Abstract
Korean red ginseng (steam treated Panax ginseng C.A. Meyer), among most prized traditional herbal remedies, has been clinically shown to improve cardiovascular disease (CVD) risk factors. Whether this holds true for the dried non-steamed variety, known as Korean white ginseng (KWG) is unclear. This study therefore, investigated the efficacy and safety of escalating doses of KWG on vascular and glycemic parameters in type 2 diabetes (T2DM). Using an acute, randomized, placebo-controlled, double-blind, crossover design, 25 participants with well-controlled T2DM (12-males: 13-females, age: 63 ± 9 years, A1c: 6.9 ± 0.7%, BMI: 29.3 ± 4.3 kg/m2) underwent five visits during which they received 1 g, 3 g, or 6 g KWG or 3 g wheat-bran control (twice) together with 50 g-glucose load. For the duration of 240 minutes, augmentation index (AI), and central blood pressure were measured at baseline and at 60 min-intervals, and ambulatory blood pressure was assessed at baseline and at 10 min-intervals. Additionally, capillary blood was collected at time zero and at 15, 30, 45, 60, 90, 120, and 180 minutes post-treatment. A symptoms questionnaire was used to assess safety and adverse events. Two-way ANOVA demonstrated a significant time-treatment interaction effect on AI (p = 0.01) with one-way ANOVA showing significant reductions in AI with 3 g KWG relative to control (p = 0.04). Compared to control, acute administration of KWG appeared to be safe, but did not affect any other postprandial, vascular or glycemic parameters. KWG might have a beneficial effect on AI, a cumulative indicator of arterial health. However, these results are preliminary and highlight the need for long-term investigation with a focus on its accountable components. Clinical Trial Registration: NCT01699074
Keywords: Clinical trial, Korean white ginseng, Type 2 diabetes, Postprandial blood glucose, Augmentation index
Introduction
The incidence of type 2 diabetes mellitus (T2DM) has been rising at an alarming rate over the last 2 decades and it is now considered to be a global epidemic. Latest statistics indicate that it affects 382 million people worldwide, with projections of a 55% increase by 2035 [1]. T2DM compounds an individual's risk for developing cardiovascular disease (CVD) [2]. Prolonged hyperglycemia and vascular abnormalities, the physiological hallmarks of diabetes, result in diabetes related micro- and macro-vascular complications [3]. As such, tight glycemic control and well managed blood pressure (BP) are fundamental to achieving optimal diabetes care [4]. However, these treatment goals generally go unmet [5], as recent estimates revealed that only half of the diagnosed individuals with diabetes are meeting their glycemic targets, and only 36% are achieving goals for blood pressure [6]. The progressive nature of the disease, along with the unmet treatment challenges generates a compelling argument for safe, effective, and affordable alternative treatments that can be used as an adjunct to current medical therapies. Concurrent with the increasing demand for more effective medications is the recent upsurge in the use of herbal remedies amongst the general public [7].
Ginseng, a traditional medicinal plant, embodies an important position in the oriental pharmacopeia. Traditionally, it is used primarily for treating illness, restoring homeostasis, and promoting longevity [8], but more recently it has been identified as the most commonly used herb for controlling CVD factors [8]. Of the thirteen ginseng species identified, the most commonly consumed and well established therapeutic herb is Asian ginseng (Panax ginseng) [9]. More specifically, varieties of Korean red ginseng (KRG), a type of Asian ginseng, have been shown to improve glycemic control and certain cardiovascular parameters when used in addition to conventional medication [10,11,12]. However, despite it being the most popular form of ginseng used in traditional dishes [13], scant clinical evidence exists on the health benefits of the white, non-steamed cultivars of Korean ginseng, Korean white ginseng (KWG). It has been suggested that steaming results in a more pronounced biological effect compared to raw dried ginseng [14], however the the process significantly alters the profile of dammarane type saponins, a pharamacologically active fraction of ginseng, also known as ginsenosides [15], and also results in loss of malonyl type ginsenosides [16,17], all of which may possibly alter its therapeutic benefits. Given the favorable metabolic properties of KWG seen in animal models [18,19] along with the added cost of steaming of KRG [20], KWG may hold promising potential in improving certain CVD risks, and thus, serve as a less expensive therapeutic alternative to KRG. As clinical data on its medicinal properties is relatively scarce, there is a compelling need for exploring its CVD health benefits. Therefore, the objective of this exploratory study was to investigate a dose-response relationship between escalating doses of KWG and its effects on vascular and glycemic parameters, which may lead into potential long term benefit exploration.
Materials and Methods
Subjects
Thirty participants with T2DM were recruited through newspaper advertisement and research database. All gave informed written consent to take part in the study, which was approved by the Research Ethics Board at St Michael's Hospital. Inclusion criteria included individuals with presence of T2DM (A1c 6.5-8.5%) for at least 1 year, treated with diet alone or diet and oral hypoglycemic medication that was unchanged starting at least three months prior to the study. In addition, eligible participants were between the ages 18-75 years, body mass index (BMI) between 25-35 kg/m2, systolic blood pressure (SBP) < 160 mmHg and diastolic blood pressure (DBP) < 100 mmHg, clinically euthyroid, normal renal and hepatic functions; no major illness, non-pregnant, not taking herbs or supplements, no excessive alcohol (> 3 drinks/day) or cigarette use (> 10 cigarettes/day), no allergy or sensitivity to the study interventions or gelatin used in the capsules.
Design
This study followed a randomized, double-blind, placebo-controlled, crossover design. On five separate occasions, each participant received one of 4 single-dose interventions in random order: 1 g, 3 g, 6 g KWG, and 3 g wheat bran (administered twice). Randomization to treatment was done using a computer-generated random number table.
Interventions
Four-year old dried powdered whole root samples of KWG were provided by the Department of Herbal Crop Research; National Institute of Horticultural & Herbal Science, RDA, Korea. All treatments were administrated in a set of twelve opaque #00 white gelatin capsules. An individual otherwise not involved in the study performed treatment concealment and randomization.
Protocol
Participants attended the Clinical Nutrition and Risk Factor Modification Center, St. Michael's Hospital (Toronto, ON, Canada) on outpatient basis on five separate occasions in the morning after a 10-12h fast. Each participant was instructed to maintain the same dietary and exercise patterns in the evening before each test. Compliance with these conditions was assessed at each study visit using a preclinical information questionnaire. Subjects were asked to refrain from taking their diabetes and antihypertensive medications on the morning of the visits. Each visit was at least 4 days apart to minimize carry-over effects. At the start of each study visit, anthropometric measurements including weight, body mass index (BMI), waist circumference, and body fat percentage were taken and a preclinical questionnaire was filled out. Baseline measurements of augmentation index (AI) and central blood pressure (CBP) followed using SphygmoCorVx instrument (AtCor Medical, Sydney, Australia). Subjects were then fitted with an ambulatory blood pressure monitor (ABPM) (Spacelabs Inc., Redmond, WA, USA) which automatically measured two BP readings, each 5 minutes apart. The mean of the two ambulatory BP readings were used as baseline BP. A capillary baseline blood sample preceded vascular measurements after which subjects consumed orally 12 capsules containing either wheat bran or a KWG intervention along with a 50-g available carbohydrate oral glucose challenge. Finger prick blood samples were subsequently taken at 15, 30, 45, 60, 90, 120 and 180 minutes post-treatment and analyzed for plasma glucose levels. AI and CBP were measured at 60, 120,180 and 240 minutes post-treatment. The ABPM obtained automatic blood pressure readings every 10 minutes for 240 minutes following treatment administration. After the third hour post-treatment, subjects consumed a standardized snack, which included 15 g (1 tbsp) low fat cream cheese (Philadelphia, Kraft Canada Inc., North York, ON, Canada), 1 slice whole toast (Dempster's 100% whole wheat bread) and 200 mL water. For the duration of the visit, occurrence of adverse events was documented on a symptoms questionnaire using a 100 mm visual analog scale. At the end of each visit, a 24-hour symptoms form was given which was returned at the next scheduled visit.
Ginseng analyses
The ginsenoside profile of the 4 yr-old KWG root was analyzed by the Department of Herbal Crop Research; National Institute of Horticultural & Herbal Science; RDA; Korea, using thin-layer chromatography (TLC) technique. The total ginsenoside concentrations in the 4yr-old KWG root was 1.8%. The individual concentrations for the protopanaxadiol (PPD) ginsenosides Rb1, Rc, Rb2, and Rd were 0.24%, 0.13%, 0.07%, and 0.03% respectively, and for the protopanaxatriol (PPT) ginsenosides Rg1 and Re were 0.30% and 0.91%, respectively. The PPD:PPT ratio was 0.35.
Blood glucose analysis
All samples were analyzed within three days of collection. Finger prick blood samples were collected in 7 mL flat base polystyrene tubes (Sarstedt Inc., Montreal, QC, Canada) containing potassium oxalate and sodium fluoride inhibitor and immediately stored at -20℃ pending analysis. Samples were analyzed for glucose concentration using the YSI 2300D STAT Plus Glucose & Lactate Analyzer (YSI Inc., Yellow Springs, OH, USA) which utilizes a glucose oxidase method. The YSI was calibrated with a standard 10 mmol/L glucose solution prior to and during analysis of each set of seven samples. Measurements were expressed in mmol/L.
Study outcomes and statistical analyses
Change in AI, the primary outcome, normalized for a heart rate of 75 beats/minute, was calculated at 60, 120, and 180, and 240 minutes. Similarly, change in CBP was calculated at each hour time point across the 4 hr visit duration. Change in ABP was calculated at the 10 minute intervals from 10 to 240 minutes inclusive. Incremental areas under the glucose curve (iAUC) were calculated geometrically ignoring areas below the 0 value for each subject and were averaged for each intervention. Changes in postprandial blood glucose (PPBG) measures were calculated at each capillary blood sampling time point (15, 30, 45, 60, 90, 120, and 180 minutes). Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) release 21.0 (SPSS Inc., Chicago, IL, USA). A box plot method was applied for the detection of any potential outliers in the data. For normally distributed data, two-way repeated measures GLM ANOVA assessed the independent and interactive effects of treatments and protocol time on change in AI, CBP, ABP, and PPBG. One-way repeated measures GLM ANOVA with Tukey's test determined differences between treatment-associated means at each time point. All data was considered statistically significant at p < 0.05. Given that the study employs a crossover design, and based on our previous observations with similar studies [11], a treatment difference of 4% in AI (SD = 5%) was used for determination of sample size, indicating that approximately 21 individuals would be required (α = 0.05 and 1-β = 0.8). Assuming a 30% attrition rate, a total of 30 subjects were to be recruited.
Results
Subject characteristics, compliance and symptoms
A total of 30 subjects with T2DM provided written informed consent and were enrolled in the study, of which five dropped out due to time conflicts. Twenty-five subjects completed the study, 12 males and 13 females, mean ± SD age: 63 ± 9 years, BMI: 29.3 ± 4.3 kg/m2, waist circumference: 101.7 ± 10.5 cm, fasting blood glucose: 7.4 ± 0.05 mmol/L, A1C: 6.9 ± 0.7 %, resting SBP/DBP: 133 ± 15.9/74 ± 7.5 mmHg. The mean duration of diabetes was 9 ± 7 years while the onset of hypertension was 14 ± 12 years, with 15 subjects taking glucose lowering and 9 subjects taking anti-hypertensive medications. Inclusion criteria were maintained throughout the duration of the study, and there were no differences in reported symptoms between KWG treatments and control during the clinic visits or the washout periods.
Effects of KWG on Augmentation Index, central and ambulatory blood pressure
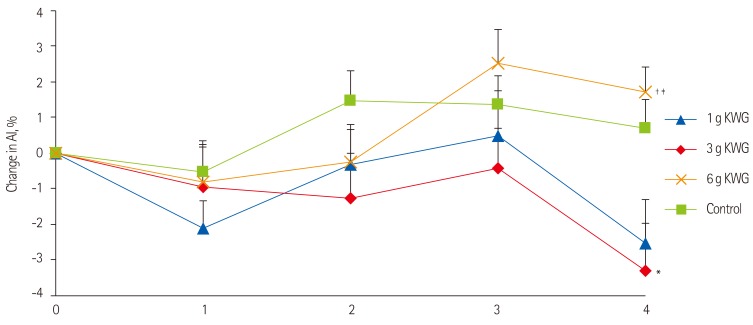
Two-way repeated measures GLM ANOVA demonstrated a significant time-treatment interactive effect on AI (p = 0.01). Thus, the effect of treatment was explored at individual time points of the AI curves using repeated measures oneway ANOVA. The Tukey's post hoc test showed that 3g KWG elicited significant reduction in AI at 240 minutes relative to control (p = 0.035) (Figure 1). There was no effect of treatment or time-treatment interaction on change in mean central (Table 1) and ambulatory systolic and diastolic BP (data not shown). There were also no significant differences in the mean absolute AI and BP values between any of the treatments at baseline.
Figure 1.
Acute dose effects of KWG treatments on Augmentation Index relative to control. Acute effects of treatment dose 1 g, 3 g, 6 g KWG or control on AI. Values are presented as the mean change from baseline in AI over 4hr post-intervention (n = 25). Control represents the mean response to two placebos. Values are mean ± SEM. KWG, Korean white ginseng; AI, Augmentation index. Two-way ANOVA: Significant time x treatment interaction (p = 0.01). *One-way ANOVA: Significant treatment effect between control and 3 g KWG (p = 0.035); †One-way ANOVA: Significant treatment effect between 3 g KWG and 6 g KWG (p = 0.005); ‡One-way ANOVA: Significant treatment effect between 1 g KWG and 6 g KWG (p = 0.02).
Table 1.
Mean acute changes in central blood pressure from baseline following KWG consumption
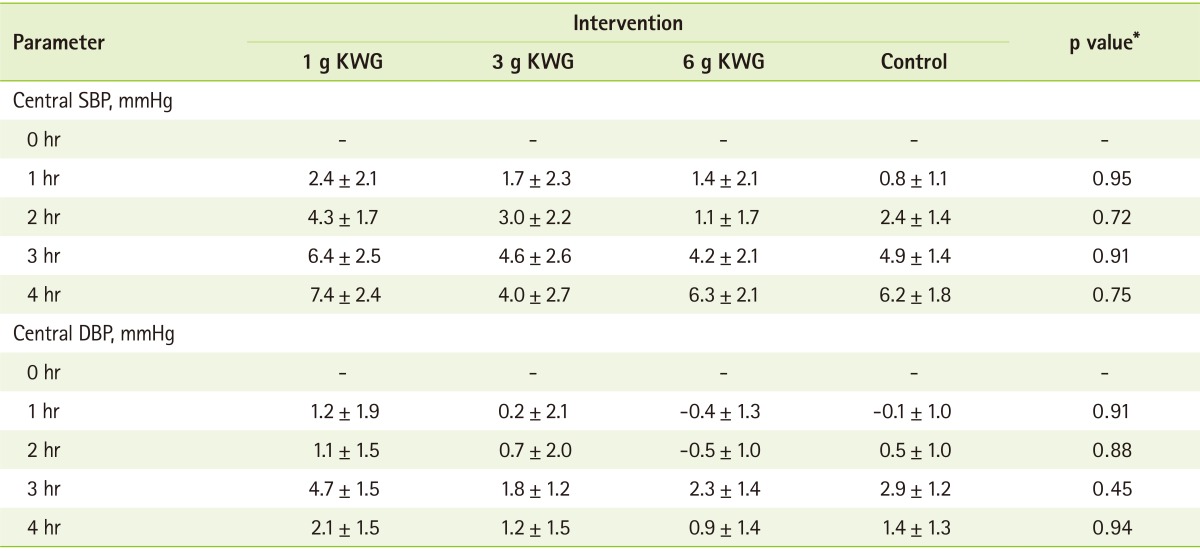
All data are mean ± SEM, all data are mean changes from baseline.
DBP: diastolic blood pressure, KWG: Korean white ginseng, SBP: systolic blood pressure.
*One-way ANOVA assessing between treatment differences at individual time points.
Effects of KWG treatments on post-prandial glycemia
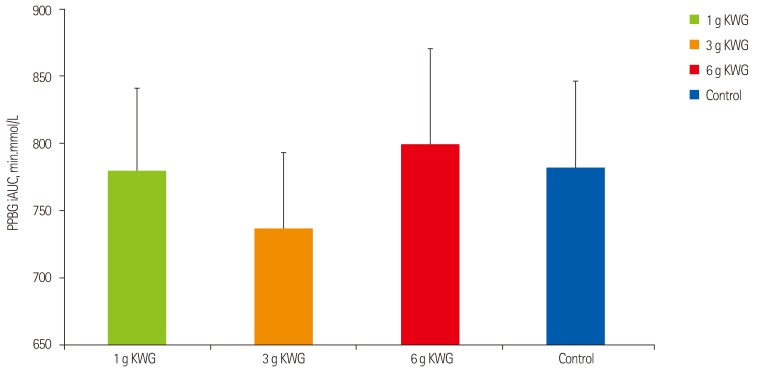
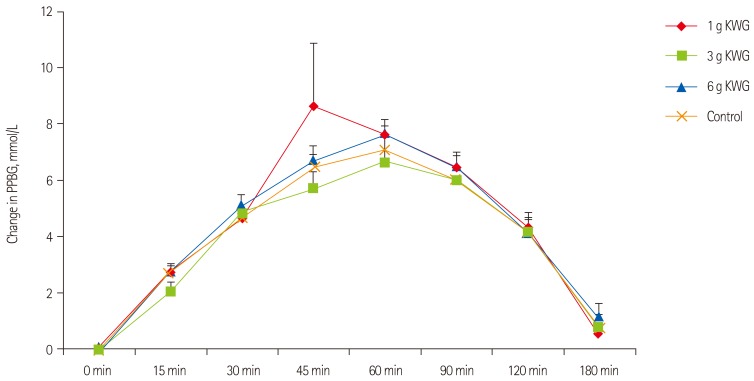
After eliminating an outlier from the data, repeated measures one-way ANOVA did not demonstrate a significant effect in blood glucose iAUC of KWG doses relative to control (Figure 2A). As well, two-way repeated measures GLM ANOVA did not show a significant time-treatment interactive effect on PPBG (p = 0.38) (Figure 2B).
Figure 2A.
Acute dose effects of KWG treatments versus control on post-prandial blood glucose incremental area under the curve. Acute effect of treatment dose 1 g, 3 g, 6 g KWG, or control PPBG iAUC (n = 24) after 3hr of intervention. Control represents the mean response to two placebos. Values are mean ± SEM. PPBG: post prandial blood glucose, iAUC: incremental area under the curve, KWG: Korean white ginseng. One-way ANOVA: (p = 0.92)
Figure 2B.
Acute dose effects of KWG treatments compared to control on post-prandial blood glucose levels. Acute effects of treatment dose 1 g, 3 g, 6 g KWG, or control on incremental PPBG. Values are presented as the mean change from baseline in PPBG over 3 hr post-intervention (n = 24). Control represents the mean response to two placebos. Values are mean ± SEM. PPBG: post prandial blood glucose, KWG: Korean white ginseng. Two-way ANOVA: (p = 0.29).
Discussion
We present here, for the first time, that KWG - the most commonly consumed form of ginseng in Korea [13] - exhibits a favorable acute effect on AI, a marker of arterial stiffness, in individuals with T2DM. More specifically, we show that this effect is associated with the consumption of 3 g KWG dose, and the effect appeared to not be dose dependent. These findings are of interest given the well-established predictive ability of AI in determining future CVD events [21,22]. Our results also indicate that KWG does not significantly reduce postprandial glycemia relative to control. A U-shaped relationship was observed between escalating doses of KWG and PPBG, with 3 g KWG showing the greatest reduction, and 6 g KWG eliciting an increase, albeit not significant.
Arterial stiffness has emerged as a novel marker of CVD risk, displaying a clear independent predictive value [22,23]. As such, acute improvements in AI demonstrated with 3 g KWG may have a clinical potential, and could aid in broadening our understanding on the hemodynamic potential of ginseng.
The acute effects seen on AI add to those from previous clinical trials though different ginseng species and varieties were explored. In an acute randomized controlled trial, 3 g KRG significantly lowered arterial stiffness in healthy individuals, relative to control, with no improvements in BP [11]. Another recent study conducted by our group in individuals with T2DM and concomitant hypertension showed that 3 g American ginseng (AG) significantly lowered radial AI and attenuated systolic BP after 12 weeks of consumption [24]. Conversely, our results are not in line with data from two studies where 3 g and 4.5 g of KRG administered daily for 3 months did not improve arterial stiffness in subjects with metabolic syndrome and hypertension [25,26]. The inconsistency in the findings could be possibly attributed to the differences in the method by which arterial stiffness was assessed in the current (radial pulse wave analysis) versus the two studies (brachialankle pulse wave velocity).
Accumulating data from animal and in vitro work have demonstrated that certain ginsenosides, such as Rb1, Re, and Rg1, display cardioprotective effects, chiefly on vascular endothelial function via endothelium dependent release of nitric-oxide (NO) [27,28,29]. In view of the clinical implications linking arterial stiffness and endothelial dysfunction to decreased NO generation and increased NO inactivation [30,31], together with evidence from preclinical data, the beneficial AI findings seen with 3 g KWG may be possibly explained by NO driven mechanism stimulated by these ginsenosides, including Rb1, Re, or Rg1, two of which (Re and Rg1) were found to be present in fairly similar concentrations as in our efficacious KRG roots [11]. While it is tempting to attribute our favorable vascular observation to these ginsenosides, the possibility that other potential active fractions of ginseng, including unmeasured ginsenosides, polysaccharides, peptides, fatty acids, and polyacetylenic alcohols might have played a role, cannot be eliminated [8].
The lack of a significant amelioration in peripheral systolic BP is not unexpected and is partly in line with our previous observations, where KRG showed either neutral or moderate effects [11,32]. Although KWG did not lower BP relative to control, the neutral BP observations are significant in light of the concern in the literature that ginseng may increase blood pressure [33], which resulted in advice given to individuals with hypertension to avoid ginseng products. Thus, this study adds further information on the safety of ginseng in hypertension.
An extensive number of clinical, laboratory and animal studies have investigated the therapeutic potential of ginseng in improving glycemia, with much focus being placed on two species: Asian, typically the steamed variety of Korean ginseng, and AG [34,35]. Presently, a number of reports from animal models reveal a possible anti-diabetic potential of the non-steamed variety of Asian ginseng [18,36,37,38]. Yet, to date, no randomized controlled trials have explored the acute glycemic and vascular effects of such varieties in T2DM. With respect to findings seen with other ginseng species and types, our results do not demonstrate a favorable acute glycemic effect as observed with the original efficacious batch of AG following standard oral glucose tolerance test (OGTT) in both healthy and subjects with T2DM [39]. As well, our findings are inconsistent with the favorable postprandial glycemic results of Panax ginseng extracts (Ginsana G115) and KRG rootlets, seen either alone or with standard glucose tolerance tests in healthy individuals [40,41]. On the other hand, we are only aware of two studies that have assessed the acute glycemic effects of the non-steamed Asian ginseng variety in healthy subjects. In these studies, both null and opposing glycemic effects were observed with single and combined ginseng doses following a 75 g-OGTT [42]. In relation to these two studies, we show similar null but not increasing glycemic effects following acute administration of escalating KWG doses. Comparing the total ginsenoside profile of the present ginseng to the one used in these two studies reveals that the concentration in the present sample is nearly twice as much (1.8% vs. 0.8% ginsenosides). However, despite having a greater ginsenoside concentration, as well as including subjects with T2DM, no glycemic lowering benefits were observed. On the other hand, our neutral glycemic observations are in contrast with evidence from animal studies [18,36]. In a recent 3 week investigation, it was found that the malonyl ginsenosides, a type of ginsenosides that exist in both fresh and air dried (non-steamed) root, significantly lowered fasting blood glucose when given at 50 and 100 mg/kg/wt doses to high fat diet fed and streptozotocin-induced diabetic rats [43]. As well, a previous study showed that 4-week oral administration of both white ginseng radix and rootlet lowered fasting blood glucose levels in KKAy mice compared to controls [18].
Reasons for the discrepancies in our glycemic findings are unclear. Variability in the ginsenoside profile might partially provide some explanation [44]. Analysis of ginsenoside profile revealed that the total ginsenoside content (1.8%) of the current ginseng variety was almost half of that found in our original efficacious batch of AG (3.2%) [39]. The optimal PPD:PPT ratio was found to be lower by 8-folds (0.35 vs. 2.44), and the relevant glycemic lowering ratios for Panax ginseng C.A Meyer (Rg1:Re and Rb2:Rc < 1) were not met [45]. Additionally, the concentration of individual PPD ginsenosides, including Rb1 and Rc, that have previously shown a glycemia lowering potential in animal models when provided at levels greater than 1, was not satisfied [46]. The implication is that it is possible that the main ginsenosides that have previously demonstrated hypoglycemic effects were not meeting their efficacy range. In line with this paradigm, we can speculate that the observed U-shaped dose-response relationship may be allocated to the level of available bioactive components, representing the fundamental nature of a pharmacological agent where biological activity is either below the threshold, as observed with 1 g KWG, or above the threshold with the upper dose of 6 g KWG. It is possible that substrate saturation is reached at a higher dose of KRG where no further benefit, followed by a possible counteractive effect, may be observed. Taken together, 3 g KWG appears to be the most optimal dose of KWG tested that can potentially improve glucose metabolism, which is in line with the dose range suggested within ginseng monographs based on traditional recommendations [47].
Two limitations to this work must be addressed. First, we assessed the vascular and glycemic health effects of KWG in an acute design. It is thus unclear whether continuous long term administrations would yield similar results. Secondly, the subjects were taking diabetes and antihypertensive medications. Though these medications were not taken on morning visits, they were taken 12-24 hrs prior to the visits. Hence, they or their metabolites might have been present in the blood on test mornings, and could have potentially confounded the participants' vascular and glycemic measures.
Conclusion
In conclusion, the current study is the first to explore the vascular and glycemic effects of KWG in subjects with T2DM. It showed that acute administration of 3 g KWG elicited modest but significant reductions in arterial stiffness, as measured by AI, while BP and glycemia were not affected. Interestingly, the optimal dose where greatest improvements were seen across vascular and glycemic parameters appears to be the 3 g dose which were not observed with either the 1 or 6 g doses. In light of such findings, future studies should aim at exploring the long term vascular and glycemic effects of the optimal dose identified herein, 3 g, with a main focus in determining its contributing constituents. Should the beneficial results on AI observed in this study be validated in longer term trials, the 4-yr old KWG root may be a promising candidate that is comparable in efficacy to the typically endorsed and valued 6-yr old KRG, creating significant economic possibilities in the area of evidence-based remedies for CVD risk reduction.
Acknowledgments
We would like to thank Danielle Vindua, Shirley Quach, and Stacy Da Silva, for their great assistance in the conduct of the clinical trial.
Footnotes
This research was supported by a grant from the Department of Herbal Crop Research; National Institute of Horticultural and Herbal Science, RDA, Korea.
Vladimir Vuksan is a holder of an American (No. 7,326,404 B2) and Canadian (No. 2,410,556) patent for use of viscous fiber blend in diabetes, metabolic syndrome and cholesterol lowering; currently holds grant support for ginseng research from the Canadian Diabetes Association, Canada and the National Institute of Horticultural & Herbal Science, RDA, Korea. Vladimir Vuksan received a travel grant from BTGin Co, Yuseong-gu, Daejeon, Republic of Korea. Vladimir Vuksan and Alexandra L. Jenkins are part owners of Glycemic Index Laboratories, Inc. a contract research organization. For the remaining authors, no conflicts of interested were declared.
References
- 1.Webber S. International Diabetes Federation News. 2014. Jan 16, Lack of consistency and uniformity in screening for gestational diabetes in India. [Google Scholar]
- 2.Vlasakakis G, Pasqua OD. Cardiovascular disease: the other face of diabetes. CPT Pharmacometrics Syst Pharmacol. 2013;2:e81. doi: 10.1038/psp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Imran SA, Rabasa-Lhoret R, Ross S. Targets for glycemic control. Can J Diabetes. 2013;37(Suppl 1):S31–S34. doi: 10.1016/j.jcjd.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Levy P. The current unmet need in type 2 diabetes mellitus: addressing glycemia and cardiovascular disease. Postgrad Med. 2009;121:7–12. doi: 10.3810/pgm.2009.05.suppl53.287. [DOI] [PubMed] [Google Scholar]
- 6.Leiter LA, Berard L, Bowering CK, Cheng AY, Dawson KG, Ekoé JM, Fournier C, Goldin L, Harris SB, Lin P, Ransom T, Tan M, Teoh H, Tsuyuki RT, Whitham D, Woo V, Yale JF, Langer A. Type 2 diabetes mellitus management in Canada: is it improving? Can J Diabetes. 2013;37:82–89. doi: 10.1016/j.jcjd.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 7.Harris PE, Cooper KL, Relton C, Thomas KJ. Prevalence of complementary and alternative medicine (CAM) use by the general population: a systematic review and update. Int J Clin Pract. 2012;66:924–939. doi: 10.1111/j.1742-1241.2012.02945.x. [DOI] [PubMed] [Google Scholar]
- 8.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 9.Panax ginseng. Monograph. Altern Med Rev. 2009;14:172–176. [PubMed] [Google Scholar]
- 10.Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Di Buono M, Lee KS, Leiter LA, Nam KY, Arnason JT, Choi M, Naeem A. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18:46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Jovanovski E, Jenkins A, Dias AG, Peeva V, Sievenpiper J, Arnason JT, Rahelic D, Josse RG, Vuksan V. Effects of Korean red ginseng (Panax ginseng C.A. Mayer) and its isolated ginsenosides and polysaccharides on arterial stiffness in healthy individuals. Am J Hypertens. 2010;23:469–472. doi: 10.1038/ajh.2010.5. [DOI] [PubMed] [Google Scholar]
- 12.Vuksan V, Sievenpipper J, Jovanovski E, Jenkins AL. Current clinical evidence for Korean red ginseng in management of diabetes and vascular disease: a Toronto's Ginseng Clinical Testing Program. J Ginseng Res. 2010;34:264–273. [Google Scholar]
- 13.Choi KT. The situation and prospect of Korean ginseng industry. Korean J Food Preserv. 2009;8:26–46. [Google Scholar]
- 14.Kim WY, Kim JM, Han SB, Lee SK, Kim ND, Park MK, Kim CK, Park JH. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 15.XiangGao L, Li F, Qi L, Xiang L. Study on hydrolysis reaction of ginsenoside and products in red ginseng processing. J Jillin Agric Univ. 2000;22:1–9. [Google Scholar]
- 16.Xie YY, Luo D, Cheng YJ, Ma JF, Wang YM, Liang QL, Luo GA. Steaming-induced chemical transformations and holistic quality assessment of red ginseng derived from Panax ginseng by means of HPLC-ESI-MS/MS(n)-based multicomponent quantification fingerprint. J Agric Food Chem. 2012;60:8213–8224. doi: 10.1021/jf301116x. [DOI] [PubMed] [Google Scholar]
- 17.Guo XY, Liu D, Ye M, Han J, Deng S, Ma XC, Zhao Y, Zhang B, Shen X, Che QM. Structural characterization of minor metabolites and pharmacokinetics of ganoderic acid C2 in rat plasma by HPLC coupled with electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. 2013;75:64–73. doi: 10.1016/j.jpba.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Chung SH, Choi CG, Park SH. Comparisons between white ginseng radix and rootlet for antidiabetic activity and mechanism in KKAy mice. Arch Pharm Res. 2001;24:214–218. doi: 10.1007/BF02978260. [DOI] [PubMed] [Google Scholar]
- 19.Lee YS, Cha BY, Yamaguchi K, Choi SS, Yonezawa T, Teruya T, Nagai K, Woo JT. Effects of Korean white ginseng extracts on obesity in high-fat diet-induced obese mice. Cytotechnology. 2010;62:367–376. doi: 10.1007/s10616-010-9288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia L, Zhao Y. Current evaluation of the millennium phytomedicine--ginseng (I): etymology, pharmacognosy, phytochemistry, market and regulations. Curr Med Chem. 2009;16:2475–2484. doi: 10.2174/092986709788682146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 22.Pereira T, Maldonado J, Pereira L, Conde J. Aortic stiffness is an independent predictor of stroke in hypertensive patients. Arq Bras Cardiol. 2013;100:437–443. doi: 10.5935/abc.20130079. [DOI] [PubMed] [Google Scholar]
- 23.Cecelja M, Chowienczyk P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis. 2012;1:pii: cvd.2012.012016. doi: 10.1258/cvd.2012.012016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mucalo I, Jovanovski E, Rahelić D, Božikov V, Romić Z, Vuksan V. Effect of American ginseng (Panax quinquefolius L.) on arterial stiffness in subjects with type-2 diabetes and concomitant hypertension. J Ethnopharmacol. 2013;150:148–153. doi: 10.1016/j.jep.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Park BJ, Lee YJ, Lee HR, Jung DH, Na HY, Kim HB, Shim JY. Effects of Korean red ginseng on cardiovascular risks in subjects with metabolic syndrome: a double-blind randomized controlled study. Korean J Fam Med. 2012;33:190–196. doi: 10.4082/kjfm.2012.33.4.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee MY, Kim YS, Bae JH, Nah DY, Kim YK, Lee MM, Kim HY. Effect of Korean red ginseng on arterial stiffness in subjects with hypertension. J Altern Complement Med. 2011;17:45–49. doi: 10.1089/acm.2010.0065. [DOI] [PubMed] [Google Scholar]
- 27.Gao Y, Deng J, Yu XF, Yang DL, Gong QH, Huang XN. Ginsenoside Rg1 inhibits vascular intimal hyperplasia in balloon-injured rat carotid artery by down-regulation of extracellular signal-regulated kinase 2. J Ethnopharmacol. 2011;138:472–478. doi: 10.1016/j.jep.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 28.Scott GI, Colligan PB, Ren BH, Ren J. Ginsenosides Rb1 and Re decrease cardiac contraction in adult rat ventricular myocytes: role of nitric oxide. Br J Pharmacol. 2001;134:1159–1165. doi: 10.1038/sj.bjp.0704377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karmazyn M, Moey M, Gan XT. Therapeutic potential of ginseng in the management of cardiovascular disorders. Drugs. 2011;71:1989–2008. doi: 10.2165/11594300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stavro PM, Woo M, Vuksan V. Korean red ginseng lowers blood pressure in individuals with hypertension. Am J Hypertens. 2004;17:S33. [Google Scholar]
- 33.Siegel RK. Ginseng abuse syndrome. Problems with the panacea. JAMA. 1979;241:1614–1615. [PubMed] [Google Scholar]
- 34.Sievenpiper JL, Jenkins AL, Dascalu A, Stavro PM, Vuksan V. Chapter 12. Ginseng in type 2 diabetes mellitus: a review of the evidence in humans. In: Pasupuleti VK, Anderson JW, editors. Nutraceuticals, glycemic health and type 2 diabetes. Oxford: Wiley-Blackwell; 2009. pp. 245–292. [Google Scholar]
- 35.Xie JT, McHendale S, Yuan CS. Ginseng and diabetes. Am J Chin Med. 2005;33:397–404. doi: 10.1142/S0192415X05003004. [DOI] [PubMed] [Google Scholar]
- 36.Dey L, Xie JT, Wang A, Wu J, Maleckar SA, Yuan CS. Anti-hyperglycemic effects of ginseng: comparison between root and berry. Phytomedicine. 2003;10:600–605. doi: 10.1078/094471103322331908. [DOI] [PubMed] [Google Scholar]
- 37.Kimura M, Waki I, Chujo T, Kikuchi T, Hiyama C, Yamazaki K, Tanaka O. Effects of hypoglycemic components in ginseng radix on blood insulin level in alloxan diabetic mice and on insulin release from perfused rat pancreas. J Pharmacobiodyn. 1981;4:410–417. doi: 10.1248/bpb1978.4.410. [DOI] [PubMed] [Google Scholar]
- 38.Waki I, Kyo H, Yasuda M, Kimura M. Effects of a hypoglycemic component of ginseng radix on insulin biosynthesis in normal and diabetic animals. J Pharmacobiodyn. 1982;5:547–554. doi: 10.1248/bpb1978.5.547. [DOI] [PubMed] [Google Scholar]
- 39.Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U, Xu Z, Vidgen E. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med. 2000;160:1009–1013. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- 40.Reay JL, Kennedy DO, Scholey AB. The glycaemic effects of single doses of Panax ginseng in young healthy volunteers. Br J Nutr. 2006;96:639–642. [PubMed] [Google Scholar]
- 41.Sievenpiper JL, Sung MK, Di Buono M, Seung-Lee K, Nam KY, Arnason JT, Leiter LA, Vuksan V. Korean red ginseng rootlets decrease acute postprandial glycemia: results from sequential preparation- and dose-finding studies. J Am Coll Nutr. 2006;25:100–107. doi: 10.1080/07315724.2006.10719519. [DOI] [PubMed] [Google Scholar]
- 42.Sievenpiper JL, Arnason JT, Leiter LA, Vuksan V. Null and opposing effects of Asian ginseng (Panax ginseng C.A. Meyer) on acute glycemia: results of two acute dose escalation studies. J Am Coll Nutr. 2003;22:524–532. doi: 10.1080/07315724.2003.10719331. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z, Wang LJ, Li X, Hu JN, Chen Y, Ruan CC, Sun GZ. Hypoglycemic effects of malonyl-ginsenosides extracted from roots of Panax ginseng on streptozotocin-induced diabetic mice. Phytother Res. 2009;23:1426–1430. doi: 10.1002/ptr.2796. [DOI] [PubMed] [Google Scholar]
- 44.Harkey MR, Henderson GL, Gershwin ME, Stern JS, Hackman RM. Variability in commercial ginseng products: an analysis of 25 preparations. Am J Clin Nutr. 2001;73:1101–1106. doi: 10.1093/ajcn/73.6.1101. [DOI] [PubMed] [Google Scholar]
- 45.Chan TW, But PP, Cheng SW, Kwok IM, Lau FW, Xu HX. Differentiation and authentication of Panax ginseng, Panax quinquefolius, and ginseng products by using HPLC/MS. Anal Chem. 2000;72:1281–1287. doi: 10.1021/ac990819z. [DOI] [PubMed] [Google Scholar]
- 46.Hasegawa H, Matsumiya S, Murakami C, Kurokawa T, Kasai R, Ishibashi S, Yamasaki K. Interactions of ginseng extract, ginseng separated fractions, and some triterpenoid saponins with glucose transporters in sheep erythrocytes. Planta Med. 1994;60:153–157. doi: 10.1055/s-2006-959440. [DOI] [PubMed] [Google Scholar]
- 47.Blumenthal M, Busse WR, Goldberg A, Gruenwald J, Hall T, Riggins CW, Rister RS. The complete German commission E monographs: therapeutic guide to herbal medicines. Austin (TX): American Botanical Council; 1998. [Google Scholar]