Abstract
Purpose
To investigate the prevalence and causes of blindness and partial sight among a population of Tunisian diabetic patients.
Methods
A cross-sectional study of 2320 randomly identified patients with diabetes mellitus. Patient's characteristics as well as data from the last ophthalmic examination were reviewed.
Results
Of all patients examined, 60.2% were females and 39.8% were males. Mean age of patients was 54.5 years. Mean duration of diabetes was 7.6 years. Diabetic retinopathy (DR) was recorded in 26.3% of patients, and was proliferative in 3.4% of patients. The prevalence of visual impairment was 22.2%, with 4.4% patients legally blind and 17.8% partially sighted. Visual impairment was significantly associated with age ≥60 years (P<0.001), duration of diabetes >10 years (P<0.001), body mass index >25 (P=0.014), hypertension (P<0.001), heart disease (P<0.001), peripheral neuropathy (P=0.03), vegetative neuropathy (P=0.002), macroalbuminuria (P<0.001), cataract (P<0.001), DR (P<0.001), diabetic macular edema (P<0.001), open angle glaucoma (P<0.001), intravitreal hemorrhage (P<0.001), rubeosis iridis (P<0.001), neovascular glaucoma (P<0.001), and tractional retinal detachment (P<0.001).
Conclusion
The current report is the largest study of DR in North African region. It provides a baseline data against which future progress can be assessed. Screening and treatment can greatly reduce the incidence of visual impairment due to diabetes.
Introduction
Visual deficiency and blindness are major public health problems in diabetics, particularly in developing countries mainly due to an increase in the number of old diabetics and the insufficiency of early tracing routines of diabetics at risk of blinding complications.1
The overall diabetes prevalence in Tunisia is 9.9%,2 and is estimated to be 11.2% in 2030.3 The prevalence of visual impairment and blindness due to diabetic retinopathy (DR) and diabetic eye complications is on the rise worldwide and more specifically in North Africa and the Middle East region,1 and DR is a priority disease in the VISION 2020 initiative for the global elimination of avoidable blindness, and the World Health Organization has made prevention of visual impairment and blindness an international priority.4
Numerous studies on the patterns of DR in various geographic regions have been published, showing similarities and distinct differences in the epidemiologic profile.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Data about the prevalence of visual impairment in diabetic population in North African region are scarce. The aim of our study was to identify prevalence and causes of blindness and partial sight in diabetic patients in a referral center in Tunisia, North Africa.
Materials and methods
This is a cross-sectional study including 2320 diabetic patients seen at Fattouma Bourguiba University Hospital, Monastir, Tunisia, between April 2007 and February 2011. All participants were primarily recruited by diabetologists or general practitioners.
Inclusion criteria included type 1 diabetes defined as age of onset <30 years and insulin required from onset, and type 2 diabetes (all other patients).
History and clinical data were defined by structured and standardized questionnaire. Age, gender, type and duration of diabetes, insulin use, presence of one or more general comorbidities, and presence of diabetic complications other than ocular including heart disease, nephropathy, and neuropathy were noted. Biological assessment included last fasting plasma glucose level (mmol/l), cholesterol level (mg/dl), systolic and diastolic blood pressure (mmHg), body mass index (BMI) (kg/m2), smoking, and alcohol consumption status.
All patients underwent a comprehensive ophthalmic examination including best-corrected Snellen visual acuity (VA), slit lamp examination, tonometry, dilated biomicroscopic fundus examination, and fundus photography. Fluorescein angiography was performed when necessary.
Only VA criteria were used to define visual impairment in our study. Blindness and partial sight were determined according to criteria used in the US definitions, where partial sight is defined as 20/200 (6/60)<VA<20/40 (6/12) and blindness is defined as VA of ≤20/200 (6/60) in the better eye. When describing both blindness and partial sight, the term visual impairment is used.
DR and diabetic macular edema (DME) were classified according to the Early Treatment of Diabetic Retinopathy Study (ETDRS) criteria.17, 18 DR is classified into non-proliferative DR (NPDR) and proliferative DR (PDR). NPDR is further subdivided into mild, moderate, and severe. DME is classified into clinically significant DME (CSDME) and clinically nonsignificant DME (CNSDME).
The study followed the tenets of the Declaration of Helsinki, and was approved by the local ethical committee of our hospital.
The data were recorded and analyzed by means of software SPSS version 18.0 for Windows (Chicago, IL, USA). Chi-square statistics were used to assess associations. A P-value of <0.05 was considered statistically significant.
Results
A total of 2320 diabetic patients (4628 eyes) were included in the study. The demographic data of patients are summarized in Table 1. Of our patients, 1396 (60.2%) were females and 924 (39.8%) were males. The sex ratio (M/F) was 0.66. Mean age of patients was 54.5 years (range, 10–92). One hundred and thirteen patients (4.9%) were aged <30 years, 1361 patients (58.7%) were aged between 30 and 50, and 846 patients (36.4%) were aged over 60.
Table 1. General characteristics and distribution of patients.
| Parameters | Number of eyes N=4628 | % |
|---|---|---|
| Mean age | 54.5 years (range, 10–92) | |
| Gender | ||
| Male | 924 | 39.8 |
| Female | 1396 | 60.2 |
| Visual acuity | ||
| Mean | 20/63 (range, LP-20/20) | |
| ≤20/200 | 366 | 7.9 |
| 20/200–20/40 | 940 | 20.3 |
| ≥20/40 | 3322 | 71.8 |
| Diabetic retinopathy | 1217 | 26.3 |
| Mild NPDR | 530 | 11.5 |
| Moderate NPDR | 280 | 6 |
| Severe NPDR | 251 | 5.4 |
| Proliferative DR | 156 | 3.4 |
| Diabetic macular edema | 402 | 8.7 |
| CSDME | 196 | 4.2 |
| CNSDME | 206 | 4.5 |
Abbreviations: CNSDME, clinically non-significant diabetic macular edema; CSDME, clinically significant diabetic macular edema; DR, diabetic retinopathy; LP, light perception; NPDR, non proliferative diabetic retinopathy.
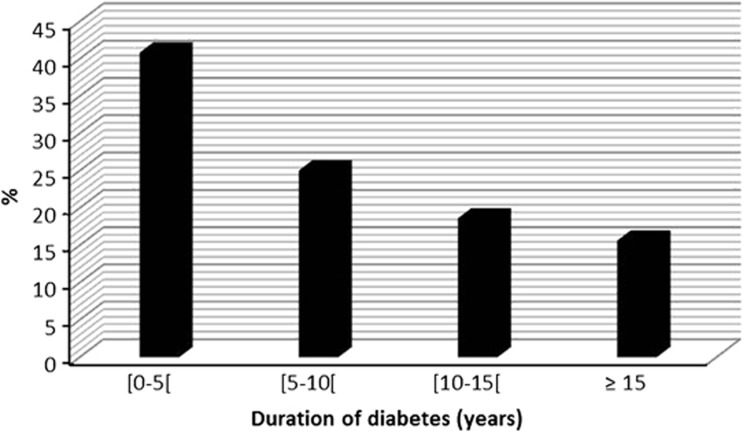
Type 1 diabetes was recorded in 255 patients (11%) and type 2 diabetes in 2065 patients (89%). Mean age at the onset of diabetes was 46.8 years. The mean duration of diabetes was 7.6 years. The diabetes duration was <5 years among 947 patients (40.8%) and >10 years among 794 patients (34.2%) (Figure 1).
Figure 1.
Duration of diabetes in our patients.
Systemic diseases associated with diabetes are summarized in Table 2. A BMI >30 was recorded in 588 patients (25.3%).
Table 2. Systemic diseases associated with diabetes.
| Number of patients N=2320 | % | |
|---|---|---|
| Hypertension | 871 | 37.5 |
| Dyslipidemia | 487 | 21 |
| Heart disease | 203 | 8.8 |
| Peripheral neuropathy | 433 | 18.7 |
| Vegetative neuropathy | 113 | 4.9 |
| Microalbuminuria | 66 | 2.8 |
| Macroalbuminuria | 14 | 0.6 |
| Obesity (BMI>30) | 588 | 25.3 |
Abbreviation: BMI, body mass index.
VA ranged from light perception (LP) to 20/20 (mean 20/63). Cataract was recorded in 1509 eyes (32.6%) and open angle glaucoma in 123 eyes (2.7%). DR was recorded in 1217 eyes (26.3%). It was mild NPDR in 530 eyes (11.5%), moderate NPDR in 280 eyes (6%), severe NPDR in 251 eyes (5.4%), and proliferative DR in 156 eyes (3.4%).
DME was recorded in 402 eyes (8.7%). Of these eyes, 206 had CNSDME (51.2%) and 196 had CSDME (48.8%).
The most common complication of DR was intravitreal hemorrhage (59 eyes; 1.3%), followed by rubeosis iridis (32 eyes; 0.6%), tractional retinal detachment (20 eyes; 0.4%), and neovascular glaucoma (14 eyes; 0.3%).
Blindness was recorded in 101 patients (4.4%) and partial sight in 413 patients (17.8%).
The frequency of eyes with blindness was 3.9% (114 eyes) in patients younger than 60 years old and 15% (252 eyes) in patients older than 60 years. The difference was statistically significant (P<0.001).
Visual impairment in our patients was significantly associated with age ≥60 years (P<0.001), duration of diabetes >10 years (P<0.001), BMI >25 (P=0.014), hypertension (P<0.001), heart disease (P<0.001), peripheral neuropathy (P=0.03), vegetative neuropathy (P=0.002), and macroalbuminuria (P<0.001) (Table 3).
Table 3. Factors associated with visual impairment.
|
Blindness |
Partial sight |
P | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age ≥60 years | 252 | 15 | 622 | 37 | <0.001 |
| Duration ≥10 years | 197 | 15 | 433 | 32.9 | <0.001 |
| Type 1 diabetes | 56 | 11 | 69 | 13.5 | 0.147 |
| Male gender | 163 | 44.5 | 347 | 36.9 | 0.248 |
| Hypertension | 174 | 10 | 429 | 24.8 | <0.001 |
| Heart disease | 61 | 15.1 | 219 | 27.2 | <0.001 |
| Obesity | 119 | 10.1 | 266 | 22.6 | 0.014 |
| Microalbuminuria | 14 | 10.6 | 30 | 22.7 | 0.513 |
| Macroalbuminuria | 3 | 21.4 | 6 | 42.9 | <0.001 |
| Hyperlipidemia | 93 | 9.5 | 214 | 22 | 0.091 |
| Vegetative neuropathy | 39 | 17.3 | 31 | 13.8 | 0.002 |
| Peripheral neuropathy | 57 | 6.6 | 219 | 25.3 | 0.03 |
| Tobacco | 93 | 9 | 265 | 25.7 | 0.860 |
| Alcohol | 15 | 4.1 | 351 | 8.2 | 0.178 |
The prevalence of blindness increased with the severity of DR. Actually, the frequency of blindness increased from 5.3% (43 eyes) in mild and moderate NPDR to 18.7% (47 eyes) in severe NPDR, and reached 46.8% (73 eyes) in PDR.
Visual impairment was significantly associated with cataract (P<0.001), DR (P<0.001), DME (P<0.001), open angle glaucoma (P<0.001), intravitreal hemorrhage (P<0.001), rubeosis iridis (P<0.001), neovascular glaucoma (P<0.001), and tractional retinal detachment (P<0.001) (Table 4).
Table 4. Prevalence of visual impairment according to ocular findings.
|
VA≤20/200
blindness |
20/200<VA<20/40
partial sight |
VA≥20/40 |
P | ||||
|---|---|---|---|---|---|---|---|
| N=366 | % | N=940 | % | N=3322 | % | ||
| Cataract | 259 | 70.8 | 538 | 57.2 | 797 | 24 | <0.001 |
| Diabetic retinopathy | 163 | 44.5 | 349 | 37.1 | 512 | 15.4 | <0.001 |
| Diabetic macular edema | 58 | 15.8 | 151 | 16.1 | 209 | 6.3 | <0.001 |
| Intravitreal hemorrhage | 33 | 9 | 18 | 1.9 | 51 | 1.5 | <0.001 |
| Tractional retinal detachment | 15 | 4.1 | 4 | 0.4 | 19 | 0.6 | <0.001 |
| Neovascular glaucoma | 12 | 3.3 | 1 | 0.1 | 13 | 0.4 | <0.001 |
| Open angle glaucoma | 13 | 3.6 | 45 | 4.8 | 58 | 1.7 | <0.001 |
| High myopia | 2 | 0.5 | 37 | 3.9 | 45 | 1.4 | <0.001 |
| Corneal opacity | 2 | 0.5 | 7 | 0.7 | 6 | 0.2 | <0.001 |
Abbreviation: VA, visual acuity.
Discussion
Diabetes is considered to be a major public health challenge worldwide. Its incidence is in strong increase, with important variations according to ethnic and/or racial groups. Diabetes represents a leading cause of visual impairment in individuals between the age of 20 and 60 years in the industrialized countries.19, 20 There is a need for reliable estimates of vision loss due to diabetes. Such estimates are important for providing baseline levels for planning public health programs to reduce the risk of visual impairment and the impact of diabetic complications, and for evaluating the results of the programs.
To the best of our knowledge, our study is the largest to provide data on causes and prevalence of visual impairment in diabetics from the North African region.
In the present study, the prevalences of DR (26.3%) as well as proliferative DR (3.4%) were lower than those reported in a previous Tunisian study (45.2% and 5.7%, respectively).21 Longer duration of diabetes in this cohort (mean 11.7 years) could explain higher prevalence compared with our study. Higher frequencies are also found in an American population (45.2 and 3.4%, respectively),12 in Afro-American patients (63.9 and 18.9%, respectively),11 in the population-based Wisconsin Epidemiologic Study of Diabetic Retinopathy22 for whites with type 1 diabetes (63.9 and 8.3%, respectively), and in a Canadian population study (40 and 10%, respectively).23
In our study, the results show that 22.2% of our patients presented a visual impairment, of them 4.4% had a legal blindness and 17.8% had a partial sight. Thus in our series, 1 diabetic in 23 is blind and 1 diabetic in 6 is a partially sighted person.
Rate of blindness in our series (4.4%) was close to that recorded in South Africa (4.4%)5 and lower than that recorded in Nigeria (18%).6 In American studies, prevalences of blindness were lower than ours.11, 12 Review of the literature shows that in Europe, the prevalence of blindness ranged from 1.1 to 7.5%.13, 14, 15, 16 In North African and the Middle East countries, the rate of blindness ranges between 5 and 15.7%.7, 8, 9, 10
In our study, 17.6% of patients had partial sight. It was higher than a previous Tunisian series (12.5%).21 Partial sight was recorded in 11.3–30% in African studies5, 6 and 10.1–39.3% in countries from North Africa and the Middle East.7, 9, 10 A lower prevalence of partial sight was found in Europe (2.8–7.5%)13, 15, 16 and in the Unites states (6.7–11%).11, 12
The frequency of visual impairment in Tunisian patients was not significantly different between the sexes, but it increased with increasing age. Other associated risk factors that had significant correlation with visual impairment in diabetic Tunisian patients included obesity, heart disease, high blood pressure, macroalbuminuria, and peripheral and vegetative neuropathy. However, smoking, alcohol consumption, and hyperlipidemia were not associated with visual impairment, in contrast with the results of the ETDRS where high levels of cholesterol were associated with persistent severe visual loss.24
Frequency of visual impairment was statistically associated with duration of diabetes. In fact, duration of >10 years of diabetes doubles the risk of visual deficiency. Visual impairment was associated with the presence of DR, the presence of DME, and DR complications notably cataract, rubeosis iridis, neovascular glaucoma, intravitreal hemorrhage, and tractional retinal detachment. Duration of diabetes, DME, complicated PDR, and cataract were found to be closely associated with visual impairment in other studies.14, 24 In recent studies, visual disability was significantly associated with female sex and DR in a population of Central African diabetics,25 and with tractional retinal detachment and optic atrophy in a Thai diabetic population.26 In a Jordanian diabetic population, visual impairment was significantly associated with age, treatment of diabetes, and DR, whereas only age and retinopathy were significantly related to blindness.7
This study had some limitations. First, data were based on known diabetes only. Patients with undiagnosed diabetes might lead to underestimation of prevalence of visual impairment. Second, the study was conducted in a single center. However, all patients were referred by diabetologists or general practitioners regardless of presence or not of visual complaints. In addition, Monastir is a tourist and industrial region on the central coast of Tunisia in which people with origins from different regions of the country live. As a consequence, and in our opinion, our sample is representative of all patients with diabetes mellitus in Tunisia and provides useful data. Other limitations include its cross-sectional design with no follow-up, glycated hemoglobin values and results of optical coherence tomography were not available for the majority of patients.
In summary, data of the present study show that the frequency of DR and associated visual impairment in Tunisian diabetic patients are relatively high and increase with increasing age and duration of diabetes. It provides baseline data that will be useful for the prevention of burden of visual impairment related to diabetes mellitus. Public health policies with educational programs and promotion of telemedicine-based digital retinal imaging are needed to ensure the appropriate screening and timely management of DR that greatly reduce the incidence of visual impairment due to diabetes. Further investigations are needed to assess the process and outcomes of care.
Acknowledgments
This work has been supported by the Ministry of Higher Education and Scientific Research of Tunisia.
The authors declare no conflict of interest.
References
- Khandekar R. Screening and public health strategies for diabetic retinopathy in the Eastern Mediterranean region. Middle East Afr J Ophthalmol. 2012;19:178–184. doi: 10.4103/0974-9233.95245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouguerra R, Alberti H, Salem LB, Rayana CB, Atti JE, Gaigi S, et al. The global diabetes pandemic: the Tunisian experience. Eur J Clin Nutr. 2007;61:160–165. doi: 10.1038/sj.ejcn.1602478. [DOI] [PubMed] [Google Scholar]
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011. 94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- World Health Organization and International Agency for the Prevention of Blindness . Vision 2020 the Right to Sight: Global Initiative for the Elimination of Avoidable Blindness Action Plan 2006–2011. World Health Organization: Geneva, Switzerland; 2007. Diabetic retinopathy; pp. 34–36. [Google Scholar]
- Read O, Cook C. Retinopathy in diabetic patients evaluated at a primary care clinic in Cape Town. S Afr Med J. 2007;97:941–942. [PubMed] [Google Scholar]
- Nwosu SN. Low vision in Nigerians with diabetes mellitus. Doc Ophthalmol. 2000;101:51–57. doi: 10.1023/a:1002799506855. [DOI] [PubMed] [Google Scholar]
- Al-Till MI, Al-Bdour MD, Ajlouni KM. Prevalence of blindness and visual impairment among Jordanian diabetics. Eur J Ophthalmol. 2005;15:62–68. doi: 10.1177/112067210501500110. [DOI] [PubMed] [Google Scholar]
- Herman WH, Aubert RE, Engelgau MM, Thompson TJ, Ali MA, Sous ES, et al. Diabetes mellitus in Egypt: glycaemic control and microvascular and neuropathic complication. Diabet Med. 1998;15:1045–1051. doi: 10.1002/(SICI)1096-9136(1998120)15:12<1045::AID-DIA696>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Khandekar R, Mohammed AJ. Visual disabilities among diabetics in Oman. Saudi Med J. 2005;26:836–841. [PubMed] [Google Scholar]
- Al-Akily SA, Bamashmus MA, Gunaid AA. Causes of visual impairment and blindness among Yemenis with diabetes: a hospital-based study. East Mediterr Health J. 2011;17:831–837. doi: 10.26719/2011.17.11.831. [DOI] [PubMed] [Google Scholar]
- Roy MS. Diabetic retinopathy in African Americans with type 1 diabetes: The New Jersey 725: I. Methodology, population, frequency of retinopathy, and visual impairment. Arch Ophthalmol. 2000;118:97–104. doi: 10.1001/archopht.118.1.97. [DOI] [PubMed] [Google Scholar]
- Fong DS, Sharza M, Chen W, Paschal JF, Ariyasu RG, Lee PP. Vision loss among diabetics in a group model Health Maintenance Organization (HMO) Am J Ophthalmol. 2002;133:236–241. doi: 10.1016/s0002-9394(01)01364-2. [DOI] [PubMed] [Google Scholar]
- Romero-Aroca P, Fernández-Balart J, Baget-Bernaldiz M, Martinez-Salcedo I, Méndez-Marín I, Salvat-Serra M, et al. Changes in the diabetic retinopathy epidemiology after 14 years in a population of Type 1 and 2 diabetic patients after the new diabetes mellitus diagnosis criteria and a more strict control of the patients. J Diabetes Complications. 2009;23:229–238. doi: 10.1016/j.jdiacomp.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Jeppesen P, Bek T. The occurrence and causes of registered blindness in diabetes patients in Arhus County, Denmark. Acta Ophthalmol Scand. 2004;82:526–530. doi: 10.1111/j.1600-0420.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- Olafsdottir E, Andersson DK, Stefansson E. Visual acuity in a population with regular screening for type 2 diabetes mellitus and eye disease. Acta Ophthalmol Scand. 2007;85:40–45. doi: 10.1111/j.1600-0420.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- Prasad S, Kamath GG, Jones K, Clearkin LG, Phillips RP. Prevalence of blindness and visual impairment in a population of people with diabetes. Eye. 2001;15 (Pt 5:640–643. doi: 10.1038/eye.2001.200. [DOI] [PubMed] [Google Scholar]
- Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98 (5 Suppl:823–833. [PubMed] [Google Scholar]
- Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- Cormack TG, Grant B, Macdonald MJ, Steel J, Campbell IW. Incidence of blindness due to diabetic eye disease in Fife 1990-9. Br J Ophthalmol. 2001;85:354–356. doi: 10.1136/bjo.85.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massin P, Kaloustian E. The elderly diabetic's eyes. Diabetes Metab. 2007;33 (Suppl 1:S4–S9. doi: 10.1016/s1262-3636(07)80052-8. [DOI] [PubMed] [Google Scholar]
- Zghal-Mokni I, Nacef L, Letaief I, Mahjoub S, Bouguila H, Blouza S, et al. Ocular manifestations of diabetes: 285 cases. Tunis Med. 2008;86:1004–1007. [PubMed] [Google Scholar]
- Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859–1868. doi: 10.1016/j.ophtha.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, McKenna A, Mozejko S, Fick GH. Diabetic retinopathy in native and nonnative Canadians. Exp Diabetes Res. 2007;2007:76271. doi: 10.1155/2007/76271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong DS, Ferris FL, 3rd, Davis MD, Chew EY. Causes of severe visual loss in the early treatment diabetic retinopathy study: ETDRS report no. 24. Early Treatment Diabetic Retinopathy Study Research Group. Am J Ophthalmol. 1999;127:137–141. doi: 10.1016/s0002-9394(98)00309-2. [DOI] [PubMed] [Google Scholar]
- Mvitu Muaka M, Longo-Mbenza B. Causes of visual disability among Central Africans with diabetes mellitus. Afr Health Sci. 2012;12:193–197. doi: 10.4314/ahs.v12i2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singalavanija A, Luangsawang K, Chotikavanich S, Tanterdtham J, Samsen P. Causes of visual impairment in Thai diabetic patients in the visual rehabilitation clinic. J Med Assoc Thai. 2012;95 (Suppl 4:S24–S29. [PubMed] [Google Scholar]