Abstract
[Purpose] A wide variety of accelerometer tools are used to estimate human movement, but there are no adequate data relating to gait symmetry parameters in the context of knee osteoarthritis. This study’s purpose was to evaluate a 3D-kinematic system using body-mounted sensors (gyroscopes and accelerometers) on the trunk and limbs. This is the first study to use spectral analysis for data post processing. [Subjects] Twelve patients with unilateral knee osteoarthritis (OA) (10 male) and seven age-matched controls (6 male) were studied. [Methods] Measurements with 3-D accelerometers and gyroscopes were compared to video analysis with marker positions tracked by a six-camera optoelectronic system (VICON 460, Oxford Metrics). Data were recorded using the 3D-kinematic system. [Results] The results of both gait analysis systems were significantly correlated. Five parameters were significantly different between the knee OA and control groups. To overcome time spent in expensive post-processing routines, spectral analysis was performed for fast differentiation between normal gait and pathological gait signals using the 3D-kinematic system. [Conclusions] The 3D-kinematic system is objective, inexpensive, accurate and portable, and allows long-term recordings in clinical, sport as well as ergonomic or functional capacity evaluation (FCE) settings. For fast post-processing, spectral analysis of the recorded data is recommended.
Key words: Gait, Accelerometer, Gyroscope
INTRODUCTION
Osteoarthritis (OA), the most common form of arthritis, is also the most common type of musculoskeletal disorder. While OA can affect virtually all joints, knee OA is of particular interest since it has the potential to severely limit mobility1,2,3,4). Severity of knee OA can be determined radiographically using the Kellgren-Lawrence criteria5). Current treatment strategies fail to prevent progression of knee OA, and therefore the current therapeutic goal is to limit pain and disability6). For assessing functional disability in OA an objective measurement method is desired. Accelerometry is a practical, cost-effective and repeatable method of reliably evaluating human movement of individual body parts or the whole body without any radiation exposure7,8,9). Accelerometers can be used for observing a range of different movements such as postural sway and falls, sit-to-stand transfer and gait9). Equal values of gait variables on both sides of the body are the definition of gait symmetry10, 11). In this context, pathological gait patterns are reflected by a certain amount of gait asymmetry. However, there is no general agreement about when gait asymmetry should be considered pathological, although it is frequently assessed and described in the pre- and postoperative clinical evaluation of patients, as well as in general rehabilitation. For example, Vogt et al.11) pointed out that gait asymmetry is often present in patients with hip OA. An arbitrary cut-off value of 10% difference from perfect symmetry has been reported as a criterion of asymmetry in gait12, 13). This 10% criterion, however, has been considered invalid because of its non-parameter-specific nature13). Visual gait analysis is used in the context of functional capacity evaluation (FCE). It is known as a clinical tool used internationally for the purpose of predicting a safe return to work. FCEs are standardized batteries of tests in which a participant’s functional ability is tested, analyzed and compared to the required physical job demands11, 13, 14). For quantitative analyses of gait symmetry or asymmetry, statistical differences between groups or limbs are used11). However, gait symmetry or asymmetry should be evaluated in footfall measures and the movement of the upper body. Accelerometer-based gait analyses of knee OA has to be established as a valid criterion of gait asymmetry that is easily measured and interpreted, making it possible to use in the rehabilitation of individual patients in clinical practice as well as in research. A wide variety of accelerometer tools are used to estimate human movement, but there are no adequate data relating to gait symmetry parameters in the context of knee OA. The aim of our study was to assess and describe 3D-accelerometer/3D-gyroscope based parameters that are able to differentiate between pathological and normal gait patterns as well as distinguish gait symmetry from gait asymmetry.
SUBJECTS AND METHODS
Twelve patients (2 female, 10 male) with knee OA diagnosed by a physician, confirmed radiographically15, 16), and graded with the Kellgren-Lawrence scale (1 to 4; Grade III (7 patients) and Grade IV (5 patients)) participated in this study. Patients were excluded if they had neurological, vestibular or musculoskeletal disorders, fracture of the lower extremity, rheumatoid arthritis, generalized osteoarthritis, limping gait or any condition that may have influenced a treadmill walking evaluation. The characteristics of the included patients were (mean ± SD): age, 44.4 ± 7.6 years; body weight, 75.5 ± 11.1 kg; height, 171.1 ± 6.5 cm; and body mass index, (BMI): 26.9 ± 3.2 kg/m2. A control group of 7 healthy persons was also tested (1 female, 6 male). Their characteristics were (mean ± SD): age, 41.7 ± 8.8 years; body weight, 73.7 ± 9.8 kg; height, 175.6 ± 7.2 cm; and body mass index, 26.1 ± 2.9 kg/m2. This study was approved by the Ethics Committee of the University Medical Center of Goettingen. Patients and controls gave their written consent to participation in this study. The method of this study conformed to the principles of the Declaration of Helsinki.
First, kinematics data were obtained using a six-camera optoelectronic system (VICON 460, Oxford Metrics). Using Kistler force plates (Type 9287A, Kistler Instrumente AG, Switzerland), kinetic data was obtained. When the magnitude of vertical ground reaction forces exceeded 2% of a subject’s body weight, gait cycles were normalized (0–100%) between two successive foot contacts. Second, accelerometers and gyroscopes were attached to the subjects for comparive measurements. They measure the tendency of an object to resist a change in motion. Accelerometers operate on a “spring-mass” principle. The motion sensors (Freescale semiconductor, Type MMA76260Q) were three tri-axial accelerometers and three gyroscopes (ADXRS 300, Analog Devices) connected to a portable data logger (Glonner Electronic Noraxon OY). Body acceleration and angular velocity were recorded three-dimensionally (3D) in the anteroposterior, vertical and mediolateral directions. The accelerometers are piezo-electric sensors that were coupled to amplifiers (± 2 g). Angular velocity is measured by a rate gyroscope through the Coriolis force and can be measured directly11, 15). The data logger was attached to the back or side of the body. Data were sampled at 1 kHz. Each study participant was advised to wear light clothing to allow comfortable movement. The measurement sensors, the accelerometer and gyroscope, were placed inside a container that was firmly attached to the individual by means of a flexible belt around the waist over the middle part of the lower back over the L3 process so that it was attached close to the centre of gravity of the human body. Participants had to feel free to move, with respect to the skin. According to the marker positioning of the optoelectronic system, the other two sensors were fixed to rigid bodies which were attached by belts to the lateral malleoli of both legs, as described in previously published studies16, 17). The 31 reflective markers of the optoelectronic system were attached to anatomical locations according to the VICON Plug-in-Gait marker placement protocol. Additionally, six reflective markers were attached to the rigid plates of the combined accelerometers and gyroscopes for correlation and evaluating accuracy with the gold standard video analysis. After calibration of the systems, all participants walked 500 m on a treadmill (HP Cosmos, Germany) at self-selected speed while acceleration and angular velocity were measured using the setup described above. After the experiment, all obtained data (500 m walking distance) were downloaded to a computer and converted into earth acceleration units (g) following a standard calculation. Subsequently, data processing was conducted with Noraxon Software (Noraxon MyoReasearch 2.02, Noraxon, Germany). Data acquisition and post-processing for the optoelectronic system was done on a Vicon-PC using Vicon software (Vicon Body-Builder 3.5). The generated acceleration data was post-processed using a lowpass filter, fast Fourier transformation (FFT), and spectral analysis to analyze differences in trunk and locomotor limb acceleration. We used gravity as a known constant acceleration for calibrating the accelerometers. The output of a stationary accelerometer’s sensing axis aligned with the global vertical must correlate to 9.81 m/s2. In this context, a two-point linear calibration can be performed that transforms the raw output to units of acceleration once a raw accelerometer output has been obtained for the static condition of 9.81 m/s2. Calibration procedures were similar to the two-point method with a slope-intercept and zero-span, assuming linearity between the raw output and acceleration. For the gyroscopes, dynamic calibrations were performed by sensor rotations of 90° and 180° within a known time. Drift correction and calibration was done according to the literature17, 18, 20). For further details on calibration, see Veltink et al20). According to previous studies11, 13, 14), symmetry parameters were calculated for footfall and trunk measurements. The angular velocity (ω) was recorded. By integrating the angular velocity over time, the angle can be determined [α = ∫ ω × δτ]. Accelerometers primarily measure accelerations (a), but integration over time allows determination of velocities [ ν = ∫ a × δτ (m/s)] and paths [s = ∫ ∫ a × δτ = ∫ ν × δτ (m)]. Following these principles, we measured accelerations and angles as described above. Spatiotemporal walking speed (distance per walking time) and cadence (number of steps per walking time) were determined. The step time asymmetry was calculated to determine differences between left and right leg movements. Using FFT, front acceleration data as well as mediolateral angular velocities were evaluated as a power spectrum on a spectral frequency scale (1/sec). It is a known fact that many gait variables change with walking speed14). To differentiate between gait abnormality and the effect of different walking speeds, variables need to be controlled for the effect of speed. For this reason, every participant was asked to walk back and forth at three steady-state speeds ranging from slow to fast (a total of six walks), allowing individual linear trend lines to be calculated over the speed range demonstrated by the individual participants18). This data normalization allowed inter-individual comparisons among the study participants, even though the actual walking speeds were self chosen. Quantitative variables were expressed as the mean ± standard deviation. The patient group (n = 12) and the control group (n = 7) were compared using unpaired t-test with a significance level of p = 0.05. Statistical analyses were performed using commercially available software (SPSS 17 for Windows, SPSS Inc., Chicago, IL, USA). The fast Fourier transform function within the analysis package of MATLAB (Matlab 7.0, Mathworks, Inc., Concord, MA) was used in the evaluation of power spectra. To detect linear correlations between the recorded parameters, a linear regression model was utilized.
RESULTS
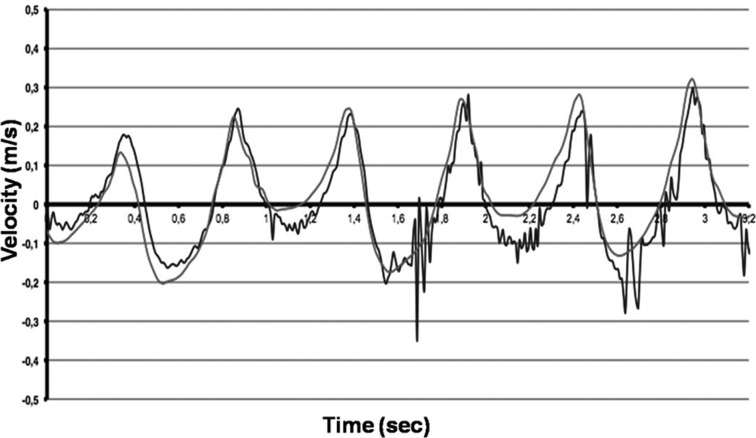
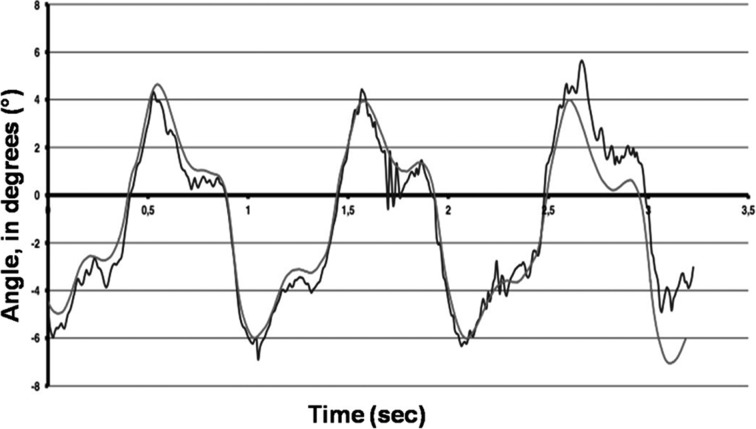
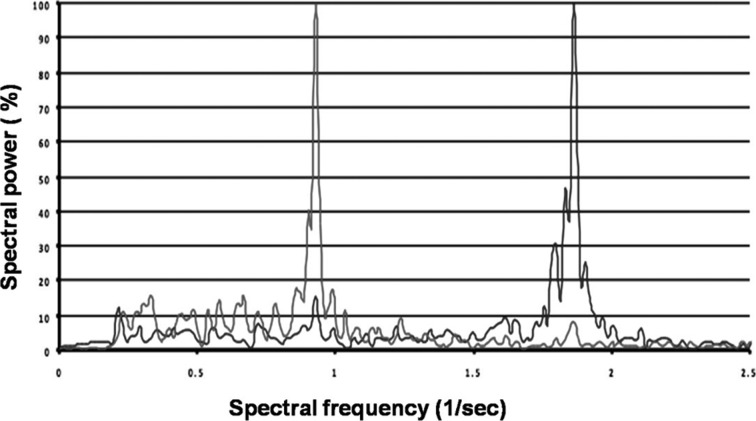
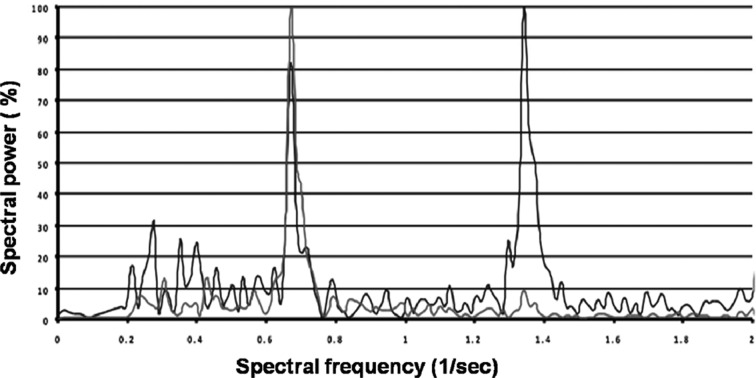
Figure 1 shows the comparison of the velocities measured by accelerometers in the lumbar region (L3) with those derived from video analysis measured by the Vicon® System. Figure 2 shows the comparison of mediolateral lumbar (L3) angles measured by gyroscopes with those derived from measurements with the Vicon® video analysis system. The root mean square difference was used to evaluate the closeness of the results of video analysis (Vicon®) with those of the accelerometer/gyroscope-based analysis. The results of the Vicon® system were used as the gold standard. Linear regression analysis revealed a significant correlation between video analysis results and the 3D-gyroscopes and accelerometers analysis results (r2=0.69; p=0.01; Fig. 1 and Fig. 2). As shown in Figs. 1 and 2, errors increased at the highest walking speed for the accelerometer data and peaks of angular velocities measured by gyroscopes, probably due to the sensors being jarred or vibrated by heel strike. The patients in the knee OA group were older, heavier and shorter than the subjects in the control group, but no significant differences in the significance level (p values > 0.05) were found between the two groups. Preferred gait velocity was significantly lower in the knee OA group (0.74 ± 0.08 meters per second) than in the control group (1.27 ± 0.14 meters per second) (p < 0.001). Controlling for differences in age, the patients’ walking speeds were significantly reduced at higher ages (p < 0.01). The other parameters showed no age-related differences. Cadence was significantly (p < 0.001) reduced in the knee OA group (97.8 ± 11.5 steps per minute) compared to the control group (116.3 ± 7.4 steps per minute). For trunk symmetry, the mediolateral (ML) angles of the lumbar region (L3) determined by gyroscopes were significantly (p < 0.001) higher in the knee OA group (9.4 ± 2.2°) than in the control group (4.3 ± 1.9°). For locomotor limb symmetry of the footfall (FF) measures, angular differences between both shanks were significantly (p < 0.001) higher in the knee OA group (11.7 ± 1.6°) than in the control group (3.1 ± 2.3°). Despite this, FF time differences between the limbs were significantly (p < 0.001) higher in the knee OA group (5.7 ± 2.8 msec) than in the control group (1.2 ± 0.9 msec). Gait asymmetries were significantly higher (p < 0.001) in the knee OA group than in the controls. Using the 10% criterion, values were still significant (p < 0.01). The knee OA patients walked with lower cadence (p < 0.001), showed more asymmetric trunk acceleration (ML) (p < 0.001) and the stance phase of the affected limb was longer than that of the unaffected limb (p < 0.001), as shown by the FF time differences between both legs. The FFT is a method of calculating the component frequencies of a signal. The summation of an infinite number of sine and cosine waves of different frequencies and amplitudes created from the movement of different anatomical structures can be thought of as a frequency signal resulting from ground reaction forces. FFT calculates the amount of movement (amplitude) at each frequency. The collection of all the component frequencies is here termed the spectrum15). Figure 3 shows the spectral visualization of normal human gait derived from measurements by 3D-accelerometry and gyroscopes. It shows two peaks, whereas the trace of the knee OA group, Fig. 4, demonstrates an additional peak, which was representative of asymmetric gait.
Fig. 1.
The comparison of 3D-accelerometry (blue lines) with video analysis (Vicon®, red lines) shows a significant correlation over time.
Fig. 2.
The comparison of 3D-gyroscopes (blue lines) with video analysis (Vicon®, red lines) shows a significant correlation.
Fig. 3.
Spectral visualization of normal gait (control group, a 46-year-old male). The blue lines show 3D-accelerometer measurements in the anteroposterior direction, and the red lines show 3D-gyroscope measurements in the mediolateral direction. The plot shows two peaks, one each for gyroscope and accelerometry.
Fig. 4.
Spectral visualization of abnormal gait (knee OA group, a 50 year-old male). The blue lines show 3D-accelerometer measurements in the anteroposterior direction, and the pink lines show 3D-gyroscope measurements in the mediolateral direction. There are three peaks, two measured by 3D-accelerometry, and one measured with a 3D-gyroscope, indicating abnormal values due to an asymmetric gait pattern.
DISCUSSION
The aim of this study was to explore 3D-accelerometer and 3D-gyroscope parameters that have the potential to differentiate between normal gait and pathological gait. Spectral analysis provides a simple evaluation method, which is able to differentiate between normal and pathological gait. This sensor-based method showed good measurement reliability of the five parameters that exhibited significant differences between the knee OA and control groups.
According to previous studies16, 17), body-mounted accelerometers and gyroscopes give results in kinematics analyses that are comparable to those of Vicon ® video analyses. As shown in Figs. 1 and 2, errors increased at the highest speed for the accelerometer data or peaks of angular velocities measured by gyroscopes, probably due to the sensors being jarred or vibrated by heel strike. This should be considered when designing future applications for accelerometers and gyroscopes. However, using body-mounted accelerometers and rate gyroscopes is as accurate as the gold standard video analysis17), as a physical method for collecting kinematic data of healthy subjects as well as patients with knee OA. In addition, accelerometers and gyroscopes used as body-mounted sensors are inexpensive and, combined with a portable data-logger, fulfill all criteria for a fully portable system that can be used in almost any environment. Comparable systems have been used in prior biomechanical studies16, 17, 19, 21). Our body-mounted system could also be used in sports situations with accelerations similar to those of walking because of its portability. Furthermore, this system could be attached to participants undergoing FCE observations to obtain quantitative data. Huisinga et al.22) reported that acceleration of the trunk during walking by patients with multiple sclerosis (MS) had larger frequency dispersion in the ML direction, than in the AP direction. This agrees with our result that ML differences from normal gait are indicative of a pathological gait pattern. Unsteady walking speeds can lead to an increase in trunk acceleration. In the present study, however, participants walked at a constant speed during the testing. According to the study by Turcot et al.17), high accelerations in the ML direction may be a consequence of joint instability due to knee OA and an unstable limb alignment. Ogata et al.23) reported lateral acceleration generated by initial foot contact was caused by varus deformity in medial knee OA patients. Therefore, ML differences in patients with unilateral knee OA measured with 3D gyroscopes and represent compensatory posture, as observed in the present (Table 1). Linear accelerations observed in this study were estimated at the same functional location for each participant rather than an arbitrary or changing location on the segment as in previous studies11, 17, 23). Estimation of linear accelerations is less affected by angular components induced by the movement of segments during gait when transposing accelerations close to joint contact surfaces17). Estimation of internal acceleration may be affected by the vector joining sensor to bone. To verify the sensitivity of the method, we compared the data of the sensor-based, body-mounted system with Vicon® video analysis. The results show a difference in 3D-acceleration and gyroscope measurements at peak magnitude and angles less than 5° (Figs. 1 and 2). These results are in line with the results of Mayagoitia et al16). In the present study, between-limb differences in FF measures were significantly higher in the knee OA group than in the controls. Furthermore, our results showed a certain amount of gait asymmetry was also present in the controls (Table 1), due to the fact that values of either the 3D accelerometer or 3D gyroscope measurements would tend to zero representing perfect symmetry16, 17, 23). Sadeghi et al.24) showed that a certain amount of gait asymmetry is also present in healthy subjects and may reflect functional between the limbs differences. For differentiating subjects with pathological gait asymmetry from subjects without pathological gait asymmetry, the reported cutoff criteria of gait asymmetry are too strict. In our present study, a general 10% criterion was used to assess differences between groups gait asymmetry. This is preferred because it is easily derived in clinical and research settings16, 24). The lack of standardization of the placement location of accelerometers can explain the variability of the results reported in previous studies16, 17, 23, 25, 30). Benoit et al.25) reported that skin movement artifacts originate from the skin moving over the musculoskeletal structure. For this reason, the movement of a subject’s skeleton may not exactly correlate with the measured movement on the skin where the sensors are attached. To address this, a non-invasive technique is needed. Applying an algorithm, as used in the present study, to the measured movement data during post-processing, can remove most of the skin movement25,26,27). Sensors can be applied in different ways to the participants using medical tape, wrapping bandages, Velcro, plastic plates, elastic straps and bone pins28). In line with previous studies, we used double-sided medical tape to attach the sensors to plastic plates and then fixed them to the subjects with tape17, 27, 28). The relaxation and contraction of the muscles underneath the straps can cause their positions to change during locomotion when sensors are fixed to elastic straps or Velcro29). Previous studies8, 16, 17) have used accelerometers to measure the acceleration experienced at impact at sampling rates of 10 kHz. These high sampling rates are not required when the measurement of the impact transmitted by a subject’s limb is done proximal to the point of contact. In line with the study by Henriksen et al.30) we used a sample rate of 1 kHz, significantly less than 10 kHz. For data post-processing of longer distance walks as used in the present study, of about 400 m and more, a considerable amount of data is collected. In this context, acceleration data can be post-processed using a low pass filter, FFT, and spectral analysis to observe differences in trunk acceleration to distinguish normal from pathological gait (Figs. 3 and 4). Our study data support the hypothesis that differences in FF measurements occur due to unilateral knee OA, and the consequent adoption of a compensatory posture. The 3D-kinematic system is objective, inexpensive, accurate, and portable, and allows long-term recordings in clinical, sport, ergonomic and FCE settings. For fast post-processing, spectral analysis of the obtained data is recommended.
Table 1. Comparison of gait parameters between the study groups .
| Parameter | Knee OA group (12 patients) | Control group (7 participants) |
|---|---|---|
| Gait velocity (m/s)* | 0.74 ± 0.08 | 1.27 ± 0.14 |
| Cadence (steps/min)* | 97.8 ± 11.5 | 116.3 ± 7.4 |
| ML trunk symmetry (angular degree)* | 9.4° ± 2.2° | 4.3° ± 1.9° |
| Limb symmetry (angular degree)* | 11.7° ± 1.6° | 3.1° ± 2.3° |
| Limb symmetry (time, msec)* | 5.7 ± 2.8 | 1.2 ± 0.9 |
* p<0.001
References
- 1.Fahlman L, Sangeorzan E, Chheda N: Older subjects without radiographic knee osteoarthritis: weight, height, and body mass index. Aging Dis, 2013, 4: 201–209 [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma L, Kapoor D, Issa S: Epidemiology of osteoarthritis: an update. Curr Opin Rheumatol, 2006, 18: 147–156 [DOI] [PubMed] [Google Scholar]
- 3.Goekoop RJ, Kloppenburg M, Kroon HM, et al. : Determinants of absence of osteoarthritis in old age. Scand J Rheumatol, 2011, 40: 68–73 [DOI] [PubMed] [Google Scholar]
- 4.Felson DT, Goggins J, Niu J, et al. : The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum, 2004, 50: 3904–3909 [DOI] [PubMed] [Google Scholar]
- 5.Bagge E, Bjelle A, Edén S, et al. : Factors associated with radiographic osteoarthritis: results from the population study 70-year-old people in Göteborg. J Rheumatol, 1991, 18: 1218–1222 [PubMed] [Google Scholar]
- 6.Kumar A, Djulbegovic B, Lohmander S, et al. : Myeloma (multiple). Clin Evid, 2006, 15: 1–29 [PubMed] [Google Scholar]
- 7.Mathie MJ, Coster AC, Lovell NH, et al. : Accelerometry: providing an integrated, practical method for long-term, ambulatory monitoring of human movement. Physiol Meas, 2004, 25: R1–R20 [DOI] [PubMed] [Google Scholar]
- 8.Tsivgoulis SD, Papagelopoulos PJ, Efstathopoulos N, et al. : Accelerometry for evaluation of gait pattern in healthy soccer athletes. J Int Med Res, 2009, 37: 1692–1700 [DOI] [PubMed] [Google Scholar]
- 9.Culhane KM, O’Connor M, Lyons D, et al. : Accelerometers in rehabilitation medicine for older adults. Age Ageing, 2005, 34: 556–560 [DOI] [PubMed] [Google Scholar]
- 10.Kim MK, Lee YS: Kinematic analysis of the lower extremities of subjects with flat feet at different gait speeds. J Phys Ther Sci, 2013, 25: 531–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogt L, Banzer W, Bayer I, et al. : Overground and walkway ambulation with unilateral hip osteoarthritis: comparison of step length asymmetries and reproducibility of treadmill mounted force plate readings. Physiother Theory Pract, 2006, 22: 73–82 [DOI] [PubMed] [Google Scholar]
- 12.Costa RV, Grecco LA, Neto HP, et al. : Analysis of the Applicability of an Ankle-Foot Orthosis during Gait in Poststroke Patients. J Phys Ther Sci, 2013, 25: 1001–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung EJ, Kim JH, Lee BH: The effects of core stabilization exercise on dynamic balance and gait function in stroke patients. J Phys Ther Sci, 2013, 25: 803–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodt-Billington C, Helbostad JL, Vervaat W, et al. : Criteria of gait asymmetry in patients with hip osteoarthritis. Physiother Theory Pract, 2012, 28: 134–141 [DOI] [PubMed] [Google Scholar]
- 15.Wurdeman SR, Huisinga JM, Filipi M, et al. : Multiple sclerosis affects the frequency content in the vertical ground reaction forces during walking. Clin Biomech (Bristol, Avon), 2011, 26: 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayagoitia RE, Nene AV, Veltink PH: Accelerometer and rate gyroscope measurement of kinematics: an inexpensive alternative to optical motion analysis systems. J Biomech, 2002, 35: 537–542 [DOI] [PubMed] [Google Scholar]
- 17.Turcot K, Aissaoui R, Boivin K, et al. : New accelerometric method to discriminate between asymptomatic subjects and patients with medial knee osteoarthritis during 3-d gait. IEEE Trans Biomed Eng, 2008, 55: 1415–1422 [DOI] [PubMed] [Google Scholar]
- 18.Eppeland SG, Myklebust G, Hodt-Billington C, et al. : Gait patterns in subjects with rheumatoid arthritis cannot be explained by reduced speed alone. Gait Posture, 2009, 29: 499–503 [DOI] [PubMed] [Google Scholar]
- 19.Isernhagen Work Systems: Functional capacity procedure manual. Duluth, MN, 1997 [Google Scholar]
- 20.Lötters JC, Schipper J, Veltink PH, et al. : Procedure for in-use calibration of triaxial accelerometers in medical applications. Sens Actuators, 1998, 68: 221–228 [Google Scholar]
- 21.Nene A, Mayagoitia R, Veltink P: Assessment of rectus femoris function during initial swing phase. Gait Posture, 1999, 9: 1–9 [DOI] [PubMed] [Google Scholar]
- 22.Huisinga JM, Mancini M, St George RJ, et al. : Accelerometry reveals differences in gait variability between patients with multiple sclerosis and healthy controls. Ann Biomed Eng, 2013, 41: 1670–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogata K, Yasunaga M, Nomiyama H: The effect of wedged insoles on the thrust of osteoarthritic knees. Int Orthop, 1997, 21: 308–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadeghi H, Allard P, Prince F, et al. : Symmetry and limb dominance in able-bodied gait: a review. Gait Posture, 2000, 12: 34–45 [DOI] [PubMed] [Google Scholar]
- 25.Benoit DL, Ramsey DK, Lamontagne M, et al. : Effect of skin movement artifact on knee kinematics during gait and cutting motions measured in vivo. Gait Posture, 2006, 24: 152–164 [DOI] [PubMed] [Google Scholar]
- 26.Ramsey DK, Lamontagne M, Wretenberg PF, et al. : Assessment of functional knee bracing: an in vivo three-dimensional kinematic analysis of the anterior cruciate deficient knee. Clin Biomech (Bristol, Avon), 2001, 16: 61–70 [DOI] [PubMed] [Google Scholar]
- 27.Südhoff I, Van Driessche S, Laporte S, et al. : Comparing three attachment systems used to determine knee kinematics during gait. Gait Posture, 2007, 25: 533–543 [DOI] [PubMed] [Google Scholar]
- 28.Leardini A, Chiari L, Della Croce U, et al. : Human movement analysis using stereophotogrammetry. Part 3. Soft tissue artifact assessment and compensation. Gait Posture, 2005, 21: 212–225 [DOI] [PubMed] [Google Scholar]
- 29.Lee IH, Park SY: A comparison of gait characteristics in the elderly people, people with knee pain, and people who are walker dependent people. J Phys Ther Sci, 2013, 25: 973–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henriksen M, Lund H, Moe-Nilssen R, et al. : Test-retest reliability of trunk accelerometric gait analysis. Gait Posture, 2004, 19: 288–297 [DOI] [PubMed] [Google Scholar]