Abstract
Background
In women, anxiety symptoms are common and increase during midlife, but little is known about whether these symptoms predict onsets of major depressive disorder (MDD) episodes. We examined whether anxiety symptoms are associated with subsequent episodes of MDD in midlife African-American and Caucasian women, and whether they confer a different risk for first versus recurrent MDD episodes.
Method
A longitudinal analysis was conducted using 12 years of data from the Study of Women’s Health Across the Nation (SWAN) Mental Health Study (MHS). The baseline sample comprised 425 Caucasian (n=278) and African American (n=147) community-dwelling women, aged 46.1±2.5 years. Anxiety symptoms measured annually using a self-report questionnaire were examined in relation to MDD episodes in the subsequent year, assessed with the SCID. Multivariable models were estimated with random effects logistic regression.
Results
Higher anxiety symptoms scores were associated with a significantly higher adjusted odds of developing an episode of MDD at the subsequent annual visit [odds ratio (OR) 1.47, p=0.01], specifically for a recurrent episode (OR 1.49, p=0.03) but non-significant for a first episode (OR 1.32, p=0.27). There were no significant racial effects in the association between anxiety symptoms and subsequent MDD episodes.
Conclusions
Anxiety symptoms often precede MDD and may increase the vulnerability of midlife women to depressive episodes, particularly recurrences. Women with anxiety symptoms should be monitored clinically during the ensuing year for the development of an MDD episode.
Keywords: Anxiety, longitudinal, major depressive disorder, midlife women, race
Introduction
Although not part of the diagnostic criteria for major depressive disorder (MDD; DSM-IV-TR), depressed individuals commonly report anxiety symptoms, which may be a major feature of their illness (Fawcett & Kravitz, 1983). Anxiety and depressive symptoms frequently coexist, and the distinction between these two sets of symptoms is not always clear. For example, the high co-morbidity of MDD and generalized anxiety disorder (GAD) is strongly influenced by four overlapping symptoms in their respective diagnostic criteria (sleep difficulties, concentration difficulties, fatigue, psychomotor agitation/ restlessness). A history of anxiety disorder (AD) increases the risk for MDD at all ages (Breslau et al. 1995; Wittchen et al. 2000) and controlling for prior ADs decreases the gender difference in lifetime depression prevalence (Breslau et al. 1995). However, the role of anxiety symptoms, independent of an AD diagnosis and history of MDD, has been little studied as a specific contributing factor for developing a depressive disorder in women.
Women may be particularly vulnerable to developing depressive disorders during midlife and the menopausal transition (Kessler et al. 1994; Bijl et al. 2002; Cohen et al. 2006; Freeman et al. 2006; Bromberger et al. 2009), during which time significant factors related to the onset of depressive episodes include fluctuating reproductive hormone levels, severe premenstrual symptoms, poor sleep, hot flashes and lack of employment (Accortt et al. 2008). Similar to depression, anxiety symptoms are more prevalent during midlife, at least for a subset of women (Bromberger et al. 2013). In a 10-year prospective study, Bromberger et al. (2013) observed that premenopausal women with low levels of anxiety were significantly more likely to report high levels of anxiety symptoms during peri- or postmenopause, and women with high levels at baseline continued to have high anxiety levels during peri- and postmenopause. In this context, anxiety symptoms may be an important indicator that a woman is vulnerable to subsequently developing a depressive episode during midlife. By determining whether anxiety symptoms are a proximal indicator of risk for a depressive episode, those susceptible to depression can be identified in advance, permitting early intervention and prevention strategies to be implemented more effectively and efficiently.
We examined the hypothesis that anxiety symptoms, independent of ADs and other factors associated with depression, would predict the onset of an episode of MDD during the subsequent follow-up visit 1 year later. We further examined whether anxiety symptoms confer a different risk for first versus recurrent MDD episodes. Because the Study of Women’s Health Across the Nation (SWAN) was designed to examine racial/ethnic differences during the menopausal transition (Sowers et al. 2000), we also examined whether the effect of anxiety symptoms differentially predicted MDD in African-American versus Caucasian women.
Method
Study design and participants
SWAN is a multi-ethnic, community-based, cohort study of the menopausal transition. Initiated in 1996, 3302 women were enrolled at seven SWAN sites: Boston, MA, Chicago, IL, Detroit area, MI, Los Angeles and Oakland, CA, Newark, NJ and Pittsburgh, PA. Study design and recruitment of the SWAN cohort have been described in detail (Sowers et al. 2000). In brief, each site recruited Caucasian women and a minority group sample. Eligible women were aged 42–52 years, premenopausal or early perimenopausal, had an intact uterus and at least one ovary, had had at least one menstrual period in the previous 3 months, had not been using any sex steroid hormone in the previous 3 months and were not pregnant. Following baseline assessments, annual assessments were conducted.
The Mental Health Study (MHS) is a SWAN ancillary study conducted initially at three sites (Pittsburgh, Chicago and New Jersey). Follow-up longitudinal data were collected only from Pittsburgh participants, who constitute our sample. Baseline data collection occurred during 1996–1997, with follow-up to January 2009.
The Pittsburgh SWAN site enrolled 463 women (162 African-American and 301 Caucasian), who were recruited using a random digit dialing sampling frame that was supplemented by a voter’s registration list (Bromberger et al. 2009, 2011). In Pittsburgh, 3540 telephone numbers called were of unknown usability (e.g. busy signals, never home, moved), 2148 contacts yielded unknown cohort eligibility (e.g. incomplete screening interview, refusal to be screened), 12 027 women were ineligible to be screened (e.g. out of age range, no period in past 3 months), and 2604 completed the screening interview. Of the latter, 1050 were eligible and 463 of the eligible women entered the Pittsburgh cohort. Participants and those who were eligible but did not participate did not vary by ethnicity, marital status, parity, quality of life, social support, perceived stress or reports of feeling ‘blue or depressed’ in the prior 2 weeks measured during the screener. The 443 MHS participants (95.7%) and the 20 non-participants (4.3%) did not differ significantly on sociodemographic variables or percentage with Center for Epidemiologic Studies Depression (CES-D) scale (Radloff, 1977) scores ≥16. Of the 443 women, data from 417 were used in the longitudinal analytic models because 18 women were missing all follow-up Structured Clinical Interview for the Diagnosis of DSM-IV Axis I Disorders (SCID) data and eight women were missing covariate data and/or did not have consecutive visits.
Institutional review board approval was obtained, and after complete description of the study to the subjects, written informed consent was obtained.
Procedures and measures
The SCID (Spitzer et al. 1992) was administered to assess lifetime psychiatric disorders at baseline, and current (past month) and recent (past year) diagnoses at each visit. All interviewers had extensive clinical experience and training, and were supervised throughout the study (by J.T.B.) to ensure consistency of SCID administration and diagnostic decision making. Interviewers demonstrated very good to excellent inter-rater reliability for both lifetime (κ= 0.81) and past year (κ =0.76–0.89, at several follow-ups) MDD, and for phobia or panic disorders (κ =0.82).
Questionnaires regarding medical/health status, reproductive and menstrual history, psychological symptoms, psychosocial factors and lifestyle characteristics were administered either orally or as written self-report at baseline and at each annual follow-up visit.
Dependent variable
This was a SCID-diagnosed first onset or recurrent episode of MDD, occurring at any time during follow-up visits 1–11 in the year subsequent to each anxiety symptoms assessment.
Independent variable
The anxiety symptoms score consisted of four items (irritability or grouchiness, feeling tense or nervous, heart pounding or racing, feeling fearful for no reason) derived from a menopausal symptom questionnaire (Neugarten & Kraines, 1965) that assessed symptom frequency in the previous 2 weeks (coded 0 =none to 4= daily) at each visit. Using factor analyses (Johnson & Wichern, 1982), these four items loaded onto a single item (summed score 0–16) with α Cronbach’s α of 0.77 in the full SWAN cohort and 0.72 in Pittsburgh. Scores in the highest quintile (20%) indicated significant anxiety (i.e. scores ≥4) (Bromberger et al. 2013); therefore, a high anxiety symptoms score was defined as a summed score ≥4. At baseline, the anxiety symptoms score showed good discriminant validity with the CES-D (Spearman r = 0.57) and convergent validity with the seven-item Generalized Anxiety Disorder (GAD-7) questionnaire (Spearman r =0.71) (Bromberger et al. 2013), a measure of generalized anxiety and anxiety symptoms (Spitzer et al. 2006; Löwe et al. 2008). Thus, the anxiety symptoms score was a valid measure to demonstrate an independent association with subsequent MDD episodes and allowed use of the CES-D and ADs as covariates.
Covariates
Sociodemographics
Age, self-identified race (African-American or Caucasian), highest level of education attained, difficulty paying for basics (financial strain), and employment status were obtained at baseline. Marital status was measured at each visit.
Psychiatric diagnoses
MDD and AD (panic disorder, agoraphobia, social phobia, specific phobia, obsessive– compulsive disorder, GAD, and AD not otherwise specified) were assessed annually with the SCID. At baseline, lifetime history was defined as the occurrence of an MDD or AD at any time prior to that month. At each visit (time T), ‘cumulative AD’ was defined as occurrence of an episode more than 1 month prior to that visit (thus not overlapping with the assessment time frame of the anxiety symptoms score). ‘Cumulative MDD’ was defined as occurrence of an episode more than 1 year prior to that visit [thus not overlapping with the outcome, subsequent MDD episode (time T+1), because the subsequent episode onset may have begun at time T]. ‘Recent MDD’ was defined as occurrence any time in the prior year, so that anxiety symptoms could be evaluated in conjunction with a known predictor of recurrent episodes. ‘Current’ MDD or AD refers to an episode that was present at the time of the interview or within the previous month.
Depressive symptoms, trait anxiety, stressors and social support
Depressive symptoms in the previous week were assessed with the well-validated CES-D (range 0–60) screening scale (Radloff, 1977). A modified version of the 10-item Trait Anxiety scale (Spielberger, 1979; Spielberger & Reheiser, 2009) was administered at baseline, in which the four-point response options scale was coded from ‘not like’ to ‘a lot like’ rather than the original frequency rating (‘almost never’ to ‘almost always’). Stressful life events (none, one, or two or more ‘very upsetting’ events since their previous visit) were assessed with a checklist of 18 life events (Bromberger et al. 2007). Social support was measured as a summed score (ranging from 0 to 16) of four items that indicated how often emotional and instrumental supports were available (Sherbourne & Stewart, 1991).
Menopausal status
This was determined using vaginal bleeding criteria, and change in status and exogenous sex steroid hormone use were assessed annually, consistent with previous SWAN analyses (WHO, 1996).
Vasomotor symptoms (VMS)
VMS (hot flashes/flushes, cold sweats and/or night sweats) were examined as a two-level composite variable (<6 days v. ≥6 days in the past 2 weeks) (Gold et al. 2006).
Data analysis
Standard descriptive statistics were used to characterize the study sample at baseline, and women with onset of at least one MDD episode (first onset or recurrent) during follow-up and those without were compared by χ2, Wilcoxon and Student’s t tests. Women with current MDD at baseline were included in all analyses. Anxiety symptoms and CES-D scores required natural logarithm transformation to reduce skewness. Paired t tests were used to compare means of unadjusted log anxiety symptoms scores at each follow-up visit by MDD at the subsequent visit.
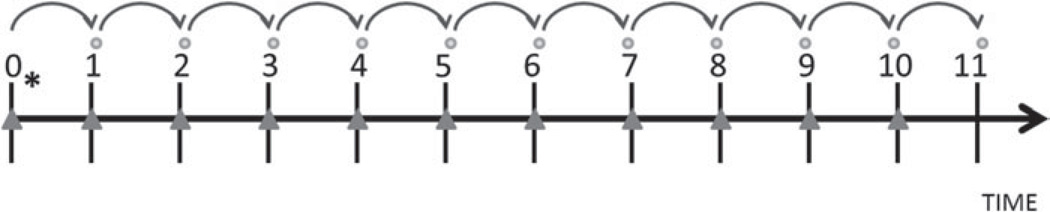
For all longitudinal modeling, lagged analyses were used such that anxiety symptoms at the current visit (time T) were used to predict the onset of an MDD episode at the subsequent annual visit (time T+1) (Fig. 1). Similarly, all covariates were time-varying from the current visit (time T), with the exception of variables measured only at baseline (age, race, education, employment, financial strain, and trait anxiety). Hence, longitudinal modeling used a maximum of 11 observations for each subject. Inclusion of data from each visit in models required complete data during consecutive visits (i.e. independent and covariate data from time T and dependent variable from time T+1). If a visit was missed, data from the preceding visit were not used to predict an episode of MDD at the next available visit.
Fig. 1.
Data analysis sequence. ● indicates the outcome, major depressive disorder (MDD), occurring at the subsequent visit. * indicates baseline (visit 0) age and race. ▲ indicates time-varying variables measured at visits 0–10, including cumulative anxiety disorders (ADs) and cumulative MDD.
Using random effects logistic regression controlling for baseline age, time since baseline (i.e. aging), and race, bivariate associations between covariates and MDD episode at the subsequent visit were examined in separate models. Characteristics associated with an MDD episode at the p <0.15 level were further evaluated by including the log anxiety symptoms score variable in each covariate model. Variables whose association with MDD episodes persisted at the p <0.15 level (race, time since baseline, financial strain, trait anxiety, social support, upsetting life events, CES-D, VMS, and cumulative/recent/current MDD and cumulative AD) were considered for inclusion as covariates in multivariable longitudinal models. Using a process of backward elimination, covariates with p< 0.05 were retained for the final fully adjusted model (which also adjusted for baseline age, race and time) to ensure optimal covariates were retained, and to reduce confounding and minimize redundancy. By allowing covariates with stronger statistical associations to be retained in the model, this method assisted in reducing confounding while maintaining adjustment for known predictors of MDD. These multivariable models were confirmed using forward and stepwise selection methods. Thus we could examine whether anxiety symptoms predicted subsequent MDD, independent of ADs, prior MDD, and other factors associated with depression.
Stratified analyses were conducted to examine whether anxiety symptoms predicted first-onset or recurrent MDD. Covariates were determined in the same manner as described previously. It was necessary to analyze these outcomes separately because covariates differed for the models. For analyses examining first-onset MDD (n=269), subjects with a lifetime history of MDD (n=147) or current MDD at baseline (n=4) and five subjects missing covariate/consecutive visit data were excluded. Analyses for recurrent MDD included 417 subjects. For the 274 subjects with no lifetime or current MDD at baseline, the first onset of MDD occurring during follow-up was censored and only subsequent episodes were counted as a recurrence. Eight women missing covariate data or not having consecutive visits were excluded from recurrent MDD analyses.
To examine race differences in the effect of anxiety symptoms on predicting an MDD episode, race interaction terms with anxiety symptoms scores, cumulative MDD, and a three-way interaction with anxiety symptoms score and cumulative MDD were used in each model.
Analyses were run using SAS version 9.3 (SAS Institute Inc., USA) and Stata version 9 (Stata Corporation, USA) for Windows. Two-tailed p values <0.05 were considered statistically significant.
Results
Sample characteristics
The baseline sample (n=425) comprised 278 Caucasian and 147 African-American women, mean age 46.1 (s.d. =2.5) years. Two-thirds (66%) were married or living as married. Education obtained included 24% with a high-school degree or less, 37% with some vocational/college schooling, and 39% with a college degree. Most women were employed (84%) and only 7% reported significant financial strain (‘very hard’ to pay for basics). At baseline, 52% were premenopausal and 48% were early perimenopausal, none used exogenous hormones, and 11% reported frequent VMS. Women who did and did not develop an MDD episode during follow-up differed significantly only on frequent VMS, which were more prevalent in the MDD group (15% v. 9%; χ2 =3.87, df =1, p<0.05). By visit 11, 80% of the women had transitioned to postmenopausal status and 11% had surgically induced menopause.
During the follow-up, 156 (36.7%) women developed at least one MDD episode, with a total of 428 distinct MDD episodes (71 first-onset +357 recurrent), and 269 (63.3%) did not develop an MDD episode. At baseline, compared with the group that did not have a subsequent MDD episode, the group with MDD episode(s) during follow-up had more prevalent (35% v. 18%) high anxiety symptoms scores, lifetime (32% v. 19%) and current (18% v. 8%) AD, and lifetime (53% v. 24%) and current (6% v. 1%) MDD (Table 1). Of those with high anxiety symptoms scores (n=103), 31% did and 69% did not have lifetime AD (p= 0.04), and 18% did and 82% did not have current AD (p=0.02). The median CES-D and trait anxiety scores were higher and upsetting life events were more prevalent in the MDD group.
Table 1.
Baseline demographic and clinical characteristics for the entire sample (n=425) and by occurrence of an episode of major depressive disorder (MDD) during 11 years of follow-up
| Onset of episode of MDD during follow-up |
||||
|---|---|---|---|---|
| Baseline characteristic | All (n=425)a | Yes (n=156)b | No (n=269)c | p value |
| Age (years), mean (s.d.) | 46.1 (2.5) | 45.8 (2.4) | 46.3 (2.6) | 0.08 |
| Race, n (%) | ||||
| African-American | 147 (35) | 58 (37) | 89 (33) | 0.39 |
| Caucasian | 278 (65) | 98 (63) | 180 (67) | |
| Very upsetting life events (past year), n (%) | ||||
| None | 202 (48) | 51 (33) | 151 (56) | |
| One | 95 (22) | 38 (24) | 57 (21) | <0.001 |
| Two or more | 127 (30) | 67 (43) | 60 (22) | |
| High anxiety symptoms, sum ≥4, n (%) | 103 (24) | 55 (35) | 48 (18) | <0.001 |
| CES-D score (past week), range 0–60, median (IQR) | 9 (3–16) | 12.5 (6–19.5) | 6 (2–13) | <0.001 |
| Anxiety symptoms score (past 2 weeks), range 0–16, median (IQR) | 2 (1–3) | 3 (2–4) | 2 (1–3) | <0.001 |
| Spielberger trait anxiety, range 10–40, median (IQR) | 16 (12–21) | 19.5 (15–25) | 15 (12–19) | <0.001 |
| Social support, range 0–16, median (IQR) | 13 (11–15) | 13 (11–15) | 13 (11–15) | 0.42 |
| SCID lifetimed history of ADe, n (%) | 100 (24) | 50 (32) | 50 (19) | 0.002 |
| SCID lifetimed history of MDD, n (%) | 147 (35) | 83 (53) | 64 (24) | <0.001 |
| SCID currentf ADe, n (%) | 50 (12) | 28 (18) | 22 (8) | 0.003 |
| SCID currentf MDD, n (%) | 13 (3) | 10 (6) | 3 (1) | 0.002 |
CES-D, Center for Epidemiologic Studies Depression scale; IQR, interquartile range; SCID, Structured Clinical Interview for the Diagnosis of DSM-IV Axis I Disorders; AD, anxiety disoder; s.d., standard deviation.
Includes three women who did not have consecutive visits and five women who were missing covariate data. Category numbers for each characteristic vary slightly from group totals due to random missing data. Column percentages may not sum to 100% due to rounding. The percentages are calculated after excluding participants with missing data. Means, percentages and medians were compared by Student t, χ2 and Wilcoxon tests respectively.
Includes 10 women with current MDD at baseline [eight subjects who had a recurrent lifetime history of MDD (prior to baseline) and had additional episode(s) during follow-up; and for two subjects baseline was their first episode of MDD and they had additional episode(s) during follow-up].
Includes three women with current MDD at baseline (for two subjects, baseline was their only episode of MDD; and for one subject baseline was the second MDD episode lifetime with none during follow-up).
Lifetime history at baseline was defined as the occurrence of AD or MDD at any time more than 1 month prior to baseline (i.e. study entry).
Includes panic disorder (5% lifetime, 1% current), agoraphobia (0.2% lifetime, 0 current), social phobia (6% lifetime, 2% current), specific phobia (6% lifetime, 4% current), obsessive–compulsive disorder (0.5% lifetime, 0.2% current), generalized anxiety disorder (1% current), and AD not otherwise specified (7% lifetime, 4% current).
Current at baseline refers to an episode of AD or MDD that was present at the time of the baseline Mental Health Study (MHS) interview or within the previous month.
Anxiety symptoms and MDD
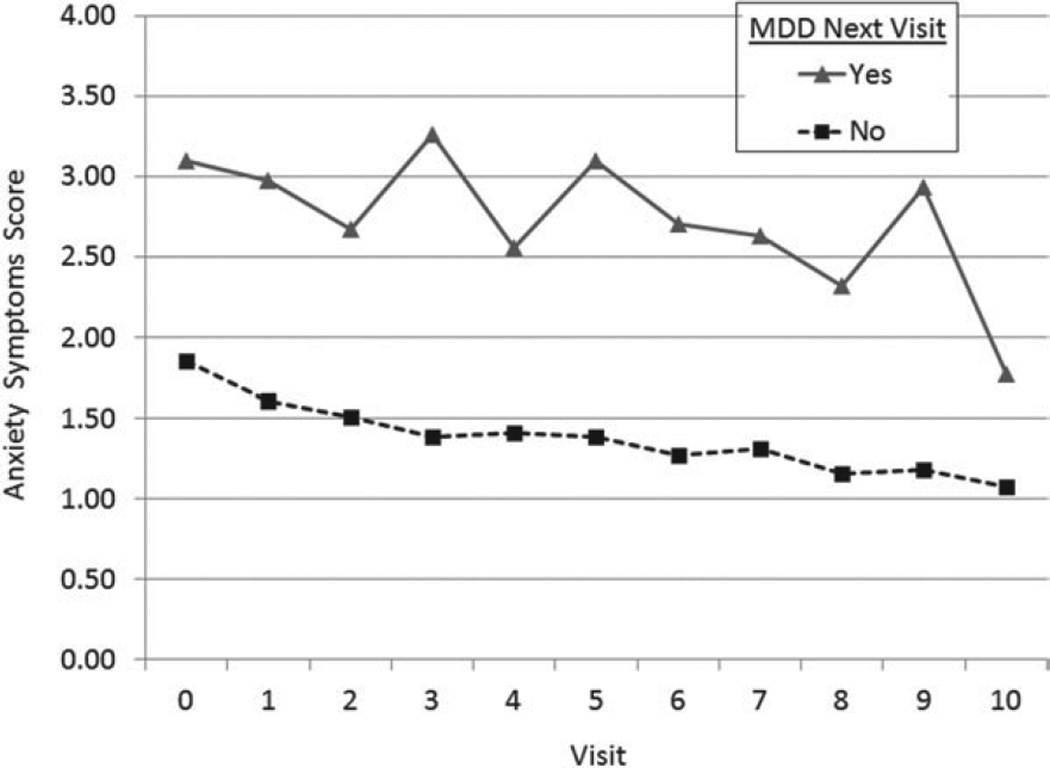
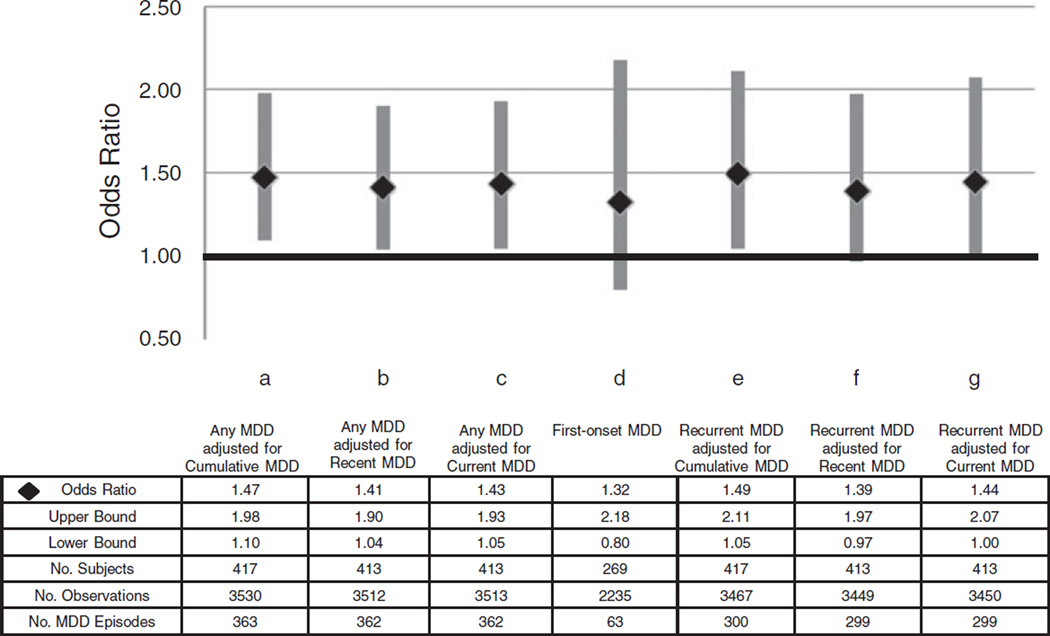
Figure 2 shows that, in unadjusted analyses, women who developed an MDD episode at their subsequent visit had higher anxiety symptoms scores (log values exponentiated) at the preceding annual visit than women who did not (all p<0.01 for log values). In the fully adjusted model shown in Table 2 (n=417), which represents 3530 observations and 363 MDD episodes, higher anxiety symptoms scores were associated with a higher odds of developing an episode of MDD during the subsequent year (OR 1.47, 95% CI 1.10–1.98, p= 0.01; Fig. 3a). Controlling for relevant covariates, each one-unit increase in the log anxiety symptoms score was associated with an increase of 47% in the odds of developing an MDD episode in the subsequent year.
Fig. 2.
Unadjusted means of anxiety symptoms scores (log values exponentiated, y axis) at current visit (time T) by major depressive disorder (MDD) group at the subsequent visit (T+1) (x axis). Paired t tests p<0.001 for log values except visit 4 (p=0.005).
Table 2.
Odds ratios (ORs) for predictors of an episode of major depressive disorder (MDD) at subsequent (T+1) visit, n=417a
| Variables | OR (95% CI) | p value |
|---|---|---|
| Anxiety symptoms scorelog | 1.47 (1.10–1.98) | 0.01 |
| Baseline age | 0.98 (0.90–1.07) | 0.66 |
| Timeb | 1.06 (1.01–1.11) | 0.02 |
| African-American | 1.41 (0.93–2.13) | 0.11 |
| Baseline trait anxiety | 1.07 (1.03–1.11) | < 0.001 |
| Very upsetting life events | < 0.001 | |
| 1 event v. 0 events | 1.28 (0.87–1.88) | 0.21 |
| 2 or more events v. 0 events | 2.14 (1.52–3.00) | < 0.001 |
| Cumulative history of ADc | 1.89 (1.29–2.77) | 0.001 |
| Cumulative history of MDDd | 2.55 (1.73–3.75) | < 0.001 |
| CES-Dlog | 1.63 (1.33–1.99) | < 0.001 |
AD, Anxiety disoder; CI, confidence interval; CES-D, Center for Epidemiologic Studies Depression scale.
Based on 3530 observations and 363 episodes of MDD (63 first-onset +300 recurrent); eight of the original 425 subjects were missing covariate data or consecutive visits. Variables are listed at time T with the exception of baseline age, trait anxiety and race.
Time since baseline (i.e. aging).
Cumulative history is defined as the occurrence of an AD at any time more than 1 month prior to the current visit. Note that histories are time-varying covariates, so the coding for each value can change across visits for subjects who had no lifetime history of AD at baseline. AD includes panic disorder, agoraphobia, social phobia, specific phobia, obsessive–compulsive disorder, generalized anxiety disorder, and AD not otherwise specified.
Cumulative history is defined as the occurrence of an MDD at any time more than 1 year prior to the current visit. Note that histories are time-varying covariates, so the coding for each value can change across visits for subjects who had no lifetime history of MDD at baseline.
Fig. 3.
Odds ratio and 95% confidence interval of anxiety symptomslog score associated with a major depressive disorder (MDD) episode (Any, First or Recurrent) at the subsequent visit in seven separate models each adjusted for multiple covariates (see text).
Higher anxiety symptoms scores remained significantly associated with MDD at the subsequent annual visit if recent or current MDD was substituted for cumulative MDD (Fig. 3b, c), or if cumulative AD was redefined to include current diagnosis (i.e. in the past month) (OR 1.46, 95% CI 1.09–1.96, p =0.01). ORs were slightly attenuated when subjects with life-time (OR 1.36, 95% CI 0.94–1.95, p=0.10), cumulative (OR 1.32, 95% CI 0.86–2.02, p= 0.20) or recent (OR 1.41, 95% CI 1.02–1.94, p=0.04) AD were omitted, when subjects with cumulative (OR 1.56, 95% CI 0.88–2.76, p=0.13) or recent (OR 1.34, 95% CI 0.97– 1.86, p=0.08) MDD were omitted, and when models were stratified by high/low anxiety symptoms scores (data not shown).
In post-hoc analyses, we looked at anxiety symptoms at subsequent visits (T+1) in women with high versus low anxiety symptoms (i.e. summed score ≥4 v.<4 at T) and MDD during the subsequent year. We found that women with high anxiety symptoms who had a subsequent MDD were likely to continue having high anxiety symptoms at time T+ 1 (59%), whereas women with low anxiety symptoms prior to subsequent MDD were less likely to have high anxiety symptoms at time T+1 (28%, p<0.0001).
Anxiety symptoms and first-onset or recurrent MDD
The first-onset model included 2235 observations and 63 MDD episodes. Adjusting for baseline age, time since baseline, race, baseline trait anxiety and CES-D score, anxiety symptoms scores were not associated with a first episode of MDD in the subsequent year (OR 1.32, 95% CI 0.80–2.18, p=0.27; Fig. 3d). The results were similar (OR 1.27, 95% CI 0.77–2.10, p=0.34) if cumulative AD was redefined to include current diagnosis (i.e. in the past month).
The recurrent MDD model included 3467 observations and 300 MDD episodes. In the fully adjusted (i.e. baseline age, time since baseline, race, baseline trait anxiety, upsetting life events, CES-D score, and cumulative MDD and AD) model, higher log anxiety symptoms scores were associated with significantly higher odds for a recurrent episode at the subsequent visit (OR 1.49, 95% CI 1.05–2.11, p =0.03; Fig. 3e). Higher log anxiety symptoms scores remained significantly associated with recurrent MDD at the subsequent annual visit if recent or current MDD was substituted for cumulative MDD (Fig. 3f, g). Similarly, higher anxiety symptoms scores remained significantly associated with recurrent MDD at the subsequent annual visit (OR 1.49, 95% CI 1.05–2.11, p=0.03) if cumulative AD was redefined to include current diagnosis (i.e. in the past month).
Anxiety symptoms and MDD episodes in Caucasian and African-American women
Both Caucasian and African-American women who developed an episode of MDD at their subsequent visit (time T+1) had higher anxiety symptoms scores at the current visit (time T) than women who did not [paired t tests p<0.05 for log values, except among African Americans at visit 7 (p=0.08)]. All two- and three-way race interaction terms were non-significant (p>0.05), so associations between anxiety symptoms and MDD episodes were not examined separately by race.
Discussion
The results supported our primary hypothesis that anxiety symptoms predict the subsequent development of an MDD episode within the year following the onset of anxiety symptoms, independent of clinical and psychosocial risk factors, and other confounders, in a large cohort of community-based midlife women. This association was observed in those with and without prior cumulative (i.e. excluding past month) ADs, and independent of prior MDD. Stratified analyses revealed that anxiety symptoms predicted a recurrent episode of MDD in the subsequent year, but did not predict a first lifetime onset of MDD.
Our data are unique in examining predictive associations of anxiety symptoms while controlling for prior ADs at time T (i.e. not concurrent AD, at time T+1) and prior MDD. Moreover, sensitivity analyses showing that the results were similar when subjects with lifetime, cumulative or recent AD were omitted suggest that anxiety symptoms are probably not a proxy for subthreshold AD. Thus, our findings suggest that midlife women with prominent anxiety symptoms should be monitored closely during the subsequent year for onset of an MDD episode.
We have shown previously that the prevalence of depressive symptoms (Bromberger et al. 2007, 2010) and MDD episodes (Bromberger et al. 2009, 2011) increase across the menopausal transition. We have also found that midlife women with low anxiety symptoms levels premenopausally are susceptible to developing high levels of anxiety during and after the menopausal transition (Bromberger et al. 2013). Although it is known that ADs increase the risk for subsequent MDD, particularly in women (e.g. Alloy et al. 1990; Breslau et al. 1995), and that co-morbid anxiety symptoms and disorders are associated with worse depression outcomes in terms of poorer response to treatment, longer times to recovery, greater depressive morbidity over time, and a larger proportion of time with depressive symptoms during long-term followup (e.g. Coryell et al. 2012; Goldberg & Fawcett, 2012), we are not aware of other published studies that show a close temporal relationship between anxiety symptoms and subsequent MDD episodes. Our results are specific to women during midlife, a period of risk for anxiety symptoms and MDD.
We found no race differences in the association between anxiety symptoms and subsequent MDD episodes, but our Caucasian and African-American sample sizes were relatively small. In National Comorbidity Survey Replication (NCS-R) data, non- Hispanic blacks (men and women combined) had lower lifetime risks for both anxiety and mood disorders (Kessler et al. 2005a) than did non-Hispanic whites. Twelve-month prevalence data showed strong correlations between co-morbid anxiety and MDDs, with women having significantly higher odds than men, and blacks having significantly lower odds than non-Hispanic whites (Kessler et al. 2005b). However, the NCS-R examined only disorders and not symptoms or subsyndromal disorders. Thus, our data remain unique in looking at predictive associations of anxiety symptoms while controlling for ADs and prior MDD, and our associations were not moderated by race.
Limitations of the current study should be noted. Annual assessments did not permit precise descriptions of temporal patterns (onset/offset), episode duration or course of MDD. Our small number of first-onset MDD episodes may have limited our ability to find a statistically significant association with anxiety symptoms. Similarly, with only 35% of Pittsburgh women being African-American, our single-site sample may have been inadequate for assessing racial differences. Data from Wittchen et al. (2000) suggested that certain ADs may be more strongly associated with the development of MDD, but frequencies in our sample were not high enough for us to examine individual ADs. We included trait anxiety but did not ascertain negative affect or neuroticism, which may also be determinants of both current anxiety and episodes of MDD (Clark et al. 1994; Mineka et al. 1998; Weinstock & Whisman, 2006), so we cannot comment on how these latter personality characteristics could contribute to MDD risk. Notably, the presence of frequent VMS was not a significant predictor of subsequent MDD in our analyses. It is plausible that collinearity among anxiety, depression and VMS during the menopausal transition (Freeman et al. 2005, 2006) limited our ability to examine VMS as a predictor of MDD. Finally, SWAN participants are all middle-aged women, and this sample comprised only Caucasians and African-Americans, limiting the ability to generalize these results to men, younger or older women, or other race/ethnic groups.
Nevertheless, there are important strengths to consider. Foremost is that our prospective longitudinal analyses suggest that anxiety symptoms can predict MDD, particularly recurrent episodes, within 1 year of measuring these symptoms, independent of prior ADs and MDD, indicating a close temporal proximity between anxiety symptoms and an MDD episode within the subsequent year. As reviewed by Goldberg & Fawcett (2012), research efforts directed toward determining the associations between depression and anxiety tend to focus on questions regarding episode outcome or prognosis rather than on predicting subsequent episodes. We focused on anxiety symptoms and many women with high anxiety did not have a history of an AD. Of the 103 women with high anxiety symptoms at baseline, 32 (31%) had a lifetime AD and 71 (69%) did not. Moreover, the predominant research and clinical focus has been on co-morbidity and temporal associations between these two disorders rather than on the predictive value of anxiety symptoms per se. Our anxiety symptoms cluster had good construct validity, both convergent with the GAD-7 and discriminant with the CES-D. Finally, in a secondary analysis of NCS-R data,Martin et al. (2013) observed that women endorsed alternative expressions of depression, such as anxiety, significantly more frequently than men did (51.3% v. 41.6%, p ≤ 0.001), so for women anxiety may be a relevant proxy symptom to consider.
To conclude, our results suggest that, independent of an AD, high levels of anxiety symptoms in midlife women warrant consideration as a factor related to subsequent development of an MDD. As an important precursor of MDD, monitoring anxiety symptoms can be a useful clinical tool for predicting the onset of a new episode of MDD by clinicians treating midlife women. Although further studies with larger samples are needed, the ability to predict a high proportion of MDD episodes by the presence of anxiety symptoms would suggest the usefulness of educating health-care professionals to recognize these symptoms. Clinicians can routinely include a rating of anxiety when assessing MDD because of anxiety’s association with prognosis and the potential risk for suicide (Goldberg & Fawcett, 2012). This is consistent with the decision to include an anxiety severity dimension in DSM-5, because anxiety severity may be a stronger predictor of MDD treatment response than any symptoms that make up the diagnostic criteria for mood disorders (Fawcett, 2013).
Acknowledgments
The SWAN has grant support from the National Institutes of Health (NIH), the Department of Health and Human Services (DHHS), through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The MHS is funded by the National Institute of Mental Health (NIMH; R01MH59689). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or NIH.
We thank the study staff at each site and all the women who participated in the SWAN.
Declaration of Interest
Dr Joffe has an investigator-initiated grant from Cephalon/Teva, is a consultant for Noven Pharmaceuticals, and is an unpaid consultant to Sunovion. This was not an industry-supported study (see Acknowledgments for NIH support). There were no off-label or investigational uses of medications or technologies.
References
- Accortt EE, Freeman MP, Allen JJ. Women and major depressive disorder: clinical perspectives on causal pathways. Journal of Women ’s Health. 2008;17:1583–1590. doi: 10.1089/jwh.2007.0592. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Kelly KA, Mineka S, Clements CM. Comorbidity of anxiety and depressive disorders: a helplessness-hopelessness perspective. In: Maser JD, Cloninger RC, editors. Comorbidity of Mood and Anxiety Disorders. Washington, DC: American Psychiatric Press; 1990. pp. 499–543. [Google Scholar]
- Bijl RV, De Graaf R, Ravelli A, Smit F, Vollebergh WA. Gender and age-specific first incidence of DSM-III-R psychiatric disorders in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Social Psychiatry and Psychiatric Epidemiology. 2002;37:372–379. doi: 10.1007/s00127-002-0566-3. [DOI] [PubMed] [Google Scholar]
- Breslau N, Schultz L, Peterson E. Sex differences in depression: a role for preexisting anxiety. Psychiatry Research. 1995;58:1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Kravitz HM, Chang Y, Randolph JF Jr, Avis NE, Gold EB, Matthews KA. Does risk for anxiety increase during the menopausal transition? Study of Women’s Health Across the Nation (SWAN) Menopause. 2013;20:488–495. doi: 10.1097/GME.0b013e3182730599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger JT, Kravitz HM, Chang Y-F, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN) Psychological Medicine. 2011;41:1879–1888. doi: 10.1017/S003329171100016X. Erratum in: Psychological Medicine (2011), 41, 2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger JT, Kravitz HM, Matthews K, Youk A, Brown C, Feng W. Predictors of first lifetime episode of major depression in midlife women. Psychological Medicine. 2009;39:55–64. doi: 10.1017/S0033291708003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, Everson-Rose SA, Gold EB, Sowers MF, Randolph JF Jr. Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN) Journal of Affective Disorders. 2007;103:267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger JT, Schott LL, Kravitz HM, Sowers M, Avis NE, Gold EB, Randolph JF Jr, Matthews KA. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN) Archives of General Psychiatry. 2010;67:598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103:103–116. [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow B. Risk for new onset of depression during the menopausal transition. The Harvard Study of Moods and Cycles. Archives of General Psychiatry. 2006;63:385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- Coryell W, Fiedorowicz JG, Solomon D, Leon AC, Rice JP, Keller MB. Effects of anxiety on the long-term course of depressive disorders. British Journal of Psychiatry. 2012;200:210–215. doi: 10.1192/bjp.bp.110.081992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J. Similarities and differences in psychiatry and medicine. Psychiatric Annals. 2013;43:50–51. [Google Scholar]
- Fawcett JF, Kravitz HM. Anxiety syndromes and their relationship to depressive illness. Journal of Clinical Psychiatry. 1983;44:8–11. [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Gracia CR, Kapoor S, Ferdousi T. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause. 2005;12:258–266. doi: 10.1097/01.gme.0000142440.49698.b7. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Archives of General Psychiatry. 2006;63:375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, Sternfeld B, Matthews K. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation (SWAN) American Journal of Public Health. 2006;96:1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D, Fawcett J. The importance of anxiety in both major depression and bipolar disorder. Depression and Anxiety. 2012;29:471–478. doi: 10.1002/da.21939. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. Englewood Cliffs, NJ: Prentice-Hall; 1982. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005a;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005b;62:617–627. doi: 10.1001/archpsyc.62.6.617. Erratum in: Archives of General Psychiatry (2005), 62, 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H-U, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Study. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Löwe B, Decker O, Müller S, Brähler E, Herzog W, Schellberg D, Herzberg Y. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Medical Care. 2008;4:266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- Martin LA, Neighbors HW, Griffith DM. The experience of symptoms of depression in men vs women: analysis of the National Comorbidity Survey Replication. Journal of the American Medical Association Psychiatry. 2013;70:1100–1106. doi: 10.1001/jamapsychiatry.2013.1985. [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Neugarten BL, Kraines RJ. ‘Menopausal symptoms’ in women of various ages. Psychosomatic Medicine. 1965;27:266–273. doi: 10.1097/00006842-196505000-00009. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS social support survey. Social Science and Medicine. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Sowers MF, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. Lobo R, Marcus R, Kelsey J. Menopause: Biology and Pathobiology. San Diego, CA: Academic Press; 2000. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition; pp. 175–188. [Google Scholar]
- Spielberger CD. Preliminary Manual for the State-Trait Personality Inventory (STPI) Tampa, FL: University of South Tampa; 1979. [Google Scholar]
- Spielberger CD, Reheiser EC. Assessment of emotions: anxiety, anger, depression, and curiosity. Applied Psychology: Health and Well-Being. 2009;1:271–302. [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, William JBW, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Weinstock LM, Whisman MA. Neuroticism as a common feature of the depressive and anxiety disorders: a test of the revised integrative hierarchical model in a national sample. Journal of Abnormal Psychology. 2006;115:68–74. doi: 10.1037/0021-843X.115.1.68. [DOI] [PubMed] [Google Scholar]
- WHO. Research on the menopause in the 1990s. Report of a WHO Scientific Group. World Health Organization Technical Report Series. 1996;866:1–107. [PubMed] [Google Scholar]
- Wittchen H-U, Kessler RC, Pfister H, Lieb M. Why do people with anxiety disorders become depressed? A prospective-longitudinal community study. Acta Psychiatrica Scandinavica. 2000;102(Suppl. 406):14–23. [PubMed] [Google Scholar]