Abstract
[Purpose] It is well known that, in both in vivo and in vitro tests, muscle fatigue is produced by severe exercise, electrical stimulation, and so on. However, it is not clear whether or not low-frequency and high-amplitude modulation specifically affects serum myoglobin or urine myoglobin. The purpose of the present study was to determine the effect of low-frequency and high-amplitude modulation on serum myoglobin and urine myoglobin. [Methods] The study used whole blood samples and urine produced over 24 hours from the thirteen healthy subjects. [Results] There was a significant increase in serum myoglobin following electrical stimulation at a frequency of 10 Hz compared with the control group. Furthermore, within 24 hours, urine myoglobin also showed a significant increase for the test volunteers subjected to electrical stimulation at the 10 Hz frequency compared with the control group. However, there were no significant differences in the concentrations of hematologic results in subjects treated with electrical stimulation. [Conclusion] These results suggest that increased myoglobin related to muscle fatigue from electrical stimulation, particularly with a current of 10 Hz combined with a high-amplitude, may be partially related to increased muscle damage.
Key words: Myoglobin, Pain, High-amplitude electrical stimulation
INTRODUCTION
Myoglobin, which was discovered in 1897, consists of 153 amino acids and 17,500 dalton of monomeric heme protein1). Structurally, it consists of a single chain protein and a heme group1). Unlike hemoglobin, which is a tetramer, a molecule of myoglobin only has one iron atom. It has a high bonding affinity for oxygen, carbon monoxide, and nitric oxide1, 2). Myoglobin is found in muscle tissue including within muscle fiber cytoplasm and is described as an oxygen storage protein1, 3). When the oxygen supply becomes limited, myoglobin dissociates the combined oxygen and thus facilitates access to oxygen for the mitochondria. Finally, it promotes energy creation through oxidative phosphorylation3, 4). It is well known that inhibiting the function of myoglobin induces decreased oxygen uptake, decreased work output, and the production of adenosine triphosphate3, 4). However, pain and the excretion of myoglobin at the muscle are important biomarkers of muscle damage5,6,7,8). Myoglobin has a small molecular mass unlike creatine kinase, which leaks into blood (half-life: 5.5 ± 3.2 h)9, 10). It is also reported that myoglobin is useful for early diagnosis of myocardial infarction because it is rapidly excreted in urine9, 10). Some studies on pain control through limiting of sympathetic nerves by electric and hot stimuli have been carried out11, 12). It is known that applying improper electrotherapy can induce adverse effects, such as pain and muscle damage7, 13). However, no study has examined the effect of electric stimuli on myoglobin itself. Therefore, this study produced muscle contraction in subjects, confirmed visually, by applying frequencies of 1 Hz, 3 Hz, and 10 Hz, which were tolerable for participants, and examined any resulting pain response and changes in the volume of myoglobin in blood and urine.
SUBJECTS AND METHODS
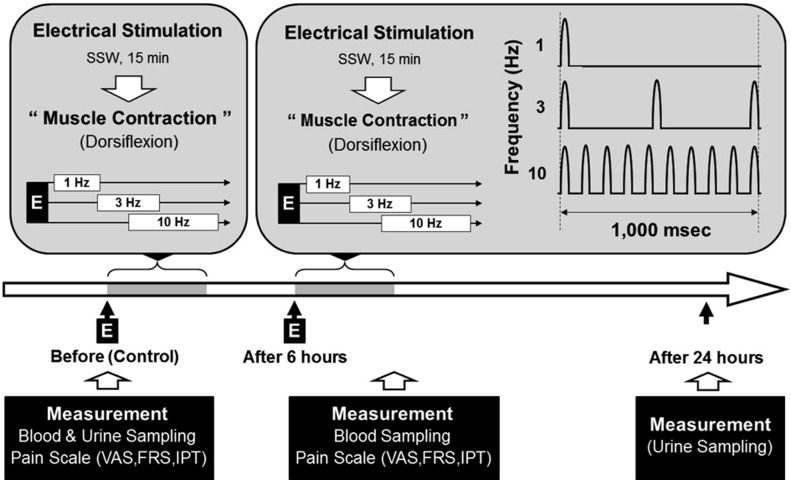
A total of thirteen men who were healthy (age, 21–27; weight, 63.3 ± 3.3 kg; height, 172.1 ± 2.7 cm) were randomly selected. Before taking part in this study, no participants had been involved in similar experiments. The temperature was maintained at 24 ± 1 °C, and all measurements were taken with the participant in the sitting position. To reduce variable use of mechanisms of the body, the participants were housed together for 24 hours and provided limited food and drink. The participants were divided into a study group, to whom the electric stimuli were applied, and a control group. The stimulus applied was a low-frequency semisinusoidal wave, and measurements were taken (Neuromed III STT-100, Stratek Co., Ltd., Anyang, Republic of Korea) after an one-week interval. The first set of stimuli, electrical stimulation at 1 Hz, 3 Hz, and 10 Hz, respectively, was applied for 15 minutes. After 6 hours, the second set of stimuli, electrical stimulation at 1 Hz, 3 Hz, and 10 Hz, was applied for 30 minutes (Fig. 1). To induce dorsiflexion of the hand joint, the disperse electrode was placed on the dorsal hand, and the activated electrode set on the lateral region of the elbow joint and epicondyle of the humerus. During the test, all participants were in the sitting position with the humerus sustained comfortably on a table. The stimulation range was 16 to 30 mA, which was endurable for the participants (Fig. 1). Muscle contraction was confirmed visually. The visual analogue scale (VAS), faces pain rating scale (FRS), and Iowa pain thermometer (IPT) were used to measure the induced pain. Measurements were taken three times because of the disadvantage of using subjective measurement tools12). The participants’ urine was sampled over 24 hours from 8:00 a.m. to 8:00 a.m. the next day (Fig. 1). To prevent decomposition of the urine samples, 10 mL of 6N HCL was added. Forty milliliters of urine was placed in serum separator tubes and stored in a freezer before analysis. Whole blood samples of 8–10 ml were taken from the cubital vein after the electrical stimulus of each participant while they remained in their sitting position. All samples were taken within 1–2 minutes of application of the stimulus, between 4:00 p.m. and 6:00 p.m., to take account of myoglobin’s half-life14). The whole blood samples were placed in glass EDTA tubes in volumes of 2–3 ml. The serum samples were left for 30–60 minutes at room temperature, and then separated using a centrifuge for 10 minutes at 3,000 rpm. Finally, the separated serum was stored in a refrigerator prior to measurement. The analysis of myoglobin in urine and in serum was performed by radioimmunoassay (Seoul Clinical Laboratories, Seoul, Republic of Korea) (Fig. 1). The SAS (version 6.12) statistical software was used for the statistical analysis. A t-test and one-way ANOVA were performed, and the level of statistical significance was set at p < 0.05. All data in the results are presented as means ± SEM. The protocol for the study was approved by the Committee of Ethics in Research of the University of Yongin, in accordance with the terms of Resolution 5-1-20, December 2006. Furthermore, all subjects provided informed consent for participation in the study.
Fig. 1.
Schematic representation of the experimental protocol for the normal healthy volunteers. Serum and urine myoglobin values were determined as described in the SUBJECTS AND METHODS section. SSW, semisinusoidal wave; E, electrical stimulation; dorsiflexion, extension of wrist joint; VAS, visual analogue scale; FRS, faces pain rating scale; IPT, Iowa pain thermometer
RESULTS
Following the application of an electric stimulus, there was no change in the level of red blood cells, white blood cells, hemoglobin, and hematocrit (Table 1). However, changes were produced in serum and in urine. The values of myoglobin in serum and in urine showed a time-dependent increase in the case group to which electric stimuli had been applied in comparison with the control group (Table 2). This was especially evident in the case of the application of the electric stimulus at the 10 Hz frequency (Table 2). Furthermore, the measured pain in the case group increased in the same time-dependent pattern following the electric stimulation in contrast with the control group (Table 2). This was again most evident with the use of electric stimulation at the 10 Hz frequency (Table 2).
Table 1. Hematologic changes due to electrical stimulation in healthy volunteers.
| Electrical stimulation with a
semisinusoidal wave |
||||
|---|---|---|---|---|
| Control | 1 Hz | 3 Hz | 10 Hz | |
| RBC (× 106/mm3) | 4.9 ± 0.7 | 4.9 ± 0.2 | 4.9 ± 0.2 | 4.9 ± 0.2 |
| Hb (g/dl) | 15.5 ± 0.3 | 15.5 ± 0.5 | 15.2 ± 1.0 | 16.0 ± 1.0 |
| Hct (%) | 44.4 ± 1.7 | 44.6 ± 2.0 | 46.3 ± 2.0 | 46.0 ± 2.3 |
| MCV (fl) | 92.0 ± 8.0 | 93.6 ± 2.2 | 94.0 ± 9.4 | 90.7 ± 8.1 |
| MCH (pg) | 31.9 ± 0.5 | 31.9 ± 1.1 | 32.7 ± 1.5 | 32.1 ± 1.3 |
| MCHC (g/dl) | 34.4 ± 0.4 | 34.9 ± 0.8 | 34.1 ± 1.1 | 34.2 ± 1.0 |
| SGOT (IU/l) | 15.8 ± 0.8 | 18.0 ± 1.8 | 18.7 ± 1.5 | 19.5 ± 1.6 |
| SGPT (IU/l) | 9.0 ± 0.8 | 11.0 ± 1.2 | 11.2 ± 0.4 | 12.3 ± 1.8 |
| Uric Acid (mg/dl) | 6.1 ± 2.1 | 6.1 ± 2.0 | 6.0 ± 1.9 | 6.1 ± 2.3 |
| BUN (mg/dl) | 12.2 ± 3.1 | 11.6 ± 3.1 | 13.0 ± 2.8 | 13.2 ± 3.9 |
The data are expressed as means±SEM. RBC, red blood cell or erythrocyte; Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamate pyruvate transaminase; BUN, blood urea nitrogen
Table 2. The effects of phase duration-dependent electrical stimulation on pain and myoglobin in healthy volunteers.
| Electrical stimulation with a
semisinusoidal wave |
||||
|---|---|---|---|---|
| Control | 1 Hz | 3 Hz | 10 Hz | |
| Serum myoglobin (ng/ml) | 7.7 ± 1.5 | 7.7 ± 2.2 | 8.8 ± 4.0 | 17.3 ± 4.7* |
| Urine myoglobin (ng/ml) | 5.1 ± 0.4 | 4.0 ± 0.5 | 4.2 ± 0.4 | 8.3 ± 0.8* |
| Visual analogue scale | 2.7 ± 0.9 | 3.0 ± 1.5 | 4.3 ± 1.8 | 6.7 ± 2.3* |
| Faces pain rating scale | 2.3 ± 0.9 | 2.7 ± 1.2 | 3.7 ± 0.9 | 6.0 ± 1.5* |
| Iowa pain thermometer | 2.3 ± 0.9 | 2.7 ± 0.9 | 3.3 ± 0.7 | 6.7 ± 1.9* |
| Total pain scale | 2.4 ± 0.4 | 2.8 ± 0.6 | 3.8 ± 0.6 | 6.4 ± 0.8* |
The data are expressed as mean±SEM. * p < 0.05
DISCUSSION
In rehabilitation, electrical stimulation is commonly applied to stimulate nerves and muscle15). However, fatigue resistance, density of capillaries, age, and medical history differ in each patient. When electric stimuli are applied without taking this into account, the resulting muscle fatigue can induce the accumulation of lactic acid and metabolites16, 17). Muscle fatigue is the condition in which force-generating ability is decreased and power output cannot be sustained. It therefore means a decrease in performance18). It is known that the accumulation of lactic acid and the related decrease in pH is the mechanism of muscle fatigue, as this inhibits reactions between Ca2+ and troponin C19). In a previous study, it was found that inhibiting Ca2+ uptake of the sarcoplasmic reticulum or decreases in Ca2+ release of the sarcoplasmic reticulum can lead to fatigue20). It has been reported that increases in extracellular K+ and intracellular Na+, caused by decreases in the function of Na+/K+-ATPase, are related to muscle fatigue21). The freeing of bradykinin and increasing activation of cyclooxygenase cause these elements to accumulate and can induce pain22, 23). The concentration of myoglobin is approximately 0.05–5 mmol/kg; it exists in skeletal muscle, cell membranes, and cytoplasm3). The function of myoglobin is storage and provision of oxygen for the tricarboxylic acid cycle of mitochondria. It is important in the production of adenosine triphosphate4). Diffusion occurs because cytochrome has an oxygen bonding affinity 50 times higher than that of myoglobin23). In other words, oxygen diffuses from myoglobin to mitochondria during muscle contractions, and this process is reversed during relaxation24). The relevance of myoglobin being found in blood serum following exercise or muscle tissue damage is evident6, 25). Sabria et al. reported that serum myoglobin increased according to exercise intensity, while there was no change during moderate exercise26). In a study by Kirwan et al., the value of both creatine kinase and myoglobin increased after unilateral isometric knee extension exercise with forty maximal voluntary contractions27). Roti et al. measured the levels of creatine phosphokinase, lactate dehydrogenase, and myoglobin in blood in 33 football players during the 24 hours following a football game28). The myoglobin value was greatly increased immediately after the game and then gradually decreased over time28). Also, Paul et al.29) reported results for measures taken from 17 weight lifters, stating that creatine kinase and myoglobin were increased after 3 sets of 6 weight lifting exercises with intensities of 70–80%. This indicates muscle damage29). Similar results were found in studies on soldiers, which showed that serum myoglobin was greatly increased after intense exercise30). Viitasalo et al. said that serum myoglobin increased in case groups to which a water jet massage had been applied for 20–30 minutes, in contrast with control groups31). In this study, the values of myoglobin in serum and urine were significantly increased according to the increasing frequency of electrical stimuli. It is reported that myoglobin leaks into the blood of patients with cardiac infarction, renal failure, Duchenne type muscular dystrophy, and polymyositis, and so its presence can be used for diagnosis9, 10, 32). Terrados et al. compared normobaric and hypobaric conditions and found that myoglobin increased in the latter condition33). Cole suggested that myoglobin is important in maintaining muscle function and O2 consumption3, 34). He reported that the myoglobin of the dog gastrocnemius-plantaris muscle under conditions of hypoxia increased after isometric exercise with twitch stimulation at 3 Hz3, 34). We indirectly confirmed that electric stimuli do not affect liver function based on the consistency of SGOT and SGPT values35). Given the consistency of the BUN values, it also appears that electric stimuli do not affect kidney function either36). However, the values of serum and urine myoglobin showed a statistically significant increase for the 24 hours following application of the electric stimuli. Thus, based on the results of this and previous studies, it is important that electric stimuli are applied solely within the appropriate range of pain. Also, physical therapists must carefully manage use of electric stimuli according to the subjective experience of their patient7). However, it is difficult to be more specific with these limited results: more systematic and multilateral studies are needed37).
REFERENCES
- 1.Hendgen-Cotta UB, Flögel U, Kelm M, et al. : Unmasking the Janus face of myoglobin in health and disease. J Exp Biol, 2010, 213: 2734–2740 [DOI] [PubMed] [Google Scholar]
- 2.Hazarika S, Angelo M, Li Y, et al. : Myocyte specific overexpression of myoglobin impairs angiogenesis after hind-limb ischemia. Arterioscler Thromb Vasc Biol, 2008, 28: 2144–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole RP: Myoglobin function in exercising skeletal muscle. Science, 1982, 216: 523–525 [DOI] [PubMed] [Google Scholar]
- 4.Wittenberg BA, Wittenberg JB: Myoglobin-mediated oxygen delivery to mitochondria of isolated cardiac myocytes. Proc Natl Acad Sci USA, 1987, 84: 7503–7507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascensão A, Rebelo A, Oliveira E, et al. : Biochemical impact of a soccer match - analysis of oxidative stress and muscle damage markers throughout recovery. Clin Biochem, 2008, 41: 841–851 [DOI] [PubMed] [Google Scholar]
- 6.Lippi G, Schena F, Salvagno GL, et al. : Acute variation of biochemical markers of muscle damage following a 21-km, half-marathon run. Scand J Clin Lab Invest, 2008, 68: 667–672 [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Lee JU, Kim IH, et al. : Noxiousness of hypertension-related norepinephrine and upregulation of norepinephrine induced by high intensity electrical stimulation in healthy volunteers. J Phys Ther Sci, 2012, 24: 795–800 [Google Scholar]
- 8.Lee JU, Kim JH, Kim MY, et al. : Increase of myoglobin in rat gastrocnemius muscles with immobilization-induced atrophy. J Phys Ther Sci, 2013, 25: 1617–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groth T, Sylvén C: Myoglobin kinetics in patients suffering from acute myocardial infarction in its early phase -as studied by the single injection method. Scand J Clin Lab Invest, 1981, 41: 79–85 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Komiyama N, Koizumi T, et al. : Usefulness of rapid quantitative measurement of myoglobin and troponin T in early diagnosis of acute myocardial infarction. Circ J, 2004, 68: 639–644 [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Kim JH: The effects of physical therapy on activation of sympathetic nerve system among older adults participating to welfare centers for the elderly. J Kor Gerontol Soc, 2010, 30: 311–322 [Google Scholar]
- 12.Kim MY, Kim JH, Lee JU, et al. : Temporal changes in pain and sensory threshold of geriatric patients after moist heat treatment. J Phys Ther Sci, 2011, 23: 797–801 [Google Scholar]
- 13.Nosaka K, Aldayel A, Jubeau M, et al. : Muscle damage induced by electrical stimulation. Eur J Appl Physiol, 2011, 111: 2427–2437 [DOI] [PubMed] [Google Scholar]
- 14.Ellis AK, Saran BR: Kinetics of myoglobin release and prediction of myocardial myoglobin depletion after coronary artery reperfusion. Circulation, 1989, 80: 676–683 [DOI] [PubMed] [Google Scholar]
- 15.Lee WD, Kim JH, Lee JU, et al. : Differences in rheobase and chronaxie between the paretic and non-paretic sides of hemiplegic stroke patients: a pilot study. J Phys Ther Sci, 2013, 25: 717–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jorfeldt L, Juhlin-Dannfelt A, Karlsson J: Lactate release in relation to tissue lactate in human skeletal muscle during exercise. J Appl Physiol, 1978, 44: 350–352 [DOI] [PubMed] [Google Scholar]
- 17.Tesch PA, Wright JE: Recovery from short term intense exercise: its relation to capillary supply and blood lactate concentration. Eur J Appl Physiol Occup Physiol, 1983, 52: 98–103 [DOI] [PubMed] [Google Scholar]
- 18.Fitts RH: Cellular mechanisms of muscle fatigue. Physiol Rev, 1994, 74: 49–94 [DOI] [PubMed] [Google Scholar]
- 19.Robertson SP, Kerrick WG: The effects of pH on Ca2+-activated force in frog skeletal muscle fibers. Pflugers Arch, 1979, 380: 41–45 [DOI] [PubMed] [Google Scholar]
- 20.Chin ER, Balnave CD, Allen DG: Role of intracellular calcium and metabolites in low-frequency fatigue of mouse skeletal muscle. Am J Physiol, 1997, 272: C550–C559 [DOI] [PubMed] [Google Scholar]
- 21.Sjøgaard G, Adams RP, Saltin B: Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol, 1985, 248: R190–R196 [DOI] [PubMed] [Google Scholar]
- 22.Stebbins CL, Carretero OA, Mindroiu T, et al. : Bradykinin release from contracting skeletal muscle of the cat. J Appl Physiol 1985, 1990, 69: 1225–1230 [DOI] [PubMed] [Google Scholar]
- 23.Gayeski TE, Honig CR: Direct measurement of intracellular O2 gradients; role of convection and myoglobin. Adv Exp Med Biol, 1983, 159: 613–621 [DOI] [PubMed] [Google Scholar]
- 24.Wittenberg BA, Wittenberg JB, Caldwell PR: Role of myoglobin in the oxygen supply to red skeletal muscle. J Biol Chem, 1975, 250: 9038–9043 [PubMed] [Google Scholar]
- 25.Koz M, Erbaş D, Bilgihan A, et al. : Effects of acute swimming exercise on muscle and erythrocyte malondialdehyde, serum myoglobin, and plasma ascorbic acid concentrations. Can J Physiol Pharmacol, 1992, 70: 1392–1395 [DOI] [PubMed] [Google Scholar]
- 26.Sabriá M, Ruibal A, Rey C, et al. : Influence of exercise on serum levels of myoglobin measured by radioimmunoassay. Eur J Nucl Med, 1983, 8: 159–161 [DOI] [PubMed] [Google Scholar]
- 27.Kirwan JP, Clarkson PM, Graves JE, et al. : Levels of serum creatine kinase and myoglobin in women after two isometric exercise conditions. Eur J Appl Physiol Occup Physiol, 1986, 55: 330–333 [DOI] [PubMed] [Google Scholar]
- 28.Roti S, Iori E, Guiducci U, et al. : Serum concentrations of myoglobin, creatine phosphokinase and lactic dehydrogenase after exercise in trained and untrained athletes. J Sports Med Phys Fitness, 1981, 21: 113–118 [PubMed] [Google Scholar]
- 29.Paul GL, DeLany JP, Snook JT, et al. : Serum and urinary markers of skeletal muscle tissue damage after weight lifting exercise. Eur J Appl Physiol Occup Physiol, 1989, 58: 786–790 [DOI] [PubMed] [Google Scholar]
- 30.Ritter WS, Stone MJ, Willerson JT: Reduction in exertional myoglobinemia after physical conditioning. Arch Intern Med, 1979, 139: 644–647 [PubMed] [Google Scholar]
- 31.Viitasalo JT, Niemelä K, Kaappola R, et al. : Warm underwater water-jet massage improves recovery from intense physical exercise. Eur J Appl Physiol Occup Physiol, 1995, 71: 431–438 [DOI] [PubMed] [Google Scholar]
- 32.Pöche H, Hopfenmüller W, Hoffmann M: Detection and identification of myoglobin in serum by immunoblotting. Effect of exercise on patients with Duchenne muscular dystrophy. Clin Physiol Biochem, 1987, 5: 103–111 [PubMed] [Google Scholar]
- 33.Terrados N, Jansson E, Sylvén C, et al. : Is hypoxia a stimulus for synthesis of oxidative enzymes and myoglobin? J Appl Physiol 1985, 1990, 68: 2369–2372 [DOI] [PubMed] [Google Scholar]
- 34.Cole RP: Skeletal muscle function in hypoxia: effect of alteration of intracellular myoglobin. Respir Physiol, 1983, 53: 1–14 [DOI] [PubMed] [Google Scholar]
- 35.Wanachiwanawin W, Luengrojanakul P, Sirangkapracha P, et al. : Prevalence and clinical significance of hepatitis C virus infection in Thai patients with thalassemia. Int J Hematol, 2003, 78: 374–378 [DOI] [PubMed] [Google Scholar]
- 36.Cai SX, Huang MY, Chen Z, et al. : Subjective symptom increase among dry-cleaning workers exposed to tetrachloroethylene vapor. Ind Health, 1991, 29: 111–121 [DOI] [PubMed] [Google Scholar]
- 37.Lee WD, Lee JU, Kim J: Differences in amplitude of functional electrical stimulation between the paretic and nonparetic sides of hemiplegic stroke patients. Toxicol Environ Health Sci, 2013, 5: 82–85 [Google Scholar]