Abstract
Clostridium perfringens is an anaerobic pathogen known to cause vast number of diseases in mammals and birds. Various toxins and hydrolysing enzymes released by the organism are responsible for the necrosis of soft tissues. Due to serious safety issues associated with current vaccines against C. perfringens, there is a need for new drug or vaccine targets. C. perfringens is extremely dependent on its host for nutrition which can be targeted for vaccine development or drug design. Therefore, it is of interest to identify the unique transport systems used by C. perfringens involved in uptake of essential amino acids that are synthesized by the host, so that therapeutic agents can be designed to target the specific transport systems. Use of bioinformatics tools resulted in the identification of a protein component of the glutamate transport system that is not present in the host. Analysis of the conservation profile of the protein domain indicated it to be a glutamate binding protein which also stimulates the ATPase activity of ATP Binding Cassettes (ABC) transporters. Homology modelling of the protein showed two distinct lobes, which is a characteristic of substrate binding proteins. This suggests that the carboxylates of glutamate might be stabilized by electrostatic interactions with basic residues as is observed with other binding proteins. Hence, the homology model of this potential drug target can be employed for in silico docking studies by suitable inhibitors.
Keywords: Clostridium perfringens, Essential amino acids, Glutamate ABC transporter system, Domain profiling, Drug targets
Background
The genus Clostridium comprises of over 150 different species of Gram positive, spore-forming, anaerobic rod shaped bacteria belonging to the fermicutes. Pathogenic species for humans and animals primarily include Clostridium perfringens, Clostridium botulinum, Clostridium difficile and C. tetani. C. perfringens is responsible for various diseases; some of the major ones are food poisoning, wound and surgical infections that lead to gas gangrene, and severe uterine infections [1]. C. perfringens produces various toxins - alpha, beta, iota, epsilon and theta. Each type of toxin is associated with a specific disease [2]. After the appearance of clinical signs of the disease caused by C. perfringens, progression of disease is very rapid, and therefore the use of antibiotics offers limited help [3]. Thus, vaccination offers a promising defence against the disease. Conventional vaccines are usually toxoid based and hence have several inherent issues. The toxoid-based vaccines contain significant amounts of undesirable proteins that often generate inflammatory response in the host. Besides, there is a risk of reversion of the toxin if the inactivation is improper or partial. Therefore, it would be beneficial to find drug targets or vaccine candidates that target the Clostridium species itself instead of the toxins that are produced by the organism.
Clostridium species are obligate fermenters of sugars and/or amino acids. Unlike most other Clostridia, Clostridium perfringens is non-motile and cannot grow in an environment where amino acid supply is limited. This is due to the fact that it lacks several genes that are required to biosynthesize many amino acids (arginine, aromatic amino acids, branched-chain amino acids, glutamate, histidine, lysine, methionine, serine, and threonine) essential to its existence [4]. Thus the bacteria rely completely on the effective transport process of a variety of essential amino acids from extracellular milieu [5]. This rate limiting step to bacterial growth offers a potential target to combat Clostridium perfringens, as reduction in intracellular metabolic precursors subsequently results in nutrient starvation. In the present study, we have identified a component of the glutamate transport system using domain conservation analysis. This component is essential for C. perfringens but does not exist in its host and thus is a potential drug target and vaccine candidate.
Methodology
Comparison of nonessential/essential amino acids of host-pathogen:
In order to identify putative drug targets, the essential amino acids for C. perfringens and nonessential amino acids for Homo sapiens were extracted from the literature [6– 8].
Domain profile conservation in periplasmic glutamate binding bacterial proteins:
Amino acid sequences of experimentally characterized solute specific binding protein of glutamate ABC-transporter system from three different bacterial species (E. coli K 12, P. putida KT2440 and Campylobacter jejuni NCTC 11168) [9– 13] were taken from Uniprot Knowledge Base (ID: P37902, Q88NY2, Q0P9X8).
The aligned consensus obtained after multiple sequence alignment of these three sequences was used to search Clostridium homologue using BLASTP analysis in C. perfringens Type-A strains. The domain consensus sequence for glutamate and aspartate transporter subunit was derived from NCBI CDD database entry PRK10797, a member of the superfamily cl19131. The T-coffee sequence alignment was used to confirm the conservation of domain profile in C. perfringens protein sequences (http://tcoffee.crg.cat/) [14]. Further, STRING database search was used to predict the functional partners of the aligned consensus of putative proteins from C. perfringens type-A strains (http://string-db.org/) [15].
Homology modeling and glutamate binding site prediction of CPR_1324:
The protein sequence of the CPR_1324 from C. perfringens TypeA-SM101 was used for structure prediction based on the availability of the crystal structure of a polar amino acid ABC uptake transporter substrate binding protein from Streptococcus thermophilus (PDB 3HV1). The sequence was submitted to SWISS MODEL SERVER [16] and the homology model was built with ProMod Version 3.70. The global and per-residue model quality was assessed using QMEAN scoring function [17]. Molegro Virtual Docker was for active site prediction (active site region was considered as the region in the 2-6 Å radius of the bound ligand). The residues within the cavity, which are important for glutamate binding, were also analysed to confirm its function.
Results & Discussion
Clostridium perfringens is an anaerobic flesh eater which is incapable of synthesizing many of the amino acids required for its growth. These essential amino acids are extracted from the host by releasing various toxins and hydrolyzing enzymes that cause necrosis of soft tissues. These amino acids are then either actively transported through ATP hydrolysis (ABC Importers) or facilitated by sodium motive force (MFS Symporters). ABC transporters encoded in bacterial genomes correlate with this requirement for survival in physiological niches. Therefore, it is not surprising that these transport systems play either a direct or indirect role in the virulence of bacteria. There is increasing evidence for the virulence associated with the genes encoding the permease components involved in the uptake of amino acids and oligopeptides [18]. ABC-transporters, a protein of the permease component of C. perfringens, has been identified as one of genes unique to a number of pathogenic bacteria Including C. perfringens [19]. An additional tier of specificity can be introduced by exploiting the essential amino acids required for bacterial growth for which mammalian host cells lack ABC import systems. To this end, a comparison of nonessential/essential amino acids need of humans and C. perfringens was carried out to identify the target transporter system for an amino acid which is essential for the pathogen and non-essential for the human host Table 1 (see supplementary material). Based on literature search, it was evident that humans essentially lack extracytoplasmic solute receptor (ESR) dependent uptake systems for their nonessential amino acids such as glutamic acid, aspartic acid and alanine [8]. Previous studies on the amino acids requirements of C. perfringens show that alanine and aspartic acid are not stringently essential for some strains of C. perfringens. Glutamic acid, however, is an essential amino acid across various strains of C. perfringens. It is one of the most important metabolites in bacterial cells, playing a central link between the metabolism of carbon and nitrogen. It serves as the amino group donor for nearly all nitrogen-containing metabolites of the cell and thus its metabolism is tightly controlled under all conditions of nutrient supply. This makes its transport system a very attractive target for vaccine and drug development. In the absence or in the presence of a low sodium ion concentration (<20 mM), that slows down the activity of sodium-driven secondary transporters, the glutamate is taken predominantly by polar amino acid transporter (PAAT) [20]. This is a typical prokaryote extracellular solute binding protein-dependent uptake system. The specificity of such transporters mainly depends on the nature of their periplasmic ligand binding protein. This solute specific component also stimulates the ATPase activity of ATP Binding Cassettes (ABC) of the importer, thereby initiating the translocation process. Very few such solute specific binding proteins of glutamate ABC- transporter system in different bacterial species have been experimentally characterized. Three such proteins from E. coli K 12, Pseudomonas putida KT2440 and Campylobacter jejuni NCTC 11168 are GltI, AatJ (PP1071) and PEB1a (CJ0921c) respectively [11– 13]. The aligned consensus of these three sequences revealed putative gene/ORF for periplasmic glutamate binding protein in C. perfringens Type-A strains Table 2 (see supplementary material). The score of T-coffee sequence alignment between the experimentally proven glutamate binding proteins and the NCBI domain consensus sequence for glutamate and aspartate transporter subunit was 84 (Figure 1A). This score was used as a threshold for confirming the conservation of domain profile in C. perfringens protein sequences. A score of 92 was obtained when the consensus of putative sequences of C. perfringens Type-A strains was aligned with standard domain sequence (Figure 1). As the conservation score is significantly higher than that of well-established periplasmic glutamate receptors, this confirms the presence of homologue sequences in Clostridium. The STRING database search predicts that these homologue sequences belong to the COG0834 family with functional partners of ABC-type amino acid transport. This further predicts a functional glutamate importer in Clostridium (Table 2).
Figure 1.
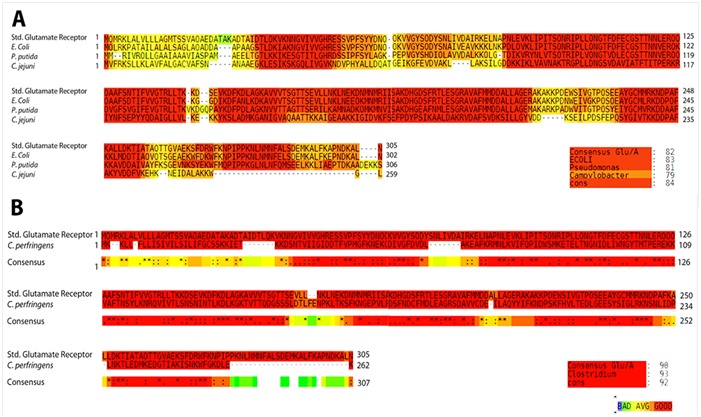
A) Multiple sequence t-coffee alignment of the glutamate binding consensus sequence from domain profile (CDD PRK10797) and sequences of characterized bacterial components of glutamate permease. Color coding indicates the extent of conservation; B) The t-coffee alignment of putative C. perfringens glutamate binding sequence of ABC transporter and standard domain consensus (CDD PRK10797).
Sequence homology search of C. perfringens TypeA- SM101 CPR_1324 from other species revealed PDB 3HV1 to be the best template for homology modeling of the target sequence as both shared 41% identity (Figure 2). PDB 3HV1 is the crystal structure of a polar amino acid ABC uptake transporter substrate binding protein from Streptococcus thermophilus. This belongs to bacterial extracellular solute-binding proteins, family 3 (PFAM Family SBP_bac_3). In all known bacterial solute-binding proteins, the bound ligand is situated in a cleft between two major protein domains which close over the solute. As shown in Figure 2, the homology modeled structure of the CPR_1324 from C. perfringens shows distinct two lobes, a characteristic of substrate binding proteins. Comparative sequence analyses and motif search prediction indicated that residues 118-KNRQVIV-124 lie in the substrate binding cleft (Figure 2). Thus, it becomes evident from the model that the carboxylate of glutamate is mainly stabilized by electrostatic interactions with basic residues in the same way as observed with other binding proteins. The quality of the model was evaluated using the QMEAN program and assessed using its score of -3.73 which shows that the predicted model is of good quality. It is evident from the figure that the substrate binding cleft is conserved between two structural lobes. Thus, the predicted model structure of C. perfringens CPR_1324 is comparable to the structurally resolved PDB 3HV1, a standard glutamate ABC uptake transporter substrate binding protein.
Figure 2.
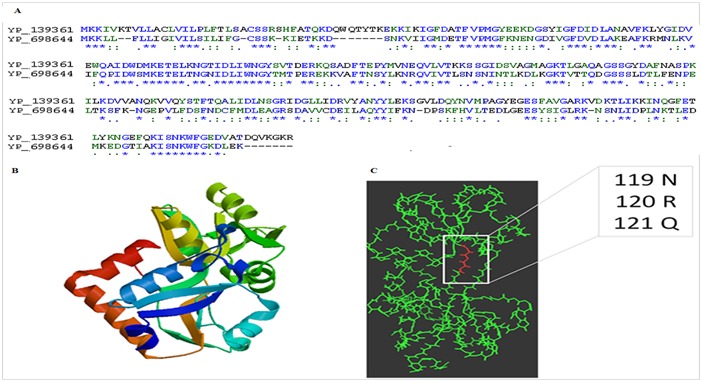
A) Amino acid sequence alignment of the target, amino acid ABC transporter (binding protein) of C. perfringens SM101 (Accession No. YP_698644) and the template i.e. polar amino acid ABC uptake transporter substrate binding protein of Streptococcus thermophilus LMG 18311 (Accession No. YP_139361). Amino acid sequence of the the template (3HV1) shows 41% sequence identity, “*” - single, fully conserved residue; “:” - conservation of strong groups; “.” - conservation of weak groups; - no consensus; B) Homology modelled structure of the amino acid ABC transporter (binding protein) of C. perfringens SM101 (Accession No. YP_698644) derived from SWISS-MODEL server; C) The putative glutamate binding site (a stretch from amino acids 118 to 124) on the modelled structure are shown. Only three amino acids are shown in red.
Conclusion
Glutamate is an essential amino acid to almost all strains of Clostridium perfringens. Its transport through importers is critical in regulation of amino acid metabolism. Inhibition of the solute specific component of this permease offers an attractive drug target to prevent bacterial growth. We identified periplasmic glutamate binding protein of C. perfringens Type-A strains to be a possible immunogenic virulent factor. It offers a strong putative target for vaccine development as its close homologue PEB1a from C. jejuni has already been reported to confer protection when used as antibacterial vaccine [21].
Supplementary material
Acknowledgments
Financial assistance from the Department of Biotechnology, New Delhi, India is acknowledged. BB and AKS acknowledge the Council of Scientific and Industrial Research, New Delhi, India, and the Department of Biotechnology, New Delhi, India for research respectively fellowships.
Footnotes
Citation:Bhatia et al, Bioinformation 10(7): 401-405 (2014)
References
- 1.Songer SJ, et al. Clin Microbiol Rev. 1996;9:216. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatheway CL, et al. Clin Microbiol Rev. 1990;3:66. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tweten RK. Vet Microbiol. 2001;82:1. doi: 10.1016/s0378-1135(01)00372-8. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu T, et al. Proc Natl Acad Sci. USA. 2002;99:996. [Google Scholar]
- 5.Sengupta N, et al. Infect Immun. 2010;78:3957. doi: 10.1128/IAI.00374-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs AR, Bonde GJ. J Gen Microbiol. 1957;16:317. doi: 10.1099/00221287-16-2-317. [DOI] [PubMed] [Google Scholar]
- 7.Goldner SB, et al. Appl Environ Microbiol. 1985;50:202. doi: 10.1128/aem.50.2.202-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Payne SH, Loomis WF. Eukaryot Cell. 2006;5:272. doi: 10.1128/EC.5.2.272-276.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pei Z, et al. Infect Immun. 1998;66:938. doi: 10.1128/iai.66.3.938-943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosie AH, Poole PS. Res Microbiol. 2001;152:259. doi: 10.1016/s0923-2508(01)01197-4. [DOI] [PubMed] [Google Scholar]
- 11.Leon-Kempis Mdel R, et al. Mol Microbiol. 2006;60:1262. doi: 10.1111/j.1365-2958.2006.05168.x. [DOI] [PubMed] [Google Scholar]
- 12.Fan CP, et al. Protein Pept Lett. 2006;13:513. doi: 10.2174/092986606776819646. [DOI] [PubMed] [Google Scholar]
- 13.Singh B, Rohm KH. Microbiology. 2008;154:797. doi: 10.1099/mic.0.2007/013185-0. [DOI] [PubMed] [Google Scholar]
- 14.Notredame C, et al. J Mol Biol. 2000;302:205. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 15.Franceschini A, et al. Nucleic Acids Res. 2013;41:D808. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwede T, et al. Nucleic Acids Res. 2003;31:3381. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benkert P, et al. Bioinformatics. 2011;27:343. doi: 10.1093/bioinformatics/btq662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garmory HS, Titball RW. Infect Immun. 2004;72:6757. doi: 10.1128/IAI.72.12.6757-6763.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chhabra G, et al. Bioinformation. 2010;4:278. doi: 10.6026/97320630004278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson AL, Chen J. Annu Rev Biochem. 2004;73:241. doi: 10.1146/annurev.biochem.73.011303.073626. [DOI] [PubMed] [Google Scholar]
- 21.Buckley AM, et al. Vaccine. 2010;28:1094. doi: 10.1016/j.vaccine.2009.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


