Abstract
Background
Attention is the capacity to flexibly orient behaviors and thoughts towards a goal by selecting and integrating relevant contextual information. The dorsal cingulate (dCC) and prefrontal (PFC) cortices play critical roles in attention. Evidence indicates that catechol-O-methyltransferase (COMT) modulates dopaminergic tone in the PFC and dCC.
Objective
In this study, we explored the effect of tolcapone, a CNS penetrant COMT inhibitor that increases cortical dopamine levels, on brain activity during a Variable Attentional Control (VAC) task.
Study Design
We performed a double-blinded, placebo-controlled, counter-balanced trial with tolcapone (Tasmar, tablets, 100 mg three times a day for 1 day and then 200 mg three times a day for 6 days; ClinicalTrials.gov identifier: NCT00044083).
Setting
The study was conducted in the Clinical Center of the National Institute of Mental Health from 2005 to 2009. Patients Twenty healthy volunteers (11 males; mean age = 32.7 years) with good imaging and performance data on both arms of the study were investigated.
Intervention
Participants underwent 3T blood-oxygen-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) while performing the event-related VAC task, which varies attention over three levels of load: LOW, INT (intermediate), and HIGH.
Main Outcome Measure
Changes in behavioral data and individual contrast images were analyzed using ANOVA with drug and task load as co-factors.
Results
There was a significant main effect of increasing task load, with resulting decreased accuracy and increased reaction time. While there was no significant effect of tolcapone on these behavioral measures, the neuroimaging data showed a significant effect on load-related changes in dCC, with significantly lower dCC activation on tolcapone compared with placebo. Further, neural activity in dCC correlated positively with COMT enzyme activity (i.e., lower COMT activity and presumably more dopamine was associated with lower activation in dCC, i.e., more efficient information processing).
Conclusion
Our results show that pharmacological reduction of COMT activity modulates the engagement of attentional mechanisms, selectively enhancing the efficiency of dCC processing in healthy volunteers, reflected as decreased activity for the same level of performance.
1 Introduction
Attention is the ability to flexibly orient and allocate resources towards a goal by selecting and integrating relevant contextual information. Its primary components include the detection of conflicting information and the allocation of attentional resources in order to bias processing in favor of task-relevant stimuli [1]. Some studies show that increasing the amount of irrelevant information generates greater cognitive conflict and thereby increases the level of attention [2, 3]. Attention is supported by a network of brain regions which includes the dorsal anterior cingulate [3–5], the dorsolateral prefrontal cortex (DLPFC) [1–3], and the inferior parietal cortex [2, 3].
Lesions to specific attention regions have been shown to impair different functional components, suggesting that each region serves a specific, complimentary role in the attention network. Lesions in the dorsal anterior cingulate result in inefficient monitoring of irrelevant information within a stimulus set [6, 7]; lesions in the prefrontal cortex (PFC) result in impaired attentional regulation [8–12]; and lesions to the inferior parietal cortex result in the inability to allocate sufficient resources to stimuli for adequate perception [13, 14] and to update mental models [15].
Altered physiology and function of the attention network have been implicated during attentional processing in neuropsychiatric disorders such as schizophrenia [16–22], Parkinson’s disease [23], and attention deficit hyperactivity disorder [24]. Dysfunction of dopamine has been implicated in the attention deficits observed in these neuropsychiatric disorders [25].
In primates, the cingulate and prefrontal cortices receive mesocortical dopaminergic projections from the substantia nigra and ventral tegmental area [26]. Mesocortical dopamine inputs increase physiological ‘efficiency’ by enhancing pyramidal cell response to sustained and excitatory inputs and modulating the excitability of inhibitory GABA neurons, thereby increasing the cortical neuro-physiological signal-to-noise ratio [26, 27]. At least in part by increasing synaptic dopamine, methylphenidate has been shown to improve working memory, with enhanced neuronal responsiveness in rats [28], and in humans, both dextroamphetamine and methylphenidate have been shown to modulate neurophysiological response underlying executive function, logical reasoning, spatial working memory and attention [29–32].
Dopamine methylation by the enzyme catechol-O-methyltransferase (COMT) is one of the critical mechanisms through which cortical dopamine is inactivated from the synaptic cleft in the frontal cortex [33], where synaptic dopamine transporters are scarce [34]. Studies involving COMT knockout mice and pharmacological administration to animals and humans show that COMT disruption increases prefrontal dopamine concentrations and enhances memory. [35–38]. Furthermore, functional magnetic resonance imaging (fMRI) studies show that a common functional polymorphism in the COMT gene that affects COMT activity accounts for variability in the physiological efficiency of cortical function on a variety of cognitive tests, including attention [39] and working memory [40, 41]. These findings suggest that a specific pharmacological manipulation of prefrontal cortical dopamine levels through the inhibition of COMT activity should lead to improved cognitive processing via enhanced dopamine-mediated prefrontal cortical efficiency.
The moderately CNS-penetrant drug tolcapone appears to be the most potent of COMT inhibitor compounds developed so far. Studies on humans show peak plasma concentration and substantial COMT inhibition less than 2 hours following tolcapone administration [42]. Tolcapone has been shown to improve cognitive function in a sample of patients with Parkinson’s disease [43]. Additionally, we have previously shown that it increases speed of response in the context of working memory, attention, and updating, and enhances prefrontal cortical information measured with fMRI processing during the n-back working memory task even in a sample of normal volunteers [38]. The effect of tolcapone on prefrontal cognition has been further substantiated recently by Farrell et al. [44]. In this study, the authors also report that tolcapone effects on cognition are modulated by the COMT val158 met polymorphism; tolcapone impaired n-back working memory performance in low activity met/ met subjects but enhanced performance in high activity val/ val subjects, supporting the inverted-U model of dopamine function in the DLPFC [45]. Tomasi et al. [32] showed an inverted-U response within the anterior cingulate during a variable-load attention task that was modulated by methylphenidate in such a way as to suggest an enhanced capacity at higher load due to reduced activation. The results from these studies suggest that tolcapone may improve attention and prefrontal cortical efficiency. We therefore explored the effect of tolcapone specifically on cortical information processing during attention in a sample of healthy volunteers while they performed a Variable Attentional Control (VAC) task during blood-oxygen-level-dependent (BOLD) fMRI. Based on prior evidence, we predicted that the drug would enhance efficiency of prefrontal cortical information processing during this cognitive operation.
2 Methods
2.1 Participants
Healthy control subjects were recruited from local and national resources as volunteers for the ‘CBDB/NIMH Sibling Study’ (D.R. Weinberger, Principal Investigator), as previously described [40]. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients included in the study. Participants underwent a structured clinical interview to rule out active Axis I diagnosis and completed a questionnaire for evaluation of personality disorder to rule out Axis II diagnosis (Diagnostic and Statistical Manual of Mental Disorders—IV Edition). Participants were aged 18–50 years. To take part in the study, participants needed to be off all medications for 6 weeks. Individuals with significant medical conditions known to affect the central nervous or cardiovascular system, a history of head trauma with loss of consciousness for longer than 5 min, a history of drug or alcohol abuse within the past 3 months prior to recruitment or cumulative history of substance abuse for over 5 years were excluded. Subjects with a history of alcohol dependence were also excluded because of the potential toxic effect of tolcapone on liver function. Of the 30 subjects who were administered the VAC task, ten subjects had bad-quality imaging data on at least 1 of the 2 scan days. Further analysis was conducted on the data from the remaining 20 subjects. Five of these subjects were homozygous for the valine allele of COMT val158met polymorphism (val/val), ten were homozygous for the methionine allele (met/met), and five subjects were heterozygous (val/met) as determined by standard COMT genotype assays [38]. COMT genotype information was included in the analysis exploring the effect of tolcapone on COMT enzyme activity, but given the small number of subjects in each genotype group, no analysis was conducted on neuroimaging and behavioral data based on genotype.
2.2 Treatment
We performed a randomized, double-blind, placebo-controlled, crossover study lasting 3 weeks (ClinicalTrials.gov identifier: NCT00044083). Both tolcapone and placebo were coded. Coded tolcapone (Tasmar, tablets) was administered orally for 7 days at a dosage of 100 mg three times a day on the first day, followed by 200 mg three times a day for the next 6 days. We performed neuropsychological testing and fMRI on the seventh day. After the first arm, a 1-week washout period followed. At the beginning of the third week, subjects who had received coded tolcapone during the first arm received coded placebo, while those who started on coded placebo during the first arm received coded tolcapone. In order to avoid potential withdrawal reactions, we discontinued the administration of coded compounds over a 3-day period at the end of both arms. During both arms, participants had to take a coded capsule of 100 mg of riboflavin twice daily to mask the urine discoloration produced after tolcapone administration.
2.3 Test Schedule
fMRI scans were performed 2 h after tolcapone administration on day 7 of each arm. Behavioral measures were evaluated by the Hamilton Anxiety Scale (HAM-A) [46] and the Profile of Mood State (POMS) questionnaire [47] the same day of testing. Subjects performed other cognitive tasks during the fMRI scan; however, for the purpose of this paper, we focused only on data from the VAC task. Time of testing was based on pharmacokinetic data showing that plasma levels of tolcapone peak 1.5–2 h after oral administration and maximum inhibition of COMT activity in red blood cells is reached between 1 and 4 h following administration of the drug [38, 42, 48, 49]. The order of presentation of the VAC task within the fMRI paradigm battery was maintained within subjects across drug conditions and across subjects as well.
2.4 VAC Task and Behavioral Data Analysis
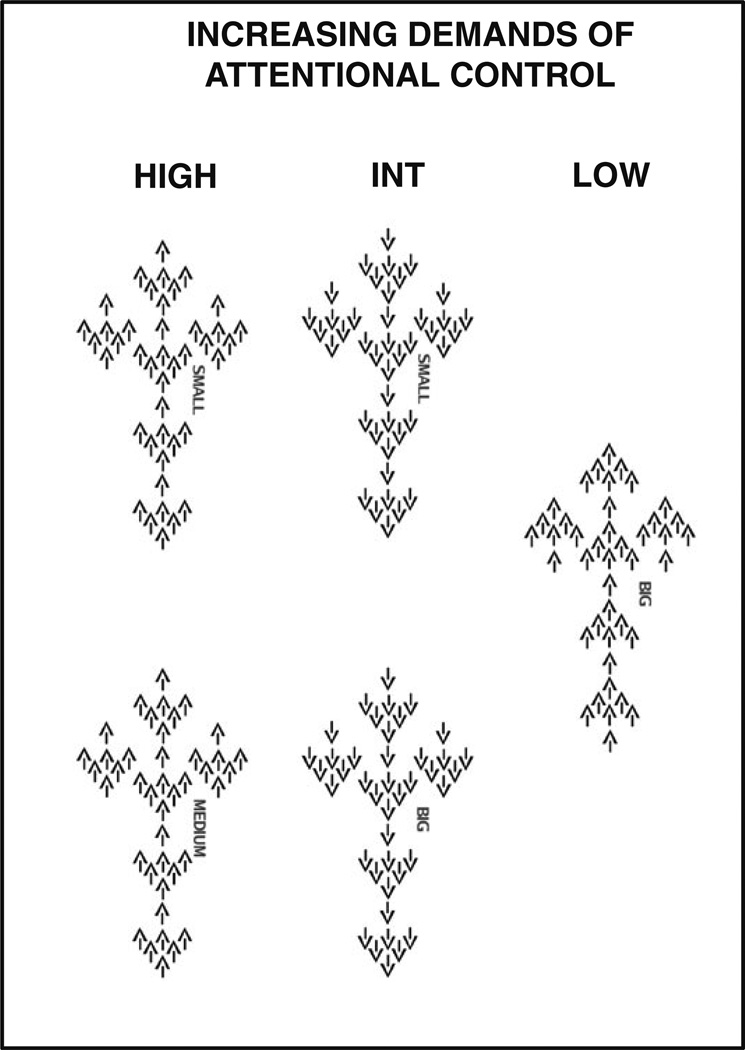
The VAC task, used to elicit increasing demands of attentional processing, has been previously described in detail [3, 39]. Briefly, each stimulus was composed of arrows of three different sizes pointing either to the right or to the left (Fig. 1). The large arrow was composed of a group of six medium-sized arrows, and medium arrows were composed of seven small arrows. A cue word (Big, Medium, or Small) was displayed above each stimulus to instruct subjects to press a button corresponding to the direction of the large, medium, or small arrows, respectively. There were three levels of attentional control: (1) low level (LOW), when all three sizes of arrows were congruent in direction with each other, and subjects were cued to attend to the big arrow; (2) intermediate level (INT), when the big arrow was incongruent in direction to the small and the medium arrows, and subjects were cued to attend to either big or small arrows; and (3) high level (HIGH), when the medium arrows were incongruent in direction to the big and the small arrows, and subjects were cued to attend to either small or medium arrows. The control condition consisted of a single bold arrow pointing to either the left or right.
Fig. 1.
Variable Attentional Control (VAC) task. The task is aimed to assess the effects of an increasing demand in attention. There were three levels of attention: (1) low level (LOW), when all three sizes of arrows were congruent in direction with each other, and subjects were cued to attend to the big arrow; (2) intermediate level (INT), when the big arrow was incongruent in direction to the small and the medium arrows, and subjects were cued to attend to either big or small arrows; and (3) high level (HIGH), when the medium arrows were incongruent in direction to the big and the small arrows, and subjects were cued to attend to either small or medium arrows
Each stimulus was presented for 800 ms, and the order of the stimuli was randomly distributed across the session [50]. The total number of stimuli presented was 241 (50 HIGH, 68 INT, 57 LOW, and 66 simple bold arrows). A fixation cross-hair was presented during the interstimulus interval, which was randomly jittered between 1.2 and 7.1 s. The total duration of the task was 10 min 8 s.
Accuracy was measured as percent correct responses. To measure the effect of tolcapone on attentional load-related change in performance, we normalized accuracy and reaction time (RT) using the formulas (INT − LOW)/ (INT + LOW) and (HIGH − INT)/(HIGH + INT). An analysis of variance (ANOVA) for repeated measures was performed using STATISTICA software (Statsoft Corp., Tulska, OK, USA) to explore the effect of drug on performance and behavioral measures, as well as on the effect of age and performance on activity measures.
2.5 fMRI Data Acquisition
Each subject performed the VAC task while undergoing BOLD fMRI using a GE Signa 3T scanner with a gradient echo-planar imaging sequence (4-mm axial slices with 1-mm gap; repetition time/echo time=2000/28; flip angle = 90°; field of view = 24 cm; matrix = 64 × 64; scan repetitions = 300). Stimuli were presented via a back-projection system, and the responses were recorded via a fiber-optic response box that allowed the measurement of the accuracy and RT for each trial. The first four scans at the beginning of each time series were discarded to allow for signal saturation.
2.6 Image Processing
Whole-brain analysis was completed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Images for each subject were slice time corrected, realigned to the first image of the scan run, spatially normalized into a standard stereotactic space [Montreal Neurological Institute (MNI) template] by using affine and nonlinear (4.5.4 basis functions) transformations, smoothed with a full-width half-maximum (FWHM) Gaussian filter (8 mm) and ratio normalized to the whole-brain global mean.
After realignment, data sets were individually examined to ensure head motion was less than 2 mm translation and less than 1.5° rotation. Quality control of images also included visual inspection of the reconstructed image data, signal variance summary images and time series diagnostic plots for image artifacts as recommended by the MRC Cognition and Brain Sciences Unit [51], and the estimation of signal-to-noise ratios (SNRs) across time series. This procedure was conducted blind to treatment and genotype information. SNR of the fMRI time series data was calculated as follows. First, voxel-wise signal intensity values were normalized by dividing each voxel intensity value by the average voxel intensity value in that volume. Voxel-wise SNR was then computed as the average of the normalized voxel intensity over the time points divided by its temporal standard deviation. The average of the voxel-wise SNR across the whole brain volume was then defined as the SNR of the time series. This value was used as one of the measures for image quality control to ensure that it was not systematically different across diagnostic groups or drug conditions.
Regressors were created for each condition using the correct responses for each stimulus. The incorrect responses and the head motion parameters were also modeled as nuisance variables. Predetermined condition effects at each voxel for each subject were estimated using a general linear model in SPM5, producing a statistical image of significantly activated brain regions for the main effect of task at each load level as well as for all load levels combined, and for the main effect of task load (HIGH > -INT > LOW). To explore drug effects, first level contrast images for each level of attentional load (HIGH, INT, LOW), for load effect (HIGH > INT > LOW), and for all levels combined from each subject were entered into second level analyses comparing placebo with tolcapone conditions (paired t tests). To explore for a drug by load interaction, first level contrast images for each level of attentional load for both placebo and tolcapone conditions from each subject were entered into a flexible factorial model, with drug and load as factors. Furthermore, to explore the relationship between COMT enzyme activity and attentional load-related neural activity, we performed simple regression analyses within SPM5 using first level contrast images (contrasts: LOW > control condition; INT > control condition; HIGH > control condition; HIGH > INT > LOW), with COMT enzyme activity as a predictor. For this analysis, enzyme activity from the sample collected 4 hours after drug administration on the day of the fMRI scan, i.e., the seventh day of each phase of the study (see section 2.7 for more details) was used.
All results are reported in MNI coordinates and at a threshold of p < 0.05 Familywise Error (FWE)-corrected within PFC or dorsal cingulate cortex (dCC) regions of interest (ROIs) based on Blasi et al. [3] (defined using the Wake Forest University PickAtlas toolbox, version 2.0, http://www.fmri.wfubmc.edu) unless otherwise specified.
2.7 COMT Enzyme Assay
COMT enzyme assay was performed to check the effect of drug administration on enzyme activity. Blood was collected on the day of initial screening and recruitment, on the seventh day of drug administration of the first phase of the study (before and about 4 hours after drug administration), and on the seventh day of drug administration of the second phase of the study (before and about 4 hours after drug administration). The post-drug measurement refers to the level from the sample collected 4 hours after drug administration on the seventh day of each phase. The procedure is as described in a prior study [38]. A repeated measures ANOVA was performed using STATISTICA software to estimate the effect of drug and COMT genotype on COMT enzyme activity.
The demographic characteristics of the 20 subjects who completed the VAC study are presented in Table 1. There was no order effect in drug and placebo administration, i.e., the number of subjects who received tolcapone first was not statistically different than the number of subjects who received placebo first; nine of the subjects received placebo first, and 11 subjects received drug first, and these two groups had a similar demographic profile (Table 1).
Table 1.
Demographic characteristics of the sample
| Characteristics | All | Placebo firsta |
Tolcapone firsta |
|---|---|---|---|
| N | 20 | 9 | 11 |
| Gender, males | 11 | 6 | 5 |
| Age (years), mean ± SD |
32.7 ± 8.6 | 31.4 ± 8.2 | 33.3 ± 9.2 |
| IQ, mean ± SD | 106.5 ± 45.7 | 105.0 ± 7.7 | 111.8 ± 12.3 |
| Years of education, mean ± SD |
16.4 ± 2.6 | 16.0 ± 2.4 | 16.7 ± 2.8 |
| Handednessb | 81.6 ± 7.5 | 70 ± 62 | 92 ± 22 |
IQ intelligence quotient
Placebo first: the number of subjects who received placebo in the first arm of the study; tolcapone first: the number of subjects who received tolcapone in the first arm of the study
Handedness was measured using the Edinburgh Inventory [52]
3 Results
3.1 Behavioral Data
3.1.1 Accuracy
There was a main effect of level of attention on accuracy (F2, 117 = 31.872; p < 0.001). As predicted, subjects performed worse on the HIGH condition relative to the INT and LOW conditions (% correct responses ± SD: HIGH 78.20 ± 15.12; INT 89.82 ± 8.87; LOW 97.02 ± 5.66). There was no significant effect of drug on accuracy (F1, 38 = 0.00484; p = 0.945) or a drug by level of attention interaction (F1, 38 = 1.0558; p = 0.311).
3.1.2 Reaction Time
There was a main effect of level of attention on RT (F2, 117 = 23.207; p < 0.001), with subjects having slower RTs on the HIGH condition relative to the INT and LOW conditions (RT in ms ± SD: HIGH 1007.69 ± 207.5; INT 884.6 ± 166.8; LOW 733.9 ± 162.3). There was no significant effect of drug on RT (F1, 38 = 0.53908; p = 0.467) or a drug by level of attention interaction (F1, 38 = 0.56843, p = 0.456).
The above results were based on analysis of behavioral data from the 20 subjects that were included in the neuroimaging analysis. However, there was no significant effect of drug or a drug by level of attention interaction on accuracy or RT even when an analysis was performed on all 30 subjects that participated in the study, including the ten subjects with poor-quality imaging data.
3.1.3 POMS and Hamilton Anxiety Scales
Tolcapone treatment was not associated with any changes in any of the POMS subscales (paired t test all p > 0.2) or in the HAM-A (paired t test p = 0.5). These results are consistent with earlier studies which show that subjects are not aware of when they are on drug or placebo.
3.2 Imaging Data
3.2.1 Effect of Level of Attention (HIGH > INT > LOW)
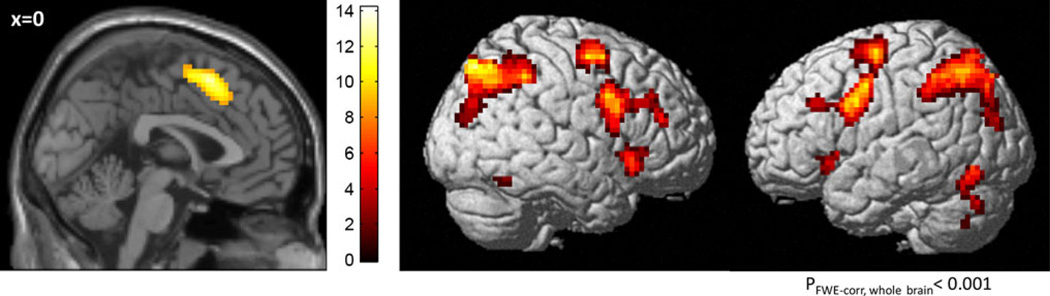
There was a main effect of increasing level of attention with greater activation during the HIGH condition in several regions, including the PFC, the dCC, and the inferior parietal cortex bilaterally (details in Fig. 2 and Table 2; all values are p < 0.001 FWE-corrected for the whole brain).
Fig. 2.
Group statistical parametric maps showing a main effect of increasing level of attention (HIGH > INT > LOW) on brain activation (p <0.001; FWE whole-brain corrected) in regions including the dorsal cingulate, parietal, and prefrontal cortices. FWE-corr, whole brain familywise error correction at whole brain level, INT intermediate
Table 2.
Activated brain regions related to increasing levels of attention (HIGH > INT > LOW) (p < 0.001 FWE whole-brain corrected)
| Brodmann areas/brain regions | ka | MNI coordinates of peak voxel |
Z value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 6, 32, 8 | 239 | 3 | 12 | 54 | Infinite |
| 9, 46, 44, 6, 45 | 284 | 51 | 6 | 33 | 7.60 |
| 47, 13, 45 | 108 | 36 | 24 | −6 | 7.81 |
| 47, 13 | 62 | −33 | 21 | 0 | 6.89 |
| 6, 9, 44 | 467 | −48 | 0 | 33 | 7.71 |
| 6 | 171 | 30 | −3 | 54 | 7.20 |
| 7, 40, 19, 39 | 656 | 36 | −69 | 45 | Infinite |
| 7, 40, 19, 39 | 648 | −30 | −66 | 57 | 7.64 |
| 37, 19 | 60 | −48 | −69 | −15 | 7.00 |
| Cerebellum | 38 | −30 | −66 | −39 | 6.51 |
| 37 | 9 | 54 | −57 | −18 | 5.92 |
| Putamen | 15 | 15 | −6 | 9 | 5.84 |
FWE familywise error, INT intermediate, MNI Montreal Neurological Institute
k = number of voxels included in the clusters that survived p < 0.001 FWE-correction at whole brain level
3.2.2 Effect of Tolcapone on All Levels of Attention
There was a main effect of drug in the dCC with lower activation during the tolcapone condition than during the placebo condition when all the tasks were considered together [peak voxel coordinates (x, y, z) = (−9, 6, 42), pFWE-corr, dCC ROI = 0.057, puncorrected = 0.001, Z = 3.00]. There was no significant difference in the inverse contrast (placebo < tolcapone) in dCC, although there was increased left inferior parietal cortex activation during tolcapone compared with placebo [(x, y, z) = (−39, −66, 45), pFWE-corr, whole brain = 0.013, puncorrected = 0.0000008, Z = 4.81].
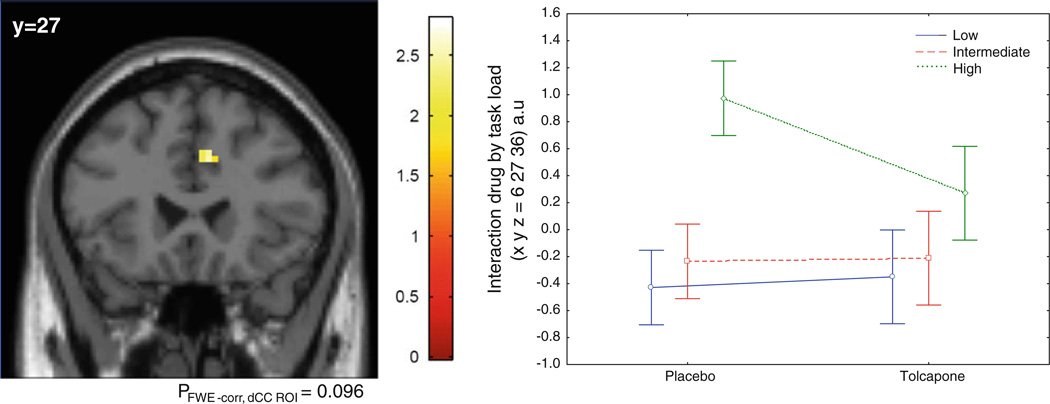
Additionally, there was a trend ‘drug by load’ interaction with decreased activation in the dCC during tolcapone compared with placebo at the high-load level compared with the other two loads [peak voxel coordinates (x, y, z) = (6, 27, 36), pFWE-corr, dCC ROI = 0.096, puncorrected = 0.003, Z = 2.74] (Fig. 3). Covarying for age and performance led to similar results.
Fig. 3.
Group statistical parametric map illustrating a drug by task-load interaction, with relatively greater attentional load-related changes in the dorsal cingulate on placebo when compared with tolcapone. There was no significant difference in the inverse contrast (placebo < tolcapone). FWE-corr, dCC ROI familywise error correction in dorsal cingulate cortex (dCC) region of interest (ROI)
Moreover, there was decreased activation in the dCC at each level of attention [LOW: peak voxel coordinates (x, y, z) = (−6, 6, 42), puncorrected = 0.003, Z = 2.76; MEDIUM: peak voxel coordinates (x, y, z) = (−12, 6, 45), puncorrected = 0.005, Z = 2.57; HIGH: peak voxel coordinates (x, y, z) = (6, 24, 36), puncorrected = 0.006, Z = 2.49] during tolcapone when compared with placebo. While these individual load effects did not survive FWE correction, there were no hints of significant differences in activation on the inverse contrast even with the threshold at p < 0.05 uncorrected (placebo < tolcapone). There was also no effect of drug (placebo > tolcapone or tolcapone > placebo) on the DLPFC.
3.2.3 COMT Enzyme Assay
COMT enzyme assay was not available for one subject during the placebo day and for two subjects for both days (placebo and tolcapone days). Repeated measures ANOVA of whole-blood COMT activity measured 4 hours after treatment (placebo vs. tolcapone) showed a significant main effect of genotype on COMT activity [F(2, 14) = 31.06, p < 0.001], with val/val individuals having higher enzyme activity when compared with val/met and met/met individuals, and val/met individuals with higher enzyme activity compared with met/met individuals (post hoc all ps < 0.004). There was also a significant main effect of drug [F(1, 14) = 6.09, p = 0.027; reduced enzyme activity after tolcapone administration] and a significant genotype × drug interaction [F(2, 14) = 4.8, p = 0.026; significantly reduced enzyme activity after tolcapone administration was observed only in the val/val individuals (p = 0.005), and not in val/met and met/met individuals (ps > 0.3)]. Means ± SD and range of COMT activity for each genotype group are reported in Table 3.
Table 3.
Catechol-O-methyltransferase (COMT) enzyme activity measured 4 h after treatment (placebo or tolcapone)
| CMT genotype |
Drug | COMT activity (cpm/mg protein) |
|
|---|---|---|---|
| Mean ± SD | Range | ||
| Val/Val | Placebo | 194606.8 ± 33248.95 | 166183.6–240754.2 |
| Val/Val | Tolcapone | 162443.3 ± 42447.9 | 98857.8–186635.7 |
| Val/Met | Placebo | 106242.8 ± 36605.0 | 79724.1–166394.7 |
| Val/Met | Tolcapone | 97566.0 ± 39667.6 | 64850.6–165318.1 |
| Met/Met | Placebo | 47434.5 ± 11837.6 | 29641.5–61988.2 |
| Met/Met | Tolcapone | 51938.4 ± 13999.1 | 38355.1–82681.4 |
3.2.4 Effect of COMT Enzyme Activity on Attention-Related Neural Activity
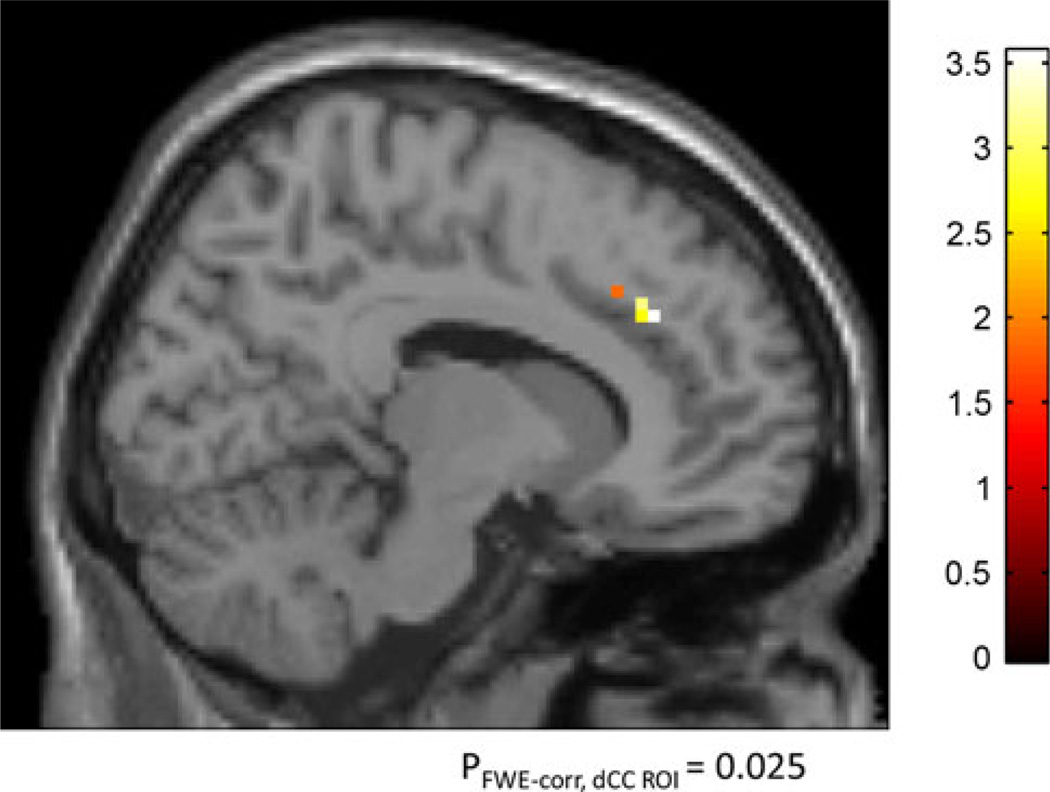
There was a significant positive correlation between COMT enzyme activity and neural activity in dCC (i.e., lower COMT activity and presumably more dopamine was associated with lower activation in dCC, i.e., more efficient information processing). This was more pronounced with increasing level of attention [INT: peak voxel coordinates (x, y, z) = (12, 21, 36), pFWE-corr, dCC ROI = 0.016, Z = 3.36; HIGH: peak voxel coordinates (x, y, z) = (9, 24, 36), pFWE-corr, dCC ROI = 0.013, Z = 3.44]. The increase of the strength of the correlation between dCC activation and COMT activity with increasing level of attention was further confirmed using the contrast HIGH > INT > LOW [peak voxel coordinates (x, y, z) = (12, 33, 30), pFWE-corr, dCC ROI = 0.025, Z = 3.24 (Fig. 4)]. No significant correlations were observed on the opposite contrasts (negative correlation).
Fig. 4.
Simple regression analyses illustrating a significant correlation between COMT activity and attentional load-related changes in the dorsal cingulate. There was no significant difference in the inverse contrast (negative correlation). COMT catechol-O-methyltransferase, FWE-corr, dCC ROI familywise error correction in dorsal cingulate cortex (dCC) region of interest (ROI)
4 Discussion
Our results show that tolcapone, a moderately CNS-penetrant COMT inhibitor, selectively affects efficiency of information processing in dCC during attention in healthy volunteers. Cortical efficiency is an operational measure of information processing based on fMRI response during a cognitive task, where more efficient information processing denotes the same behavioral output achieved with more focused cortical activation. We observed less activation in the dCC during the tolcapone condition compared with placebo for the same level of task performance during the VAC task, a task that demands an increasing level of attention. Administration of tolcapone decreased COMT enzyme activity, which putatively increases dopamine levels. Tolcapone also decreased load-related neural activity, as reflected by changes in the BOLD response during the VAC task. Interestingly, this effect of tolcapone on load-related change in neural activity was specifically observed in the dCC. Presumably by increasing the levels of dopamine via the inhibition of COMT, tolcapone decreased activity in the dCC though the level of behavioral performance did not change as compared with the placebo condition during the monitoring of conflicting information. This suggests that tolcapone may improve efficiency of the physiological response of the cingulate cortex, specifically the dorsal anterior cingulate, during attentional control.
Our data are consistent with results from numerous neuroimaging studies involving the modulation of cortical dopamine levels via the functional polymorphism in the COMT gene on similar cognitive and physiological measures in normal controls [38–41]. Particularly, Blasi et al. [39] showed an effect of the val158met functional polymorphism in the COMT gene on the efficiency of dCC activation during the VAC task. They showed that the level of difficulty in the VAC task was associated with increased activity in a network consisting of the dCC, DLPFC, and the PC; however, the effect of the COMT val158met polymorphism was restricted to the dCC. Met/met individuals, who have lower COMT activity and therefore relatively higher cortical synaptic dopamine concentrations, showed more efficient dCC activity than val/val individuals, who supposedly have higher COMT activity and therefore lower cortical synaptic dopamine concentrations. Furthermore, pharmacological manipulations through other drugs such as dextroamphetamine, modafinil, etc., known to modulate dopamine activity have also demonstrated the same efficient cortical information processing during executive cognition [41] as well as attention tasks [53]. Administration of modafinil, an arousal-enhancing agent likely acting at least in part also through the dopaminergic pathway, improved efficiency of information processing in the dorsal anterior cingulate during the VAC task [53]. In line with these results, our study showed that increased cortical synaptic dopamine concentrations via tolcapone administration compared with placebo resulted in more efficient dCC information processing.
It is interesting that we observed a relatively exclusive effect of dopamine level modulation on activity in the dCC region among other brain areas in the network underlying attention. The absence of the same effect in the DLPFC, despite dopaminergic projections in this region, could be due to a task- and region-specific effect of the drug. The VAC task was designed to manipulate the demand for conflict detection by varying the congruency/incongruency of hierarchical stimuli across levels of attention (the demand for conflict detection is greater in HIGH > -INT > LOW); whereas differential allocation of attentional resources, which was manipulated by varying the focus of the stimuli to either local or global characteristics, differed within each level and not across levels [3]. Studies trying to define the relative contribution of the different brain regions underlying the attention network implicate the dCC in handling conflict [1, 3–7] and the dorsal PFC in executive cognition, attention regulation [8–12], and working memory function [54, 55]. The lack of tolcapone effect on DLPFC information processing during the VAC task is consistent with our prior observations of more efficient information processing only in the dCC in individuals homozygous for the met allele (i.e., individuals with presumably greater cortical dopamine levels) when compared with individuals homozygous for the val allele [39]. We reasoned in that report that conflict-monitoring aspects of the task are the critical processes engaged and that the load on DLPFC processing during this task is minimal. Our current results with tolcapone would seem to support this assumption.
It is important to acknowledge that our current sample is relatively small, but it reflects the strict exclusion criteria we set for image quality. We believe that this step is critical in ensuring that the signal and noise characteristics are comparable across both scan sessions. Our final sample size is comparable to the sample sizes of other cognitive enhancer neuroimaging studies [43, 56, 57]. Moreover, although we did not find any significant differences in performance induced by tolcapone administration at the doses used in this study, this is an advantage in interpreting neuroimaging studies since the changes observed in BOLD fMRI signal reflect an improvement in cortical processing that cannot be conflated with an effect of a change in performance. A future dose-finding experiment might help identify an optimal dose that would improve performance with activation changes. Furthermore, the use of peripheral rather than central measures of COMT enzyme activity is a limitation of the current study, since functionally distinct isoforms of COMT enzyme are expressed in the brain (where the membrane bound form predominates) and periphery (where the soluble form predominates).
5 Conclusion
In conclusion, we present evidence that tolcapone decreased physiological activation in the dCC without concomitant change in performance as compared with placebo during an attentional task, suggesting that tolcapone may enhance efficiency of information processing in the dCC during attention. This selective modulation of dCC during a conflict processing task, taken together with the results from an earlier study [34] in which tolcapone was shown to selectively modulate PFC activity during a working memory task, further advocates a task- and region-specific effect of tolcapone. The effects of tolcapone on cortical information processing may also be modified by COMT genotype, warranting further research in a larger sample on a potential drug by genotype interaction.
Acknowledgments
This research was supported by direct funding of the Weinberger Lab in the Intramural Research Program of the National Institute of Mental Health, NIH, Bethesda, MD 20892, USA. and the Lieber Institute for Brain Development.
We thank Saumitra Das, MS for his help with data acquisition.
Footnotes
Sophia C. Magalona, Roberta Rasetti, Jingshan Chen, Qiang Chen, Ian Gold, Heather Decot, Joseph H. Callicott, Karen F. Berman, José A. Apud, Daniel R. Weinberger, and Venkata S. Mattay declare that they have no conflict of interest.
Contributor Information
Sophia C. Magalona, Clinical Brain Disorders Branch (CBDB), National Institute of Mental Health (NIMH), National Institutes of Health (NIH), Bethesda, MD, USA
Roberta Rasetti, Clinical Brain Disorders Branch (CBDB), National Institute of Mental Health (NIMH), National Institutes of Health (NIH), Bethesda, MD, USA.
Jingshan Chen, Clinical Brain Disorders Branch (CBDB), National Institute of Mental Health (NIMH), National Institutes of Health (NIH), Bethesda, MD, USA.
Qiang Chen, Lieber Institute for Brain Development, Johns Hopkins Medical Campus, 855 North Wolfe Street, Baltimore, MD 21205, USA.
Ian Gold, Clinical Brain Disorders Branch (CBDB), National Institute of Mental Health (NIMH), National Institutes of Health (NIH), Bethesda, MD, USA.
Heather Decot, Clinical Brain Disorders Branch (CBDB), National Institute of Mental Health (NIMH), National Institutes of Health (NIH), Bethesda, MD, USA.
Joseph H. Callicott, Clinical Brain Disorders Branch (CBDB), National Institute of Mental Health (NIMH), National Institutes of Health (NIH), Bethesda, MD, USA
Karen F. Berman, Clinical Brain Disorders Branch (CBDB), National Institute of Mental Health (NIMH), National Institutes of Health (NIH), Bethesda, MD, USA
José A. Apud, Clinical Brain Disorders Branch (CBDB), National Institute of Mental Health (NIMH), National Institutes of Health (NIH), Bethesda, MD, USA
Daniel R. Weinberger, Lieber Institute for Brain Development, Johns Hopkins Medical Campus, 855 North Wolfe Street, Baltimore, MD 21205, USA Department of Psychiatry, Johns Hopkins School of Medicine, Baltimore, MD, USA; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Solomon H. Snyder Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA; The McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Venkata S. Mattay, Email: anand.mattay@libd.org, Lieber Institute for Brain Development, Johns Hopkins Medical Campus, 855 North Wolfe Street, Baltimore, MD 21205, USA.
References
- 1.MacDonald AWI, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 2.Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, et al. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. NeuroImage. 2003;20(4):2135–2141. doi: 10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Blasi G, Goldberg TE, Elveva˚g B, Rasetti R, Bertolino A, Cohen J, et al. Differentiating allocation of resources and conflict detection within attentional control processing. Eur J Neurosci. 2007;25(2):594–602. doi: 10.1111/j.1460-9568.2007.05283.x. [DOI] [PubMed] [Google Scholar]
- 4.Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. Neuroimage. 2003;19(4):1361–1368. doi: 10.1016/s1053-8119(03)00167-8. [DOI] [PubMed] [Google Scholar]
- 5.Kerns JG, Cohen JD, MacDonald AW3, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 6.Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, Wilkinson H. Impairments of attention after cingulotomy. Neurology. 1999;53(4):819–824. doi: 10.1212/wnl.53.4.819. [DOI] [PubMed] [Google Scholar]
- 7.Danckert J, Maruff P, Ymer C, Kinsella G, Yucel M, de Graaff S, et al. Goal-directed selective attention and response competition monitoring: Evidence from unilateral parietal and anterior cingulate lesion. Neuropsychology. 2000;14(1):16–28. doi: 10.1037//0894-4105.14.1.16. [DOI] [PubMed] [Google Scholar]
- 8.Bartus RT, Levere TE. Frontal decortication in rhesus monkeys: a test of the interference hypothesis. Brain Res. 1977;119(1):233–248. doi: 10.1016/0006-8993(77)90103-2. [DOI] [PubMed] [Google Scholar]
- 9.Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25(2):359–365. doi: 10.1016/0028-3932(87)90024-8. [DOI] [PubMed] [Google Scholar]
- 10.Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, et al. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- 11.Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33(1):1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- 12.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deuel RK, Regan DJ. Parietal hemineglect and motor deficits in the monkey. Neuropsychologia. 1985;23(3):305–314. doi: 10.1016/0028-3932(85)90017-x. [DOI] [PubMed] [Google Scholar]
- 14.King VR, Corwin JV. Comparisons of hemi-inattention produced by unilateral lesions of the posterior parietal cortex or medial agranular prefrontal cortex in rats: Neglect, extinction, and the role of stimulus distance. Behav Brain Res. 1993;54(2):117–131. doi: 10.1016/0166-4328(93)90070-7. [DOI] [PubMed] [Google Scholar]
- 15.Danckert J, Sto¨ttinger E, Quehl N, Anderson B. Right hemisphere brain damage impairs strategy updating. Cereb Cortex. 2012;22(12):2745–2760. doi: 10.1093/cercor/bhr351. [DOI] [PubMed] [Google Scholar]
- 16.Yu¨cel M, Volker C, Collie A, Maruff P, Danckert J, Velakoulis D, et al. Impairments of response conflict monitoring and resolution in schizophrenia. Psychol Med. 2002;32(7):1251–1260. doi: 10.1017/s0033291702006128. [DOI] [PubMed] [Google Scholar]
- 17.Weiss EM, Golaszewski S, Mottaghy FM, Hofer A, Hausmann A, Kemmler G, et al. Brain activation patterns during a selective attention test-a functional MRI study in healthy volunteers and patients with schizophrenia. Psychiatry Res. 2003;123(1):1–15. doi: 10.1016/s0925-4927(03)00019-2. [DOI] [PubMed] [Google Scholar]
- 18.Heckers S, Weiss AP, Deckersbach T, Goff DC, Morecraft RJ, Bush G. Anterior cingulate cortex activation during cognitive interference in schizophrenia. Am J Psychiatry. 2004;161(4):707–15. doi: 10.1176/appi.ajp.161.4.707. [DOI] [PubMed] [Google Scholar]
- 19.Kerns JG, Cohen JD, MacDonald AW3, Johnson MK, Stenger VA, Aizenstein H, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162(10):1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- 20.Weiss EM, Siedentopf C, Golaszewski S, Mottaghy FM, Hofer A, Kremser C, et al. Brain activation patterns during a selective attention test-a functional MRI study in healthy volunteers and unmedicated patients during an acute episode of schizophrenia. Psychiatry Res. 2007;154(1):31–40. doi: 10.1016/j.pscychresns.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Blasi G, Taurisano P, Papazacharias A, Caforio G, Romano R, Lobianco L, Fazio L, Di Giorgio A, Latorre V, Sambataro F, Popolizio T, Nardini M, Mattay VS, Weinberger DR, Bertolino A. Nonlinear response of the anterior cingulate and prefrontal cortex in schizophrenia as a function of variable attentional control. Cereb Cortex. 2010;20(4):837–845. doi: 10.1093/cercor/bhp146. [DOI] [PubMed] [Google Scholar]
- 22.Delawalla Z, Csernansky JG, Barch DM. Prefrontal cortex function in nonpsychotic siblings of individuals with schizophrenia. Biol Psychiatry. 2008;63(5):490–497. doi: 10.1016/j.biopsych.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cools R, Rogers R, Barker RA, Robbins TW. Top-down attentional control in Parkinson’s disease: salient considerations. J Cognitive Neurosci. 2010;22(5):848–859. doi: 10.1162/jocn.2009.21227. [DOI] [PubMed] [Google Scholar]
- 24.Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67(1):53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 26.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74(1):1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Goldman-Rakic PS, Muly EC3, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31(2–3):295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 28.Devilbiss DM, Berridge CW. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol Psychiatry. 2008;64(7):626–635. doi: 10.1016/j.biopsych.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, Bigelow LB, Goldberg TE, Berman KF, Kleinman JE. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. J Neurosci. 1991;11(7):1907–1917. doi: 10.1523/JNEUROSCI.11-07-01907.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattay VS, Berman KF, Ostrem JL, Esposito G, Van Horn JD, Bigelow LB, Weinberger DR. Dextroamphetamine enhances ‘‘neural network-specific’’ physiological signals: a positron-emission tomography rCBF study. J Neurosci. 1996;16(15):4816–4822. doi: 10.1523/JNEUROSCI.16-15-04816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20(6):RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, Wong C, Jayne M, Fowler JS. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage. 2011;54(4):3101–3110. doi: 10.1016/j.neuroimage.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karoum F, Chrapusta SJ, Egan MF. 3-methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: Reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63(3):972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- 34.Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-O-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24(23):5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28(35):8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liljequist R, Haapalinna A, Ahlander M, Li YH, Mannisto PT. Catechol O-methyltransferase inhibitor tolcapone has minor influence on performance in experimental memory models in rats. Behav Brain Res. 1997;82(2):202–202. doi: 10.1016/s0166-4328(97)80989-8. [DOI] [PubMed] [Google Scholar]
- 38.Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32(5):1011–1120. doi: 10.1038/sj.npp.1301227. [DOI] [PubMed] [Google Scholar]
- 39.Blasi G, Mattay VS, Bertolino A, Elveva˚g B, Callicott JH, Das S, et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25(20):5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98(12):6912–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100(10):6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51(4):593–628. [PubMed] [Google Scholar]
- 43.Gasparini M, Fabrizio E, Bonifati V, Meco G. Cognitive improvement during tolcapone treatment in Parkinson’s disease. J Neural Transm. 1997;104(8–9):887–894. doi: 10.1007/BF01285556. [DOI] [PubMed] [Google Scholar]
- 44.Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. COMT Val(158)Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol Psychiatry. 2012;71(6):538–534. doi: 10.1016/j.biopsych.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldman-Rakic P. The cortical dopamine system: role in memory and cognition Adv. Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 47.McNair DM, Lorr M, Droppleman LF. Revised manual for the profile of mood states. San Diego: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 48.Jorga K, Fotteler B, Heizmann P, Gasser R. Metabolism and excretion of tolcapone, a novel inhibitor of catechol-O-methyl-transferase. Br J Clin Pharmacol. 1999;48(4):513–520. doi: 10.1046/j.1365-2125.1999.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jorga KM. Pharmacokinetics, pharmacodynamics, and tolerability of tolcapone: a review of early studies in volunteers. Neurology. 1998;50(5 Suppl 5):S31–S38. doi: 10.1212/wnl.50.5_suppl_5.s31. [DOI] [PubMed] [Google Scholar]
- 50.Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10(5):607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- 51.Cognition and Brain Sciences Unit. [cited 2011 Jun 23];DataDiagnostics - MRC CBU Imaging Wiki. 2009 [homepage on the Internet]. Available from: Medical Research Council. http://imaging.mrc-cbu.cam.ac.uk/imaging/DataDiagnostics.
- 52.Oldfield R. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 53.Rasetti R, Mattay VS, Stankevich B, Skjei K, Blasi G, Sambataro F, et al. Modulatory effects of modafinil on neural circuits regulating emotion and cognition. Neuropsychopharmacology. 2010;35(10):2101–2109. doi: 10.1038/npp.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9(1):20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 55.Paskavitz JF, Sweet LH, Wellen J, Helmer KG, Rao SM, Cohen RA. Recruitment and stabilization of brain activation within a working memory task; an FMRI study. Brain Imaging Behav. 2010;4(1):5–21. doi: 10.1007/s11682-009-9081-4. [DOI] [PubMed] [Google Scholar]
- 56.Spence SA, Green RD, Wilkinson ID, Hunter MD. Modafinil modulates anterior cingulate function in chronic schizophrenia. Br J Psychiatry. 2005;187:55–61. doi: 10.1192/bjp.187.1.55. [DOI] [PubMed] [Google Scholar]
- 57.Hunter MD, Ganesan V, Wilkinson ID, Spence SA. Impact of modafinil on prefrontal executive function in schizophrenia. Am J Psychiatry. 2006;163(12):2184–2186. doi: 10.1176/appi.ajp.163.12.2184. [DOI] [PubMed] [Google Scholar]