Abstract
Background
The use of lipid lowering agents is suboptimal among dual enrollees, particularly blacks.
Objectives
To determine whether the removal of restrictive drug caps under Medicare Part D reduced racial differences among dual enrollees with diabetes.
Research Design
An interrupted time series with comparison series design (ITS) cohort study.
Subjects
8,895 black and white diabetes ≥18 year old patients drawn from a nationally representative sample of fee-for-service dual enrollees (January 2004–December 2007) in states with and without drug caps before Part D.
Measures
We examined the monthly (1) proportion of patients with any use of lipid lowering therapies and (2) intensity of use. Stratification measures included age (<65, ≥65), race (white vs. black) and gender.
Results
At baseline, lipid lowering drug use was higher in no drug cap states (drug cap: 54.0% vs. non-drug cap: 66.8%) and among whites versus blacks (drug cap: 58.5% vs. 44.9%, no drug cap: 68.4% vs. 61.9%). In strict drug cap states only, Part D was associated with an increase in the proportion with any use [nonelderly: +0.07 absolute percentage points (95% CI: 0.06, 0.09), p<0.001; elderly: +0.08 (0.06,0.10), p<0.001] regardless of race. However, we found no evidence of a change in the white-black gap in the proportion of users despite the removal of a significant financial barrier.
Conclusions
Medicare Part D was associated with increased use of lipid lowering drugs, but racial gaps persisted. Understanding non-coverage-related barriers is critical to maximizing the potential benefits of coverage expansions for disparities reduction.
Introduction
Medicare beneficiaries who are simultaneously enrolled in Medicaid, also known as dual enrollees, represent less than 14% of the Medicaid population, but their care accounts for 50% of total health care expenditures.1,2 More than one-third of dual enrollees have diabetes and, compared to other Medicare beneficiaries with diabetes, dual enrollees are more than twice as likely to be hospitalized for diabetes complications.1,3 Cardiovascular disease is the leading cause of morbidity and mortality in diabetes, making management of cardiovascular risk factors such as hyperlipidemia a critical component of diabetes management.4,5 Clinical guidelines recommend the use of lipid lowering agents for diabetes patients due to the potential for these agents to reduce adverse cardiovascular events.6 However, the use of these agents among dual enrollees with diabetes is suboptimal.7 Blacks, who are overrepresented among dual enrollees, have lower rates of use of lipid lowering drugs than whites despite being at increased risk for death from heart disease.1,7,8–11 Out of pocket costs for lipid lowering therapies have been identified as a significant barrier to guideline consistent treatment and as a potential determinant of disparities in use.12
Historically, coverage for lipid lowering and other prescription drugs was provided to dual enrollees through state administered Medicaid programs. The generosity of drug coverage through these programs has been highly variable, with several states (Texas, Oklahoma, Mississippi, Arkansas) imposing restrictive caps on the number of reimbursable prescriptions per month.13–17 Drug caps have been associated with lower medication use and increase adverse events in vulnerable populations.18–21 The Medicare Modernization Act of 2003 required all dual enrollees to transition from Medicaid drug coverage to Medicare Part D, which disallowed the use of strict drug caps.22,23 Blacks are overrepresented among dual enrollees transitioning from Medicaid to Medicare Part D and are less likely to report having access to needed prescription medications under Part D.24 Yet the impact of this major change in coverage for dual enrollees with diabetes on access to cardioprotective lipid lowering therapies and disparities in the use of these agents is unknown.
Using the implementation of Medicare Part D as a natural experiment, we compared changes in the use of lipid lowering therapies among black and white dual enrollees living in states with and without restrictive drug caps. We hypothesized that the introduction of Part D would be associated with an increase in the use of lipid lowering therapies. In addition, based on findings from an earlier pre post analysis of changes in overall medication use the Medicare population, we anticipated greater response among black dual enrollees,25 resulting in a reduction in racial disparities in treatment in drug cap states. While this paper focuses on the impact of this transition on January 1, 2006, such transitions are ongoing since disabled Medicaid beneficiaries who become eligible for dual Medicare enrollment after a required two-year waiting period and Medicaid beneficiaries who age into Medicare are automatically switched from Medicaid to Part D drug coverage.22,23 Therefore, findings from this analysis have the potential to inform strategies to facilitate the smooth transition of dual enrollees from Medicaid to Part D going forward. Moreover, the identification of potential differences in response to coverage changes by race have relevance to current health reform efforts to reduce disparities in health care through the expansion of health insurance coverage.
Methods
Study Design
We used an interrupted time series with comparison series (ITS) cohort design to examine changes in use of lipid lowering drugs 24 months before and after the transition of dual enrollees from Medicaid to Medicare Part D drug coverage. As Part D affected all dual enrollees, there is no natural control group. Therefore, we exploited natural variation in the existence of drugs caps in state Medicaid programs to identify comparison groups of states based on exposure to drug caps before the introduction of Part D and heterogeneity of policy response among racial subgroups within strict drug cap and no drug cap states, respectively. We used longitudinal baseline data to allow each subgroup to act as its own control, comparing utilization after Part D to what we would have predicted in the absence of Part D, drawing on stable baseline trends. Assuming that state residency, while not random, is unrelated to the use of lipid lowering therapy among diabetes patients, this natural experimental design should provide strong evidence of Part D effects.
Our study protocol was reviewed and approved by the Institutional Review Board of the Kaiser Foundation Research Institute.
Data Sources and Study Population
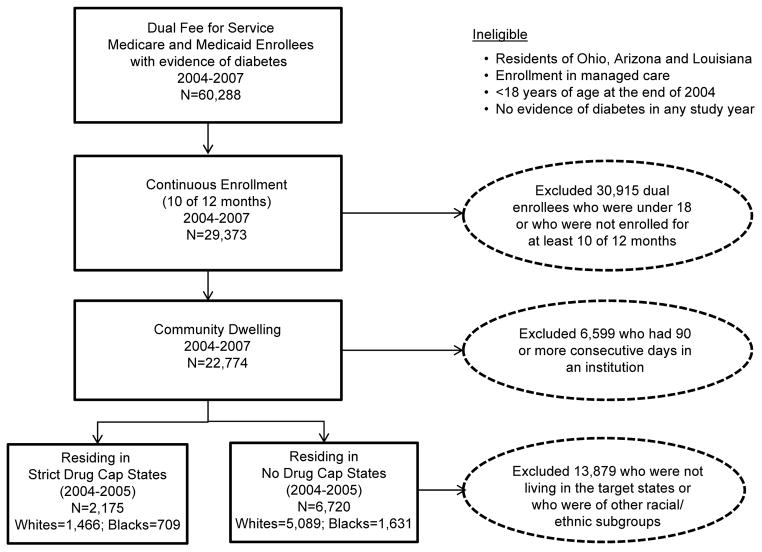
Using a merged dataset of a 5% nationally representative sample of linked Medicaid, Medicare, and Part D drug event standard analytic files for dual enrollees for the years 2004 through 2007 obtained from the Centers for Medicare and Medicaid Services, we identified fee-for-service beneficiaries who were at least 18 years old in 2004 and who had at least one hospital diagnosis or two physician diagnoses (no more than 12 months apart) of diabetes (ICD9: 250.XX) at any time during the study period (2004–2007). (Figure 1) We excluded enrollees residing in Ohio, Arizona, and Louisiana due to data anomalies in those states such as concurrent changes in coding and reporting methods.26 The resulting study population included 60,288 adults with diabetes dually enrolled in fee-for-service (i.e., excluding managed care) Medicaid and Medicare.
Figure 1.
Cohort Selection Criteria
We further required continuous enrollment for at least 10 months per year in both Medicaid and Medicare during the study period (n=29,373) and no more than 90 consecutive days in any year in an institution such as a nursing home (n=22,774; representing more than 455,000 dual enrollees nationwide).
State Restrictions on Prescription Drug Reimbursement before Part D
We assigned strict drug cap status to four states (Texas, Oklahoma, Mississippi, Arkansas) that consistently limited monthly drug coverage to five or fewer total prescriptions or to three or fewer brand name drugs during the 24-month baseline period 2004–2005 and had no evidence of overrides or exceptions based on published summaries of drug benefits.14–18,27,28 To reduce possible misclassification, we excluded from analysis 11 states (AL, CA, GA, IL, KS, KY, ME, NC, NY, PA, SC) where true exposure to drug caps was difficult to ascertain due to higher cap thresholds and generous waiver policies. In addition, we excluded Tennessee, which instituted a strict drug cap late in the baseline period.15
We identified a comparison subgroup of dual enrollees who lived in 31 states (AK, CO, CT, DE, FL, HI, IA, ID, IN, MA, MD, MI, MN, MO, MT, ND, NE, NH, NJ, NM, NV, OR, RI, SD, UT, VA, VT, WA, WI, WV, WY) and the District of Columbia where there was no evidence of a prescription drug cap during the 24-month baseline period. Our final cohort for analysis included 8,895 black and white diabetes patients living in either strict drug cap or no drug cap states.
Outcomes
Our primary outcomes were (1) the monthly proportion of patients with any use of lipid lowering therapies (statins, niacin, bile-acid resins, fibric acid, derivatives, cholesterol absorption inhibitors) and (2) the intensity of use of these medications as represented by standardized monthly doses, a validated measure used in previous policy evaluation studies.19,21,22 We spread the days’ supply of each lipid lowering medication over the days following each dispensing; overlapping dispensing of the same medication was concatenated. We then created monthly indicators of any use for each patient based on the availability of a given medication on one or more days in a month, and we used these patient-specific measures to calculate monthly prevalence of use in each patient subgroup.
To assess intensity of use, we first defined a standardized monthly dose (SMD)19,22 for each generic entity of interest, which was equal to the median number of milligrams dispensed per month across person-months with any use during the entire study period. Thus, SMDs represent the population’s “typical” monthly dose for a given medication. We then calculated average SMDs per patient per month (for specific drugs and by class) in order to evaluate changes in population intensity of use of lipid lowering agents over time, controlling for changes in prescription size. A key advantage of the SMD measure is that it allows for comparisons in the intensity of use across unique drug entities.
Policy Variables
We controlled for prior trends in the outcomes of interest using segmented time series regression, as described in previous studies.19–22,29 We included a dichotomous indicator for months before and after Part D implementation, as well as a variable to estimate changes in after-Part D trends. We identified state drug cap status in 2005 (strict drug cap vs. no drug cap) using a dichotomous indicator. Variation in Part D effect by drug cap status was evaluated by stratifying on the cap indicator and exploring interactions between it and the two Part D variables described above.
Covariates
We used Medicare administrative files to determine racial identity, age, and gender. Given higher rates of long-term disability among younger dual enrollees, we stratified all analyses by age: aged 65 or older (elderly) versus 18–65 years of age (non-elderly). Race and ethnicity data in these files are generated from Social Security Administration records based on social security card applications. Before 1980, the only options for race categorization were “white,” “black,” and “other,” resulting in low and inaccurate capture of data on Hispanics and other racial and ethnic groups.30 While geocode- and surname-enhanced race and ethnicity data are now available to link to the 5% sample, these enhancements were not available throughout our study period.31 Therefore, we included only patients with race designated as white or black, which are highly sensitive and specific.30
To compare underlying health status across subgroups at baseline, we created patient-specific comorbidity scores using the hierarchical chronic conditions method employed by the Centers for Medicare and Medicaid Services for risk adjustment and service claims from 2005.32 We also calculated the proportions of patients in each subgroup that year who had at least one hospital or two physician claims with a diagnosis of hypertension, and hospital claims related to diabetes, stroke, and ischemic heart disease.
Statistical Analysis
We used interrupted time series with comparison series to evaluate changes in the level and slope of the drug utilization outcomes, controlling for baseline trends.33 We first estimated the overall impact of transitioning to Part D by cap status using separate ITS models for the nonelderly and elderly subgroups in both strict drug cap states and in the comparison group of no drug cap states, separately. We did not control for prior cardiovascular risk factors because lipid lowering therapy is recommended for all diabetes patients over 40 years old.6
Our data indicated a period of instability during the month before and three months following implementation of Part D, which may reflect anticipatory policy effects, data anomalies or some combination of factors that have the potential to bias study results. Therefore, we excluded observations generated during this brief, four-month transition period (i.e., December 2005–March 2006) from the models. The units of analysis were state-months, with 4 strict drug cap status states (“treatment”) and 32 no drug cap states(“comparison group”) and monthly data from 23 pre-2006 months and 21 post 2006 months, leading to 44 observations (23 +21) in each group where the model is fitted. Our time series models controlled for autocorrelation by testing for first-order autoregressive processes and correcting for significant correlations. We also tested for non-linearity of the models.33
We directly evaluated the impact of Part D on racial gaps in the use of lipid lowering drugs by modeling month-to-month white-black differences in use.34 We stratified these ITS models by age, but not for cardiovascular risk as in the main models. Given higher levels of cardiovascular related disease among blacks at baseline, our models should represent conservative estimates of disparities. We then show the estimated white-black difference in the outcomes of interest at one year post intervention (April 2007) relative to what we would have expected based on prior trends. For models indicating evidence of an interaction between race and the policy effect variables, we further conducted race and age stratified, as well as race, age and gender stratified models to explore the nature of the interaction.
To account for multiple testing, we applied a Bonferroni correction to reduce the likelihood of false positives.35 For analyses of Part D impact overall and analyses of changes in the white-black gap, we conducted eight independent tests and p values of 0.006 or smaller were considered statistically significant. For race, age and gender stratified models, we used a cut off of 0.004 to evaluate statistical significance to account for 12 independent tests. All statistical analyses were conducted using the SAS system (SAS, v.9.0, Durham, NC).36
Results
Enrollee Characteristics
We identified 8895 black and white dual enrollees with diabetes living in strict drug cap (2175) and no drug cap states (6720) who met our study criteria (Table 1). Compared to dual enrollees living in no drug cap states prior to Part D, dual enrollees in strict drug cap states were more likely to be black, non-elderly, and female. The proportions of dual enrollees using diabetes-related medications were similar across the two groups with the exception of use of insulin (drug cap: 28.4% vs. no drug cap: 32.1%) and lipid lowering agents (drug cap: 54.0% vs. non-drug cap: 66.8%), both of which were more commonly used by dual enrollees in states without drug caps. In addition, dual enrollees in strict drug cap states were more likely to have comorbid hypertension (66.9% vs. 57.5%).
Table 1.
Baseline Characteristics of Dual Medicare and Medicare Enrollees in 2005 by State Drug Cap Status and Race (Black and White)a
| Strict Drug Caps | No Drug Caps | Strict Drug Caps | No Drug Caps | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=2175 | %/sd | n=6720 | % | Whites n=1466 |
% | Blacks n=709 |
% | Whites n=5089 |
% | Blacks n=1631 |
% | |
| Race | ||||||||||||
| White | 1466 | 67.4 | 5089 | 75.7 | ||||||||
| Black | 709 | 32.6 | 1631 | 24.3 | ||||||||
| Age group (%) | ||||||||||||
| 65 and older | 1459 | 67.1 | 3528 | 52.5 | 1004 | 68.5 | 455 | 64.2 | 2575 | 50.6 | 953 | 58.4 |
| less than 65 | 716 | 32.9 | 3192 | 47.5 | 462 | 31.5 | 254 | 35.8 | 2514 | 49.4 | 678 | 41.6 |
| Gender (%) | ||||||||||||
| Female | 1643 | 75.5 | 4625 | 68.8 | 1050 | 71.6 | 593 | 83.6 | 3393 | 66.7 | 1232 | 75.5 |
| Male | 532 | 24.5 | 2095 | 31.2 | 416 | 28.4 | 116 | 16.4 | 1696 | 33.3 | 399 | 24.5 |
| Any Medication Use (%) | ||||||||||||
| Oral Hypoglycemics | 1581 | 72.7 | 4822 | 71.8 | 1065 | 72.6 | 516 | 72.8 | 3656 | 71.8 | 1166 | 71.5 |
| Insulin | 617 | 28.4 | 2157 | 32.1 | 392 | 26.7 | 225 | 31.7 | 1556 | 30.6 | 601 | 36.8 |
| Oral Hypoglycemics or Insulin | 1847 | 84.9 | 5680 | 84.5 | 1225 | 83.6 | 622 | 87.7 | 4264 | 83.8 | 1416 | 86.8 |
| Antihypertensives | 1829 | 84.1 | 5644 | 84.0 | 1202 | 82.0 | 627 | 88.4 | 4162 | 81.8 | 1482 | 90.9 |
| Lipid Lowering Agents | 1175 | 54.0 | 4488 | 66.8 | 857 | 58.5 | 318 | 44.9 | 3479 | 68.4 | 1009 | 61.9 |
| Mean Comorbidity Score (sd)b | 1.51 | 1.01 | 1.52 | 1.07 | 1.52 | 1.02 | 1.47 | 0.99 | 1.49 | 1.03 | 1.61 | 1.17 |
| Hypertension Diagnosis (%)c | 1456 | 66.9 | 3866 | 57.5 | 932 | 63.6 | 524 | 73.9 | 2651 | 52.1 | 1215 | 74.5 |
| Any Hospitalization in 2005 (%) | 524 | 24.1 | 1526 | 22.7 | 350 | 23.9 | 174 | 24.5 | 1137 | 22.3 | 389 | 23.9 |
| Diabetesd | 482 | 22.2 | 1417 | 21.1 | 320 | 21.8 | 162 | 22.8 | 1057 | 20.8 | 360 | 22.1 |
| Ischemic Heart Diseasee | 103 | 4.7 | 362 | 5.4 | 70 | 4.8 | 33 | 4.7 | 256 | 5.0 | 106 | 6.5 |
| Strokef | 49 | 2.3 | 162 | 2.4 | 37 | 2.5 | 12 | 1.7 | 111 | 2.2 | 51 | 3.1 |
BOLD=Statistically significant at the 0.05 level
State Medicaid program limited monthly drug coverage to five or fewer total prescriptions or to three or fewer brand name drugs during baseline (2004–2005). The 32 no-cap states were: AK, CO, CT, DC, DE, FL, HI, IA, ID, IN, MA, MD, MI, MN, MO, MT, ND, NE, NH, NJ, NM, NV, OR, RI, SD, UT, VA, VT, WA, WI, WV, WY. The 4 strict cap states were: AR, MS, OK, TX. The following 11 states had less restrictive caps: AL, CA, GA, IL, KS, KY, ME, NC, NY, PA, SC. We excluded three states (AZ, LA, OH) due to data anomalies and one state (TN) that introduced a drug cap during the baseline period.
Based on the Centers for Medicare and Medicaid Services Hierarchical Condition Categories model.
At least one hospital or two physician claims with ICD 9= 401. 401.0, 401.1, 401.9, 402, 403, 403.1, 404, 405, 405.0, 405.01, 405.1, 405.11)
At least one hospital claim for diabetes (ICD-9:250.xx)
At least one hospital claim for ischemic heart disease (ICD-9=414.0, 414.1, 414.10, 414.11, 414.12, 414.8, 414.9);
At least one hospital claim for stroke (ICD-9=431.xx, 431.xx-434.xx, 436.01, 436.9)
Within strict drug cap states (Table 1), blacks were more likely than whites to be nonelderly, female, using insulin, any diabetes medication, have hypertension, and to be on antihypertensives. In no drug cap states, blacks were more likely to be elderly, female, have higher levels of comorbidity, including hypertension, to use diabetes and antihypertensive drugs and to have higher rates of hospitalization for ischemic heart disease and stroke. However, use of lipid lowering therapy was higher among whites compared to blacks in both strict drug cap (whites: 59.5%; blacks: 44.9%) and no drug cap states (whites: 68.4%; blacks: 61.9%).
Changes in Use of Lipid-Lowering Medications in Strict Drug Cap versus No Drug Cap States
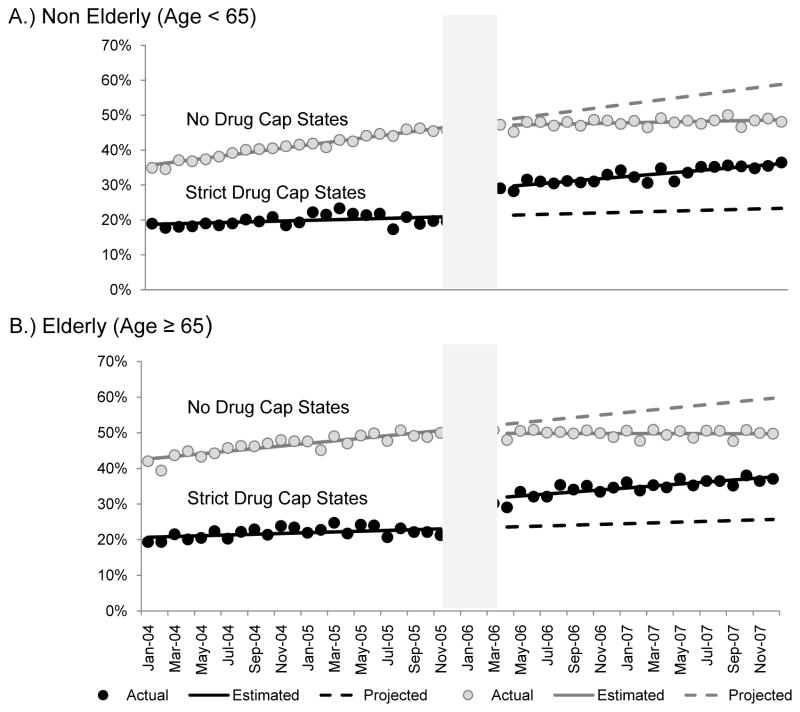
Figure 2 shows time series of changes in the proportion of dual enrollees using lipid lowering therapies in strict and no drug cap states before and after Part D implementation. Among both nonelderly and elderly dual enrollees with diabetes living in strict drug cap states, the transition from Medicaid to Medicare Part D was associated with significant increases in the proportion of patients using lipid lowering medications [nonelderly: 0.07 absolute percentage points (95% CI: 0.05, 0.09), p<0.001; elderly: 0.08 (0.05,0.10), p<0.001]. (Table 2) There was also an increasing trend in the proportion of patients with any use overtime in both age groups. In contrast, there were no statistically significant level shifts in use at the time of Part D implementation among those living in no drug cap states [nonelderly: −0.001 (−0.01, 0.01), p=0.749; elderly: −0.01 (−0.03, 0.01), p=0.205].
Figure 2. Time Series of the Proportion with Any Use of Lipid Lowering Medications, 2004–2007.
A.) Non-eldery (Age < 65)
Strict drug cap states
Actual proportion of users, solid black circles
Estimated proportion of users per month, solid black line
Projected proportion of users per month, dotted black line
B.) Elderly (Age ≥ 65)
No drug cap states
Actual proportion of users, solid grey circles
Estimated proportion of users per month, solid grey line
Projected proportion of users per month, dotted grey line
Table 2.
Estimated Impact of Part D on Use of Lipid Lowering Medications among Dual Enrollees by State Drug Cap Statusa
| Strict Drug Cap States
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Intercept | Baseline Trend | Part D | Trend Change Post Part D | |||||
|
|
|
|
|
|||||
| Estimate (95% CI) | p-valueb | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
|
|
|
|
|
|||||
| Non-Elderly | ||||||||
| Any | 0.19 (0.17, 0.20) | <0.001 | 0.001 (0.0001, 0.002) | 0.029 | 0.07 (0.05, 0.09) | <0.001 | 0.002 (0.001 (0.003) | 0.002 |
| SMD c | 0.32 (0.28, 0.35) | <0.001 | 0.002 (−0.001, 0.004) | 0.202 | 0.09 (0.03, 0.14) | 0.002 | 0.002 (−0.001, 0.01) | 0.192 |
| Elderly | ||||||||
| Any | 0.20 (0.19, 0.22) | <0.001 | 0.001 (0.00004, 0.002) | 0.042 | 0.08 (0.05, 0.10) | <0.001 | 0.002 (0.0001, 0.003) | 0.034 |
| SMD | 0.32 (0.29, 0.35) | <0.001 | 0.002 (0.0001, 0.004) | 0.036 | 0.08 (0.03, 0.12) | 0.002 | −0.0004 (−0.004, 0.003) | 0.819 |
|
| ||||||||
| No Drug Cap States | ||||||||
|
| ||||||||
| Non-Elderly | ||||||||
| Any | 0.35 (0.35, 0.36) | <0.001 | 0.005 (0.004, 0.005) | <0.001 | −0.001 (−0.01, 0.01) | 0.749 | −0.004 (−0.005, −0.003) | <0.001 |
| SMD | 0.44 (0.43, 0.46) | <0.001 | 0.01 (0.01, 0.01) | <0.001 | −0.01 (−0.03, 0.005) | 0.144 | −0.004 (−0.01, −0.003) | <0.001 |
| Elderly | ||||||||
| Any | 0.42 (0.41, 0.43) | <0.001 | 0.004 (0.003, 0.004) | <0.001 | −0.01 (−0.03, 0.01) | 0.205 | −0.004 (−0.005, −0.002) | <0.001 |
| SMD | 0.48 (0.47, 0.50) | <0.001 | 0.01 (0.01, 0.01) | <0.001 | 0.002 (−0.02, 0.03) | 0.842 | −0.01 (−0.01, −0.004) | <0.001 |
State Medicaid program limited monthly drug coverage to five or fewer total prescriptions or to three or fewer brand name drugs during the 24-month baseline period, 2004–2005. The 32 no-cap states were: AK, CO, CT, DC, DE, FL, HI, IA, ID, IN, MA, MD, MI, MN, MO, MT, ND, NE, NH, NJ, NM, NV, OR, RI, SD, UT, VA, VT, WA, WI, WV, WY. The 4 strict cap states were: AR, MS, OK, TX. The following 11 states had less restrictive caps: AL, CA, GA, IL, KS, KY, ME, NC, NY, PA, SC. Three states were excluded from the study due to data anomalies: AZ, LA, OH. We also excluded one state due to the introduction of a drug cap during the baseline period: TN.
p value < 0.006 was considered significant
Standardized monthly dose = median number of milligrams dispensed per month across person-months with any drug use
Similarly, the introduction of Part D was associated with increased intensity of use of lipid lowering medications among both non-elderly and elderly dual enrollees in strict drug cap states [nonelderly: 0.09 SMDs (0.03, 0.14), p=0.002; elderly: 0.08 (0.03, 0.12), p=0.002] but not in no drug cap states [nonelderly: −0.01 (−0.03, 0.005); p=0.144; elderly: 0.002 (−0.02, 0.03), p=0.842]. In addition, no drug cap states had a statistically significant (p<0.001) decreasing trend in both the proportion of users [nonelderly: −0.004 (−0.005, −0.003); elderly: −0.004 (−0.005, −0.002)] and in the intensity of use [nonelderly: −0.004 (−0.01, −0.003); elderly: −0.01(−0.01 −0.004)] of lipid lowering agents.
Racial Differences in Changes in Use of Lipid-Lowering Medications after Part D
The results of models estimating the white-black gap in use of lipid lowering medications, as well as estimated white-black differences in use at 12 months post-policy implementation (April 2007) relative to what would have been expected given prior trends are presented in Table 3. ITS models of monthly white-black differences did not produce statistically significant evidence of a change in the racial gap in the proportion of users, regardless of state drug cap status. We did, however, observe a slight, statistically significant declining trend in the white-black gap in the intensity of use post Part D among dual enrollees living in no drug cap states [nonelderly white-black gap: −0.004 (−0.01, −0.002), p<0.001; elderly white-black gap: −0.005 (−0.007, −0.002), p<0.001)].
Table 3.
Estimated Impact of Part D on White-Black Differences in Lipid Lowering Medication Use among Dual Enrollees by State Cap Status & Agea
| Strict Drug Cap States
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Baseline Trend | Part D | Trend Change Post Part D | White-Black Gap: 1 Year Post | ||||||
|
|
|
|
|
|
||||||
| Estimate (95% CI) | p-value b | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimatedc | Projectedd | |
|
|
|
|
|
|
||||||
| Non-Elderly | ||||||||||
| Any | 0.05 (0.04, 0.07) | <0.001 | 0.0002 (−0.001, 0.001) | 0.686 | 0.02 (−0.01, 0.04) | 0.211 | 0.001 (−0.001, 0.003) | 0.391 | 0.09e | 0.06 |
| SMDf | 0.15 (0.10, 0.20) | <0.001 | 0.003 (−0.0004, 0.01) | 0.08 | −0.06 (−0.14, 0.02) | 0.158 | 0.001 (−0.005, 0.007) | 0.695 | 0.24g | 0.28 |
| Elderly | ||||||||||
| Any | 0.01 (−0.01, 0.02) | 0.415 | 0.001 (0.00005, 0.002) | 0.042 | −0.002 (−0.03, 0.02) | 0.855 | −0.0001 (−0.002, 0.002) | 0.886 | 0.05 | 0.06 |
| SMD | 0.10 (0.07, 0.14) | <0.001 | 0.003 (−0.0001, 0.005) | 0.055 | −0.01 (−0.07, 0.05) | 0.813 | −0.003 (−0.01, 0.001) | 0.200 | 0.16 | 0.20 |
| No Drug Cap States
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Baseline Trend | Part D | Trend Change Post Part D | White-Black Gap: 1 Year Post | ||||||
|
|
|
|
|
|
||||||
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimatedc | Projectedd | |
|
|
|
|
|
|
||||||
| Non-Elderly | ||||||||||
| Any | 0.12 (0.11, 0.13) | <0.001 | 0.001 (0.0004, 0.001) | 0.001 | −0.01 (−0.02, 0.001) | 0.067 | −0.0002 (−0.001, 0.001) | 0.604 | 0.14 | 0.16 |
| SMD | 0.22 (0.20, 0.23) | <0.001 | 0.004 (0.002, 0.005) | <0.001 | 0.03 (0.003, 0.05) | 0.032 | −0.004 (−0.01, −0.002) | <0.001 | 0.32 | 0.36 |
| Elderly | ||||||||||
| Any | 0.06 (0.05, 0.07) | <0.001 | 0.001 (−0.0002, 0.001) | 0.153 | 0.01 (−0.01, 0.03) | 0.397 | 0.00002 (−0.001, 0.001) | 0.979 | 0.09 | 0.08 |
| SMD | 0.12 (0.10, 0.15) | <0.001 | 0.003 (0.001, 0.004) | 0.001 | 0.03 (−0.005, 0.07) | 0.085 | −0.005 (−0.007, −0.002) | <0.001 | 0.20 | 0.24 |
State Medicaid program limited monthly drug coverage to five or fewer total prescriptions or to three or fewer brand name drugs during the 24-month baseline period, 2004–2005. The 32 no-cap states were: AK, CO, CT, DC, DE, FL, HI, IA, ID, IN, MA, MD, MI, MN, MO, MT, ND, NE, NH, NJ, NM, NV, OR, RI, SD, UT, VA, VT, WA, WI, WV, WY. The 4 strict cap states were: AR, MS, OK, TX. The following 11 states had less restrictive caps: AL, CA, GA, IL, KS, KY, ME, NC, NY, PA, SC. Three states were excluded from the study due to data anomalies: AZ, LA, OH. We also excluded one state due to the introduction of a drug cap during the baseline period: TN.
Estimated outcome at 12 months post policy implementation (April 2007) based on time series model
Projected outcome at 12 months post policy implementation (April 2007) based on time series model
p value < 0.006 was considered significant
difference in the absolute percentage point estimate of the proportion of users per month at 12 months post policy implementation (April 2007)
Standardized monthly dose
difference in the standardized monthly dose per month in April 2007
Age-race stratified models of SMD for patient in no cap states (see Table, Supplemental Digital Content 1) suggest that the primary driver of this change was a declining trend among whites [nonelderly: −0.005 (−0.01, −0.004); elderly: −0.01 (−0.01, −0.005)]. We further observed a small, statistically significant decrease in the level of intensity of use among non-elderly blacks [−0.04 (−0.06, −0.02); p=0.001] in no drug cap states, that was not observed among whites. Further stratification by gender revealed that while the declining trend among whites was not gender specific, among blacks, only non-elderly men exhibited a declining trend in the intensity of use post-policy [−0.005 (−0.01, −0.003); p<0.001)]. In addition, the observed decrease in the level of intensity of use at the time of the policy among non-elderly blacks was primarily driven by changes among non-elderly black women [−0.04 (−0.07, −0.02);p=0.003].
Conclusions
Among fee-for-service dual enrollees with diabetes living in strict drug cap states with partial drug coverage, we found that the implementation of Part D was associated with abrupt increases in utilization of potentially life-saving lipid lowering medications compared to baseline trends that were not present in states without caps. In addition, we observed a sudden flattening of the upward baseline trend in the use of lipid lowering therapy among dual enrollees in no cap states. These findings differ from previously published studies,37,38 which show no effect or minimal effects of Part D among dual enrollees. However, these prior studies did not adjust for state-level variation in the generosity of Medicaid coverage prior to Part D, which may have masked pockets of pent-up demand39 within the larger population of patients. This pent-up demand effect may be stronger in our study due to the focus on lipid lowering drugs, medications for which there is strong evidence of cost-related underuse and that are considered clinically essential medications for most adult patients with diabetes.11 Slight declining trends in the use of lipid lowering agents among dual enrollees in no cap states may be related to other changes in coverage related to Part D that were not the focus of this particular study. Specifically, those transitioning to private Part D plans from Medicaid may have faced other, somewhat less restrictive, forms of utilization management not addressed in this study (e.g., prior authorization, copayments).23
To our knowledge, this is one of the first studies to evaluate the impact of transitioning from Medicaid to Medicare Part D on disparities in use of clinically essential services. The removal of drug caps under Medicare Part D was not associated with an immediate narrowing of racial differences in the use of lipid lowering therapy in strict cap states as we had hypothesized. Instead, we observed a declining trend in differences in the intensity of use among those in no drug cap states, particularly among whites regardless of gender or age. The decrease in the level of intensity of use among non-elderly black women in no drug cap states was also unexpected. However, these changes were relatively small in magnitude, potentially limiting their clinical significance.
Our findings in drug cap states suggest that disparities in essential medication use in this population may be due to factors other than coverage, such as racial differences in beliefs about the benefits and potential harms of therapy, differences in quality of care or prescribing practices across settings, overall medication burden due to higher rates of comorbid hypertension or differences in trust and patient engagement, including use self-management support services.40 However, evidence of racial difference in response to the policy in no drug cap states were surprising and may be related to other aspects of Part D such as changes in exposure to prior authorization and other administrative cost containment strategies or unobserved co-occurring policies in these states.
While this study focuses on patient utilization of lipid lowering therapy, it is important to note that differential response to the policy by race may also reflect variation in physician response to policy change. In their role as prescribers, physicians can change prescribing practices in an effort to take advantage of expansions in coverage or to avoid administrative barriers to medication use. Yet, prior evidence suggests that physicians may be less likely to prescribe statins for black relative to white patients.41 By extension, variation in physician advocacy based on patient characteristics such as race or characteristics of the settings where minority patients receive care may have contributed to the lower rates of beneficial policy effects in vulnerable population subgroups. More studies using data on prescribing physicians, settings of care, and patient reported outcomes are needed to fully understand this and other potential drivers of heterogeneity in policy effects by race.
Several limitations deserve discussion. First, because all dual enrollees transitioned to Part D in January 2006, no true control group exists, which precludes a direct comparison of the effect between state drug cap subgroups, which varied considerably in underlying population characteristics at baseline. In addition, our decision to include only continuously-enrolled patients in order to increase the stability of the underlying population also resulted in reduced generalizability of our findings to dual enrollees who move in and out of coverage.
A disadvantage of the ITS modeling strategy is that we could not control for confounders that occurred simultaneously with the policy, which are the only potential confounders of ITS designs.33 We assumed that sudden changes in population level characteristics such as severity of illness were highly unlikely. However, we could not control for unobservable local policy changes at the time of Part D implementation (e.g., state level efforts to educate Medicaid patients about the policy).
Our analysis of disparities is limited by the inaccuracy of racial and ethnic identifiers. Specifically, due to limited validity and reliability of race and ethnicity data during the study period,31,32 we excluded subgroups other than blacks and whites and did not further subset these groups by Hispanic ethnicity. Therefore, our analyses may mask subgroup differences based on ethnicity and do not necessarily extend to other racial groups that were excluded from this study.
Using a natural experiment, we found strong evidence that eliminating drug caps was associated with increasing the level and intensity of use of lipid lowering agents among vulnerable dual enrollees with diabetes. However, racial disparities in the use of these agents persisted post Part D despite the removal of a significant coverage related barrier to treatment. Targeted interventions to address non-coverage-related barriers to the use of lipid lowering therapy among blacks may be a necessary adjunct to ongoing policy level efforts to reduce access related treatment disparities in this highly vulnerable population. Additionally, while the selected study design provided strong evidence of differential effects by race, age and gender, alternative mixed methods designs may be needed to explore the contextual factors that drive variation in policy response.
Supplementary Material
Acknowledgments
Funding Disclosure: This study was supported by grants from the National Institute on Aging [R01AG032249] and the Agency for Health Care Research and Quality [R01 HS018577]. Drs. Adams, Ross-Degnan, and Soumerai also received support from the Health Delivery Systems Center for Diabetes Translational Research funded by the National Institute for Diabetes, Digestive and Kidney Diseases [P30DK092924]. Dr. Adams is also a co-investigator in the Learnings in Diabetes Prevention from an Integrated Delivery System [U58 DP002721] and Medication Adherence and Social Disparities in Diabetes [R01 DK080726-01] studies. Dr. Trinacty is supported by a career development award from the Agency for Health Care Research and Quality [K01 HS018072].
Contributor Information
Alyce S. Adams, Email: Alyce.S.Adams@kp.org.
Jeanne M. Madden, Email: jeanne_madden@harvardpilgrim.org.
Fang Zhang, Email: Fang_Zhang@harvardpilgrim.org.
Stephen B. Soumerai, Email: ssoumerai@hms.harvard.edu.
Dan Gilden, Email: dmg@jen.com.
Jennifer Griggs, Email: jengrigg@umich.edu.
Connie Mah Trinacty, Email: connie.mah.trinacty@kp.org.
Christine Bishop, Email: bishop@brandeis.edu.
Dennis Ross-Degnan, Email: Dennis_Ross-Degnan@hms.harvard.edu.
REFERENCE LIST
- 1.Medicare Payment Advisory Commission. [Accessed August 15, 2013.];Chapter 3: Dual eligible beneficiaries: An overview. 2004 Available at: http://www.medpac.gov/publications/congressional_reports/June04_Ch3.pdf.
- 2.The Henry J. Kaiser Family Foundation. [Accessed September 15, 2009.];Dual Eligibles: Medicaid’s Role for Low-Income Medicare Beneficiaries. 2006 Available at: www.kff.org/medicaid/upload/4091_06.pdf.
- 3.Jiang HJ, Wier LM, Potter DEB, Burgess J. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet] Rockville (MD): Agency for Health Care Policy and Research (US); 2006 Feb – 2010 Sep. Potentially Preventable Hospitalizations among Medicare-Medicaid Dual Eligibles, 2008: Statistical Brief #96. [PubMed] [Google Scholar]
- 4.Howard DL, Hakeem FB, Njue C, et al. Racially disproportionate admission rates for ambulatory care sensitive conditions in North Carolina. Public Health Rep. 2007 May-Jun;122:362–72. doi: 10.1177/003335490712200310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang HJ, Andrews R, Stryer D, et al. Racial/ethnic disparities in potentially preventable readmissions: the case of diabetes. Am J Public Health. 2005 Sep;95:1561–7. doi: 10.2105/AJPH.2004.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care for diabetes – 2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackwell S, Baugh D, Montgomery M, et al. Noncompliance in the Use of Cardiovascular Medications in the Medicare Part D Population. MMRR. 2011;1:E1–E27. doi: 10.5600/MMRR.001.04.A05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schore J, Brown R, Lavin B. Racial disparities in prescription drug use among dually eligible beneficiaries. Health Care Financ Rev. 2003;25:77–90. [PMC free article] [PubMed] [Google Scholar]
- 9.Mark TL, Axelsen KJ, Mucha L, et al. Racial differences in switching, augmentation, and titration of lipid-lowering agents by Medicare/Medicaid dual-eligible patients. Am J Manag Care. 2007:13S72–79. [PubMed] [Google Scholar]
- 10.Margolis KL, Dunn K, Simpson LM, et al. Coronary heart disease in moderately hypercholesterolemic, hypertensive black and non-black patients randomized to pravastatin versus usual care: The Antihypertensive and Lipid Lowering to Prevent Heart Attack Trial (ALLHAT-LLT) Am Heart J. 2009;158:948–55. doi: 10.1016/j.ahj.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochanek KD, Xu J, Murphy SL, et al. Deaths: Final Data for 2009. National Vital Statistics Reports. 2011;60(3) [PubMed] [Google Scholar]
- 12.Choudhry NK, Patrick AR, Antman EM, et al. Cost-Effectiveness of Providing Full Drug Coverage to Increase Medication Adherence in Post–Myocardial Infarction Medicare Beneficiaries. Circulation. 2008;117:1261–1268. doi: 10.1161/CIRCULATIONAHA.107.735605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cramer JA, Benedict A, Muszbek N, et al. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62:76–87. doi: 10.1111/j.1742-1241.2007.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowley J, Ashner D, Elam L. State outpatient prescription drug policies: findings from a national survey, 2005 update. Prepared by Health Policy Institute at Georgetown University and Kaiser Commission on Medicaid and the Uninsured for the Henry J. Kaiser Family Foundation. Washington, DC: The Henry J. Kaiser Family Foundation; 2005. [Accessed September 11, 2007.]. Available at http://kaiserfamilyfoundation.files.wordpress.com/2013/01/state-medicaid-outpatient-prescription-drug-policies-findings-from-a-national-survey-2005-update-report.pdf. [Google Scholar]
- 15.National pharmaceutical Council. [Accessed October 28, 2008.];Pharmaceutical Benefits Under State Medical Assistance Programs. 2007 Available at: www.npcnow.org/Public/Research___Publications/Publications/pub_rel_research/pub_medicaid/Pharmaceutical_Benefits_Under_State_Medical_Assistance_Programs_2007.aspx.
- 16.Martin BC, McMillan JA. The impact of implementing a more restrictive prescription limit on Medicaid recipients: Effect on cost, therapy and out-of-pocket expenditures. Med Care. 1996;24:686–701. doi: 10.1097/00005650-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Oklahoma Health Care Authority. [Accessed October 28, 2011.];Information for Providers. Available at: http://okhca.org/providers.aspx?id=1218.
- 18.Hearne J. CRS Report for Congress: Prescription Drug Coverage Under Medicaid, Updated February 6, 2008. Prepared by Congressional Research Service for Members and Committees of Congress; [Accessed May 1, 2013.]. Available at: http://aging.senate.gov/crs/medicaid16.pdf. [Google Scholar]
- 19.Soumerai SB, Avorn J, Ross-Degnan D, et al. Payment restrictions for prescription drugs under Medicaid: effects on therapy, cost, and equity. N Engl J Med. 1987;317:550–556. doi: 10.1056/NEJM198708273170906. [DOI] [PubMed] [Google Scholar]
- 20.Soumerai SB, Ross-Degnan D, Avorn J, et al. Effects of Medicaid drug-payment limits on admission to hospitals and nursing homes. N Engl J Med. 1991;325:1072–1077. doi: 10.1056/NEJM199110103251505. [DOI] [PubMed] [Google Scholar]
- 21.Soumerai SB, McLaughlin TJ, Ross-Degnan D, et al. Effects of a limit on Medicaid drug-reimbursement benefits on the use of psychotropic agents and acute mental health services by patients with schizophrenia. N Engl J Med. 1994:650–655. doi: 10.1056/NEJM199409083311006. [DOI] [PubMed] [Google Scholar]
- 22.The Henry J. Kaiser Family Foundation. [Accessed August 15, 2013.];Implications of the New Medicare Law for Dual Eligibles: 10 Key Questions and Answers. 2004 Available at: http://kaiserfamilyfoundation.files.wordpress.com/2013/01/implications-of-the-new-medicare-law-for-dual-eligibles-10-key-questions-and-answers.pdf.
- 23.Elliott RA, Majumdar SR, Gillick MR, et al. Benefits and consequences of the new Medicare drug benefit for the poor and the disabled. N Engl J Med. 2005;353:2739–2741. doi: 10.1056/NEJMp058242. [DOI] [PubMed] [Google Scholar]
- 24.Haviland AM, Elliott MN, Weech-Maldonado R, et al. Racial/Ethnic Disparities in Medicare Part D Experiences. Med Care. 2012;50:S40–S47. doi: 10.1097/MLR.0b013e3182610aa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briesacher BA, Zhao Y, Madden JM, et al. Medicare part D and changes in prescription drug use and cost burden: national estimates for the Medicare population, 2000 to 2007. Med Care. 2011 Sep;49:834–41. doi: 10.1097/MLR.0b013e3182162afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Medicare and Medicaid Services. [Accessed June 17, 2013.];MSIS State Data Characteristics/Anomalies Report. Availbable at http://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MedicaidDataSourcesGenInfo/downloads/anomalies1.pdf.
- 27.Illinois Department of Human Services. [Accessed October 28, 2011.];Limit on brand name prescription drugs. Available at www.dhs.state.il.us/page.aspx?item=19580.
- 28.Kentucky Cabinet for Health and Family Services. [Accessed October 28, 2011.];Department for Medicaid Services. Outpatient Pharmacy Programs. Available at: www.lrc.ky.gov/kar/907/001/019.htm.
- 29.Adams AS, Zhang F, LeCates RL, et al. Prior Authorization for Antidepressants in Medicaid: Effects among Disabled Dual Enrollees. Arch Intern Med. 2009;169:750–6. doi: 10.1001/archinternmed.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arday SL, Arday DR, Monroe S, et al. HCFA’s Racial and ethnic data: Current accuracy and recent improvements. Health Care Financ Rev. 2000;21:107–116. [PMC free article] [PubMed] [Google Scholar]
- 31.Agency for Healthcare Research and Quality, US Department of Health and Human Services. Creation of New Race-Ethnicity Codes and Socioeconomic Status (SES) Indicators for Medicare Beneficiaries, Final Report, January 2008. Prepared by: RTI International [CMS Contract Number: 500-00-0024, Task No. 21], AHRQ Publication Number 08-0029-EF.
- 32.Pope GC, Kautter J, Ellis RP, et al. Risk Adjustment for Medicare Capitation Payments Using the CMS-HCC Model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 34.Harper S, Lynch J, Meersman SC, et al. An overview of methods for monitoring social disparities in cancer with an example using trends in lunch cancer incidence by area-socioeconomic position and race-ethnicity, 1992–2004. Am J Epidemiol. 2008;167:889–899. doi: 10.1093/aje/kwn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teixeira-Pinto A, Siddique J, Gibbons R, et al. Statistical Approaches to Modeling Multiple Outcomes In Psychiatric Studies. Psychiatr Ann. 2009 Jul 1;39:729–735. doi: 10.3928/00485713-20090625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SAS Institute Inc. SAS OnlineDoc®, Version 9. SAS Institute Inc; Cary, NC: 2002–2006. [Google Scholar]
- 37.Basu A, Yin W, Alexander GC. Impact of Medicare Part D on Medicare-Medicaid dual-eligible beneficiaries’ prescription utilization and expenditures. Health Serv Res. 2010;45:133–51. doi: 10.1111/j.1475-6773.2009.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrank WH, Patrick AR, Pedan A, et al. The effect of transitioning to Medicare part d drug coverage in seniors dually eligible for Medicare and Medicaid. J Am Geriatr Soc. 2008;56:2304–10. doi: 10.1111/j.1532-5415.2008.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gamble VN. Under the shadow of Tuskegee: African Americans and health care. Am J Public Health. 1997;87:1773–1778. doi: 10.2105/ajph.87.11.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gellad WF, Huskamp HA, Phillips KA, et al. How the new Medicare drug benefit could affect vulnerable populations. Health Aff (Millwood) 2006;25:248–255. doi: 10.1377/hlthaff.25.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Link Charles R, Condliffe Simon, Townsend Bryan. Working Papers 08-07. University of Delaware, Department of Economics; 2008. Who receives statins? Variations in physicians’ prescribing patterns for patients with coronary heart disease, dyslipidemia, and diabetes. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.