Abstract
Mitochondrial diseases encompass a wide range of presentations and mechanisms, dictating a need to consider both broad-based and disease-specific therapies. The manifestations of mitochondrial dysfunction and the response to therapy vary between individuals. This probably reflects the genetic complexity of mitochondrial biology, which requires an excess of 2000 genes for proper function, with numerous interfering epigenetic and environmental factors. Accordingly, we are increasingly aware of the complexity of these diseases which involve far more than merely decreased ATP supply. Indeed, recent therapeutic progress has addressed only specific disease entities. In this review present and prospective therapeutic approaches will be discussed on the basis of targets and mechanism of action, but with a broad outlook on their potential applications.
Keywords: mitochondrial therapy, clinical trials, idebenone, MNGIE
Mitochondrial therapies: a vast array of staggering challenges
Disorders of the mitochondrial respiratory chain (RC) (classically referred to as ‘mitochondrial disease’; Glossary) are relatively common. Without speaking of mutations in nuclear genes encoding mitochondrial proteins, pathogenic mutations of the mitochondrial DNA (mtDNA) are found in approximately 1 in 200 individuals [1], with clinical expression seen in 1:10 000 [1] to 1:5 000 individuals [2]. Because they encompass an extraordinary number of different clinical entities, requiring therapeutic intervention from conception to the end of the life, it is often not productive to consider therapy of mitochondrial diseases in overly broad terms. Instead, focusing on the pathophysiology may provide more insight into therapeutic targets. The effect of the genetic background of an individual on the consequences of mitochondrial dysfunction and response to therapy also demand caution in drawing conclusions about natural history and therapeutic outcomes [3]. Indeed, despite remarkable progress in elucidating the molecular bases of these disorders, convincing evidence of therapeutic efficacy can only be claimed in a very limited number of circumstances [4]. This review will focus on recent and potential therapeutic approaches for mitochondrial diseases with special emphasis on their underlying molecular and biochemical targets.
Targeting the genetic causes
Mitochondria require 1500 to 2000 genes for proper function, and these are unequally distributed between the nuclear genome (>98%) and the mtDNA (<2%) [5]. Moreover, each mitochondrion contains multiple copies of mtDNA with multiple mitochondria per cell, leading to a large population of mitochondrial chromosomes in each cell (up to 300 000 in an oocyte). The end result is the ability of cells to support a burden of mutant mtDNA molecules without a resulting functional impairment below a given threshold [6]. Thus, the maintenance and transmission of even deleterious mutations in the mitochondrial genome is tolerated, a phenomenon that may in part account for the relatively high frequency of disease resulting from mtDNA mutations, despite evidence for germline selection against highly deleterious variants [7]. Worldwide, it is estimated that 700 000 to 1500 000 individuals have clinical disease related to mtDNA mutations. Targeting molecular correction to the mitochondrial and nuclear DNA involves challenges unique to each cellular compartment.
mtDNA
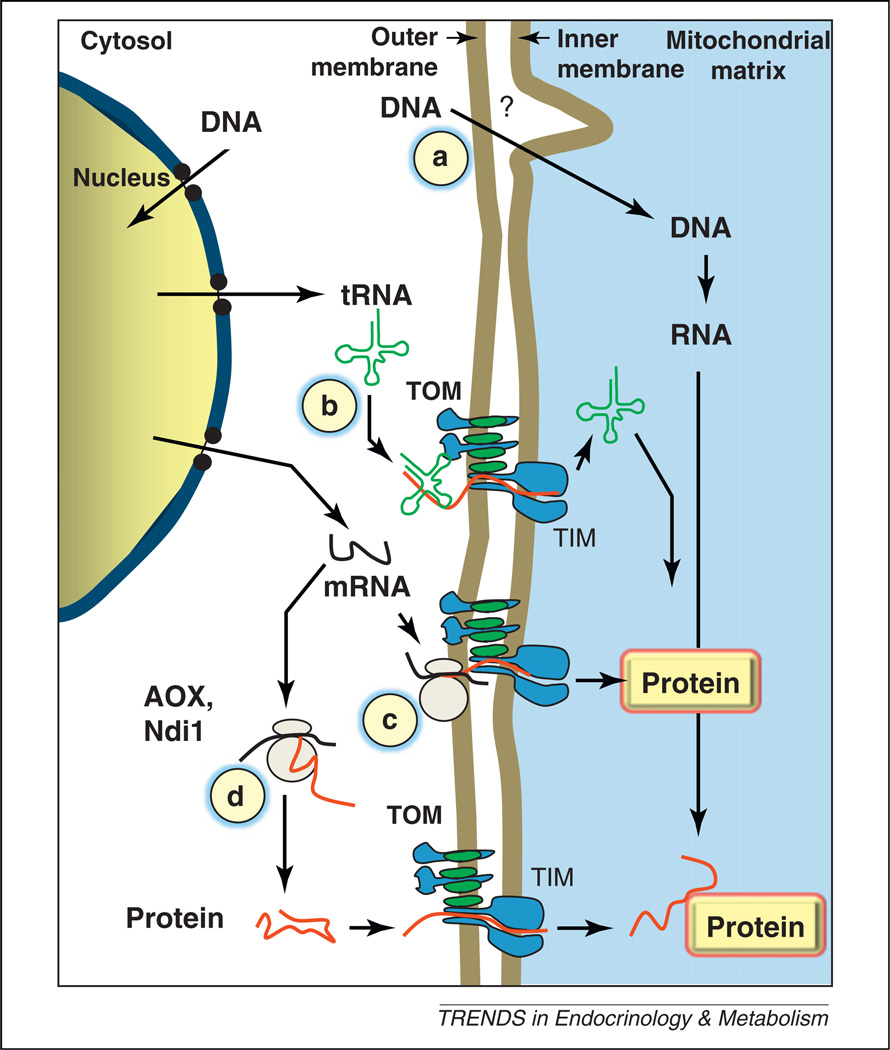
Success in targeting DNA to mitochondria for gene replacement or protein overexpression has been limited. Although many of the difficulties are common to those encountered in nuclear gene therapy, at least one major hurdle is specific to mitochondria: the targeted genetic material is enclosed in a double-membrane-enclosed organelle [8]. To date, with the exception of some microorganisms (e.g., the yeast Saccharomyces cerevisiae, or the green alga Chlamydomonas reinhardtii) whose mtDNA can be transformed by a biolistic approach (a gene gun delivery system) [9], it has proved extremely difficult to manipulate mtDNA in situ, even in model organisms or cell culture (Figure 1). Nevertheless, physiological mechanisms to exchange genetic material between mitochondria and the nucleus, in the form of both RNA and DNA, are in fact present in plants [10] and probably many other organisms, which may explain why so many copies of mitochondrial genes can be detected in the nuclear DNA and even vice versa [11]. To circumvent this difficulty, several innovative strategies have been developed. First, faulty mtDNA genes have been re-coded to be expressed in the nucleus (Figure 1). Here, a crucial parameter appears to be the perimitochondrial location of the expressed mRNA, which can be enhanced by adding the proper targeting signals, and this considerably increases the chance of success of directing the subsequent translation products to mitochondria [12]. However, the feasibility of allotopic expression of mtDNA genes in the nucleus is controversial [13]. Manipulating mtDNA is also potentially feasible through delivery to mitochondria of DNA modification enzymes such as endonucleases that target a heteroplasmic mutation [14]. tRNA molecules can be imported into mitochondria through the TIM/TOM (inner and outer membrane translocases) complex that is responsible for import of nucleus-encoded mitochondrial proteins in the mitochondrial matrix, providing a potential mechanism to deliver therapeutic molecules to reverse mitochondrial tRNA gene mutations (Figure 1) [15].
Figure 1.
Targeting defective genes in mitochondrial diseases. (a) Under specific experimental conditions, naked DNA in close contact to isolated mitochondria can be taken into the mitochondrial matrix by an unknown mechanism (?) [80]. (b) Cytosolic tRNA can be imported into mitochondria through the TIM/TOM complex responsible for mitochondrial protein import. (c) A gene normally found uniquely in mtDNA can be re-coded for cytosolic translation and the resulting mRNA targeted to the mitochondrial surface to facilitate mitochondrial import of the synthesized protein. (d) Allotopic genes (AOX, Ndi1), normally absent from human cells, can be expressed to functionally complement deficient mitochondria. Abbreviations: AOX, alternative oxidase; Ndi1, internal (rotenone-resistant) NADH dehydrogenase; TOM, translocase of the outer membrane; TIM, translocase of the inner membrane.
Nucleus-encoded mitochondrial proteins
Nucleus-encoded proteins comprise the vast majority of mitochondrial proteins and recognition of their deficiency expands rapidly. These present with clinical symptoms that bring patients to many medical specialists (neurologists, cardiologists, endocrinologists, oncologists). The challenge for molecular correction in these disorders is in directing therapeutic proteins to the mitochondria, and few successes have been realized. Expression of exogenous genes must be finely tuned to meet the needs of affected tissues, which may be limited to specific parts of the body, frequently remote sectors of the neurological system, or required throughout the whole organism. In keeping with this, successful gene therapy trials restricted to the eyes have been reported for the Harlequin mouse [16]. This mouse, with a complex I deficiency resulting from decreased expression of AIF (apoptosis-inducing factor), develops several features which include a severe optic atrophy. Recently, this has been successfully treated by adeno-associated virus (AAV2)-AIF1 gene therapy, paving the way to possible gene therapy for optic atrophy in mitochondrial diseases. In the near future, in addition to the eyes and the hematopoietic compartment (below), focused gene therapy approaches for the heart may become feasible.
Friedreich ataxia, the most common recessive ataxia in human, is a mitochondrial disease caused by trinucleotide repeat expansion (GAA) in the first intron of a nuclear gene encoding frataxin, a protein involved in iron-cluster synthesis [17]. The expansion impairs transcription due to the formation of an abnormal chromatin structure. Because DNA structure and expression are strongly dependent on histone modification status (acetylation to phosphorylation ratio), these anomalies can be somewhat modulated by the use of HDAC (histone deacetylase) inhibitors. In the case of Friedreich ataxia, the trinucleotide repeat expansion in the frataxin gene results in impaired iron–sulfur cluster synthesis and hypersensitivity to oxidative insult. To date, the low levels of frataxin in both cell and mouse models for Friedreich ataxia have proved to be significantly increased upon treatment with an HDAC inhibitor, indicating the therapeutic potential of such an approach [18]. However, concerns over the possible toxicity of such DNA-targeting drugs mean that large-scale clinical trials have to be preceded by safety validation.
The overall gene set: mitochondrial and nuclear genes
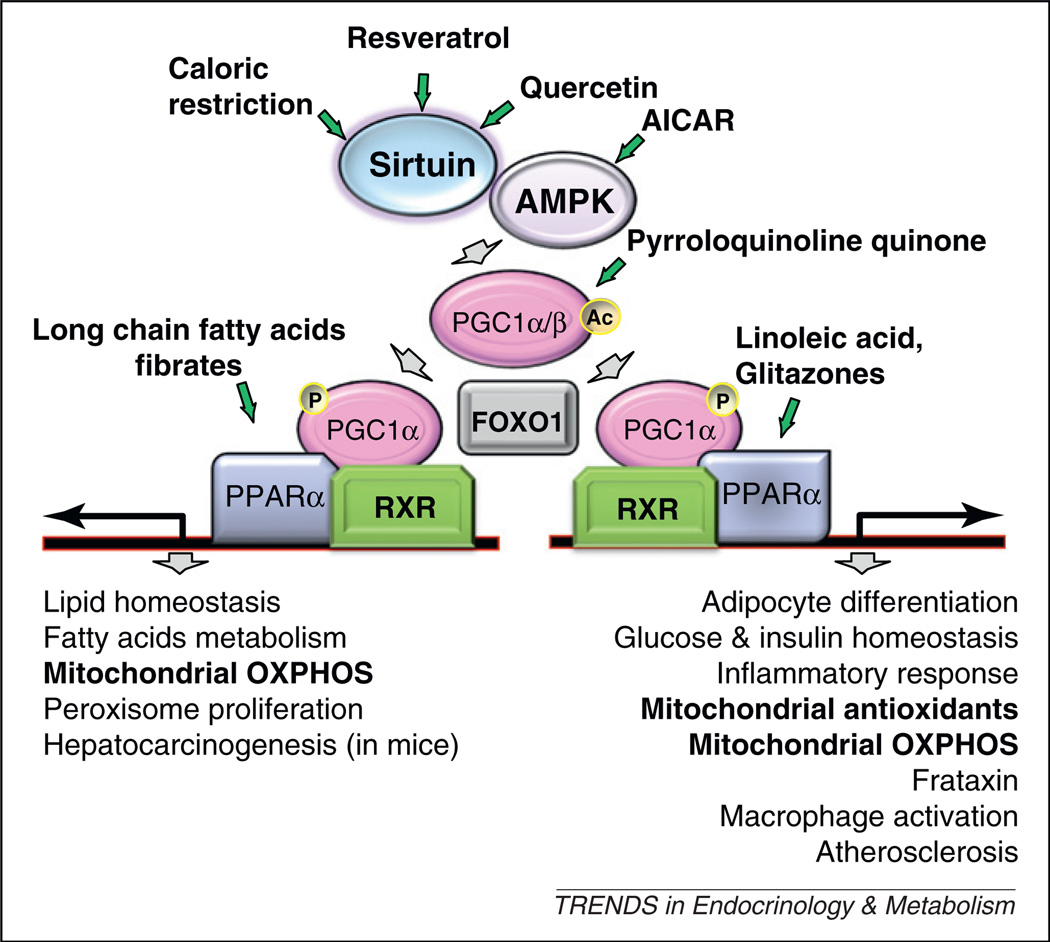
Because a minimum threshold of mitochondrial function frequently appears sufficient to balance organ dysfunction, increasing the overall activity of the ‘chondriome’ seems to be a reasonable option. Accordingly, several putative master regulators of mitochondrial biogenesis or function have been targeted to boost mitochondrial activity, including those resulting in the activation of a network of proteins including SIRT1, an NAD-dependent protein deacetylase, AMP-activated protein kinase (AMPK), an enzyme that plays a role in cellular energy homeostasis, the transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator 1 α (PGC-1α) and the nuclear hormone receptor peroxisome proliferator-activated receptor (PPAR) family [19,20] (Figure 2). A large group of molecules are proposed to interact in this network with both pleiotropic and organ-specific effects, a complexity not necessarily fully understood [21]. However, these factors represent a real hope in the context of mitochondrial diseases. In keeping with this, the AMPK agonist, 5-ami-noimidazole-4-carboxamide ribonucleoside (AICAR), an AMP analog, was recently shown to partially correct cytochrome c oxidase (COX) deficiency, in three COX-deficient mouse models, with significant motor improvement in one [21]. Bezafibrate (a PPAR panagonist, widely used as a hypolipidemic drug) has demonstrated efficacy in mild forms of human carnitine palmitoyltransferase 2 (CPT2) deficiency [19]. In vivo studies of bezafibrate in RC disorders are restricted to various mitochondrial mouse models and remain conflicting with beneficial effects [22,23], absence of effect (Surf1 knockout mouse [21]) or even acute myotoxicity (ACTA-Cox15 knockout mouse [21]). Mitochondrial biogenesis was increased [22] or unaffected by bezafibrate [21,23]. Bezafibrate induced severe hepatic side-effects (massive hepatomegaly) and dramatic weight-loss in both mutant and wild-type individuals [21,23]; these have not, however, been reported in humans and should be regarded as rodent-specific and possibly dose-dependent [24]. Resveratrol, one of the most potent natural SIRT1 activators has been shown to protect mice against high-fat induced obesity and insulin resistance through activation of mitochondrial biogenesis [25]. Further, it has been recently shown that resveratrol markedly induced mitochondrial fatty acid oxidation capacities in CPT2 or very long-chain Acyl-CoA dehydrogenase (VLCAD)-defective human fibroblasts [26].
Figure 2.
The Sirt1/AMPK/PGC1/PPAR signaling network. A schematized and partial view of the signaling pathway indicating its major downstream targets (bottom) and factors susceptible to interfere with signaling (green arrows). Abbreviations: AICAR, 5-aminoimidazole-4-carboxamide ribonucleoside; AMPK, AMP-activated protein kinase; FOXO, forkhead box O; OXPHOS, oxidative phosphorylation; PGC1, peroxisome proliferator-activated receptor γ coactivator 1; PPAR, peroxisome proliferator-activated receptor; RXR, retinoid X receptor.
Finally, as discussed further, diet is a central modulating factor in this regulatory network, offering a potentially natural way to cope with pathological manifestations [27].
Expression of allotopic genes
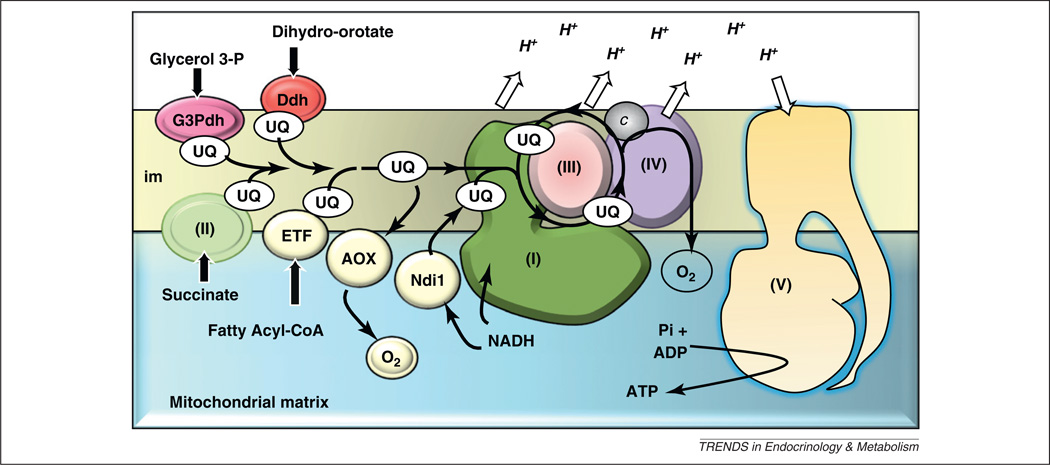
In several organisms there are natural ways to avoid mitochondrial electron-transfer blockade (Figure 3). A major attraction of these alternative pathways stems from their physiological action as safety valves: in the main, they appear to function only when needed – in other words, in case of blockade of the RC. In keeping with this, the alternative oxidase (AOX) that is present in plants, several microorganisms and a subset of animals can convey electrons from the ubiquinone CoQ10 pool directly to oxygen when the cytochrome segment of the RC is blocked. It has been shown that AOX from the urochordate Ciona intestinalis can be safely expressed in cultured human cells [28] and in flies [29], and complements genetically-triggered COX deficiency [30]. Similarly, expression of the S. cerevisiae Ndi, a monomeric protein whose activity can replace the main redox function of the 45-subunit complex I, has been shown to complement complex I deficiency in cells and flies successfully [31]. The possible use of these by-passes in therapy needs next to be validated in whole-organism mammalian models.
Figure 3.
Protection of mitochondria by alternative proteins. A scheme of the respiratory chain (RC) in mammalian mitochondria featuring the respirasome which in the inner mitochondrial membrane associates complexes I, III and IV (I, III, IV) and channels electrons from the quinone pools reduced by the various dehydrogenases: complex I, complex II (II, SDH), the electron transfer flavoprotein (ETF), the glycerol-3-phosphate dehydrogenase (G3Pdh) and the dihydroorotate dehydrogenase (Ddh). The ATPase (complex V) uses the proton gradient established during electron transfer to generate ATP from ADP and phosphate. Acting as safety valves when expressed as allotopic proteins in the RC of mammalian cells, the internal rotenone-resistant NADH dehydrogenase (Ndi1) and the alternative oxidase (AOX) proteins avoid the accumulation of mitochondrial matrix NADH and over-reduction of the RC, as in their organisms of origin.
Targeting the cellular consequences
Mitochondrial dysfunction has numerous predictable effects on cell functions, depending on its nature, its severity, the cell or tissue affected, and the genetic background of the individual [3]. A primary consequence of most oxidative phosphorylation (OXPHOS) impairments is decreased ATP production. Under several circumstances glycolytic ATP can, however, be compensatory. Cultured human cells in vitro, as well as cancer cells in vivo, grow and divide – even preferentially – despite defective OXPHOS function, provided that glucose is available: the so-called Warburg effect. In other tissues, such as rat heart cardiomyocytes, induction by glucocorticoid therapy of creatine kinase, under the control of the PPARγ receptor, modulates ATP production [32]. This observation provides a rationale for the use of creatine as a therapy for mitochondrial disease. Creatine is a frequent component of the therapeutic ‘cocktail’ supposed to be beneficial for patients with these disorders [33]. This cocktail also contains a variety of antioxidant molecules because oxidative stress is frequently assumed to result from RC dysfunction [5]. This assumption must, however, be qualified by the fact that superoxide radical formation by the RC largely depends on (i) the flux of electrons through the chain, which is often significantly decreased by dysfunction, and (ii) the reduction of specific auto-oxidizable components, especially CoQ10 and flavin cofactors, which is not always the result of RC dysfunction. Accordingly, the efficacy of these otherwise harmless cocktails has yet to be established, despite years of clinical recommendations. Interestingly, a substantial blockade of the RC often results in pyruvate and α-ketoglutarate accumulation, two potent antioxidant molecules that may be involved in the physiological turnover of H2O2 [34]. As a rule, the incidence of oxidative stress in mitochondrial RC dysfunction has yet to be demonstrated in the vast majority of cases, probably accounting for the limited effect of antioxidant therapy in most mitochondrial diseases.
Instead of increasing production of oxidative compounds, RC dysfunction can result in hypersensitivity to oxidative stress, as described in COX deficiency [30] and Friedreich ataxia [35], albeit by different mechanisms. In this situation, antioxidant therapy seems a promising option and some limited successes have been reported. Idebenone, a potent antioxidant derivative of CoQ10 (Box 1), has been trialed in Friedreich ataxia [36] and Leber hereditary optic neuropathy (LHON) [37]. This short-chain CoQ10 homolog is known to distribute throughout the organism, and possibly functions in a cyclic fashion, being reoxidized by the RC after reducing radical species. Idebenone may also shuttle electrons to the RC [38]. Unfortunately, in spite of several attempts at open and double-blind controlled trials, the efficacy of idebenone in Friedreich ataxia remains disputed [39]. Although there is indeed some evidence of a significant effect on a subset of patients with Friedreich ataxia [40], the limited cohorts of available patients compromise the power of these studies, and additional trials may be needed to achieve statistical significance. In addition, the potential existence of subsets of patients (true responders versus non-responders to idebenone) has been demonstrated in the case of LHON patients [41]. In practice, with few therapeutic alternatives available for these disorders, it appears reasonable to test the otherwise harmless idebenone in each patient because this might at least slow the progression of the disease. Supplementation with CoQ10 is frequently used to treat mitochondrial diseases. This of course is the treatment of choice in the primary deficiencies of CoQ10 synthesis where spectacular effects can be observed [42]. However, it must be noted that the decreased CoQ10 content paralleling the loss of mitochondrial function that is observed in several conditions (including aging) might well reflect an adaptation to a decreased need rather than a deleterious loss [43]. Indeed, despite a long history of use of CoQ10 alone or as a part of a therapeutic cocktail, no convincing demonstration has been provided of its efficacy under these conditions except for the well-being of its manufacturers and distributors.
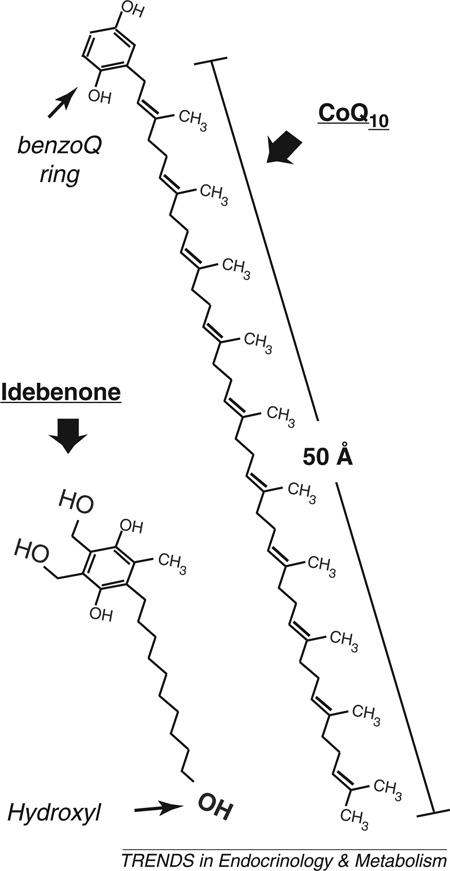
Box 1. CoQ10 or idebenone?
CoQ10 is an extremely hydrophobic molecule, consisting of a benzoquinone ring (able to exchange electrons) and a huge 50-carbon unsaturated side-chain (Figure I). CoQ10 is synthesized in all cells and distributed in the various cell compartments, with only 20% being found in the mitochondria. It can act as an antioxidant by providing electrons to neutralize unstable radical molecules. However, due to its high hydrophobicity it is fairly difficult to handle and acts mostly in the core of cellular membranes which more hydrophilic radicals readily escape. CoQ10 was modified to keep its antioxidant properties but decrease its hydrophobicity. Idebenone is a homolog of CoQ10 with a short (10-carbon) side-chain ending with a hydroxyl group. Being much more hydrophilic, this synthetic molecule is relatively readily distributed in the organism where it targets many more compartments than CoQ10. The two molecules, distributed in the US as food supplements, have no recognized side effects (up to 45 mg/kg/day for idebenone).
Figure I. Comparison of the chemical structures of ubiquinone (CoQ10) and idebenone. Note the different lengths of the side chains, the unsaturated double-bonds of the CoQ10 side-chain, and the additional charges of idebenone. Abbreviation: BenzoQ, benzoquinone.
Mitochondrial dysfunction has been shown to lead to some unexpected consequences that have therapeutic implications. For example, the accumulation of succinate and fumarate, resulting from deficiency of RC complex II (SDH) and fumarase deficiency respectively, readily inhibits prolylhydroxylase activity, favoring stabilization and nuclear translocation of the hypoxia inducing factor 1α (HIF1α) [44,45]. This triggers angiogenesis and might contribute to tumor development (paraganglioma for SDH deficiency [44], uterine and skin leiomyomata and papillary renal cell cancer for fumarase deficiency [46], even if a causal role for HIF in tumor development is debatable). Under these circumstances, α-ketoglutarate supplementation of SDH- [45] or fumarase-deficient [47] cells restores the standard location of the HIF protein, providing further evidence of the role of the metabolic imbalance between succinate and α-ketoglutarate in the control of the prolylhydroxylase and of a potential therapeutic strategy.
Targeting organism/organ dysfunction
Although the most effective therapies remain supportive care and symptomatic treatment, nutritional therapy, exercise and organ transplantation deserve special attention.
Nutrition
The central role of mitochondria in intermediary metabolism makes them a key interface between the environmental calorie supply and the energy requirements of each organ. Consequently, targeted dietary handling may be a valuable therapeutic option in an attempt to increase mitochondrial function, or to bypass mitochondrial dysfunction [2]. Most if not all of the available in vivo data address the increase of dietary fats [27].
An isocaloric high-fat diet (HFD) may improve some patients with mitochondrial diseases, most notably those with complex I deficiency [48–51]. This beneficial effect had initially been attributed to the ability of fatty acid β-oxidation to feed electrons to electron transfer flavoprotein (ETF), thereby bypassing complex I (Figure 3). Another explanation is that fatty acids might act as natural ligands for the PPAR family of receptors, resulting in a generalized stimulation of mitochondrial biogenesis [52] (Figure 2). It has been reported that wild-type rats fed an HFD show a gradual increase in muscle mitochondrial biogenesis, with increases in various mitochondrial enzyme levels, mtDNA copy number, and mitochondrial capacity to oxidize fatty acids [53]. These changes were ascribed to activation, by fatty acids, of PPARδ, resulting in a gradual post-transcriptionally regulated increase in PGC-1α protein expression with no increase in PGC-1α mRNA [53]. The ability of fatty acids to stimulate mitochondrial biogenesis in muscle may explain the apparently beneficial effects of an HFD in mitochondrial diseases. The ketogenic diet has been used for years in children with seizures resistant to conventional antiepileptic drugs, and has also been reported to be successful for treating epilepsy in children with mitochondrial diseases [54]. During fasting, ketone bodies are generated in the liver by β-oxidation of fatty acids and subsequent ketogenesis. Ketone bodies are delivered to, and oxidized by, the brain. Thus, ketone bodies serve as a non-glucose energy substrate that protects against muscle proteolysis and spares glucose. The ketogenic diet induces ketosis by increasing the amount of dietary fat to approximately 90% of the calorie intake. The mechanisms involved in the neuroprotective effects of the ketogenic diet are still unclear, but there is evidence that this diet increases brain energy metabolism by upregulating mitochondrial biogenesis [55], increasing ATP and adenosine concentrations and neuron–glia interactions [56]; inhibiting ROS production [57], and even shifting the level of heteroplasmy in cell cultures harboring single mtDNA deletions [58].
Ketogenic diet was beneficial in a mouse model with late-onset progressive mitochondrial myopathy. The results showed that the diet induced mitochondrial biogenesis, slowed histological disease progression, and diminished the amount of COX-negative muscle fibers [59]. Similarly, an HFD significantly slowed clinical disease progression in the RC complex I-deficient Harlequin mice [60]. The HFD-fed mice exhibited a less severe neurodegenerative course than the animals fed the control diet. Moreover, a sub-group of highly responsive animals in a genetically mixed population was identified [60], echoing the high variability of the disease progression in Harlequin mice [61].
Use of the anaplerotic compound triheptanoin, a triglyceride with heptanoate side-chains, represents a dietary approach to the treatment of disorders of mitochondrial long-chain fatty acid oxidation that may have relevance to RC deficiency. Anaplerosis refers to metabolic pathways that replenish the citric acid cycle (CAC) intermediates, which are essential to energy metabolism. This therapy has been reported to improve cardiomyopathy dramatically in patients with VLCAD deficiency and muscle symptoms in CPT2 deficiency patients who were previously treated with standard dietary supplementation of even-carbon medium-chain triglycerides [62]. Triheptanoin induced a rapid plasma increase in C4- and C5-ketone bodies, the latter being a precursor of propionyl-CoA that is then converted to succinyl-CoA, a CAC intermediate. The potential benefits of a triheptanoin anaplerotic therapy in patients with long-chain mitochondrial fatty acid oxidation defects are currently undergoing formal clinical trials in the US. Triheptanoin therapy may also provide benefit in the treatment of RC defects given the effects of the ketogenic diet in these disorders, as well as the possible secondary defects in mitochondrial fatty acid oxidation [63] and the CAC cycle [64] possibly associated with some RC defects.
Exercise and physical activity
The benefits of exercise therapy for RC defects remain a matter of debate and controversy [65]. On the one hand, aerobic training could prevent muscle deconditioning and wasting and increase exercise capacity [66], but concern remains that it could trigger myolysis of already compromised muscle fibers. Proof of concept of the benefits of exercise was provided by studies showing that exercise increased wild-type mtDNA in muscle fibers in patients with mtDNA mutations, probably by stimulating muscle regeneration [67]. This concept was further evaluated in vivo in adult-onset myopathy with chronic progressive external ophthalmoplegia associated with a single heteroplasmic deletion of mtDNA (ΔmtDNA) in skeletal muscle. The potential therapeutic effects of prolonged (12 weeks) progressive overload leg resistance training in eight patients with muscle-restricted heteroplasmic ΔmtDNAs were evaluated. At the end of the training program all patients exhibited increased muscle strength and improved muscle oxidative capacity, albeit with a limited fall of ΔmtDNA mutation load [68].
It has also been shown that increased PGC-1α levels in skeletal muscle in a mitochondrial myopathy mouse model (MLC1F-Cox10 knockout mouse) stimulated residual OXPHOS capacity by increasing mitochondrial mass, resulting in delayed and less severe course of the disease and increased lifespan [22]. Furthermore, in skeletal muscle of these COX-10 deficient mice, endurance exercise enhanced ATP production via increased mitochondrial mass [69]. Interestingly, it has recently been shown that, in wild-type mice, endurance exercise induced a generalized increase of mitochondrial biogenesis (as determined by PGC1-α and citrate synthase mRNA levels) in various brain regions, suggesting that endurance exercise could be beneficial in the many diseases with impaired brain OXPHOS [70]. In another study, endurance exercise for 5 months had a spectacular effect on the polymerase γ mutant mice (mtDNA mutator mice) [71]. Exercise induced systemic mitochondrial biogenesis, prevented mtDNA depletion and mutations, increased mitochondrial oxidative capacity and RC assembly, restored mitochondrial morphology, and attenuated pathological levels of apoptosis in multiple tissues. Moreover, it was shown that these adaptations conferred complete phenotypic protection and prevented premature death [71].
Finally of note, the level of habitual physical activity in 100 adult patients affected by mitochondrial diseases has been shown to be significantly lower in patients than in control subjects, suggesting that physical activity may constitute a modifiable risk factor in mitochondrial disease [72].
Overall, it seems reasonable to conclude that endurance exercise therapy is beneficial in mitochondrial disorders, although care must be taken for patients known to experience acute episodes of rhabdomyolysis.
Transplantation
Transplantation is a controversial issue in the treatment of mitochondrial diseases, principally because the diseases typically involve multiple organs and manifest asynchronously. Single organ transplantation is usually not appropriate. However, in particular clinical situations (e.g., persistently isolated organ failure) transplantation may be a valuable option. Heart transplantation was successfully performed in six children with severe and isolated mitochondrial cardiomyopathy due to heart RC enzyme deficiency [73]. Three of these six patients are reported to be symptom-free with a follow-up of 2.6–6.5 years. Because liver disease may stabilize or even sometimes regress, liver transplantation should only be performed in cases of life-threatening liver failure when no extra-hepatic symptoms are present. Unfortunately, the latter condition may be difficult to document, especially concerning neurologic signs in young children. It is also crucial to remember that, even if no extra-hepatic symptoms are present at the time of transplantation, they can develop afterwards. In one multicenter study of 11 patients receiving liver transplants for RC defects, five were alive and well at last follow-up (ranging from 5 months to 8 years), whereas six died, including three after neurological deterioration post-transplantation [74]. Mitochondrial depletion syndrome due to deoxyguanosine kinase (DGUOK) deficiency often presents with isolated liver failure, and thus liver transplantation may be a therapeutic option. In one study of DGUOK deficiency, three of four patients undergoing liver transplant remain alive. Two of the patients are reported to have no post-transplant symptoms whereas one patient has exhibited neurological deterioration [75].
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE), due to mutations in the thymidine phosphorylase (TP) gene, is probably the only mitochondrial disease in which stem cell transplantation may replace the missing enzyme activity and lead to long-term clinical improvement. MNGIE is a disorder of nucleotide utilization that results in severe gastrointestinal dysmotility, external ophthalmoplegia with ptosis, cachexia, peripheral neuropathy and leukoencephalopathy [76]. The defective thymidine phosphorylase leads to an increase in the level of dTTP and produces an imbalance in the nucleotide pool, thereby altering mtDNA replication and repair. The reduction of circulating nucleosides, as achieved transiently by hemodialysis [77] and platelet infusion [78], and on a long-term basis by successful allogeneic stem cell transplantation [79], have been shown to be beneficial therapeutic options. Preliminary data for enzyme replacement by allogeneic hematopoietic stem cell transplantation have shown biochemical improvement, with rapid restoration of enzyme activity and reduction or disappearance of plasma thymidine and deoxyuridine. Moreover, a considerable clinical improvement up to 3.5 years post-transplantation has been reported in all the nine engrafted patients [76].
Concluding remarks
Several strategies have been devised towards therapies for mitochondrial disease. Several trials have been performed on cultured cells and animal models with some success, but very little has been actually brought to clinical practice. With the exceptions of primary CoQ10 deficiency and, more recently, MNGIE disease, our capacity to counteract or reduce the effect of RC defects and slow disease progression is limited. However, the renewed interest in nutritional modulation, and in the use of chemicals acting on diet-associated regulatory pathways (SIRT1/AMPK/PGC1/PPARs), illustrated by the accumulation of recent data (especially on mouse models), should lead to clinical trials based on this promising approach. Accordingly, the implementation of trials that take into account the limited cohorts of frequently heterogeneous patients and the inter-individual variability of the therapeutic response will be crucial to advancing the field.
Acknowledgments
The authors gratefully acknowledge the constant support of the Association contre les Maladies Mitochondriales (AMMi), the Association Française contre les Myopathies (AFM), the Association Française contre l’Ataxie de Friedreich (AFAF). M.S. received grant support from the INSERM, the Société Française de Pédiatrie (SFP), the Institut Servier and the Fulbright Scholar Program (Monahan Foundation). P.R. and P.B. are supported by the Agence Nationale de la Recherche (ANR) projects MitOxy and AIFinter. J.V. was supported in part from National Institutes of Health grants R01DK78775 and DK54936. The research of H.T.J. is supported by Academy of Finland, The European Research Council, Sigrid Juselius Foundation and Tampere University Hospital Medical Research Fund.
Glossary
- Allogeneic hematopoietic stem cell transplantation
the transplantation of hematopoietic stem cells from a donor to a recipient.
- Allotopic gene expression
expression and synthesis of an exogenous wild-type version of a mutated protein in the nuclear–cytosolic compartment and its importation into mitochondria to replace the mutated protein. Allotopically expressed genes may include both human mtDNA genes as well as genes from other organisms, although the latter is most correctly referred to as xenotopic gene expression.
- Chondriome
also termed chondrioma, the total mitochondrial content of a cell, taken as a functional unit.
- Cytochrome c oxidase (COX)
also known as complex IV of the respiratory chain (RC).
- Friedreich ataxia
the most prevalent form of autosomal recessive cerebellar ataxia in Caucasians. In the vast majority of cases it is caused by a GAA trinucleotide repeat expansion in the first intron of the frataxin-encoding gene (FXN), which results in decreased gene expression and partial loss of function of the frataxin protein in the mitochondrial matrix. Frataxin has been shown to interact with the iron–sulfur cluster (ISC) assembly machinery. Frataxin loss of function therefore can result in ISC-containing protein deficiency, decreasing aconitase and mitochondrial RC activity, but it also results in hypersensitivity to oxidative stress and iron accumulation in affected organs.
- Homoplasmy
refers to a situation where all mtDNA molecules in a cell, tissue or organism are identical. Because several hundred mtDNA molecules are present in a cell, mutations can either affect all the mtDNA molecules (homoplasmy) or part of them (heteroplasmy).
- Leber hereditary optic neuropathy (LHON)
the most common mitochondrial disorder. It causes progressive irreversible blindness. Over 90% of European and North American patients harbor one of three pathogenic mutations in mtDNA (m.3460G>A, m.11778G>A, and m.14484T>C) affecting complex I of the mitochondrial RC.
- Mitochondrial diseases
a vast group of human disorders resulting from mitochondrial dysfunction. Classically, the term mitochondrial disease (also termed RC defects or oxidative phosphorylation (OXPHOS) diseases) refers to any dysfunction of the RC/OXPHOS system. However, mitochondrial dysfunction is not only observed in monogenic RC defects (resulting from mutations in mtDNA or nuclear DNA) but is also associated with more common conditions, such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, Huntington’s disease, cancer, diabetes and obesity. Additionally, a progressive decline in the expression of mitochondrial genes is an essential feature of normal human aging. Thus, mitochondrial diseases conceptually, encompass not only defects of the RC but the broader set of disorders with mitochondrial dysfunction.
- Ophthalmoplegia or external ophthalmoplegia
a paralysis of one or more of the muscles that control eye movement (extraocular muscles). This condition is associated with several neurologic disorders.
- Rhabdomyolysis
breakdown of skeletal muscle causing myoglobin and other intracellular proteins and electrolytes to leak into the circulation. Rhabdomyolysis is associated with a wide variety of diseases including some inherited disorders of glycolysis, glycogenolysis, mitochondrial disorders, and long-chain fatty acid oxidation defects, as well as injuries, medications and toxins.
References
- 1.Chinnery PF, et al. Epigenetics, epidemiology and mitochondrial DNA diseases. Int. J. Epidemiol. 2012;41:177–187. doi: 10.1093/ije/dyr232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace DC, et al. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benit P, et al. Genetic background influences mitochondrial function: modeling mitochondrial disease for therapeutic development. Trends Mol. Med. 2010;16:210–217. doi: 10.1016/j.molmed.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Hirano M, et al. CoQ(10) deficiencies and MNGIE: two treatable mitochondrial disorders. Biochim. Biophys. Acta. 2012;1820:625–631. doi: 10.1016/j.bbagen.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koopman WJ, et al. Monogenic mitochondrial disorders. N. Engl. J. Med. 2012;366:1132–1141. doi: 10.1056/NEJMra1012478. [DOI] [PubMed] [Google Scholar]
- 6.Larsson NG, Clayton DA. Molecular genetic aspects of human mitochondrial disorders. Annu. Rev. Genet. 1995;29:151–178. doi: 10.1146/annurev.ge.29.120195.001055. [DOI] [PubMed] [Google Scholar]
- 7.Stewart JB, et al. Strong purifying selection in transmission of mammalian mitochondrial DNA. PLoS Biol. 2008;6:e10. doi: 10.1371/journal.pbio.0060010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen NM, et al. An update on targeted gene repair in mammalian cells: methods and mechanisms. J. Biomed. Sci. 2011;18:10. doi: 10.1186/1423-0127-18-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnefoy N, Fox TD. Genetic transformation of Saccharomyces cerevisiae mitochondria. Methods Cell Biol. 2001;65:381–396. doi: 10.1016/s0091-679x(01)65022-2. [DOI] [PubMed] [Google Scholar]
- 10.Rustin P, et al. Targeting allotopic material to the mitochondrial compartment: new tools for better understanding mitochondrial physiology and prospect for therapy. Med. Sci. (Paris) 2007;23:519–525. doi: 10.1051/medsci/2007235519. (in French) [DOI] [PubMed] [Google Scholar]
- 11.Alverson AJ, et al. Origins and recombination of the bacterial-sized multichromosomal mitochondrial genome of cucumber. Plant Cell. 2011;23:2499–2513. doi: 10.1105/tpc.111.087189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corral-Debrinski M. mRNA specific subcellular localization represents a crucial step for fine-tuning of gene expression in mammalian cells. Biochim. Biophys. Acta. 2007;1773:473–475. doi: 10.1016/j.bbamcr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Perales-Clemente E, et al. Allotopic expression of mitochondrial-encoded genes in mammals: achieved goal, undemonstrated mechanism or impossible task? Nucleic Acids Res. 2011;39:225–234. doi: 10.1093/nar/gkq769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacman SR, et al. Modulating mtDNA heteroplasmy by mitochondria-targeted restriction endonucleases in a ‘differential multiple cleavage-site’ model. Gene Ther. 2007;14:1309–1318. doi: 10.1038/sj.gt.3302981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Entelis N, et al. A glycolytic enzyme, enolase, is recruited as a cofactor of tRNA targeting toward mitochondria in Saccharomyces cerevisiae . Genes Dev. 2006;20:1609–1620. doi: 10.1101/gad.385706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouaita A, et al. Downregulation of apoptosis-inducing factor in Harlequin mice induces progressive and severe optic atrophy which is durably prevented by AAV2-AIF1 gene therapy. Brain. 2012;135:35–52. doi: 10.1093/brain/awr290. [DOI] [PubMed] [Google Scholar]
- 17.Campuzano V, et al. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- 18.Sandi C, et al. Prolonged treatment with pimelic O-aminobenzamide HDAC inhibitors ameliorates the disease phenotype of a Friedreich ataxia mouse model. Neurobiol. Dis. 2011;42:496–505. doi: 10.1016/j.nbd.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnefont JP, et al. Bezafibrate for an inborn mitochondrial beta-oxidation defect. N. Engl. J. Med. 2009;360:838–840. doi: 10.1056/NEJMc0806334. [DOI] [PubMed] [Google Scholar]
- 20.Marmolino D, et al. PGC-1alpha down-regulation affects the antioxidant response in Friedreich’s ataxia. PLoS ONE. 2010;5:e10025. doi: 10.1371/journal.pone.0010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viscomi C, et al. In vivo correction of COX deficiency by activation of the AMPK/PGC-1alpha Axis. Cell Metab. 2011;14:80–90. doi: 10.1016/j.cmet.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenz T, et al. Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab. 2008;8:249–256. doi: 10.1016/j.cmet.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Yatsuga S, Suomalainen A. Effect of bezafibrate treatment on late-onset mitochondrial myopathy in mice. Hum. Mol. Genet. 2012;21:526–535. doi: 10.1093/hmg/ddr482. [DOI] [PubMed] [Google Scholar]
- 24.Djouadi F, Bastin J. Species differences in the effects of bezafibrate as a potential treatment of mitochondrial disorders. Cell Metab. 2011;14:715–716. doi: 10.1016/j.cmet.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Bastin J, et al. Exposure to resveratrol triggers pharmacological correction of fatty acid utilization in human fatty acid oxidation-deficient fibroblasts. Hum. Mol. Genet. 2011;20:2048–2057. doi: 10.1093/hmg/ddr089. [DOI] [PubMed] [Google Scholar]
- 27.Schiff M, et al. Mitochondrial response to controlled nutrition in health and disease. Nutr. Rev. 2011;69:65–75. doi: 10.1111/j.1753-4887.2010.00363.x. [DOI] [PubMed] [Google Scholar]
- 28.Hakkaart GA, et al. Allotopic expression of a mitochondrial alternative oxidase confers cyanide resistance to human cell respiration. EMBO Rep. 2006;7:341–345. doi: 10.1038/sj.embor.7400601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Ayala DJ, et al. Expression of the Ciona intestinalis alternative oxidase (AOX) in Drosophila complements defects in mitochondrial oxidative phosphorylation. Cell Metab. 2009;9:449–460. doi: 10.1016/j.cmet.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Dassa EP, et al. Expression of the alternative oxidase complements cytochrome c oxidase deficiency in human cells. EMBO Mol. Med. 2009;1:30–36. doi: 10.1002/emmm.200900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yagi T, et al. Possibility of transkingdom gene therapy for complex I diseases. Biochim. Biophys. Acta. 2006;1757:708–714. doi: 10.1016/j.bbabio.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Mizuno M, et al. Antenatal glucocorticoid therapy accelerates ATP production with creatine kinase increase in the growth-enhanced fetal rat heart. Circ. J. 2010;74:171–180. doi: 10.1253/circj.cj-09-0311. [DOI] [PubMed] [Google Scholar]
- 33.Dimauro S, Rustin P. A critical approach to the therapy of mitochondrial respiratory chain and oxidative phosphorylation diseases. Biochim. Biophys. Acta. 2008;1792:1159–1167. doi: 10.1016/j.bbadis.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Salahudeen AK, et al. Hydrogen peroxide-induced renal injury. A protective role for pyruvate in vitro and in vivo . J. Clin. Invest. 1991;88:1886–1893. doi: 10.1172/JCI115511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayot A, et al. Friedreich’s ataxia: the vicious circle hypothesis revisited. BMC Med. 2011;9:112. doi: 10.1186/1741-7015-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rustin P, et al. Effect of idebenone on cardiomyopathy in Friedreich’s ataxia: a preliminary study. Lancet. 1999;354:477–479. doi: 10.1016/S0140-6736(99)01341-0. [DOI] [PubMed] [Google Scholar]
- 37.Cortelli P, et al. Clinical and brain bioenergetics improvement with idebenone in a patient with Leber’s hereditary optic neuropathy: a clinical and 31P-MRS study. J. Neurol. Sci. 1997;148:25–31. doi: 10.1016/s0022-510x(96)00311-5. [DOI] [PubMed] [Google Scholar]
- 38.Geromel V, et al. Coenzyme Q(10) and idebenone in the therapy of respiratory chain diseases: rationale and comparative benefits. Mol. Genet. Metab. 2002;77:21–30. doi: 10.1016/s1096-7192(02)00145-2. [DOI] [PubMed] [Google Scholar]
- 39.Meier T, et al. Assessment of neurological efficacy of idebenone in pediatric patients with Friedreich’s ataxia: data from a 6-month controlled study followed by a 12-month open-label extension study. J. Neurol. 2012;259:284–291. doi: 10.1007/s00415-011-6174-y. [DOI] [PubMed] [Google Scholar]
- 40.Hausse AO, et al. Idebenone and reduced cardiac hypertrophy in Friedreich’s ataxia. Heart. 2002;87:346–349. doi: 10.1136/heart.87.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klopstock T, et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011;134:2677–2686. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rotig A, et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet. 2000;356:391–395. doi: 10.1016/S0140-6736(00)02531-9. [DOI] [PubMed] [Google Scholar]
- 43.Quinzii CM, Hirano M. Primary and secondary CoQ(10) deficiencies in humans. Biofactors. 2011;37:361–365. doi: 10.1002/biof.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selak MA, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 45.Briere JJ, et al. Mitochondrial succinate is instrumental for HIF1alpha nuclear translocation in SDHA-mutant fibroblasts under normoxic conditions. Hum. Mol. Genet. 2005;14:3263–3269. doi: 10.1093/hmg/ddi359. [DOI] [PubMed] [Google Scholar]
- 46.Tomlinson IP, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 47.O'Flaherty L, et al. Dysregulation of hypoxia pathways in fumarate hydratase-deficient cells is independent of defective mitochondrial metabolism. Hum. Mol. Genet. 2011;19:3844–3851. doi: 10.1093/hmg/ddq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panetta J, et al. Effect of high-dose vitamins, coenzyme Q and high-fat diet in paediatric patients with mitochondrial diseases. J. Inherit. Metab. Dis. 2004;27:487–498. doi: 10.1023/B:BOLI.0000037354.66587.38. [DOI] [PubMed] [Google Scholar]
- 49.de Meer K, et al. Increasing fat in the diet does not improve muscle performance in patients with mitochondrial myopathy due to complex I deficiency. J. Inherit. Metab. Dis. 2005;28:95–98. doi: 10.1007/s10545-005-1485-8. [DOI] [PubMed] [Google Scholar]
- 50.Roef MJ, et al. Triacylglycerol infusion does not improve hyperlactemia in resting patients with mitochondrial myopathy due to complex I deficiency. Am. J. Clin. Nutr. 2002;75:228–236. doi: 10.1093/ajcn/75.2.228. [DOI] [PubMed] [Google Scholar]
- 51.Roef MJ, et al. Triacylglycerol infusion improves exercise endurance in patients with mitochondrial myopathy due to complex I deficiency. Am. J. Clin. Nutr. 2002;75:237–244. doi: 10.1093/ajcn/75.2.237. [DOI] [PubMed] [Google Scholar]
- 52.Kliewer SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hancock CR, et al. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7815–7820. doi: 10.1073/pnas.0802057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang HC, et al. Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia. 2007;48:82–88. doi: 10.1111/j.1528-1167.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- 55.Bough KJ, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 56.Masino SA, Geiger JD. Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets? Trends Neurosci. 2008;31:273–278. doi: 10.1016/j.tins.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maalouf M, et al. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santra S, et al. Ketogenic treatment reduces deleted mitochondrial DNAs in cultured human cells. Ann. Neurol. 2004;56:662–669. doi: 10.1002/ana.20240. [DOI] [PubMed] [Google Scholar]
- 59.Ahola-Erkkila S, et al. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum. Mol. Genet. 2010;19:1974–1984. doi: 10.1093/hmg/ddq076. [DOI] [PubMed] [Google Scholar]
- 60.Schiff M, et al. Mouse studies to shape clinical trials for mitochondrial diseases: high fat diet in Harlequin mice. PLoS ONE. 2011;6:e28823. doi: 10.1371/journal.pone.0028823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benit P, et al. The variability of the harlequin mouse phenotype resembles that of human mitochondrial-complex I-deficiency syndromes. PLoS ONE. 2008;3:e3208. doi: 10.1371/journal.pone.0003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roe CR, et al. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J. Clin. Invest. 2002;110:259–269. doi: 10.1172/JCI15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watmough NJ, et al. Impaired mitochondrial beta-oxidation in a patient with an abnormality of the respiratory chain. Studies in skeletal muscle mitochondria. J. Clin. Invest. 1990;85:177–184. doi: 10.1172/JCI114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burgess SC, et al. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice. J. Biol. Chem. 2006;281:19000–19008. doi: 10.1074/jbc.M600050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeviani M. Train, train, train! No pain, just gain. Brain. 2008;131:2809–2811. doi: 10.1093/brain/awn264. [DOI] [PubMed] [Google Scholar]
- 66.Jeppesen TD, et al. Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain. 2006;129:3402–3412. doi: 10.1093/brain/awl149. [DOI] [PubMed] [Google Scholar]
- 67.Shoubridge EA, et al. Complete restoration of a wild-type mtDNA genotype in regenerating muscle fibres in a patient with a tRNA point mutation and mitochondrial encephalomyopathy. Hum. Mol. Genet. 1997;6:2239–2242. doi: 10.1093/hmg/6.13.2239. [DOI] [PubMed] [Google Scholar]
- 68.Murphy JL, et al. Resistance training in patients with single, large-scale deletions of mitochondrial DNA. Brain. 2008;131:2832–2840. doi: 10.1093/brain/awn252. [DOI] [PubMed] [Google Scholar]
- 69.Wenz T, et al. Endurance exercise is protective for mice with mitochondrial myopathy. J. Appl. Physiol. 2009;106:1712–1719. doi: 10.1152/japplphysiol.91571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Steiner JL, et al. Exercise training increases mitochondrial biogenesis in the brain. J. Appl. Physiol. 2011;111:1066–1071. doi: 10.1152/japplphysiol.00343.2011. [DOI] [PubMed] [Google Scholar]
- 71.Safdar A, et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc. Natl. Acad. Sci. U.S.A. 2011;108:4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Apabhai S, et al. Habitual physical activity in mitochondrial disease. PLoS ONE. 2011;6:e22294. doi: 10.1371/journal.pone.0022294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonnet D, et al. Heart transplantation in children with mitochondrial cardiomyopathy. Heart. 2001;86:570–573. doi: 10.1136/heart.86.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sokal EM, et al. Liver transplantation in mitochondrial respiratory chain disorders. Eur. J. Pediatr. 1999;158(Suppl. 2):S81–S84. doi: 10.1007/pl00014328. [DOI] [PubMed] [Google Scholar]
- 75.Dimmock DP, et al. Abnormal neurological features predict poor survival and should preclude liver transplantation in patients with deoxyguanosine kinase deficiency. Liver Transpl. 2008;14:1480–1485. doi: 10.1002/lt.21556. [DOI] [PubMed] [Google Scholar]
- 76.Garone C, et al. Clinical and genetic spectrum of mitochondrial neurogastrointestinal encephalomyopathy. Brain. 2011;134:3326–3332. doi: 10.1093/brain/awr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yavuz H, et al. Treatment of mitochondrial neurogastrointestinal encephalomyopathy with dialysis. Arch. Neurol. 2007;64:435–438. doi: 10.1001/archneur.64.3.435. [DOI] [PubMed] [Google Scholar]
- 78.Lara MC, et al. Infusion of platelets transiently reduces nucleoside overload in MNGIE. Neurology. 2006;67:1461–1463. doi: 10.1212/01.wnl.0000239824.95411.52. [DOI] [PubMed] [Google Scholar]
- 79.Hirano M, et al. Allogeneic stem cell transplantation corrects biochemical derangements in MNGIE. Neurology. 2006;67:1458–1460. doi: 10.1212/01.wnl.0000240853.97716.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weber-Lotfi F, et al. Developing a genetic approach to investigate the mechanism of mitochondrial competence for DNA import. Biochim. Biophys. Acta. 2009;1787:320–327. doi: 10.1016/j.bbabio.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]