Sir,
We read with great interest the article by Toprak et al1 regarding the evaluation of possible preoperative predictive factors influencing the outcome of cross-linking (CXL) in a group of 96 patients with progressive keratoconus (KC). The authors conclude that an older age (≥30 years), a worse corrected distance visual acuity (CDVA; ≤20/40), and a thinner corneal pachimetry (<450 μm) were positive predictive factors for CXL, whereas maximum K (Kmax) did not affect the postoperative change.
Although we agree that age influences the outcome of the treatment,2 our results, stratified into four subgroups (<18 years, 18–28 years, 29–39 years, and over 40 years), demonstrated a better functional and morphological outcomes in the population between 18 and 39 years of age.
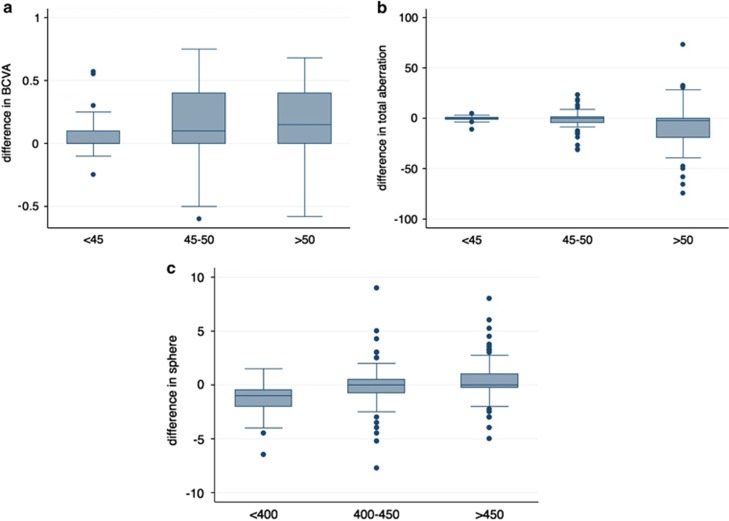
Furthermore, here we report a subset analysis of the data evaluated in Ophthalmology2 based on preoperative curvature and thinnest corneal thickness (ThCT). In particular, the data have been stratified according to preoperative Simk1 (<45 D; 45–50 D; >5 0D) and ThCT (<400 μm; 400–450 μm; >450 μm). Comparative analysis between curvature subgroups indicated the subgroup over 50 D as the best responder with a significant improvement in CDVA (P<0.05) and in total aberration outcomes (P<0.05; Figures 1a and b). Analysis based on ThCT showed a better reaction to CXL in the subgroup <400 μm with a significant difference in sphere change (P<0.05; Figure 1c).
Figure 1.
Box and whisker plot between follow-up and preoperative values with respect to preoperative curvature (a and b) and thickness (c). Differences divided by preoperative Simk1 for best-corrected visual acuity (BCVA) are shown in graph a, for total aberration outcomes are shown in graph b. Changes in sphere divided by preoperative pachymetry are shown in graph c.
These results are in partial agreement with that of Greenstein et al,3 which showed that patients with a preoperative Kmax≥55 D were more likely to have flattening ≥2 D.
Our main finding shows that eyes with progressive KC with higher curvature (>50 D) and low pachymetry (<400 μm), representing more advanced cases, are more likely to have an improvement after CXL.
Moreover, we would like to comment on the reported use of the isotonic riboflavin solution.
It has been demonstrated that, in advanced KC (with thin corneas), using a standard isotonic solution, dextran, induces a decrease of thickness (CCT) that can cause endothelial damage and deep stromal opacities.4 To avoid these complications it is advisable to check intraoperative CCT and in case of the thickness reducing <400 μm, use swelling solutions.2
Vetter et al5 in a recent report did not find a correlation between osmolality and CCT after riboflavin eye drop application but found an inverted correlation between dextran concentration and CCT. The authors explained that hypertonicity and hypotonicity of solutions do not have a significant effect on stromal thickness because only 2–3% of the corneal stroma volume consists of cells, conversely dextran possesses a high affinity for water because of its abundant hydrophilic hydroxyl groups leading to deswelling beyond the physiological level. For this reason we suggest to evaluate not only the osmolality of the solution but also the concentration of dextran.
In conclusion, our report confirmed the literature finding that a preoperative high keratometry is a positive predictive factor. Furthermore, our findings add that keratoconic corneas with very low pachymetry are more likely to improve. For this reason we suggest to treat advanced KC also and in case of ThCT <400 μm to use swelling solutions.
The authors declare no conflict of interest.
References
- Toprak I, Yaylalı V, Yildirim C. Factors affecting outcomes of corneal collagen crosslinking treatment. Eye (Lond) 2014;28 (1:41–46. doi: 10.1038/eye.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinciguerra R, Romano MR, Camesasca FI, Azzolini C, Trazza S, Morenghi E, et al. Corneal cross-linking as a treatment for keratoconus: four-year morphologic and clinical outcomes with respect to patient age. Ophthalmology. 2013;120 (5:908–916. doi: 10.1016/j.ophtha.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Greenstein SA, Hersh PS. Characteristics influencing outcomes of corneal collagen crosslinking for keratoconus and ectasia: implications for patient selection. J Cataract Refract Surg. 2013;39 (8:1133–1140. doi: 10.1016/j.jcrs.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Vinciguerra P, Albe E, Romano MR, Sabato L, Trazza S. Stromal opacity after cross-linking. J Refract Surg. 2012;28 (3:165. doi: 10.3928/1081597X-20120301-04. [DOI] [PubMed] [Google Scholar]
- Vetter JM, Brueckner S, Tubic-Grozdanis M, Vossmerbaumer U, Pfeiffer N, Kurz S. Modulation of central corneal thickness by various riboflavin eyedrop compositions in porcine corneas. J Cataract Refract Surg. 2012;38 (3:525–532. doi: 10.1016/j.jcrs.2011.09.045. [DOI] [PubMed] [Google Scholar]