Abstract
Background
The role of the innate immune system in the development of thrombotic microangiopathy (TM) after α1,3-galactosyltransferase gene-knockout (GTKO) pig organ transplantation in primates is uncertain.
Methods
Twelve organs (9 hearts, 3 kidneys) from GTKO pigs were transplanted into baboons that received no immunosuppressive therapy, partial regimens, or a full regimen based on costimulation blockade. After graft failure, histological and immunohistological examinations were carried out.
Results
Graft survival of <1 day was prolonged to 2–12 days with partial regimens (acute humoral xenograft rejection [AHXR]) and to 5 and 8 weeks with the full regimen (TM). Clinical and/or laboratory features of consumptive coagulopathy occurred in 7 of 12 baboons. Immunohistochemistry demonstrated IgM, IgG, and complement deposition in most cases. Histopathology demonstrated neutrophil and macrophage infiltrates, intravascular fibrin deposition and platelet aggregation (TM). Grafts showed expression of primate tissue factor (TF), with increased mRNA levels, and TF was also expressed on baboon macrophages/monocytes infiltrating the graft.
Conclusions
Our data suggest: (i) irrespective of the presence or absence of the adaptive immune response, early or late xenograft rejection is associated with activation of the innate immune system; (ii) porcine endothelial cell activation and primate TF expression by recipient innate immune cells may both contribute to the development of TM.
Keywords: α1,3-galactosyltransferase gene-knockout; Baboon; Co-stimulatory blockade; Consumptive coagulopathy; Heart; Kidney; Pig; Xenotransplantation
INTRODUCTION
The availability of α1,3-galactosyltransferase gene-knockout (GTKO) pigs has enabled the transplantation (Tx) of organs into nonhuman primates (1, 2). Initial studies by Kuwaki et al indicated that, with an immunosuppressive regimen that prevented the T cell-dependent adaptive immune response, heart grafts survived for 2–6 months (3, 4); graft failure was from a thrombotic microangiopathy (TM) (5).
Subsequently, however, Chen et al (6) reported both hyperacute rejection and acute humoral xenograft rejection (AHXR) in renal grafts from GTKO pigs in baboons that received an immunosuppressive regimen that did not prevent an elicited antibody response. These in vivo observations correlated with in vitro assays that indicated a significant cytotoxicity associated with the binding of antibodies to GTKO pig cells (7–10).
We here report 12 organs (9 hearts, 3 kidneys) from GTKO pigs transplanted into baboons that received no therapy, partial regimens, or full therapeutic regimen based on costimulation blockade. With or without an adaptive immune response, the histopathologic features seen were associated with an innate immune response, while TM was a constant feature.
METHODS
Animals
Baboons (Papio species, 6–20kg, of known ABO blood type, n=12: Division of Animal Resources, Oklahoma University Health Sciences Center, Oklahoma City, OK, and the Southwest Foundation for Biomedical Research, San Antonio, TX) were recipients. Homozygous GTKO pigs (n=9, of blood group O (nonA), 10–20kg, Revivicor, Blacksburg, VA), served as sources of hearts (n=9) and kidneys (n=3) (1, 11). Tissues from all major organs were stained with biotinylated GSIB4 lectin and were negative for Galα1,3Gal (Gal) expression (1).
All animal care was in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1985). Protocols were approved by the University of Pittsburgh or the University of Maryland Institutional Animal Care and Use Committee.
Surgical Procedures
Anesthesia, intravascular catheter placements in pigs and baboons, heart and kidney excision in pigs, renal Tx and heterotopic heart Tx (in the abdomen) in baboons have been described previously (12–14). Two independent observers regularly monitored cardiac graft function by palpation. Kidney graft status was monitored by serum creatinine (13–16).
In some cases, after excision of a rejected heart graft, the baboon was followed for several weeks; remnants of pig aorta and pulmonary artery remained in the baboon. After rejection of a life-supporting kidney graft, the baboon was euthanized.
Immunosuppressive and Supportive Therapy
Baboons received either no therapy (n=2), partial immunosuppression (n=7), or full immunosuppression (n=3) (Table 1). Baboons with partial immunosuppression electively received cobra venom factor only (n=2), cobra venom factor (CVF) + thymoglobulin + leflunomide (n=1), or low-dose anti-CD154mAb (ABI193; NovartisPharma, Basel, Switzerland) + low-dose CTLA4-Ig + MMF (n=2). In two others, technical problems prevented the full therapy from being administered. The full therapeutic regimen consisted of induction with thymoglobulin (Genzyme, Cambridge, MA) and maintenance with anti-CD154mAb, mycophenolate mofetil (MMF; Roche, Basel, Switzerland), and methylprednisolone (17, 18).
TABLE 1.
Immunosuppressive regimen, graft survival and complications after gtko organ transplantation in baboons
| Exp | Graft type |
Immunosuppression | Graft Survival |
Complications |
|---|---|---|---|---|
| 1 | Heart | None | 2.5 hours (5) | Heart could not be accommodated in abdomen |
| 2 | Heart | None | 1 day (5) | AHXR. CC (8) |
| 3 | Heart | CVF (1) | 7 days (5) | AHXR. CC (9) |
| 4 | Kidney | CVF (1) | 2 days (5) | AHXR. CC (8) |
| 5 | Heart | CVF + thymoglobulin+ leflunomide (2) |
12 days | AHXR. CC. Euthanized |
| 6 | Heart | Low-dose anti-CD154mAb + CTLA4-Ig + MMF (3) |
6 days (5) | AHXR. CC (9) |
| 7 | Kidney | Low-dose anti-CD154mAb + CTLA4-Ig + MMF (3) |
5 days | AHXR. CC (6); Died 1 day after excision of graft. |
| 8 | Heart | Full co-stimulation blockade regimen (4) |
6 days | Obstruction of IV catheters (6). AHXR. Euthanized |
| 9 | Heart | Full co-stimulation blockade regimen (4) |
12 days | Obstruction of IV catheters (6).AHXR. Euthanized. |
| 10 | Kidney | Full co-stimulation blockade regimen (4) |
3 days | Renal artery thrombosis (7) Euthanized |
| 11 | Heart | Full co-stimulation blockade regimen (4) |
5 weeks | Stenosis at anastomoses. TM + ischemic myopathy. CC (9). Euthanized |
| 12 | Heart | Full co-stimulation blockade regimen (4) |
8 weeks | TM. CC (9) |
CVF (starting at 100U/kg and then daily based on CH50 values).
Induction with thymoglobulin (5–10mg/kg on days −3 and −1). CVF (starting at 100U/kg on day −1, and then on days 0 and 1, based on CH50 values) + leflunomide (20mg/kg x2/day).
Anti-CD154mAb (30mg/kg on day 0, and 20mg/kg on days 3 and 7) + CTLA4-Ig (20mg/kg on day 0 followed by 10mg/kg on days 3 and 7) + MMF (30mg/kg/day IV).
Induction with thymoglobulin (5–10mg/kg on days −3 and −1). CVF (starting at 100U/kg on day −1, and continued daily until day 3, based on CH50 values) + anti-CD154mAb (25mg/kg on days −1, 0, 4, 7, 10, 14 and every 5 days thereafter) + MMF (110mg/kg/day IV) + methylprednisolone (starting at 4mg/kg/day on day 0 and tapering).
Graft excised, and baboon followed to determine immune response.
Resulted in discontinuation of drug administration.
Probably associated with inadequate heparinization in the presence of anti-CD154mAb Therapy, as reported previously (21, 22).
CC diagnosed on basis of laboratory parameters and by clinical bleeding.
CC diagnosed on basis of laboratory parameters only.
Because consumptive coagulopathy (CC) can develop in xenograft recipients (19, 20), and in view of potential anti-CD154mAb-associated thromboembolic complications (21, 22), heparin was begun on day 0 and gradually increased in an effort to maintain the aPTT at 150sec. (Previous experience demonstrated that maintenance of the aPTT >150sec was associated with an increased risk of bleeding) However, this goal was not always achieved.
Monitoring of Recipient Baboons
Blood cell counts, chemistry, and coagulation parameters were measured by standard methods. The serum trough level of the anti-CD154mAb was monitored by ELISA (maintained at >400µg/mL in the full regimen) (23), and T/B cell numbers by flow cytometry. Anti-nonGal antibody levels were determined by flow cytometry, and serum cytotoxicity was determined using GTKO peripheral blood mononuclear cells (7).
Histopathology and Immunohistopathology of Porcine Heart and Kidney Grafts
Biopsies of xenografts were obtained 30min after reperfusion, at the time of graft excision, or at the time of euthanasia. Tissues were fixed in 10% formalin and embedded in paraffin. Four-micron (4µm) sections were stained with hematoxylin and eosin and with martius-scarlet-blue for light microscopy. Immunohistochemical staining for IgM, IgG, C3, C4d, fibrin and platelets, neutrophils (myeloperoxidase), macrophages (CD68), T lymphocytes (CD3), and B lymphocytes (CD20) was performed (6). Primate tissue factor (TF) was stained using an anti-human TF antibody (American Diagnostica, Stamford, CT).
qRT PCR Measurement of Primate TF mRNA Levels in Heart Xenografts
Total RNA was extracted from the graft or native heart using Trizol (Life Technologies, Grand Island, NY). RNA content was measured using 260/280 UV spectrophotometry. Briefly, total RNA pellets were suspended in RNase-free water, followed by treatment with DNase I (Life Technologies, Rockville, MD). RNA (3µg) from each sample was used for reverse transcription with an oligo dT (Life Technologies) and Superscript II (Life Technologies). PCR mixture was prepared using SYBR Green PCR Master Mix (PE Applied Biosystems, Foster City, CA). Primers for baboon-TF were 5’-TGCTTTTACACAGCAGACACAGAGT-3’ (forward) and 5’-AAGACCCGTGCCAAGTACGT-3’ (reverse); baboon-β-actin were 5’-TGGAAGAATGCGGCTCATATT-3’ (forward) and 5’ TACTATCCAATCCTAGAAAGAACATG-3’ (reverse). Thermal cycling conditions were 10min at 95°C, followed by 40 cycles of 95°C for 15sec, and 60°C for 1min on an ABI PRISM 7000 Sequence Detection System (PE Applied Biosystems).
RESULTS
Pig Heart and Kidney Graft Survival and Complications
With no immunosuppression (n=2), graft survival was <24h, though in one case this was because the graft had to be excised because it was too large to close the abdomen (Table 1). Both hearts were excised, and both baboons were followed for monitoring of their immune response. Graft survival was prolonged to 2–12 days in baboons receiving partial immunosuppression (heart Tx, n=5; kidney Tx, n=2), with graft failure from AHXR. In baboons receiving the full regimen (n=3), with one exception, graft survival was extended to 5 and 8 weeks (Table 1). In 7 of the 12 baboons, features of CC were detected (Table 1). CC was identified either clinically, i.e., bleeding, and/or through laboratory parameters (rapidly falling platelet count, falling hematocrit, and prolongation of PT and aPTT even after the discontinuation of heparin infusion) (24), which were seen at the time of cessation of graft function or euthanasia.
Immunologic Monitoring
In those that received the full regimen, T and B cell counts remained low, as reported previously (3, 4). There was a trend for an increase in neutrophils and monocytes as rejection developed. The platelet count was variable, depending on immunosuppressive therapy and the adequacy of heparinization, but generally fell as TM developed.
Anti-nonGal IgM was present in all baboons before Tx, but only one had anti-nonGal IgG. In those baboons that received no or partial immunosuppressive therapy and were followed for >8 days (either in the presence of a functioning graft or after graft excision), there was an increase in both anti-nonGal IgM and IgG binding on flow cytometry, and increased lysis of GTKO PBMC in the serum cytotoxicity assay, indicating sensitization to nonGal antigens.
In baboons that received full therapy, there was no increase in antibody binding or serum cytotoxicity to GTKO PBMC, indicating that this regimen had prevented sensitization, as reported previously (3, 4, 17, 18, 25).
Histopathology of GTKO Pig Hearts and Kidneys
The histopathological findings at the time of graft excision or recipient euthanasia are summarized in Table 1. Of importance, as early as 1h after reperfusion, most grafts showed features of a humoral response (IgM, IgG, and/or complement deposition on the vascular endothelium). Irrespective of the period of graft survival, features of a humoral response were present in 10 out of 12 at the time of graft excision (Figures 1 and 2). Furthermore, all excised grafts showed features of TM (widespread platelet and fibrin deposition) in the interstitial capillaries and large vessels (Figure 2), except the heart graft electively excised after 2.5h.
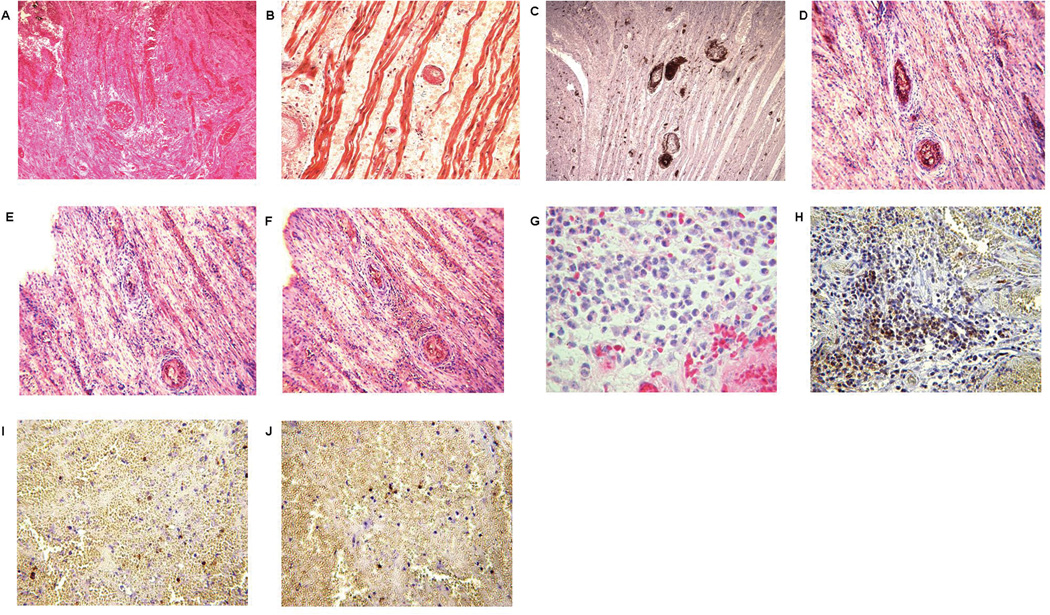
Figure 1.
Histopathologic features of failed heart grafts. Graft failure occurred after 24 hours in Exp 2, showing (A) hemorrhage, thrombosis and infarction (H&E, x400), (B) fibrin deposition (MSB, x400), and (C) platelet accumulation (IHC, x400). IgM (D), IgG (E), and complement C3 (F) deposition (all indicated by brown staining - arrows) were present in the heart of Exp 3 that underwent failure at 7 days from AHXR (x400). Graft failure occurred on day 6 in Exp 6, showing significant neutrophil (H&E, x200) (G) and macrophage (H) infiltration, with relatively less T (I) and B (J) cell infiltration (all IHC, x200, stained brown).
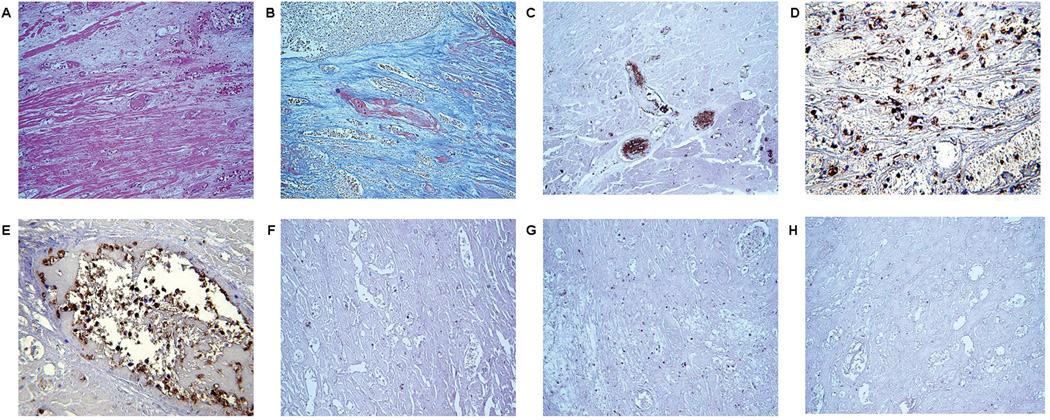
Figure 2.
Histopathologic features of the heart graft that failed after 8 weeks (Exp 12), showing (A) thrombosis and infarction (H&E, x200), (B) fibrin deposition (MSB x400), and (C) massive platelet accumulation (IHC, x400). A neutrophil infiltrate can be seen in the interstitium (IHC, x400) (D); these cells are also present in the vessels of the graft (E), but there are relatively few infiltrating macrophages (IHC,200) (F), and very few T (G) and B (H) lymphocytes (stained brown) (magnification x200).
With no immunosuppression, the main features in the failed grafts were of the humoral response and TM together with cellular infiltration in the form of neutrophils and macrophages only (Figure 1A–C). With partial immunosuppression, the grafts showed features of AHXR and TM (Figure 1D–J), but some grafts showed no or minimal antibody and complement deposition. T and B cell infiltrates were not a constant feature in these grafts. With the full immunosuppressive regimen, myocardial ischemia and TM were the prominent features (Figure 2), with features of a humoral response at the time of excision; T and B cell infiltrates were minimal.
Primate Tissue Factor Expression in Failed Xenografts
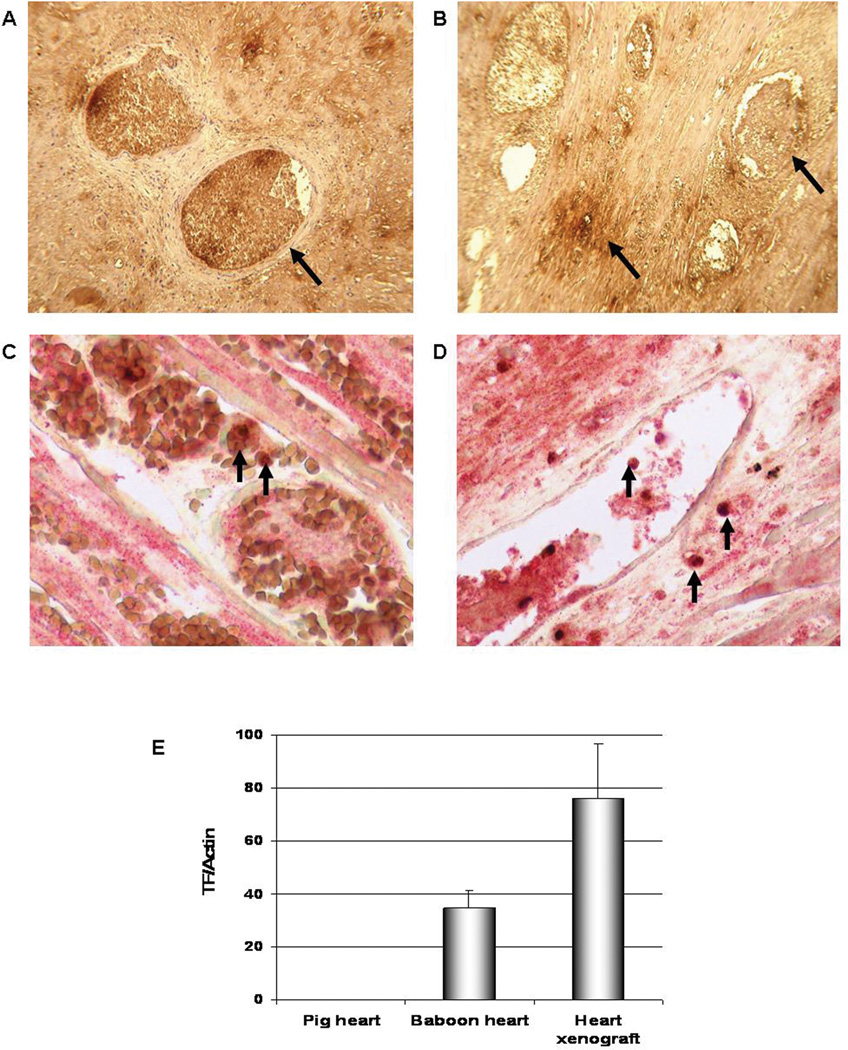
Five of the heart grafts were examined for primate TF expression. These grafts showed strong expression of TF in the thrombosed vessels and less expression in the interstitium (Figure 3A, B). To further elucidate possible sources of the primate TF in the failed pig hearts, double-staining for macrophages and primate TF was performed; this showed co-localization of TF with CD68+ cells (macrophages) in both the thrombosed vessels and in the interstitium of the graft (Figure 3C, D), suggesting a role for these cells in the initiation of TM through the expression of TF. Primate TF was detected in high levels compared to lower levels in a normal baboon heart and none in a normal pig heart (Figure 4E).
Figure 3.
Staining for primate TF in heart grafts that rejected at day 12 (A; Exp 5) and at 8 weeks (B; Exp 12), showing strong staining in the thrombosed vessels and less staining in the interstitium (arrows) (x600). Co-localization of primate TF (red stain) and macrophages (stained for CD68, brown) in heart grafts excised on day 12 (C, Exp 5) and at 8 weeks (D, Exp 12) is indicated by arrows (x600). Biopsies from 3 other rejected xenografts showed similar results. Primate TF mRNA levels in a rejected porcine heart xenograft by qRT-PCR (E); the heart failed from TM at 8 weeks (Exp 12) and showed high levels of primate TF. Tissue from a normal baboon heart was used as a positive control, while a porcine heart was used as a negative control to ensure the specificity of the primate TF primer. Real-time PCR data were plotted as the ΔRn fluorescence signal versus the cycle number. The expression of each gene was normalized to actin mRNA content and calculated relative to control using the comparative CT method.
DISCUSSION
Hyperacute rejection follows the Tx of wild-type pig organs in nonhuman primates (14, 26), and has been reported following GTKO kidney (6) and heart (27) Tx in baboons, although this complication was not seen in the initial series of studies (3, 4, 16).
In the present series, the hearts transplanted in the absence of any immunosuppressive therapy (Exp 1 and 2) showed some features of vascular injury as early as 2.5h after Tx. The heart graft excised at 24h showed typical features of antibody-mediated rejection. There was a neutrophil infiltrate in both grafts, and macrophages were present in the thrombi in the vessels. In both cases, as it was too early for an elicited antibody response to have developed, the histopathologic features must have been related to the innate immune system. This experience also indicated that CC can occur as early as 24h after Tx.
When only partial immunosuppressive therapy was administered (Exp 3–7), or when therapy was unable to be administered adequately (Exp 8 and 9), graft failure was associated with AHXR which occurred either before or after an elicited anti-nonGal antibody response, with a cellular infiltrate consisting mainly of neutrophils and macrophages, T and B lymphocytes being less obvious. Features of TM were widespread, and CC occurred in 5 of the 7 baboons. The predominant histopathologic features were therefore associated with an innate immune response. The results in these experiments correlate well with those of Chen et al (6), and demonstrate that similar histopathology is seen in hearts and kidneys.
With full immunosuppression, there was deposition of IgM, IgG, and complement, and neutrophil and macrophage infiltration, but there was minimal T and B cell infiltration, and no evidence of a T cell-dependent elicited antibody response (Exp 11 and 12). These findings confirm that an immunosuppressive regimen based on costimulation blockade can prevent sensitization to pig antigens, correlating with previous reports (3, 4). The presence of IgM and IgG antibody deposition in grafts that survived for 5 and 8 weeks might be due to minimal but continuous production of natural antibodies throughout this period. Although careful inspection of multiple sections could identify T cells in the graft, there were few, as reported previously (3–5, 25, 28). The absence of an elicited antibody response and the absence of a response in MLR (3, 4, 25) are indicators that AHXR and TM may not be associated solely with T cell activation.
Innate immunity is a prerequisite for effective adaptive immunity (reviewed in (29)), but, in contrast, there is evidence that T cells might be a prerequisite for the innate immune cellular response. It may be relevant that the heart graft that functioned for 8 weeks showed minimal T and B cell infiltrates and few macrophages.
The potential factors involved in the development of TM have been discussed elsewhere (30, 31). Endothelial cell activation with the expression of porcine TF (32), inducing a change from an anticoagulant to a procoagulant state, which is almost certainly associated with dysregulation of coagulation (33), seems a likely initial mechanism, but it has been suggested that TF expression by recipient cells might play a role in the initiation of coagulation in xenografts (34). A recent report has shown that contact with porcine aortic endothelial cells can induce TF expression on both human platelets and monocytes (35). Our own data would strongly support the conclusion that activation of recipient cells is a significant factor, though the precise role of primate TF in the development of TM in porcine xenografts has not been clarified.
Although neutrophils have been shown to play a role in the rejection of xenografts (36–39), their role in the pig-to-baboon model has not been addressed. Neutrophils can discriminate between allogeneic and xenogeneic cells, resulting in endothelial cell activation in the absence of natural antibody and complement (36). When antibody and complement are present, adhesion of neutrophils to porcine aortic endothelial cells is increased (39). These effects are not related to the expression of Gal on the porcine cells (37). Macrophage infiltration was a prominent finding following the Tx of organs from wild-type pigs into baboons reported by Wu et al (40), and has also been documented in islet xenografts (41).
Features of CC were seen in 7 out of 12 baboons irrespective of the time that had elapsed since the graft was inserted. This observation may be associated with the presence of platelets and macrophages (and possibly neutrophils) in the graft as these cells can, under certain circumstances, express TF (42). There is evidence to suggest that these recipient cells circulating within the vasculature contribute to TF expression, perhaps more than do vascular endothelial cells (43, 44). It remains possible that coagulation pathway activation can occur without a requirement for significant antibody deposition or complement activation, and is a primary trigger for early graft injury, as suggested previously (45). In several studies in both human and animal models of sepsis (which is a severe form of endothelial cell activation), TF expression on these cells and on platelets coincided with the development of systemic inflammatory and hypercoagulable states (42, 46). Coagulation and inflammation are vital elements of the innate immune system, and there is considerable interaction between the coagulation and inflammation pathways (47, 48). In the present study, we were not able to identify any specific coagulation markers that indicated that CC would or would not develop.
In conclusion, our data suggest:- (i) irrespective of the presence or absence of the adaptive immune response, early or late xenograft rejection is associated with activation of the innate immune system; (ii) porcine endothelial cell activation by immunoglobulin/complement and TF expression by innate immune cells may both contribute to the development of TM. The roles of monocytes and platelets in xenograft rejection, and particularly in the development of TM and CC, have perhaps been hitherto underestimated. Therapeutic strategies targeted at innate immunity should reduce both innate and adaptive immunity, facilitating xenograft survival, and might result in a more clinically successful immunosuppressive regimen.
TABLE 2.
Graft survival and histopathology at time of graft failure
| Exp | Antibody deposition | Complement deposition | Cellular Infiltrate | Thrombotic Microangiopathy |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | C3 | C4d | C5b-9 | T cells | B cells | MΦ | Neut | Fibrin deposition |
Platelets | |
| 1 | ( + ) | ( + ) | ( + ) | ( −− ) | ( −− ) | ( −− ) | ( −− ) | ( + ) | ( + ) | ( −− ) | ( −− ) |
| 2 | ( + + + ) * | ( + + + ) * | ( ++ ) | ( + ) | ND | ( −− ) | ( −− ) | ( −− )* | ( + + + ) | ( + + + ) | ( + + ) |
| 3 | ( + + + ) * | ( + + + ) * | ( + ) | ( + ) | ( + ) | ( −− ) | ( −− ) | ( −− )* | ( + + + ) | ( + + + ) | ( + + ) |
| 4 | ( + + + ) | ( −− ) | ( + + + ) | ND | ND | ( + ) | ( + ) | ( + + ) | ( + + + ) | ( + + + ) | ( + + + ) |
| 5 | ( + + + ) * | ( + + + ) * | ( + ) | ( + ) | ( + ) | ( −− ) | ( −− ) | ( + ) | ( + + + ) | ( + + + ) | ( + + ) |
| 6 | ( + ) | ( −− ) | ( −− ) | ND | ND | ( + ) | ( + ) | ( +++ ) | ( + + + ) | ( + + + ) | ( + + ) |
| 7 | ( −− ) | ( −− ) | ( −− ) | ND | ND | ( + ) | ( + ) | ( + ) | ( ++ ) | ( + + + ) | ( + + ) |
| 8 | ( + + ) | ( + ) | ( + + + ) | ( + + ) | ND | ( −− ) | ( −− ) | ( + ) | ( + + + ) | ( + + + ) | ( + + ) |
| 9 | ( + + + ) * | ( + + + ) * | (++ ) | ( + ) | ND | ( −− ) | ( −− ) | ( ++ ) | ( + + + ) | ( + + + ) | ( + + + ) |
| 10 | ( + + ) | ( + + ) | ( + ) | ( −− ) | ND | ( −− ) | ( −− ) | ( −− ) | ( + ) | ( + + + ) | ( + + + ) |
| 11 | ( + + ) | ( + + ) | ( + + ) | ( −− ) | ( −− ) | ( + ) | ( + ) | ( + + + ) | ( + ) | ( + + ) | ( + + ) |
| 12 | ( + + + ) | ( + ) | ( + + + ) | ( + + ) | ND | ( + ) | ( + ) | ( + ) | ( + + + ) | ( + + + ) | ( + + + ) |
(−−) none; (+) mild; (++) moderate; (+++) severe.
h = hours; d = days; w = weeks.
MΦ = macrophages; Neut = neutrophils
IgM deposition: Intensive in large vessels and less severe in the interstitial capillaries.
IgG deposition: Intensive in interstitial capillaries and less severe in the large vessels.
monocytes/macrophages seen in the vessels only.
ACKNOWLEDGEMENTS
We thank Walter Schuler, PhD, and his colleagues at the Novartis Institutes for Biomedical Research (Basel, Switzerland) for making ABI793 available to us, Roche Pharmaceuticals (Nutley, NJ, USA) for generously providing mycophenolate mofetil, and Genzyme (Cambridge, MA) for the gift of thymoglobulin. Y J Lin, MD, was a recipient of a grant from the National Cheng Kung University Hospital, Tainan City, Taiwan, and H-C Tai, M.D., was a recipient of a grant from the National Taiwan University Hospital, Taipei, Taiwan. We thank Weihua Liu from Dr. Garcia’ laboratory for expert help and technical support.
FUNDING SOURCES
Work in our laboratories is supported in part by NIH program projects U01-AI68642 (DKCC) and U01-AI66335 (RNP), by a VA Merit Award (RNP), and by a Sponsored Research Agreement between the University of Pittsburgh and Revivicor, Inc (DKCC). Most of the baboons used in the study were from the Oklahoma University Health Sciences Center, Division of Animal Resources, which is supported by NIH P40 sponsored grant RR012317-09.
ABBREVIATIONS
- AHXR
acute humoral xenograft rejection
- CC
consumptive coagulopathy
- Gal
Galα1,3Gal
- GTKO
α1,3-galactosyltransferase gene-knockout
- mAb
monoclonal antibody
- TF
tissue factor
- TM
thrombotic microangiopathy
- Tx
transplantation
REFERENCES
- 1.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolber-Simonds D, Lai L, Watt SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101(19):7335. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 4.Tseng YL, Kuwaki K, Dor FJ, et al. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80(10):1493. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 5.Houser SL, Kuwaki K, Knosalla C, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11(5):416. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11(12):1295. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezzelarab M, Hara H, Busch J, et al. Antibodies directed to pig non-Gal antigens in naive and sensitized baboons. Xenotransplantation. 2006;13(5):400. doi: 10.1111/j.1399-3089.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 8.Hara H, Ezzelarab M, Rood PP, et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation. 2006;13(4):357. doi: 10.1111/j.1399-3089.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 9.Rood PP, Hara H, Busch JL, et al. Incidence and cytotoxicity of antibodies in cynomolgus monkeys directed to nonGal antigens, and their relevance for experimental models. Transpl Int. 2006;19(2):158. doi: 10.1111/j.1432-2277.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 10.Hara H, Long C, Lin YJ, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008 doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 11.Dai Y, Vaught TD, Boone J, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20(3):251. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 12.Cooper DKCYY, Niekrasz M. Heart transplantation in primates. In: Cramer DVPL, Makowka L, editors. Handbook of Animal Models in Transplantation Research. Boca Raton: CRC Press; 1994. p. 173. [Google Scholar]
- 13.Kozlowski T, Shimizu A, Lambrigts D, et al. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation. 1999;67(1):18. doi: 10.1097/00007890-199901150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Buhler L, Yamada K, Kitamura H, et al. Pig kidney transplantation in baboons: anti-Gal(alpha)1–3Gal IgM alone is associated with acute humoral xenograft rejection and disseminated intravascular coagulation. Transplantation. 2001;72(11):1743. doi: 10.1097/00007890-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 15.Knosalla C, Gollackner B, Buhler L, et al. Correlation of biochemical and hematological changes with graft failure following pig heart and kidney transplantation in baboons. Am J Transplant. 2003;3(12):1510. doi: 10.1046/j.1600-6135.2003.00258.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 17.Buhler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69(11):2296. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 18.Buhler L, Alwayn IP, Basker M, et al. CD40-CD154 pathway blockade requires host macrophages to induce humoral unresponsiveness to pig hematopoietic cells in baboons. Transplantation. 2001;72(11):1759. doi: 10.1097/00007890-200112150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Buhler L, Basker M, Alwayn IP, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70(9):1323. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 20.Ierino FL, Kozlowski T, Siegel JB, et al. Disseminated intravascular coagulation in association with the delayed rejection of pig-to-baboon renal xenografts. Transplantation. 1998;66(11):1439. doi: 10.1097/00007890-199812150-00006. [DOI] [PubMed] [Google Scholar]
- 21.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6(2):114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 22.Knosalla C, Gollackner B, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism revisted. Transplantation. 2002;74(3):416. doi: 10.1097/00007890-200208150-00024. [DOI] [PubMed] [Google Scholar]
- 23.Tai HC, Campanile N, Ezzelarab M, Cooper DK, Phelps C. Measurement of anti-CD154 monoclonal antibody in primate sera by competitive inhibition ELISA. Xenotransplantation. 2006;13(6):566. doi: 10.1111/j.1399-3089.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 24.Ezzelarab M, Cortese-Hassett A, Cooper DK, Yazer MH. Extended coagulation profiles of healthy baboons and of baboons rejecting GT-KO pig heart grafts. Xenotransplantation. 2006;13(6):522. doi: 10.1111/j.1399-3089.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 25.Kuwaki K, Knosalla C, Dor FJ, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4(3):363. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 26.Kozlowski T, Monroy R, Xu Y, et al. Anti-Gal(alpha)1–3Gal antibody response to porcine bone marrow in unmodified baboons and baboons conditioned for tolerance induction. Transplantation. 1998;66(2):176. doi: 10.1097/00007890-199807270-00006. [DOI] [PubMed] [Google Scholar]
- 27.McGregor CG, Tazelaar HD, Rao VP, et al. Gene-knockout (GT-KO) heterotopic cardiac xenotransplantation: are GT-KO pigs essential for successful clinical xenotransplantation? Xenotransplantation. 2007;14(2):181. [Google Scholar]
- 28.McGregor CG, Teotia SS, Byrne GW, et al. Cardiac xenotransplantation: progress toward the clinic. Transplantation. 2004;78(11):1569. doi: 10.1097/01.tp.0000147302.64947.43. [DOI] [PubMed] [Google Scholar]
- 29.Fox A, Harrison LC. Innate immunity and graft rejection. Immunol Rev. 2000;173:141. doi: 10.1034/j.1600-065x.2000.917313.x. [DOI] [PubMed] [Google Scholar]
- 30.Cooper DK, Dorling A, Pierson RN, 3rd, et al. Alpha1,3-galactosyltransferase gene-knockout pigs for xenotransplantation: where do we go from here? Transplantation. 2007;84(1):1. doi: 10.1097/01.tp.0000260427.75804.f2. [DOI] [PubMed] [Google Scholar]
- 31.Tai HC, Ezzelarab M, Hara H, Ayares D, Cooper DK. Progress in xenotransplantation following the introduction of gene-knockout technology. Transpl Int. 2007;20(2):107. doi: 10.1111/j.1432-2277.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 2008;172(6):1471. doi: 10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robson SC, Cooper DK, d'Apice AJ. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 2000;7(3):166. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 34.Nagayasu T, Saadi S, Holzknecht RA, Plummer TB, Platt JL. Expression of tissue factor mRNA in cardiac xenografts: clues to the pathogenesis of acute vascular rejection. Transplantation. 2000;69(4):475. doi: 10.1097/00007890-200002270-00003. [DOI] [PubMed] [Google Scholar]
- 35.Lin CC, Chen D, McVey JH, Cooper DK, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86(5):702. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.al-Mohanna F, Collison K, Parhar R, et al. Activation of naive xenogeneic but not allogeneic endothelial cells by human naive neutrophils: a potential occult barrier to xenotransplantation. Am J Pathol. 1997;151(1):111. [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Mohanna F, Saleh S, Parhar RS, Khabar K, Collison K. Human neutrophil gene expression profiling following xenogeneic encounter with porcine aortic endothelial cells: the occult role of neutrophils in xenograft rejection revealed. J Leukoc Biol. 2005;78(1):51. doi: 10.1189/jlb.0904494. [DOI] [PubMed] [Google Scholar]
- 38.Mejia-Laguna JE, Martinez-Palomo A, Lopez-Soriano F, Garcia-Cornjeo M, Biro CE. Prolonged survival of kidney xenografts in leucopenic rabbits. Immunology. 1971;21(6):879. [PMC free article] [PubMed] [Google Scholar]
- 39.Richards AC, Tucker AW, Carrington CA, White DJ, Wallwork JD. Neutrophils and xenotransplantation. Lancet. 1996;348(9041):1596. doi: 10.1016/s0140-6736(05)66224-1. [DOI] [PubMed] [Google Scholar]
- 40.Wu G, Pfeiffer S, Schroder C, et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation. 2005;12(3):197. doi: 10.1111/j.1399-3089.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 41.Yi S, Hawthorne WJ, Lehnert AM, et al. T cell-activated macrophages are capable of both recognition and rejection of pancreatic islet xenografts. J Immunol. 2003;170(5):2750. doi: 10.4049/jimmunol.170.5.2750. [DOI] [PubMed] [Google Scholar]
- 42.Todoroki H, Nakamura S, Higure A, et al. Neutrophils express tissue factor in a monkey model of sepsis. Surgery. 2000;127(2):209. doi: 10.1067/msy.2000.103027. [DOI] [PubMed] [Google Scholar]
- 43.Drake TA, Cheng J, Chang A, Taylor FB., Jr Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol. 1993;142(5):1458. [PMC free article] [PubMed] [Google Scholar]
- 44.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134(5):1087. [PMC free article] [PubMed] [Google Scholar]
- 45.Wu G, Pfeiffer S, Schroder C, et al. Coagulation cascade activation triggers early failure of pig hearts expressing human complement regulatory genes. Xenotransplantation. 2007;14(1):34. doi: 10.1111/j.1399-3089.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 46.Lupu C, Westmuckett AD, Peer G, et al. Tissue factor-dependent coagulation is preferentially up-regulated within arterial branching areas in a baboon model of Escherichia coli sepsis. Am J Pathol. 2005;167(4):1161. doi: 10.1016/S0002-9440(10)61204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strukova S. Blood coagulation-dependent inflammation. Coagulation-dependent inflammation and inflammation-dependent thrombosis. Front Biosci. 2006;11:59. doi: 10.2741/1780. [DOI] [PubMed] [Google Scholar]
- 48.Suo Z, Citron BA, Festoff BW. Thrombin: a potential proinflammatory mediator in neurotrauma and neurodegenerative disorders. Curr Drug Targets Inflamm Allergy. 2004;3(1):105. doi: 10.2174/1568010043483953. [DOI] [PubMed] [Google Scholar]