Abstract
Aims: This study estimated the levels of glycemic control among subjects with self-reported diabetes in urban and rural areas of four regions in India.
Research Design and Methods: Phase I of the Indian Council of Medical Research–India Diabetes (ICMR–INDIAB) Study was conducted in a representative population of three states of India (Tamil Nadu, Maharashtra, and Jharkhand) and one Union Territory (Chandigarh) and covering a population of 213 million people. Using a stratified multistage sampling design, individuals ≥20 years of age were recruited. Glycemic control among subjects with self-reported diabetes was assessed by measurement of glycated hemoglobin (HbA1c), estimated by the Variant™ II Turbo method (Bio-Rad, Hercules, CA).
Results: Among the 14,277 participants in Phase I of INDIAB, there were 480 subjects with self-reported diabetes (254 urban and 226 rural). The mean HbA1c levels were highest in Chandigarh (9.1±2.3%), followed by Tamil Nadu (8.2±2.0%), Jharkhand (8.2±2.4%), and Maharashtra (8.0±2.1%). Good glycemic control (HbA1c <7%) was observed only in 31.1% of urban and 30.8% of rural subjects. Only 22.4% of urban and 15.4% of rural subjects had reported having checked their HbA1c in the past year. Multiple logistic regression analysis revealed younger age, duration of diabetes, insulin use, and high triglyceride levels to be significantly associated with poor glycemic control.
Conclusions: The level of glycemic control among subjects with self-reported diabetes in India is poor. Urgent action is needed to remedy the situation.
Introduction
India is home to the second largest number of individuals with diabetes in the world, and currently more than 65 million people are estimated to have diabetes in India.1 If uncontrolled, individuals with diabetes are at risk of developing chronic complications of diabetes such as retinopathy, nephropathy, neuropathy, foot disease, and heart disease, which have the potential to endanger sight, limb, and life. This has profound implications for the public health scenario of the country.
Good metabolic control, right from the time of diagnosis of diabetes, is key to the prevention of chronic complications. Measurement of glycated hemoglobin (HbA1c) is now universally accepted as the most reliable indicator of long-term glycemic control because it accurately reflects an individual's blood glucose levels over the preceding 2–3 months. Assessment of the level of diabetes control in a population using HbA1c is a good indicator of the quality of diabetes care available to the population.
Unfortunately, data on glycemic control among Indian patients with diabetes are scarce. Although there have been a few studies assessing the quality of diabetes care in India, they either have confined themselves to clinic outpatients or have sampled individuals from small, geographically discrete areas.2–5 There has been, to date, no nationally representative population-based study on the level of diabetes control in India. This article presents data on diabetes control, as assessed by HbA1c, from Phase I of the Indian Council of Medical Research–India Diabetes (ICMR–INDIAB) Study.
Research Design and Methods
The ICMR–INDIAB study is an ongoing cross-sectional national study on the prevalence of diabetes and related metabolic disorders such as obesity and hypertension in India. The detailed methodology of the study has been published separately.6 In brief, this is a door-to-door survey of individuals 20 years of age and above. Because of the complex logistics involved, the study is being done in phases. Phase I of the ICMR–INDIAB study was conducted from November 2008 to April 2010, in three states randomly selected to represent the south (Tamil Nadu; population, 67.4 million), west (Maharashtra; population, 112.7 million), and east (Jharkhand; population, 31.5 million) of India and one Union Territory representing northern India (Chandigarh; population, 1.4 million). The INDIAB–North East Phase involving all the eight northeastern states—Assam, Arunachal Pradesh, Manipur, Megahalaya, Mizoram, Nagaland, Tripura, and Sikkim—is currently ongoing, and in the ICMR–INDIAB–Rest of India phase, five more states are currently in progress.
Using a precision of 20% (80% power) and allowing for a nonresponse rate of 20%, the sample size was calculated to be 4,000 per state/Union Territory (2,800 rural and 1,200 urban). Thus the sample size for the entire study once completed will be 1,24,000 individuals (28 states, including two Union Territories and one National Capital Territory). For Phase I of the study, as four regions were studied, the estimated sample size was 16,000 individuals. This article is based on the results of Phase I of the ICMR–INDIAB study.
Sampling strategy
A stratified multistage sampling design was adopted. The primary sampling units were villages in rural areas and census enumeration blocks in urban areas. Three-level stratification was done based on geography, population size, and socioeconomic status in order to obtain a representative sample of the region being studied. For Phase I, in total, 16,607 individuals (5,112 urban and 11,495 rural) were selected from 363 primary sampling units (188 urban and 175 rural), of whom 14,277 individuals responded (response rate, 86%). Institutional Ethics Committee approval was obtained, and written informed consent was obtained from all study subjects in the local language.
In all study subjects, a structured questionnaire was administered to obtain data on sociodemographic parameters and behavioral aspects. Height, weight, and waist circumference were measured using standardized techniques,7 and the body mass index was calculated as the weight (in kilograms) divided by the square of the height (in meters).
Blood pressure was recorded in the sitting position in the right arm using the electronic Omron model HEM-7101 machine (Omron Corp., Tokyo, Japan). The final reading was recorded as the average of two readings taken 5 min apart. The Omron HEM-7101 blood pressure measuring device was validated in 33 subjects as per the international validation protocol. The average differences between the Omron HEM-7101 device and mercury sphygmomanometer readings were 0.3±1.9 and −0.9±1.4 mm Hg for systolic and diastolic blood pressure, respectively, which fulfilled the recommendation criteria of the international protocol.8
All subjects with self-reported diabetes were administered an additional questionnaire that elicited information on duration of diabetes, medication use, self-monitoring of blood glucose and complications, if any. In addition, a fasting capillary blood glucose was determined using a OneTouch® Ultra® glucose meter (LifeScan, a Johnson & Johnson Company, Milpitas, CA).
A venous sample was drawn in all subjects with diabetes for assessment of HbA1c, lipids, and serum creatinine. Samples were centrifuged within 1 h at the survey site, and serum was transferred to separate labeled vials and temporarily stored in −20°C freezers until they were transferred to the central laboratory of the Madras Diabetes Research Foundation at Chennai, India. All biochemical assays were carried out by the same team of laboratory technicians using the same method throughout the study period. HbA1c was estimated by high-pressure liquid chromatography using the Variant™ II Turbo machine (Bio-Rad, Hercules, CA), which is certified by the National Glycohemoglobin Standardization Program as having documented traceability to the Diabetes Control and Complications Trial reference method.9 Serum cholesterol (cholesterol esterase oxidase–peroxidase–amidopyrine method), serum triglycerides (glycerol phosphate oxidase–peroxidase–amidopyrine method), and high-density lipoprotein cholesterol (direct method; polyethylene glycol–pretreated enzymes) were measured using an autoanalyzer (model 2700/480; Beckman Coulter AU [Olympus, County Clare, Ireland]). Serum creatinine was measured using the Jaffe method. The intra- and interassay coefficients of variation for the biochemical assays ranged from 3.1% to 7.6%.
Definitions
Self-reported/known diabetes was defined by a physician diagnosis of diabetes and current use of medications for diabetes (insulin or oral hypoglycemic agents).
Hypertension was diagnosed in subjects who were on antihypertensive medications or had a systolic blood pressure of ≥140 mm Hg and/or a diastolic blood pressure of ≥90 mm Hg.10
Statistical analysis
Statistical analyses were performed using the SAS statistical package (version 9.0; SAS Institute, Inc., Cary, NC). Estimates were expressed as mean±SD. Student's t test was used to compare groups for continuous variables, and the χ2 test was used to compare proportions between two groups. Logistic regression analysis was used to examine the association between various exposures and outcomes using glycemic control as the dependent variable and those factors that had a significant association with glycemic control on univariate analysis as independent variables. A P value of <0.05 was considered significant.
Results
Of the 16,607 eligible subjects in the four regions studied, 14,277 participated in the study (response rate, 86.0%). There were 480 subjects with self-reported or “known” diabetes in these four regions, 254 of whom were from urban areas and 226 from rural areas.
Table 1 shows the general characteristics and biochemical parameters of the subjects with self-reported diabetes in the four regions studied. There was no statistically significant difference in the mean age of subjects in urban and rural areas, but mean duration of diabetes was greater among urban subjects. Subjects in urban areas were significantly more likely to have higher levels of education and to have higher annual household incomes than those in rural areas, and the prevalence of smoking was also significantly higher among them. There were no significant differences among urban and rural subjects in terms of body mass index, waist circumference, or blood pressure. However, although serum triglyceride levels were higher in urban subjects, the fasting blood glucose level was higher among rural subjects. More subjects in urban areas were on oral hypoglycemic agents. Although in the rural areas the proportion of insulin users was higher, the percentage of individuals who were not on any drug treatment for diabetes was also higher.
Table 1.
General Characteristics and Biochemical Parameters of the Subjects with Self-Reported Diabetes in All Four Regions of India Studied
| Parameter | Overall | Urban | Rural | P value |
|---|---|---|---|---|
| Number of subjects | 480 | 254 | 226 | |
| Age (years) | 53±12 | 54±11 | 53±13 | 0.757 |
| Age distribution [n (%)] | ||||
| 20–24 years | 2 (0.4) | 1 (0.4) | 1 (0.4) | 0.934 |
| 25–34 years | 21 (4.4) | 7 (2.8) | 14 (6.2) | 0.066 |
| 35–44 years | 86 (17.9) | 41 (16.1) | 45 (19.9) | 0.282 |
| 45–54 years | 146 (30.4) | 88 (34.6) | 58 (25.7) | 0.033* |
| 55–65 years | 138 (28.8) | 75 (29.5) | 63 (27.9) | 0.690 |
| 65+ years | 87 (18.1) | 42 (16.5) | 45 (19.9) | 0.338 |
| Male [n (%)] | 268 (55.8) | 148 (58.3) | 120 (53.1) | 0.255 |
| Type 1 diabetes [n (%)] | 1 (0.2) | 0 | 1 (0.4) | — |
| Education level [n (%)] | ||||
| Illiterate/less than primary school | 145 (30.2) | 58 (22.8) | 87 (38.5) | <0.001† |
| Middle or high school | 278 (57.9) | 148 (58.3) | 130 (57.5) | 0.869 |
| College or higher | 57 (11.9) | 48 (18.9) | 9 (4.0) | <0.001† |
| Household income (INR) | ||||
| <5,000 | 218 (48.8) | 81 (34.8) | 137 (64.0) | <0.001† |
| 5,000–10,000 | 105 (23.5) | 66 (28.3) | 39 (18.2) | 0.012* |
| >10,000 | 124 (27.7) | 86 (36.9) | 38 (17.8) | <0.001† |
| Alcohol user [n (%)] | ||||
| Current | 54 (11.3) | 30 (11.8) | 24 (10.6) | 0.680 |
| Former | 33 (6.9) | 18 (7.1) | 15 (6.6) | 0.846 |
| Smoking [n (%)] | ||||
| Current | 52 (10.8) | 37 (14.6) | 15 (6.6) | 0.005* |
| Former | 32 (6.7) | 16 (6.3) | 16 (7.1) | 0.732 |
| Body mass index (kg/m2) | 25.4±4.4 | 25.6±3.9 | 25.3±4.9 | 0.546 |
| Waist circumference (cm) | ||||
| Male | 92.9±11.3 | 93.8±10.1 | 91.9±12.5 | 0.180 |
| Female | 86.9±12.4 | 87.9±10.1 | 85.8±14.3 | 0.210 |
| Blood pressure (mm Hg) | ||||
| Systolic | 141±21 | 142±21 | 141±22 | 0.662 |
| Diastolic | 82±10 | 82±10 | 82±11 | 0.729 |
| Fasting blood glucose (mg/dL) | 181±83 | 179±81 | 182±85 | 0.750 |
| Total cholesterol (mg/dL) | 178±40 | 177±42 | 180±37 | 0.508 |
| Triglycerides (mg/dL)a | 166 | 168 | 164 | 0.340 |
| HDL (mg/dL) | ||||
| Male | 34±8 | 33±8 | 34±9 | 0.401 |
| Female | 40±11 | 40±12 | 40±10 | 0.924 |
| Duration of diabetes since diagnosis (years) | 6.4±6.3 | 6.8±6.9 | 5.9±5.6 | 0.135 |
| Prescribed medication [n (%)] | ||||
| OHA | 367 (76.5) | 203 (79.9) | 164 (72.6) | 0.058 |
| Insulin | 11 (2.3) | 5 (2.0) | 6 (2.7) | 0.616 |
| OHA+insulin | 39 (8.1) | 19 (7.5) | 20 (8.8) | 0.584 |
| Neither | 63 (13.1) | 27 (10.6) | 36 (15.9) | 0.086 |
Data are mean±SD values unless indicated otherwise.
Data are presented as geometric mean values.
Statistically significant differences are indicated: *P<0.05, †P<0.001, for rural compared with urban participants.
HDL, high-density lipoprotein; INR, Indian rupee; OHA, oral hypoglycemic agent.
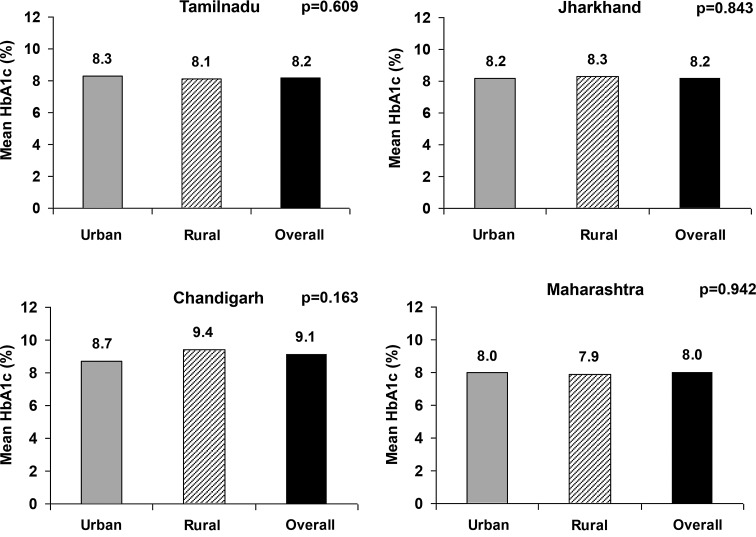
Figure 1 shows the mean HbA1c values in the subjects with self-reported diabetes in each of the four regions studied, which were highest in Chandigarh (9.1%); Tamil Nadu and Jharkhand were next at 8.2%, followed by Maharashtra (8.0%). It is interesting that there was no significant difference in the mean HbA1c among urban and rural dwellers in any of the regions studied.
FIG. 1.
Mean glycated hemoglobin (HbA1c) among subjects with self-reported diabetes (n=480) in four regions of India.
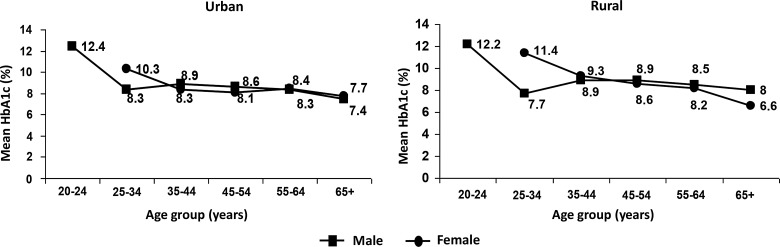
Figure 2 shows the age- and gender-specific mean HbA1c in all the four regions pooled together. The highest mean HbA1c levels were found in the youngest age group (20–24 years), whereas the lowest levels were found in the age group of 65 years and above, in both urban and rural areas and for both sexes.
FIG. 2.
Age- and gender-specific mean glycated hemoglobin (HbA1c) among subjects with self-reported diabetes. P for trend: urban, P=0.034 among female subjects; rural, P<0.001 among male subjects.
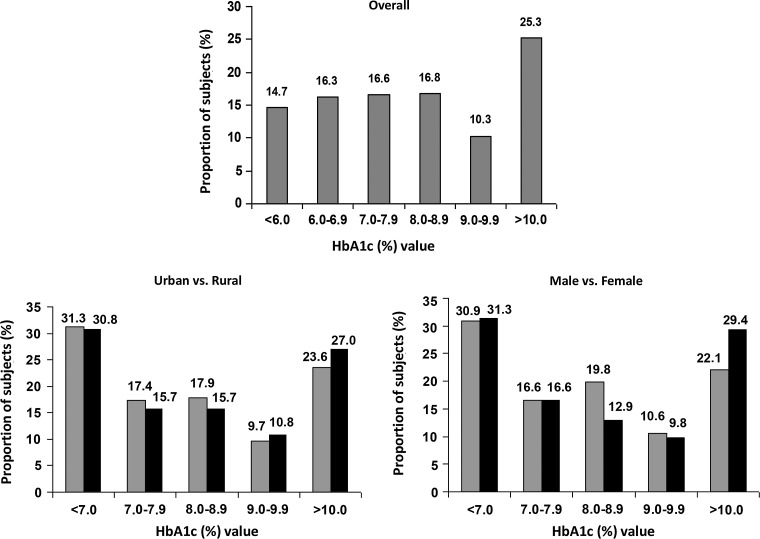
Figure 3 shows the frequency distribution of HbA1c among subjects with self-reported diabetes in all the four regions: only 31% of subjects had HbA1c levels below 7%, and 27% of rural and 23.6% of urban subjects had an HbA1c level of >10%.
FIG. 3.
Frequency distribution of glycated hemoglobin (HbA1c) among subjects with self-reported diabetes.
The reported frequency of measurement of HbA1c among self-reported diabetes subjects showed that only 19.2% of subjects reported having tested their HbA1c during the past year (22.4% urban vs. 15.4% rural; difference not significant), whereas more than 20% of subjects were unaware if their HbA1c was tested in the past year (16.5% urban vs. 24.4% rural; difference not significant).
Table 2 shows the results of multiple logistic regression analysis performed to assess the factors associated with poor glycemic control. Duration of diabetes (odds ratio [OR]=1.076; 95% confidence interval [CI], 1.031–1.123; P=0.001), insulin use (OR=2.479; 95% CI, 1.012–6.072; P=0.047), and triglyceride levels (OR=1.005; 95% CI, 1.002–1.008; P<0.001) were found to be significantly associated with poor glycemic control, whereas age (OR=0.949; 95% CI, 0.928–0.971; P<0.001) was negatively associated with poor glycemic control.
Table 2.
Multiple Logistic Regression Analysis to Show Variables Associated with Poor Glycemic Control (Glycated Hemoglobin Level of ≥7%)
| Variablea | OR (95% CI) | P value |
|---|---|---|
| Age | 0.949 (0.928–0.971) | <0.001 |
| Duration of diabetes | 1.076 (1.031–1.123) | 0.001 |
| Insulin user | 2.479 (1.012–6.072) | 0.047 |
| Triglycerides | 1.005 (1.002–1.008) | <0.001 |
The reference group for comparisons was the group of subjects with a glycated hemoglobin level of <7%.
Included all variables with P<0.20 in univariate analysis.
CI, confidence interval; OR, odds ratio.
Discussion
The results show that the mean HbA1c levels among subjects with self-reported diabetes are high in all the regions studied, with no significant differences between the urban and rural areas, and that, overall, only 31% of these subjects have good control of diabetes as defined as an HbA1c level of <7%. More than 60% of subjects in both urban and rural areas had not had their HbA1c level checked in the past year.
Several large randomized prospective trials have demonstrated that the chronic vascular complications of diabetes can be prevented or delayed by achieving and maintaining good glycemic control. The Diabetes Control and Complications Trial11 in type 1 diabetes and the United Kingdom Prospective Diabetes Study12 in type 2 diabetes showed that an HbA1c target of 7% or below was associated with significantly lower risk of diabetes-related microvascular complications. Moreover, the long-term follow-up components of these two trials have shown that good glycemic control, achieved early in the course of the disease and maintained over a period of time, has long-term protective effects on the development of complications, even if control subsequently becomes less intense.13,14 Various national and international diabetes organizations have therefore defined the targets for good glycemic control as an HbA1c level of below 7%15 or below 6.5%.16
Notwithstanding this large body of evidence, a large proportion of patients with diabetes fail to achieve their glycemic targets even in the developed countries. In the United States, the National Health and Nutrition Examination Studies estimated that, in 2001–2002, fewer than 50% of patients with diabetes had HbA1c levels below 7%, although this figure improved to 55.7% by 2003–2004.17 The situation is worse in developing countries: a recent population-based study from China showed that only 39.7% of patients treated for diabetes had adequate glycemic control.18
Previous studies from India have also highlighted the problem of poor attainment of glycemic targets among patients with diabetes; however, the majority of these have been clinic-based. The Diabcare-Asia Study assessed 24,317 patients with diabetes recruited from 230 centers across 12 Asian countries.19 The Indian component of the study included 2,269 subjects from 26 centers, and the mean HbA1c level was found to be 8.9±2.1%, with more than 83% of participants having HbA1c levels above 7%.3 Among the countries studied, only the Philippines had a lower proportion of subjects attaining glycemic goals. A follow-up of the same study was conducted in 2011, involving 6,168 subjects from India. This study showed no improvement in the mean HbA1c levels (8.97±2.2%), and the proportion of subjects with an HbA1c level of >7% also remained high, at 80.3%.2
There have been only a few small population-based studies on the level of glycemic control conducted in various parts of India. In a cross-sectional community-based survey of 3,069 adults >18 years of age from Kerala, the mean HbA1c level of the 164 subjects with previously diagnosed diabetes was found to be 8.1±2.3%, and only 40% of them had HbA1c levels of <7%.4 Another cross-sectional survey of 819 adults in Delhi belonging to the middle- and upper-income group showed that 37.8% of the subjects with known diabetes had HbA1c levels above 7%.5 Another population-based study in south India involving 7,101 subjects (524 subjects with known diabetes) estimated that 71.2% did not meet the glycemic goal.20 However, in this study, the adequacy of glycemic control was assessed using postprandial plasma glucose levels alone, and no HbA1c measurements were done.
The results of the present study show that levels of glycemic control in India remain unacceptably poor. More than 60% of subjects fail to meet the recommended HbA1c goal of <7%. Although the differences did not reach statistical significance, the proportion of subjects exhibiting poor glycemic control was higher, whereas that of subjects exhibiting good glycemic control was lower, in rural areas compared with urban areas. The large number of individuals with poorly controlled diabetes in rural areas is worrying because this could potentially translate to a high risk of complications in this segment of the population, who can least afford treatment of these complications and who live in regions where facilities for such treatment are scarce or nonexistent.
Our results also show that the highest HbA1c levels were found in the youngest age group studied (20–25 years). HbA1c levels declined with age among both males and females and in both urban and rural areas. The occurrence of high levels of HbA1c in young subjects with diabetes is of concern as these individuals are likely to be exposed to prolonged periods of hyperglycemia compared with older subjects. This accumulated glycemic burden is likely to put them at high risk of developing vascular complications during the prime of their lives unless urgent steps are taken to bring down the plasma glucose levels.
We report that fewer than 20% of the subjects with known diabetes had had their HbA1c tested during the past year. The American Diabetes Association recommends that HbA1c be tested at least semiannually in individuals with diabetes who have stable glycemic control and quarterly in patients whose therapy has changed or who are not meeting glycemic goals.15 The frequency of measurement of HbA1c has been directly linked to level of glycemic control in various populations. In a case control study of 193 subjects with type 2 diabetes seen over a 6-month period in a rural practice in the United States, good control of diabetes based on HbA1c levels was positively associated with adherence to recommendations on the frequency of monitoring of HbA1c.21 In a cross-sectional study of 1,511 patients recruited from 15 hospitals in China, poor glycemic control was found to be associated with a lower frequency of monitoring of HbA1c.22 The extremely low frequency of HbA1c testing revealed by our results is worrying as it indicates that large proportions of the population with diabetes in India do not have recent data on their status of glycemic control, leading to delay in intensification of treatment and accumulation of avoidable glycemic burden.
Our results show that duration of diabetes and insulin use are associated with a higher odds of having poor glycemic control. This is to be expected because diabetes is a progressive disease, the control of which becomes more difficult with increasing duration of disease even with the use of multiple drugs.23 The DiabCare Asia–India study also showed a similar increasing trend of HbA1c level with increasing duration of diabetes.3 Also, in most cases, insulin therapy tends to be delayed until patients have failed all the available oral antidiabetes drugs.2 Therefore insulin users tend to be patients with more severe and difficult-to-control hyperglycemia, and it is not surprising that these individuals have worse glycemic control than those on other modalities of treatment. Our results also show that age is negatively associated with poor glycemic control, that is, higher age is associated with better glycemic control. A similar association has also been noted by Nagpal and Bhartia5 in their study in Delhi. The association of higher triglyceride levels with higher HbA1c levels is also likely a reflection of poor glycemic control preceding hypertriglyceridemia.
The main strengths of the study lie in the fact that it is a large population-based survey and that the subjects have been so chosen as to provide a truly representative sample of the region studied with respect to urban–rural and geographical differences. Our study also has certain limitations. Being a cross-sectional study, no cause-and-effect relationships can be drawn from our results. The presence of variant hemoglobins or hemoglobinopathies could potentially have introduced error into HbA1c measurements in parts of the country where such variants are prevalent. However, this factor is unlikely to have affected the overall results of our study, given that hemoglobin variants are restricted to geographically discrete locations in India, even within individual states. Also, the Variant II machine we used for estimating the HbA1c level is capable of picking up the presence of most hemoglobin variants. The high prevalence of iron deficiency anemia in India is another factor that could conceivably have interfered with our results because studies have shown that this condition is likely to falsely increase HbA1c levels.24 In addition, a lower level of HbA1c need not necessarily be an indicator of better glycemic control because it could also be on account of an increase in hypoglycemic events, particularly in the elderly with an increased duration of diabetes; our results need to be interpreted in this context.
In conclusion, our results show that glycemic control among subjects with self-reported diabetes is poor in India, with less than a third of subjects exhibiting good glycemic control and a significant proportion having HbA1c levels>10%, even in urban areas. Frequency of testing of HbA1c is also far lower than recommended by the global guidelines. The findings point to the existence of a huge number of patients with uncontrolled diabetes in India, who are at grave risk of developing macro- and microvascular complications, the cost of which the country can ill afford. Thus there is a need to increase awareness among patients and healthcare providers regarding the importance of good glycemic control, so that decisions on treatment escalation can be taken and implemented at the appropriate time and patients can protected from the ill effects of the accumulated and potentially avoidable glycemic burden.
Acknowledgments
This study was funded by the Indian Council of Medical Research, New Delhi (grant number 55/1/TF/Diab./07-NCD-II). We gratefully acknowledge the ICMR–INDIAB Expert Group for their valuable suggestions and scientific input. We also thank the ICMR–INDIAB quality managers, quality supervisors, and the field team for smooth conduct of the study and the participants for their cooperation. This is the eighth article from the ICMR–INDIAB Study (ICMR–INDIAB-8).
Author Disclosure Statement
No competing financial interests exist.
R.M.A. and V.M. conceived the study and its design and were involved in implementation of the study and interpretation of the data and helped to draft and revise the manuscript. R.U., M.D., and R.P. were involved in the design and coordination of the study, interpretation of the data, and drafting the manuscript. S.R.J., A.B., V.K.D., and P.P.J. were responsible for supervision of the study in their respective states. S.V.M., P.V.R., R.L., T.K., D.K.S., and A.K.D. were part of the study expert committee and helped in revising the manuscript critically for important intellectual content. R.J., K.V., E.N., and V.V. helped in the execution of the study and were responsible for maintaining quality in the study. R.S. was responsible for data management and statistical analyses. All authors read and approved the final manuscript. V.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.International Diabetes Federation: IDF Diabetes Atlas, 6th ed. Brussels: International Diabetes Federation, 2013 [Google Scholar]
- 2.Mohan V, Shah S, Saboo B: Current glycemic status and diabetes related complications among type 2 diabetes patients in India: data from the A1chieve Study. J Assoc Physicians India 2013;61(Suppl):12–15 [PubMed] [Google Scholar]
- 3.Raheja BS, Kapur A, Bhoraskar A, Sathe SR, Jorgensen LN, Moorthi SR, Pendsey S, Sahay BK: DiabCare Asia—India Study: diabetes care in India—current status. J Assoc Physicians India 2001;49:717–722 [PubMed] [Google Scholar]
- 4.Menon VU, Guruprasad U, Sundaram KR, Jayakumar RV, Nair V, Kumar H: Glycaemic status and prevalence of comorbid conditions among people with diabetes in Kerala. Natl Med J India 2008;21:112–115 [PubMed] [Google Scholar]
- 5.Nagpal J, Bhartia A: Quality of diabetes care in the middle- and high-income group populace: the Delhi Diabetes Community (DEDICOM) survey. Diabetes Care 2006;29:2341–2348 [DOI] [PubMed] [Google Scholar]
- 6.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, Nath LM, Das AK, Madhu SV, Rao PV, Shukla DK, Kaur T, Ali MK, Mohan V: The Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) Study: methodological details. J Diabetes Sci Technol 2011;5:906–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison GG, Buskirk ER, Lindsay Carter ER, Johnston FE, Lohman TG, Pollock ML, Roche AF, Wilmore JH: Skinfold thickness and measurement technique. In: Lohman TG, Roche AF, Martorell R, eds. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics, 1988;55–70 [Google Scholar]
- 8.O'Brien E, Atkins N, Stergiou G, Karpettas N, Parati G, Asmar R, Imai Y, Wang J, Mengden T, Shennan A; Working Group on Blood Pressure Monitoring of the European Society of Hypertension: European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit 2010;1523–38 [DOI] [PubMed] [Google Scholar]
- 9.National Glycohemoglobin Standardisation Program. List of NGSP Certified Methods. www.ngsp.org/docs/methods.pdf (accessed February18, 2014)
- 10.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee: The seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC-7). JAMA 2003;289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 11.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 12.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 13.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan DM; DCCT/EDIC Research Group: The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 years: overview. Diabetes Care 2014;37:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association: Executive summary: standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl 1):S4–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jellinger PS, Davidson JA, Blonde L, Einhorn D, Grunberger G, Handelsman Y, Hellman R, Lebovitz H, Levy P, Roberts VL; ACE/AACE Diabetes Road Map Task Force: Road maps to achieve glycemic control in type 2 diabetes mellitus: ACE/AACE Diabetes Road Map Task Force. Endocr Pract 2007;13:260–268 [DOI] [PubMed] [Google Scholar]
- 17.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB: Is glycemic control improving in U.S. adults? Diabetes Care 2008;31:81–86 [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G; 2010 China Noncommunicable Disease Surveillance Group: Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–959 [DOI] [PubMed] [Google Scholar]
- 19.Chuang LM, Tsai ST, Huang BY, Tai TY; Diabcare-Asia 1998 Study Group: The status of diabetes control in Asia—a cross-sectional survey of 24 317 patients with diabetes mellitus in 1998. Diabet Med 2002;19:978–985 [DOI] [PubMed] [Google Scholar]
- 20.Ramachandran A, Mary S, Sathish CK, Selvam S, Catherin Seeli A, Muruganandam M, Yamuna A, Murugesan N, Snehalatha C: Population based study of quality of diabetes care in southern India. J Assoc Physicians India 2008;56:513–516 [PubMed] [Google Scholar]
- 21.Parcero AF, Yaeger T, Bienkowski RS: Frequency of monitoring hemoglobin A1C and achieving diabetes control. J Prim Care Community Health 2011;2:205–208 [DOI] [PubMed] [Google Scholar]
- 22.Fu C, Ji L, Wang W, Luan R, Chen W, Zhan S, Xu B: Frequency of glycated hemoglobin monitoring was inversely associated with glycemic control of patients with Type 2 diabetes mellitus. J Endocrinol Invest 2012;35:269–273 [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association: Implications of the United Kingdom Prospective Diabetes Study (position statement). Diabetes Care 2002;25(Suppl 1):S28–S32 [DOI] [PubMed] [Google Scholar]
- 24.Hardikar PS, Joshi SM, Bhat DS, Raut DA, Katre PA, Lubree HG, Jere A, Pandit AN, Fall CH, Yajnik CS: Spuriously high prevalence of prediabetes diagnosed by HbA1c in young Indians partly explained by hematological factors and iron deficiency anemia. Diabetes Care 2012;35:797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]