To the Editor:
Whole-genome data in medical contexts have become increasingly available because of technological advances and improved sequencing efficiency. As a consequence, a rise in ambiguous situations involving novel molecular alterations and their potential association with human illness can be anticipated (1). The inability to predict clinical phenotypes based on mutations and copy number variants (2) can especially complicate prenatal decision making. We encountered a compelling instance of this problem with a couple that had lost one child to a sporadic gene mutation and serendipitously faced the prospect of a second infant with a separate rare genetic disorder.
In January 2012, a 27-year-old G2P1 healthy female presented for prenatal care for her second pregnancy. Her first child was affected with Cornelia de Lange syndrome and died at 6weeks of age because of complications from premature birth and intraventricular hemorrhage. With informed parental consent, chorionic villus sampling (CVS) at 12 weeks of the second pregnancy was performed to test the Nipped-B-like (NIPBL) gene, which was mutated in the deceased child. Fetal DNA testing for NIPBL mutations was negative. Subsequently, the remaining CVS specimen was used as a quality assurance control in prenatal evaluation of an unrelated male fetus at risk for Menkes disease, a lethal X-linked recessive neurometabolic disease caused by mutation of the ATP7A gene (3). The molecular diagnostic assay used, multiplex ligation-dependent probe amplification (SALSA MLPA P104, MRC-Holland, Amsterdam, The Netherlands), is most reliable when the at-risk test specimen is matched with a same-tissue control, e.g. chorionic villus. In this case, although the primary test specimen proved negative for the familial ATP7A alteration, we identified a duplication of exons 1–7 in the control specimen. Because Menkes disease is an X-linked recessive condition and the control DNA was from a male fetus, this unexpected result contained implications for the pregnancy, and the family was recontacted.
There was no past family history of Menkes disease. One ATP7A allele with the exon 1–7 duplication was detected in DNA from the mother and maternal grandmother whose clinical examinations were unremarkable. The duplication was not present in the mother’s healthy adult brother’s X-chromosome. We had not otherwise identified this abnormality in approximately 150 assays performed with this kit in our molecular diagnostics center. On the basis of the new genomic information, and the prospect of a second infant with a potentially lethal disorder, the parents weighed carefully whether to terminate the pregnancy.
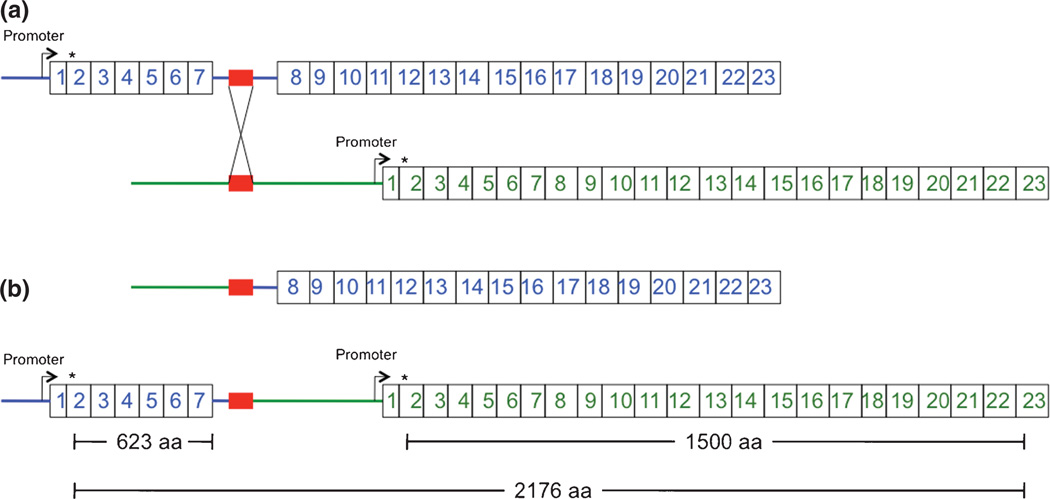
ATP7A duplications are estimated to represent the molecular cause of Menkes disease in 4–10% of affected patients (4, 5). All prior reported examples (n =24) involved intragenic ATP7A tandem duplications, predicted to disrupt the normal translational reading frame and produce nonfunctional ATP7A proteins. In contrast, the exon 1–7 duplication we detected had not been reported in the literature and occurred at the 5′ end of the ATP7A gene rather than within the gene. There are 76 copy number variants involving ATP7A listed in the dbVar/Gene database. Four (nsv869485, nsv869350, esv147665, and nsv491743) have a duplication breakpoint in the 5′ portion of ATP7A. However, none of these encompass the first 100 kb of the ATP7A gene extending to exon 7 that is involved in this duplication. We hypothesized that, if the exon 1–7 duplication was in tandem, three ATP7A molecules could be generated depending on promoter selection, mRNA splicing, and position of the 5′ breakpoint (Fig. 1). We reasoned that one or two of these would be functional and, based on potential molecular mechanisms of altered gene expression under various duplication scenarios (6), would avert Menkes disease.
Fig. 1.
Proposed molecular mechanism resulting in tandem duplication of exons 1–7 of ATP7A. (a) Chromosomal misalignment of similar DNA sequences (red blocks) in intron 7 (enlarged) and the region upstream of ATP7A during meiosis results in an unequal crossing-over event. (b) The consequence of this event would be a deletion of ATP7A exons 1–7 on one X chromosome (top) and a tandem duplication on the other (bottom). The latter rearrangement, inherited by the male infant in this report, is predicted to generate three possible protein products, of which at least one (the 1500 amino acid normal length) should be functional. This interpretation assumes that the crossing-over event did not involve the ATP7A gene promoter. Asterisks denote the translation initiation site in ATP7A exon 2. aa, amino acids.
After provided with the information and counseled concerning the potential risks and benefits, they ultimately chose to continue the pregnancy. In July 2012, at 36 weeks gestation, a 2.8 kg male infant was delivered with Apgar scores of 8 and 9. Plasma neurochemical analysis, a sensitive and specific diagnostic test for Menkes disease (7), was performed on day 1 of life, and was unequivocally normal. No other medical problems were evident, and the mother and infant were discharged home on postnatal day 3. The infant remains in good health. Further detailed characterization of the gene duplication including the final ATP7A transcript(s) and chromosomal localization is planned.
This case raised several questions relevant to the sometimes-competing requirements for informed consent in genetic testing and laboratory quality assurance practices. While the written consent document obtained at the time of this mother’s CVS procedure indicated ‘one or more genetic tests’, only evaluation of the previously affected child’s NIBPL mutation was cited specifically, and consent for ATP7A testing was obtained later. No formal guidelines exist on the nature of samples for routine use as quality assurance controls in molecular diagnostic testing. Institutions offering such testing typically derive their own individual policies. The use of anonymous DNA specimens, a mixture of samples, or references provided by kit manufacturers all represent practical alternative considerations, especially when tissue-specific controls for DNA extraction are unnecessary. Despite the anxiety of this particular situation, the family remained grateful for the molecular diagnostic information and associated genetic counseling.
In summary, we present a case in which prospective parents, their health care providers, laboratory personnel, and consultants all wrestled with the interpretation of genomic information considered perfectly accurate, but imperfectly predictive. More detailed understanding of human genomic variation should eventually converge with advances in DNA sequencing technology to enrich both molecular medicine and prenatal diagnosis of genetic disease. At present, however, we operate in an awkward interval when our capacity to capture information often surpasses our ability to fully understand it, as illustrated by this example.
Acknowledgements
We thank the family for their cooperation, Marie-Pierre Moizzard, Lisbeth Birk Møller, and Jerry Menikoff for helpful discussions, Amanda Loux for performing the MLPA analysis, and Courtney Holmes for measurement of plasma neurochemicals. This study was funded by Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Ethics approval
Informed consent for DNA and neurochemical diagnostic testing, and approvals from Institutional Review Boards were obtained from the University of Minnesota Medical Center-Fairview, the University of Chicago, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
References
- 1.Bodurtha J, Strauss JF., 3rd Genomics and perinatal care. N Engl J Med. 2012;366:64–73. doi: 10.1056/NEJMra1105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boone PM, Soens ZT, Campbell IM, et al. Incidental copy-number variants identified by routine genome testing in a clinical population. Genet Med. 2012 doi: 10.1038/gim.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaler SG. The neurology of ATP7A copper transporter disease: emerging concepts and future trends. Nat Rev Neurol. 2011;7:15–29. doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moizard MP, Ronce N, Blesson S, et al. Twenty-five novel mutations including duplications in the ATP7A gene. Clin Genet. 2010;79:243–253. doi: 10.1111/j.1399-0004.2010.01461.x. [DOI] [PubMed] [Google Scholar]
- 5.Mogensen M, Skjørringe T, Kodama H, Silver K, Horn N, Møller LB. Exon duplications in the ATP7A gene: frequency and transcriptional behaviour. Orphanet J Rare Dis. 2011;6:73. doi: 10.1186/1750-1172-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henrichsen CN, Chaignat E, Reymond A. Copy number variants, diseases and gene expression. Hum Mol Genet. 2009;18 (R1):R1–R8. doi: 10.1093/hmg/ddp011. [DOI] [PubMed] [Google Scholar]
- 7.Kaler SG, Holmes CS, Goldstein DS, et al. Neonatal diagnosis and treatment of Menkes disease. N Engl J Med. 2008;358:605–614. doi: 10.1056/NEJMoa070613. [DOI] [PMC free article] [PubMed] [Google Scholar]