Summary
Diterpene derivatives of the natural product acanthoic acid have potent anti-inflammatory effects in vivo. Través et al. (2014) report that the primary molecular mechanism of action of diterpenesstructurally related to acanthoic acidis the direct activation of PI3-kinase signaling in macrophages, which in turn inhibits NF-κB activation and suppresses pro-inflammatory gene expression.
Throughout history,humans have exploited herbal and plant remedies such as willow bark and ginseng for their therapeutic effects. The advent of organic extraction techniquesenabled the isolation of the small moleculesin these complex mixtures responsible for their effects, perhaps the best example being the identification of salicylic acid.An advantage of using natural products from traditionalremedies is that they often pose decreased likelihood of overt toxicity relative to novel chemical compounds. A greater advantage is our ability to use synthetic methods to chemically modifynaturally-derivedbioactive moleculesto increase their stability, potency and specificity, as the acetylation of salicylic acid to create aspirin showed. The disadvantages of bioactive natural products include that their often complex chemical structure poses significant synthetic chemistry challenges that hinder their mass production, and that their therapeutic effects are usually the result of simultaneous modulation of multiple biological pathways, which complicates elucidation of their mechanism of action.
Diterpenes aregeranylgeranyl pyrophosphate-based small molecules isolated from plant extracts.Examples of diterpenes include acanthoic acid, phytol, retinol, forskolin and cafestol.Several members of this large class of compounds have been shown to possess anti-inflammatory, analgesic, bactericidal, and anti-hypertensive properties (Zwenger and Basu, 2008). Acanthoic acid is a diterpene that was isolated in 1988 from the bark of the root of the Korean plant Acanthopanax koreanumtraditionally used to treat rheumatism (Kim, et al., 1988).Years later, studies showed that the anti-inflammatory properties of acanthoic acid and its derivativescould be ascribed to suppression of MAPK and NF-κB signaling, two major pro-inflammatory pathways in cells, as well as to direct activation ofliver X receptor (LXR) signaling(Chao, et al., 2005; Kim, et al., 2004; Traves, et al., 2007).Synthetic agonists of LXRs, ligand-regulated transcription factors of the nuclear receptor superfamily, had been shown to dampen the action of NF- κB in the nucleus, thus inhibiting pro-inflammatory gene expression(Joseph, et al., 2003).
In this issue, Través et al. strive to further discern the effects on macrophage activation and systemic inflammation, and the molecular mechanism of action, of diterpenes structurally related to acanthoic acid. Macrophagessense foreign invaders using Toll-like receptors (TLRs) embedded in their membrane. TLR-initiated signaling is a key feature of the innate immune response. It activates a program of pro-inflammatory gene expression in macrophages that enables them to counter extraneous intruders. Activated macrophages secrete cytokines that attract neutrophils and other immune system cells to the site of infection.Macrophages can also engulf and digest foreign bodies and dead or dying cells as part of the host defense mechanism. Activated macrophages also serve as important antigen-presenting cells that promote T-cell activation. However, because aberrant macrophage activation can also exacerbatediseases with an inflammatory component and autoimmune disorders, it is desirable to identify new approaches to curbdysregulated macrophage pro-inflammatory signaling.
Través et al. tested the effect of five acanthoic acid diterpene analogueson macrophage activation in vitro and found that several were able to significantly suppress the response to bacterial lipopolysaccharide (LPS) and other TLR agonists. Efficacy was highly dependent on the functional group present on the C-4 position of these acanthoic acid derivatives. Gene expression analysis confirmed the antagonistic effect of several diterpenes on NF-κB, MAPK, and Jak-STAT signaling, which resulted in reduced pro-inflammatory cytokine secretion. Changes in gene expression induced by diterpene treatment overlapped considerably with the transcriptional transrepression profile of ligands that activate LXRs, PPARs, and glucocorticoid receptors, nuclear receptors that can be activated to suppress inflammation(Ogawa, et al., 2005). It was not surprising that bioactive diterpenes shared gene expression changes with LXR ligands, since Travéset al. had previously shown that these diterpenes could act as direct LXR agonists to suppress LXR-dependent anti-inflammatory pathways (Traves, et al., 2007). However, there were also distinct gene expression changes in diterpene-treated cells that seemed independent of LXR activation. Using LXRαβ null macrophages, Través et al. showed that the diterpene analogues retained the bulk of their anti-inflammatory properties in the absence of LXR. Notably, these diterpenes were protective in vivo in three different models of acute inflammation (TPA-induced ear edema, circulating TNF-α levels following LPS injection, and protection from LPS and D-GalN induced lethality). These effects were largely retained in LXRαβ null mice.
To discern the LXR-independent mechanism of action of anti-inflammatory diterpenes, these investigators focused on their effects on NF-κB signaling. The bioactive diterpenes decreased phosphorylation and thus activation of the cytoplasmic IKK complex that controls NF-κB translocation to the nucleus and pro-inflammatory gene expression, something that synthetic LXR ligands are unable to do. But they did not do so directly. Instead, Través et al. noticed that the diterpenes promoted rapid phosphorylation of Akt in vivo while decreasing phosphorylation of IKK, and that this was accompanied by an increase in phosphorylated PIP2. This last finding turned their attention to PI3K signaling. Significantly, the effect of diterpenes on inflammatory signaling was greatly dampened in the presence of the general PI3K inhibitor LY294002, revealing the importance of activated PI3K-Akt signaling for the anti-inflammatory effects of bioactive diterpenes. Treatment of LPS-stimulated LXRα/β null macrophages with LY294002 also attenuated the diterpene-induced decrease in expression of enzymes (nitric oxide synthase-2, cyclooxygenase-2) and cytokines (CXCL1, CXCL10) involved in inflammatory signaling, indicating that the LXR-independent anti-inflammatory activity of diterpenes was indeed mediated by stimulation of PI3K signaling. In a series of elegant experiments using isoform-specific pharmacological inhibitors as well as siRNA knockdowns, the authors then pinpointed the p110δ and p110γ isoforms of the PI3K catalytic subunit as the two main effectors of diterpene action on PI3K-Akt signaling. Intriguingly, the diterpenes appeared to act as direct activators of these PI3K isoforms, for the kinase activity of in vitro translated p110δ, but not p110α, was enhanced upon diterpene treatment. Although the role of the PI3K-Akt pathway in modulating macrophage inflammatory signaling was previously established (Weichhart and Saemann, 2008), the discovery of anti-inflammatory acanthoic acid-based diterpenes as PI3K direct activators is significant. These molecules represent the first non-peptide-derived, direct activators of PI3K.
In summary, this novel and valuable study has established the anti-inflammatory properties of several acanthoic acid-related diterpenes and shown that direct activation of the PI3K-Akt signaling cascade is theirmainmechanism of action, not activation of LXRs. The molecular details of the interaction between bioactive diterpenes and p110δ/γ remain to be elucidated, as is whether direct PI3K activation is also their mode of action in vivo, although this seems likely. It is worth noting that the effect of diterpenes on Akt phosphorylation, and presumably their activation of PI3K signaling, occurs even in the absence of strong pro-inflammatory stimuli. Elaboration of this finding may suggest applications for these diterpenes in chronic low-grade inflammatory states (e.g., atherosclerosis, diabetes) quite distinct from the acute inflammation settings that were used to test diterpene action in vivo in this study. Given the multiple roles of PI3K and Akt signalingin cell growth, survival, and cancer, the extent to which these acanthoic acid-based diterpenes maybe useful and safe in chronic conditions remains to be established and may ultimately depend on the predominant PI3K catalytic subunit present in the cells in question.
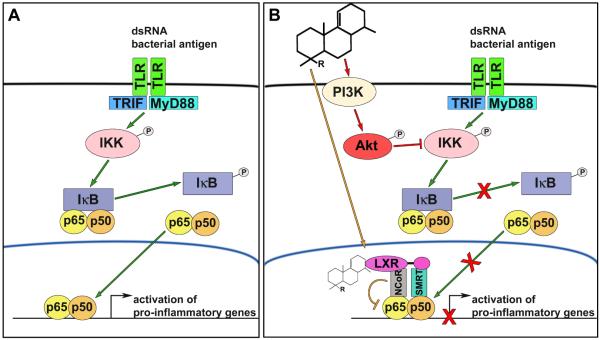
Figure 1. Anti-inflammatory actions of acanthoic acid derivatives on macrophage NF-κB signaling.
(A) Foreign antigens such as dsRNA and lipopolysaccharide bind to Toll-like receptors (TLR) on the membrane of macrophages, leading to TLR subtype-dependent recruitment of myeloid differentiation primary response 88 (MyD88) and/or TIR-domain-containing adapter-inducing interferon-β (TRIF)-dependent pathways. Downstream activation of the IκB kinase complex (IKK) promotes inhibitor of κB (IκB) phosphorylation and dissociation from the p65-p60 nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB ) complex. NF-κB subsequently translocates to the nucleus where it binds to DNA response elements and promotes pro-inflammatory gene expression. (B) Acanthoic acid derivatives had shown to have anti-inflammatory activity by directly activating liver X receptors (LXR) that transrepress NF-κB-dependent gene transcription by complexing with nuclear receptor corepressor 1 (NCoR) and silencing mediator for retinoid or thyroid hormone-receptors (SMRT). In this study, Travéset al. demonstrate that acanthoic acid-based diterpenes primarily inhibit NF-κB signaling pathway through activation of the phosphoinositide 3-kinase (PI3K)-Akt pathway.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chao TH, Lam T, Vong BG, Traves PG, Hortelano S, Chowdhury C, Bahjat FR, Lloyd GK, Moldawer LL, Bosca L, et al. A new family of synthetic diterpenes that regulates cytokine synthesis by inhibiting IkappaBalpha phosphorylation. Chem. Biochem. 2005;6:133–144. doi: 10.1002/cbic.200400089. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- Kim JA, Kim DK, Jin T, Kang OH, Choi YA, Choi SC, Kim TH, Nah YH, Choi SJ, Kim YH, et al. Acanthoic acid inhibits IL-8 production via MAPKs and NF-kappaB in a TNF-alpha-stimulated human intestinal epithelial cell line. Clin. Chim. Acta. 2004;342:193–202. doi: 10.1016/j.cccn.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Kim YH, Chung BS, Sankawa U. Pimaradiene Diterpenes from Acanthopanax koreanum. J. Nat. Prod. 1988;51:1080–1083. [Google Scholar]
- Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traves PG, Hortelano S, Zeini M, Chao TH, Lam T, Neuteboom ST, Theodorakis EA, Palladino MA, Castrillo A, Bosca L. Selective activation of liver X receptors by acanthoic acid-related diterpenes. Mol. Pharmacol. 2007;71:1545–1553. doi: 10.1124/mol.106.031906. [DOI] [PubMed] [Google Scholar]
- Través, et al. Chem. Biol. 2014;21:xxx–xxx. [Google Scholar]
- Weichhart T, Saemann MD. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Ann. Rheum. Dis. 2008;67(Suppl 3):iii70–74. doi: 10.1136/ard.2008.098459. [DOI] [PubMed] [Google Scholar]
- Zwenger S, Basu C. Plant terpenoids: applications and future potentials. Biotechnol. Mol. Biol. Rev. 2008;3:1–7. [Google Scholar]