Abstract
Purpose
Lestaurtinib (CEP-701), a multi-kinase inhibitor with potent activity against the Trk family of receptor tyrosine kinases, has undergone early phase clinical evaluation in children with relapsed neuroblastoma. We studied the interaction of CEP-701 with isotretinoin (13cRA) and fenretinide (4HPR), two retinoids that have been studied in children with high-risk neuroblastoma.
Methods
In vitro growth inhibition was assessed following a 72-hour drug exposure using the sulforhodamine B (SRB) assay in eight neuroblastoma cell lines with variable TrkB expression. When appropriate, the combination index (CI) of Chou-Talalay was used to characterize the interaction of 13cRA (non-constant ratio) or 4HPR (constant ratio) with CEP-701.
Results
The median (range) IC50 of single-agent CEP-701 across all cell lines was 0.09 (0.08–0.3) μM. The combination of 13cRA and CEP-701 resulted in additive to synergistic interactions in four of the five cell lines studied. Addition of 1 or 5 μM of 13cRA decreased the median (range) CEP-701 IC50 1.5-fold (1.1–2.8-fold) and 1.7-fold (1.5–1.8-fold), respectively. With 10 μM 13cRA, less than 50% of cells survived when combined with various concentrations of CEP-701. The combination of 4HPR and CEP-701 trended toward being antagonistic, with a median (range) CI at the ED50 of 1.3 (1.1–1.5).
Conclusions
The combination of 13cRA and CEP-701 was additive or synergistic in a spectrum of neuroblastoma cell lines, suggesting that these agents can be potentially studied together in the setting of minimal residual disease following intensive chemoradiotherapy for children with high-risk neuroblastoma.
Keywords: Neuroblastoma, Retinoid, Lestaurtinib (CEP-701), In vitro
Introduction
Neuroblastoma is the most common extra-cranial childhood solid tumor, accounting for 8–10% of all pediatric cancers [2]. It is derived from the sympaticoadrenal lineage of the neural crest and can develop anywhere along the sympathetic chain [20]. The prognosis of children diagnosed with neuroblastoma varies widely with age, stage of tumor, biology, and histology. Children less than 1 year of age at diagnosis with a stage 1 tumor with favorable features have an estimated survival of 95% [19]. However, children diagnosed at greater than 18 months of age with stage IV tumors with unfavorable features have an estimated survival of 25–40% and experience significant short and long-term toxicities associated with intensive therapy [6].
A feature of unfavorable neuroblastomas is the expression of the neurotrophin receptor, TrkB, and its ligand, brain derived neurotrophic factor (BDNF) [24]. The BDNF/TrkB loop may up-regulate growth and promote survival of unfavorable neuroblastoma through autocrine or paracrine pathways [1, 24]. In addition, unfavorable neuroblastoma cell lines with high expression of TrkB have been shown to be more resistant to chemotherapy than neuroblastoma cell lines with lower expression of TrkB [13].
Lestaurtinib (CEP-701), a novel tyrosine kinase inhibitor, is a potent inhibitor of the Trk tyrosine kinase receptor family. Like its parent compound, CEP-751, CEP-701 is a synthetic derivative of K252a, but CEP-701 can be given orally. While CEP-701 has been shown to inhibit multiple kinases [33], its ability to inhibit TrkB makes it potentially relevant for the treatment of children with neuroblastoma. CEP-701 has been studied in a spectrum of adult cancers [4, 17, 22, 23, 28, 29, 32] and has undergone investigation in acute myelogenous leukemia (AML) because of its ability to inhibit FLT3, inducing apoptosis in FLT3/ITD-expressing cell lines and leukemic blasts [3, 16, 17, 28]. CEP-701 is currently undergoing evaluation in combination with conventional cytotoxic therapy for infants with leukemia. A phase 1 study of CEP-701 is also being conducted in children with refractory/relapsed neuroblastoma.
Isotretinoin (13cis retinoic acid, 13cRA) is a synthetic retinoid that causes differentiation and decreased proliferation of neuroblastoma cells [25, 26, 30]. When incorporated into treatment following myeloablative therapy with autologous stem cell transplantation, 13cRA increases 3-year event-free survival in children with high-risk neuroblastoma [21] and is now a standard component of treatment for patients with high-risk neuroblastoma. Fenretinide (4HPR) is a synthetic retinoid that triggers apoptosis in neuroblastoma cells [7, 18] that has completed early phase clinical trials for patients with relapsed/refractory neuroblastoma [10, 11, 31].
We evaluated the in vitro profiles of CEP-701, 13cRA, and 4HPR when given as single agents and in combination in a panel of neuroblastoma cell lines. Our goal was to explore the integration of CEP-701 for potential clinical use following chemoradiotherapy in children with high-risk neuroblastoma. Based on prior preclinical evaluations of CEP-701 and CEP-751 [8, 9, 14, 22, 23], we hypothesized that the single-agent efficacy of CEP-701 would be related to TrkB expression status.
Materials and methods
A panel of five neuroblastoma cell lines was initially used: CHP-134, IMR-5, E6-NBLS, SH-SY5Y, and the SH-SY5Y-BR6. The SH-SY5Y-BR6 line has been engineered from SH-SY5Y to express high levels of full-length, functional TrkB receptors [13, 14]. Based on our initial results, we expanded our analysis to include three additional neuroblastoma cell lines: NLF, NLF-A, and NLF-B. The NLF-A and NLF-B lines have been engineered from NLF to express high levels of functional TrkA and TrkB levels, respectively. CHP-134, IMR-5, E6-NBLS, SH-SY5Y, and NLF cell lines were grown in RPMI-1640 containing 10% fetal bovine serum, 1% glutamine, and 1 mM oxaloacetate, 0.45 mM pyruvate, and 0.2 U/ml insulin. The SH-SY5Y-BR6, NLF-A, and NLF-B lines were grown the same media with the addition of 0.3 mg/mL G418. All cell lines were tested for mycoplasma contamination using MycoAlert® mycoplasma detection assay from Cambrex (East Rutherford, NJ). 13cRA and 4HPR were purchased from Sigma (St. Louis, MO). CEP-701 was provided by Cephalon (Frazer, PA).
Growth inhibition assays
Each cell line was studied in growth inhibition experiments using 96-well microtiter plates. Twenty-four hours after cell plating, cell lines were exposed to CEP-701, 13cRA, 4HPR or their combination for 72 h (three replicates per experiment). Cells were then washed in the appropriate media and grown for an additional 72 h. To ensure that a complete sigmoidal survival-concentration curve could be observed, the following drug concentrations were studied: CEP-701 (0.01–0.5 μM), 13cRA (0.001–200 μM), and 4HPR (0.1–10 μM). Experiments were repeated at least twice.
Survival-concentration curves were generated using the SRB assay [15, 27]. Following 72-hour drug exposure and subsequent 72-hour growth, 50 μl of cold 50% trichloroacetic acid (TCA) (4°C) was added to the wells at the liquid air interface of each well to produce a final TCA concentration of 10%. The cell culture plates were incubated for 30 min at 4°C and then washed three times with distilled water. Once plates were air dried, 100 μl of 0.4% SRB stain containing 1% acetic acid was added to each well. The plates were then incubated for 30 min at room temperature and washed with 1% acetic acid. Once the stained plates were air dried, 100 μl of 10 mM Tris Base was added to each well and plates were gently agitated for 5 min. The optical density was then read using a Molecular Devices VERSAmax (Sunnyvale, CA) microplate reader at 520 nm. The background signal from media-alone controls was subtracted and data were normalized to untreated cells. The 50% growth inhibitory concentration (IC50) was determined by fitting a four parameter logistic equation to the data:
where X is the drug concentration, Emax and Emin are the concentrations at which maximum and minimum cytotoxicity are observed, respectively, and EC50 is the concentration at which 50% of the maximum cytotoxicity is attained (Emin + Emax)/2. The IC50 was then calculated using the fitted parameters and solving for X with survival set to 50%.
Determination of synergy
The interaction between CEP-701 and 4HPR was characterized using a constant drug ratio (based on IC50) and analyzed with the combination index (CI) method [5]. The CI method is based on the median effect equation, and determination of synergy using this method is independent of the mechanism of inhibition of the two drugs. This analysis yields two parameters that describe the interactions among drugs in a given combination: the combination index (CI) and the dose reduction index (DRI). The combination index is calculated by the equation:
A CI of<1 indicates synergism, a CI of 1 indicates additive effects, and a CI of >1 indicates antagonism.
Because the IC50 of 13cRA is not clinically achievable, pharmacologically relevant fixed concentrations of 13cRA (previously determined to result in ≤25% growth inhibition) were added to cells exposed to CEP-701 in order to evaluate the interaction between 13cRA and CEP-701.
Results
We initially conducted our experiments in five cell lines (SH-SY5Y, SH-SY5Y-BR6, IMR-5, CHP-134, E6-NBLS). TrkA and B expression were determined using Illumina expression, semi-quantitative PCR, or TaqMan® gene expression assays. Qualitative expression of TrkA and TrkB in each cell line is presented in Table 1. Because of the finding that expression of TrkB did not appear to predict for in vitro drug sensitivity, we expanded our study to include 3 additional cell lines (NLF, NLF-A, NLF-B). The individual cytotoxicity profiles for each agent are shown in Table 1. The median (range) IC50 of single-agent CEP-701 across all cell lines was 0.09 (0.08–0.3) μM. The median (range) IC50 of 13cRA and 4HPR were 25 (2 to > 100) and 1.0 (0.4–2.4) μM, respectively.
Table 1.
TrkA and B status for each cell line studied (Ø no expression, ⇑⇑ high expression, ⇑ moderate expression, ⇓ low expression). Single- agent IC50 data for CEP-701, 13cRA, and 4HPR. CI data for the CEP-701 and 4HPR combination
| Cell line | Trk status | IC50μM (SD) |
Combination index [CI (range)] 4HPR + CEP-701 | ||
|---|---|---|---|---|---|
| CEP-701 | 13cRA | 4HPR | |||
| SH-SY5Y-BR6 | Ø TrkA ⇑⇑TrkB | 0.17 (0.03) | 2.3 (1.2) | 2.0 (0.4) | 1.1 (0.9–1.3) |
| SH-SY5Y | ØTrkA ØTrkB | 0.08 (0.02) | 2.1 (1.9) | 1.0 (0.3) | 1.3 (1.2–1.7) |
| IMR-5 | ⇑TrkA ⇑TrkB | 0.08 (0.02) | 25 (13) | 0.4 (0.04) | 1.3 (1.2–1.4) |
| CHP-134 | ⇑TrkA ⇑TrkB | 0.09 (0.03) | 25 (10) | 0.5 (0.1) | 1.1 (1.1–1.1) |
| E6-NBLS | ⇓TrkA ØTrkB | 0.16 (0.01) | 99 (10) | 2.4 (0.5) | 1.5 (1.3–1.7) |
| NLF-A | ⇑⇑TrkA ØTrkB | 0.3 (0.003) | >100 | - | - |
| NLF-B | ØTrkA ⇑⇑TrkB | 0.08 (0.01) | 18 (4) | - | - |
| NLF | ØTrkA ØTrkB | 0.09 (0.02) | 14 (3) | - | - |
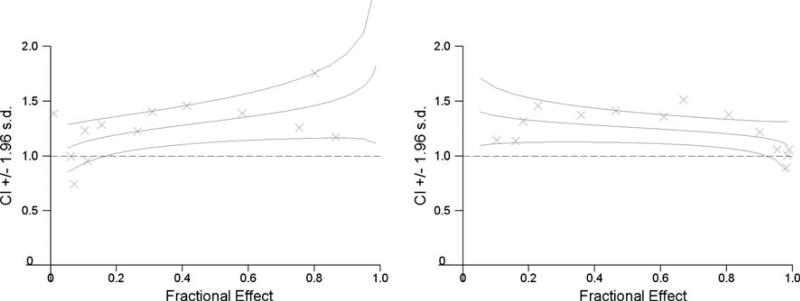
The combination of 4HPR and CEP-701 trended toward being antagonistic, with a median (range) CI at the ED50 of 1.3 (1.1–1.5) (Table 1). Combination plots illustrating the negative interaction between CEP-701 and 4HPR are shown in Fig. 1.
Fig. 1.
Combination index plots illustrating the interaction between CEP-701 and 4HPR in SH-SY5Y (right) and SH-SY5Y-BR6 (left) cell lines. The points on the graph delineate the CI for each experimental value. The middle line represents the estimated CI associated with the fractional effect and the upper and lower lines represent the bounds of the 95% confidence interval for the estimated CI. Both graphs demonstrate that the interaction trends toward antagonistic (CI >1)
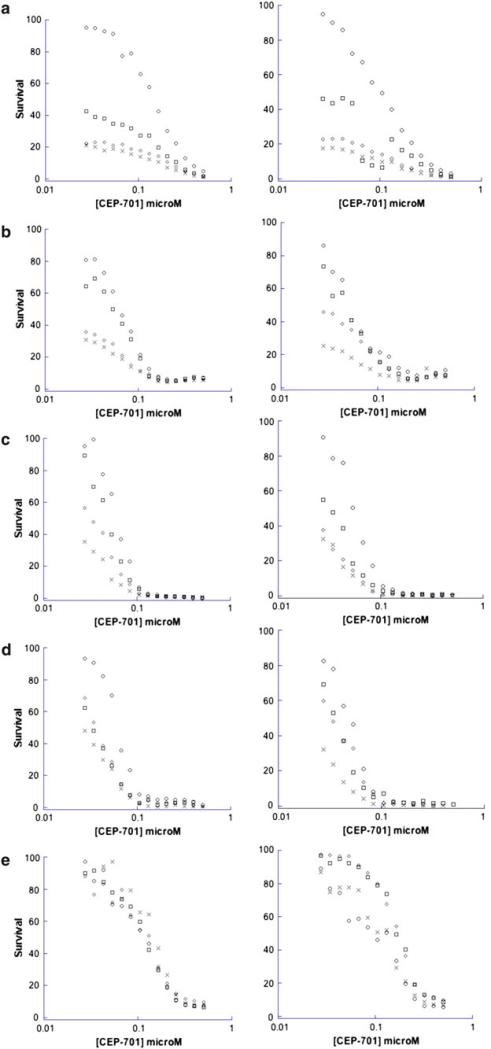
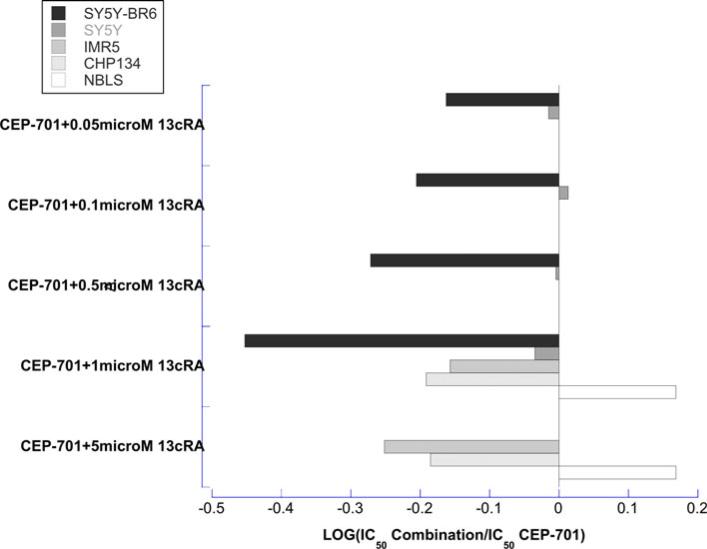
The combination of 13cRA and CEP-701, however, resulted in additive to synergistic interactions in four of the five cell lines studied. Addition of 1 and 5 μM of 13cRA decreased the median (range) CEP-701 IC50 1.5-fold (1.1–2.8-fold) and 1.7-fold (1.5–1.8-fold), respectively (Figs. 2, 3, and Supplemental Table). With 10 μM 13cRA, less than 50% of cells survived when combined with various concentrations of CEP-701. Due to the increased sensitivity of SH-SY5Y-BR6 and SH-SY5Y lines to 13cRA, 0.05, 0.1, and 0.5 μM of 13cRA was added to CEP-701. These combinations also demonstrated additive to synergistic interactions (Fig. 3).
Fig. 2.
Dose-effect plots illustrating the interaction between CEP-701 and 13cRA in the a SH-SY5Y-BR6, b SH-SY5Y, c IMR-5, d CHP-134, and e E6-NBLS cell lines. ○ CEP-701, □ CEP-701 and 1 microM 13cRA, ◇ CEP-701 and 5 microM 13cRA, × CEP-701 and 10 microM 13cRA
Fig. 3.
Bar graph illustrating the effect of 13cRA on the cytotoxicity (as measured by the IC50) of CEP-701. In the SH-SY5Y and SH-SY5Y-BR6 lines, less than 50% of cells survived when 5 μM was added to various concentrations of CEP-701. Due to observed increased sensitivity of SH-SY5Y and SH-SY5Y-BR6 lines to 13cRA, the interaction was also assessed using 0.5, 0.1, and 0.05 μM 13cRA
Discussion
We evaluated the in vitro cytotoxicity of CEP-701, 13cRA, and 4HPR as single agents and when used in combination in a panel of neuroblastoma cell lines. Evaluation of CEP-701 as a single agent demonstrated that neither TrkA nor TrkB expression appeared to correlate with CEP-701 single-agent growth inhibition. While CEP-701 is a promiscuous tyrosine kinase inhibitor, its activity in neuroblastoma has been thought to be through inhibition of the Trk family of kinases. For this reason, we hypothesized that CEP-701 would have similar activity to its parent compound, CEP-751, in competitively inhibiting the ATP-binding sites of the Trk family of tyrosine kinases. Evans et al. [9] evaluated the in vivo antitumor activity of CEP-751 in xenograft models using IMR-5, CHP-134, E6-NBLS, SH-SY5Y, and SH-SY5Y-TrkB(G12) cell lines. The greatest activity of CEP-751 was seen in the SH-SY5Y-TrkB(G12) cell line, the line with the highest TrkB expression. Subsequent evaluations confirmed that activation of TrkB by BDNF in SH-SY5Y-TrkB(G12) cells was inhibited by CEP-751 in a dose-dependent fashion [8]. A recent publication by Iyer et al. [14] confirmed that CEP-701 can inhibit the phosphorylation of TrkB in the SH-SY5Y-BR6 cell line at concentrations of 10–250 nM in the presence of exogenous BDNF, however, complete inhibition of phosphorylation was not demonstrated. This study also demonstrated that there was no TrkB phosphorylation in the SH-SY5Y-BR6 line in the absence of exogenous BDNF. Therefore, one possible reason that our results did not demonstrate that activity of CEP-701 could be predicted based on expression of TrkB is that exogenous BDNF is needed in in vitro models to activate the TrkB pathway. It cannot be excluded, however, that the activity of CEP-701 on the Trk B pathway may be different than its parent compound CEP-751 or that the antitumor effects of CEP-701 in neuroblastoma are due to interactions with other tyrosine kinases.
Our analysis demonstrates complementary approaches that can be used to evaluate interaction (antagonism, additivity, synergy) of chemotherapeutic agents. We evaluated the interaction between CEP-701 and 4HPR using a fixed ratio of the compounds based on their IC50. We then characterized the interaction using the CI as proposed by Chou and Talalay. Using this approach, we found that the interaction of CEP-701 and 4HPR trended toward antagonistic in all of the cell lines studied. These results are supported by the recent evaluation of the combination of CEP-701 and 4HPR in an SH-SY5Y-BR6 xenograft model of neuroblastoma [14]. This study found that while both 4HPR and CEP-701 demonstrated activity as single agents compared to vehicle control, when combined their activity was no greater than that of CEP-701 alone.
Because the IC50 of 13cRA in each cell line is not clinically achievable, we used fixed concentrations of 13cRA that result in ≤25% cytotoxicity to determine the interaction between CEP-701 and 13cRA. The single-agent cytotoxicity of 13cRA presented in Table 1 demonstrates a high degree of variability in each of the cell lines studied. It should be noted that in a subset of experiments at high 13cRA concentrations, the method to accurately estimate Emax and hence IC50 is limited; these values should be interpreted with caution. Overall the addition of 13cRA to CEP-701 resulted in at least additive interactions in four of the five cell lines studied with the greatest effect seen in the cell line with the highest expression of TrkB. 13cRA likely exerts its effects on neuroblastoma cells via isomerization to all-trans retinoic acid or 9-cis retinoic acid, retinoids that have both been shown to increase the expression of TrkB [12]. Therefore, one possible mechanism for the observed synergy of these two compounds is that 13cRA up-regulates the TrkB pathway leading to increased sensitivity to CEP-701, even in the absence of exogenous BDNF. Because of the promiscuity of CEP-701, further testing of 13cRA with a potent and selective inhibitor of TrkB is needed to confirm the hypothesis that the observed favorable interaction of CEP-701 and 13cRA is via inhibition of the TrkB pathway. Nonetheless, our results suggest that the combination of CEP-701 and 13cRA may have the potential to further eliminate minimal residual disease following intensive chemoradiotherapy.
Supplementary Material
Acknowledgments
This work was supported by the CTSA K12-KL2RR024134 grants from the National Institute of Health and the Alex's Lemonade Stand Young Investigator Grant.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00280-011-1623-y) contains supplementary material, which is available to authorized users.
Contributor Information
Robin E. Norris, Division of Oncology, The Children's Hospital of Philadelphia, Philadelphia, PA, USA Division of Clinical Pharmacology and Therapeutics, The Children's Hospital of Philadelphia, Colket Translational Research Building, 3501 Civic Center Blvd., Room 4014, Philadelphia, PA 19104, USA.
Jane E. Minturn, Division of Oncology, The Children's Hospital of Philadelphia, Philadelphia, PA, USA
Garrett M. Brodeur, Division of Oncology, The Children's Hospital of Philadelphia, Philadelphia, PA, USA
John M. Maris, Division of Oncology, The Children's Hospital of Philadelphia, Philadelphia, PA, USA
Peter C. Adamson, Division of Oncology, The Children's Hospital of Philadelphia, Philadelphia, PA, USA Division of Clinical Pharmacology and Therapeutics, The Children's Hospital of Philadelphia, Philadelphia, PA, USA.
References
- 1.Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 2.Brodeur GM, Maris JM. Neuroblastoma. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. Lippincott Williams & Wilkins; Philadelphia: 2006. pp. 933–970. [Google Scholar]
- 3.Brown P, Meshinchi S, Levis M, Alonzo TA, Gerbing R, Lange B, Arceci R, Small D. Pediatric AML primary samples with FLT3/ITD mutations are preferentially killed by FLT3 inhibition. Blood. 2004;104:1841–1849. doi: 10.1182/blood-2004-03-1034. [DOI] [PubMed] [Google Scholar]
- 4.Chan E, Mulkerin D, Rothenberg M, Holen KD, Lockhart AC, Thomas J, Berlin J. A phase I trial of CEP-701 + gemcitabine in patients with advanced adenocarcinoma of the pancreas. Invest New Drugs. 2008;26:241–247. doi: 10.1007/s10637-008-9118-3. [DOI] [PubMed] [Google Scholar]
- 5.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 6.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, Mosseri V, Simon T, Garaventa A, Castel V, Matthay KK. The International Neuroblastoma Risk Group (INRG) classification system: an INRG task force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Vinci A, Geido E, Infusini E, Giaretti W. Neuroblastoma cell apoptosis induced by the synthetic retinoid N-(4-hydroxyphenyl) retinamide. Int J Cancer. 1994;59:422–426. doi: 10.1002/ijc.2910590322. [DOI] [PubMed] [Google Scholar]
- 8.Evans AE, Kisselbach KD, Liu X, Eggert A, Ikegaki N, Camoratto AM, Dionne C, Brodeur GM. Effect of CEP-751 (KT-6587) on neuroblastoma xenografts expressing TrkB. Med Pediatr Oncol. 2001;36:181–184. doi: 10.1002/1096-911X(20010101)36:1<181::AID-MPO1043>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 9.Evans AE, Kisselbach KD, Yamashiro DJ, Ikegaki N, Camoratto AM, Dionne CA, Brodeur GM. Antitumor activity of CEP-751 (KT-6587) on human neuroblastoma and medulloblastoma xenografts. Clin Cancer Res. 1999;5:3594–3602. [PubMed] [Google Scholar]
- 10.Formelli F, Cavadini E, Luksch R, Garaventa A, Villani MG, Appierto V, Persiani S. Pharmacokinetics of oral fenretinide in neuroblastoma patients: indications for optimal dose and dosing schedule also with respect to the active metabolite 4-oxofenretinide. Cancer Chemother Pharmacol. 2008;62:655–665. doi: 10.1007/s00280-007-0649-7. [DOI] [PubMed] [Google Scholar]
- 11.Garaventa A, Luksch R, Lo Piccolo MS, Cavadini E, Montaldo PG, Pizzitola MR, Boni L, Ponzoni M, Decensi A, De Bernardi B, Bellani FF, Formelli F. Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin Cancer Res. 2003;9:2032–2039. [PubMed] [Google Scholar]
- 12.Giannini G, Dawson MI, Zhang X, Thiele CJ. Activation of three distinct RXR/RAR heterodimers induces growth arrest and differentiation of neuroblastoma cells. J Biol Chem. 1997;272:26693–26701. doi: 10.1074/jbc.272.42.26693. [DOI] [PubMed] [Google Scholar]
- 13.Ho R, Eggert A, Hishiki T, Minturn JE, Ikegaki N, Foster P, Camoratto AM, Evans AE, Brodeur GM. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62:6462–6466. [PubMed] [Google Scholar]
- 14.Iyer R, Evans AE, Qi X, Ho R, Minturn JE, Zhao H, Balamuth N, Maris JM, Brodeur GM. Lestaurtinib enhances the anti-tumor efficacy of chemotherapy in murine xenograft models of neuroblastoma. Clin Cancer Res. 2010;16:1478–1485. doi: 10.1158/1078-0432.CCR-09-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HM, Han SB, Kim MS, Kang JS, Oh GT, Hong DH. Efficient fixation procedure of human leukemia cells in sulforhodamine B cytotoxicity assay. J Pharmacol Toxicol Methods. 1996;36:163–169. doi: 10.1016/s1056-8719(96)00113-x. [DOI] [PubMed] [Google Scholar]
- 16.Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, Chopra R, Clark R, Levis MJ, Small D. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 17.Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, Smith BD, Jones-Bolin S, Ruggeri B, Dionne C, Small D. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99:3885–3891. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 18.Mariotti A, Marcora E, Bunone G, Costa A, Veronesi U, Pierotti MA, Della Valle G. N-(4-hydroxyphenyl) retinamide: a potent inducer of apoptosis in human neuroblastoma cells. J Natl Cancer Inst. 1994;86:1245–1247. doi: 10.1093/jnci/86.16.1245. [DOI] [PubMed] [Google Scholar]
- 19.Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr. 2005;17:7–13. doi: 10.1097/01.mop.0000150631.60571.89. [DOI] [PubMed] [Google Scholar]
- 20.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 21.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 22.Miknyoczki SJ, Chang H, Klein-Szanto A, Dionne CA, Ruggeri BA. The Trk tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits significant antitumor efficacy in preclinical xenograft models of human pancreatic ductal adenocarcinoma. Clin Cancer Res. 1999;5:2205–2212. [PubMed] [Google Scholar]
- 23.Miknyoczki SJ, Dionne CA, Klein-Szanto AJ, Ruggeri BA. The novel Trk receptor tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits antitumor efficacy against human pancreatic carcinoma (Panc1) xenograft growth and in vivo invasiveness. Ann N Y Acad Sci. 1999;880:252–262. doi: 10.1111/j.1749-6632.1999.tb09530.x. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawara A, Azar CG, Scavarda NJ, Brodeur GM. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol Cell Biol. 1994;14:759–767. doi: 10.1128/mcb.14.1.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds CP, Kane DJ, Einhorn PA, Matthay KK, Crouse VL, Wilbur JR, Shurin SB, Seeger RC. Response of neuroblastoma to retinoic acid in vitro and in vivo. Prog Clin Biol Res. 1991;366:203–211. [PubMed] [Google Scholar]
- 26.Sidell N, Altman A, Haussler MR, Seeger RC. Effects of retinoic acid (RA) on the growth and phenotypic expression of several human neuroblastoma cell lines. Exp Cell Res. 1983;148:21–30. doi: 10.1016/0014-4827(83)90184-2. [DOI] [PubMed] [Google Scholar]
- 27.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 28.Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, Murphy KM, Dauses T, Allebach J, Small D. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 29.Strock CJ, Park JI, Rosen M, Dionne C, Ruggeri B, Jones-Bolin S, Denmeade SR, Ball DW, Nelkin BD. CEP-701 and CEP-751 inhibit constitutively activated RET tyrosine kinase activity and block medullary thyroid carcinoma cell growth. Cancer Res. 2003;63:5559–5563. [PubMed] [Google Scholar]
- 30.Thiele CJ, Reynolds CP, Israel MA. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. Nature. 1985;313:404–406. doi: 10.1038/313404a0. [DOI] [PubMed] [Google Scholar]
- 31.Villablanca JG, Krailo MD, Ames MM, Reid JM, Reaman GH, Reynolds CP. Phase I trial of oral fenretinide in children with high-risk solid tumors: a report from the Children's Oncology Group (CCG 09709). J Clin Oncol. 2006;24:3423–3430. doi: 10.1200/JCO.2005.03.9271. [DOI] [PubMed] [Google Scholar]
- 32.Weeraratna AT, Dalrymple SL, Lamb JC, Denmeade SR, Miknyoczki S, Dionne CA, Isaacs JT. Pan-trk inhibition decreases metastasis and enhances host survival in experimental models as a result of its selective induction of apoptosis of prostate cancer cells. Clin Cancer Res. 2001;7:2237–2245. [PubMed] [Google Scholar]
- 33.Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, Karaman MW, Pratz KW, Pallares G, Chao Q, Sprankle KG, Patel HK, Levis M, Armstrong RC, James J, Bhagwat SS. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood. 2009;114:2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.