) (Table 1). Under all 3 conditions, the illusion intensity and range of illusion were significantly correlated (p < 0.05) (Table 2).
Table 1. Range of illusion and illusion intensity under all conditions.
| Condition 1 | Condition 2 | Condition 3 | |
|---|---|---|---|
| Range of illusion (°) | 42.8 ± 16.8 | 38.3 ± 13.2 | 38.8 ± 14.0 |
| Illusion intensity (VAS; mm) | 75.2 ± 21.9 | 70 ± 20.4 | 70.9 ± 19.9 |
Mean ± SD. VAS: Visual Analog Scale
Table 2. Correlation between range of illusion and illusion intensity.
| Range of illusion | |||
| Condition 1 | Condition 2 | Condition 3 | |
| Condition 1 | |||
| Condition 2 | 0.83** | ||
| Condition 3 | 0.85** | 0.95** | |
| *p<0.05; **p<0.01 | |||
| Illusion intensity (VAS) | |||
| Condition 1 | Condition 2 | Condition 3 | |
| Condition 1 | |||
| Condition 2 | 0.83** | ||
| Condition 3 | 0.87** | 0.92** | |
*p<0.05; **p<0.01. VAS: Visual Analog Scale
Analysis of neural activity showed a significant increase in oxyHb level in the left premotor cortex (p < 0.05), left and right sensory motor cortices, and parietal area (p < 0.01) under all 3 conditions (Table 3). Multiple comparison analysis revealed no significant difference in brain activity among the different vibration stimulation devices used.
Table 3. Comparison of effect size in regions of interest (ROIs) under the 3 conditions.
| ROI | Illusion | Control | |
|---|---|---|---|
| Right parietal association area | Condition 1 | 0.133 ± 0.317** | −0.350 ± 0.472 |
| Condition 2 | 0.152 ± 0.306** | −0.330 ± 0.454 | |
| Condition 3 | 0.119 ± 0.318** | −0.208 ± 0.469 | |
| Right primary sensorimotor area | Condition 1 | 0.030 ± 0.369** | −0.323 ± 0.433 |
| Condition 2 | 0.101 ± 0.342** | −0.349 ± 0.432 | |
| Condition 3 | 0.003 ± 0.345** | −0.384 ± 0.456 | |
| Right premotor area | Condition 1 | 0.006 ± 0.358 | 0.314 ± 0.687 |
| Condition 2 | 0.020 ± 0.357 | 0.153 ± 0.688 | |
| Condition 3 | 0.057 ± 0.378 | 0.102 ± 0.704 | |
| Left parietal association area | Condition 1 | 0.149 ± 0.360** | −0.325 ± 0.403 |
| Condition 2 | 0.046 ± 0.359** | −0.202 ± 0.398 | |
| Condition 3 | 0.106 ± 0.328** | −0.240 ± 0.420 | |
| Left primary sensorimotor area | Condition 1 | 0.118 ± 0.278** | −0.192 ± 0.256 |
| Condition 2 | 0.114 ± 0.278** | −0.164 ± 0.229 | |
| Condition 3 | 0.188 ± 0.295** | −0.256 ± 0.243 | |
| Left premotor area | Condition 1 | 0.147 ± 0.415* | −0.149 ± 0.382 |
| Condition 2 | 0.168 ± 0.397* | −0.107 ± 0.382 | |
| Condition 3 | 0.106 ± 0.416* | −0.198 ± 0.399 | |
Mean ± SD. *p<0.05; **p<0.01
DISCUSSION
In this study, we found no significant difference in the intensity of the evoked illusion or in the range of the illusion of motion between the 3 conditions; furthermore, the illusion intensity and range of illusion of motion were significantly correlated under all 3 conditions. This indicates that each one of the vibration stimulation devices used elicited the same illusory effect. We observed a significant increase in oxyHb concentrations in the left premotor cortex, left and right sensory motor cortices, and parietal area under each of the 3 conditions during the illusion of motion. We also observed neural activation in the same brain areas as previously reported1, 3, 6).
Previous studies investigating vibration stimulation of the tendon consistently reported the optimal frequency for eliciting the illusion of motion as being between 70 and 100 Hz2,3,4,5) however, these studies used different vibration stimulation devices. The majority of studies used a stimulation frequency of approximately 80 Hz22,23,24,25,26,27). The stimulation frequencies of the 2 handy massagers used in this study were between 70 and 91.7 Hz, which is within the range reported to be optimal for eliciting the illusion of motion. In the current study, we demonstrated that these devices could reliably evoke not only the same illusion intensity and range of illusion of motion but also the same patterns of neural activity as that produced using more sophisticated vibration stimulation devices.
Previous studies have shown that the illusion of motion evoked by vibration stimulation of the tendon activates the same brain areas as those activated during both active movement and motor imagery7, 8). During active movement, efferent sensory information is input to the brain as the muscle contracts, and information from the muscle spindles plays an important role in the perception of movement and of changes in limb position. The primary motor cortex is crucial for kinesthetic information processing and perception of movement and position1, 22). Here, we observed increased activity in the primary motor cortex during the illusion of motion. This was most likely due to afferent input from muscle spindles, as well as the illusion of motion caused by vibration stimulation of the wrist tendon. Thus, we think that active movement and illusion of motion are associated with similar patterns of brain activity.
In addition, previous studies showed similar brain activity patterns for motor imagery and the illusion of motion evoked by vibration stimulation applied to a tendon8). Notably, it has been reported that motor imagery can be effective in promoting functional recovery of the upper limb in patients with stroke28). Further, the intensity of the illusion can be decreased or increased by actively evoking motor imagery and applying vibration stimulation to the appropriate tendon at the same time8, 26). Thus, by employing a combination of the illusion of motion evoked by vibration stimulation and active motor imagery, the potential beneficial effects in a clinical setting can be maximized for both the techniques. Previous studies have also shown that illusory movements prevent the cortical disruption normally caused by immobilization in healthy people11) and that the pain symptoms and range of motion of patients with CRPS type I improved after they experienced the illusion of motion evoked by vibrating the tendon of the affected limb9).
Given the above findings, we believe that the illusion of motion evoked by vibration stimulation of a tendon could be effectively utilized in a clinical setting for prophylactic and rehabilitative purposes. Furthermore, our result showing that the illusion of motion can be reliably evoked using a handy massager within the optimal stimulation frequency indicates the possibility of making the technique easily accessible and affordable for a large number of patients in a variety of clinical settings.
Acknowledgments
We would like to thank all the volunteers for participating in this study.
REFERENCES
- 1.Naito E: Sensing limb movements in the motor cortex: how humans sense limb movement. Neuroscientist, 2004, 10: 73–82 [DOI] [PubMed] [Google Scholar]
- 2.Goodwin GM, McCloskey DI, Matthews PB: The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain, 1972, 95: 705–748 [DOI] [PubMed] [Google Scholar]
- 3.Roll JP, Vedel JP: Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res, 1982, 47: 177–190 [DOI] [PubMed] [Google Scholar]
- 4.Naito E, Ehrsson HH: Kinesthetic illusion of wrist movement activates motor-related areas. Neuroreport, 2001, 12: 3805–3809 [DOI] [PubMed] [Google Scholar]
- 5.Naito E, Ehrsson HH, Geyer S, et al. : Illusory arm movements activate cortical motor areas: a positron emission tomography study. J Neurosci, 1999, 19: 6134–6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casini L, Romaiguère P, Ducorps A, et al. : Cortical correlates of illusory hand movement perception in humans: a MEG study. Brain Res, 2006, 1121: 200–206 [DOI] [PubMed] [Google Scholar]
- 7.Keinrath C, Wriessnegger S, Müller-Putz GR, et al. : Post-movement beta synchronization after kinesthetic illusion, active and passive movements. Int J Psychophysiol, 2006, 62: 321–327 [DOI] [PubMed] [Google Scholar]
- 8.Naito E, Kochiyama T, Kitada R, et al. : Internally simulated movement sensations during motor imagery activate cortical motor areas and the cerebellum. J Neurosci, 2002, 22: 3683–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gay A, Parratte S, Salazard B, et al. : Proprioceptive feedback enhancement induced by vibratory stimulation in complex regional pain syndrome type I: an open comparative pilot study in 11 patients. Joint Bone Spine, 2007, 74: 461–466 [DOI] [PubMed] [Google Scholar]
- 10.Goble DJ, Coxon JP, Van Impe A, et al. : Brain activity during ankle proprioceptive stimulation predicts balance performance in young and older adults. J Neurosci, 2011, 31: 16344–16352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roll R, Kavounoudias A, Albert F, et al. : Illusory movements prevent cortical disruption caused by immobilization. Neuroimage, 2012, 62: 510–519 [DOI] [PubMed] [Google Scholar]
- 12.Hagura N, Oouchida Y, Aramaki Y, et al. : Visuokinesthetic perception of hand movement is mediated by cerebro-cerebellar interaction between the left cerebellum and right parietal cortex. Cereb Cortex, 2009, 19: 176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasdzewski G, Strangman G, Wagner J, et al. : Differences in the hemodynamic response to event-related motor and visual paradigms as measured by near-infrared spectroscopy. Neuroimage, 2003, 20: 479–488 [DOI] [PubMed] [Google Scholar]
- 14.Miyai I, Tanabe HC, Sase I, et al. : Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage, 2001, 14: 1186–1192 [DOI] [PubMed] [Google Scholar]
- 15.Hoshi Y, Kobayashi N, Tamura M: Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol 1985, 2001, 90: 1657–1662 [DOI] [PubMed] [Google Scholar]
- 16.Schroeter ML, Zysset S, Kruggel F, et al. : Age dependency of the hemodynamic response as measured by functional near-infrared spectroscopy. Neuroimage, 2003, 19: 555–564 [DOI] [PubMed] [Google Scholar]
- 17.Strangman G, Franceschini MA, Boas DA: Factors affecting the accuracy of near-infrared spectroscopy concentration calculations for focal changes in oxygenation parameters. Neuroimage, 2003, 18: 865–879 [DOI] [PubMed] [Google Scholar]
- 18.D’Esposito M, Deouell LY, Gazzaley A: Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci, 2003, 4: 863–872 [DOI] [PubMed] [Google Scholar]
- 19.Hesselmann V, Zaro Weber O, Wedekind C, et al. : Age related signal decrease in functional magnetic resonance imaging during motor stimulation in humans. Neurosci Lett, 2001, 308: 141–144 [DOI] [PubMed] [Google Scholar]
- 20.Riecker A, Grodd W, Klose U, et al. : Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. J Cereb Blood Flow Metab, 2003, 23: 565–573 [DOI] [PubMed] [Google Scholar]
- 21.Winer BJ: Statistical principles in experimental design. New York: Mc Graw-Hill. 1991 [Google Scholar]
- 22.Naito E, Roland PE, Ehrsson HH: I feel my hand moving: a new role of the primary motor cortex in somatic perception of limb movement. Neuron, 2002, 36: 979–988 [DOI] [PubMed] [Google Scholar]
- 23.Naito E, Ehrsson HH: Somatic sensation of hand-object interactive movement is associated with activity in the left inferior parietal cortex. J Neurosci, 2006, 26: 3783–3790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naito E, Scheperjans F, Eickhoff SB, et al. : Human superior parietal lobule is involved in somatic perception of bimanual interaction with an external object. J Neurophysiol, 2008, 99: 695–703 [DOI] [PubMed] [Google Scholar]
- 25.Roll JP, Albert F, Thyrion C, et al. : Inducing any virtual two-dimensional movement in humans by applying muscle tendon vibration. J Neurophysiol, 2009, 101: 816–823 [DOI] [PubMed] [Google Scholar]
- 26.Thyrion C, Roll JP: Perceptual integration of illusory and imagined kinesthetic images. J Neurosci, 2009, 29: 8483–8492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kito T, Hashimoto T, Yoneda T, et al. : Sensory processing during kinesthetic aftereffect following illusory hand movement elicited by tendon vibration. Brain Res, 2006, 1114: 75–84 [DOI] [PubMed] [Google Scholar]
- 28.Sharma N, Baron JC, Rowe JB: Motor imagery after stroke: relating outcome to motor network connectivity. Ann Neurol, 2009, 66: 604–616 [DOI] [PMC free article] [PubMed] [Google Scholar]